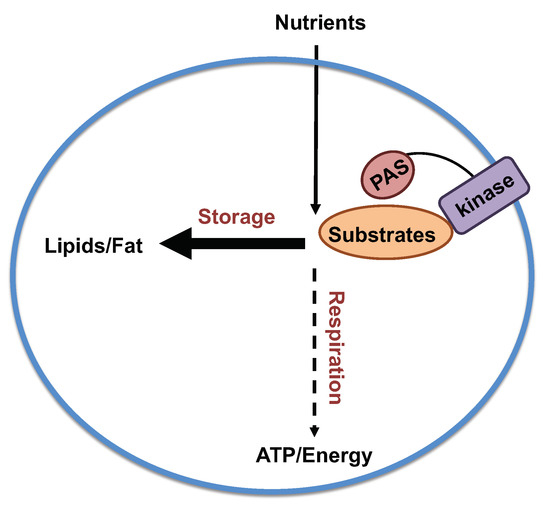
Per-Arnt-Sim Kinase (PASK) Deficiency Increases Cellular Respiration on a Standard Diet and Decreases Liver Triglyceride Accumulation on a Western High-Fat High-Sugar Diet
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. PAS Kinase-Deficient Mice Exhibit Increased Respiration When on A Normal Chow (NC) Diet
3.2. Decreased Complex I Protein is Observed on the HFHS Diet
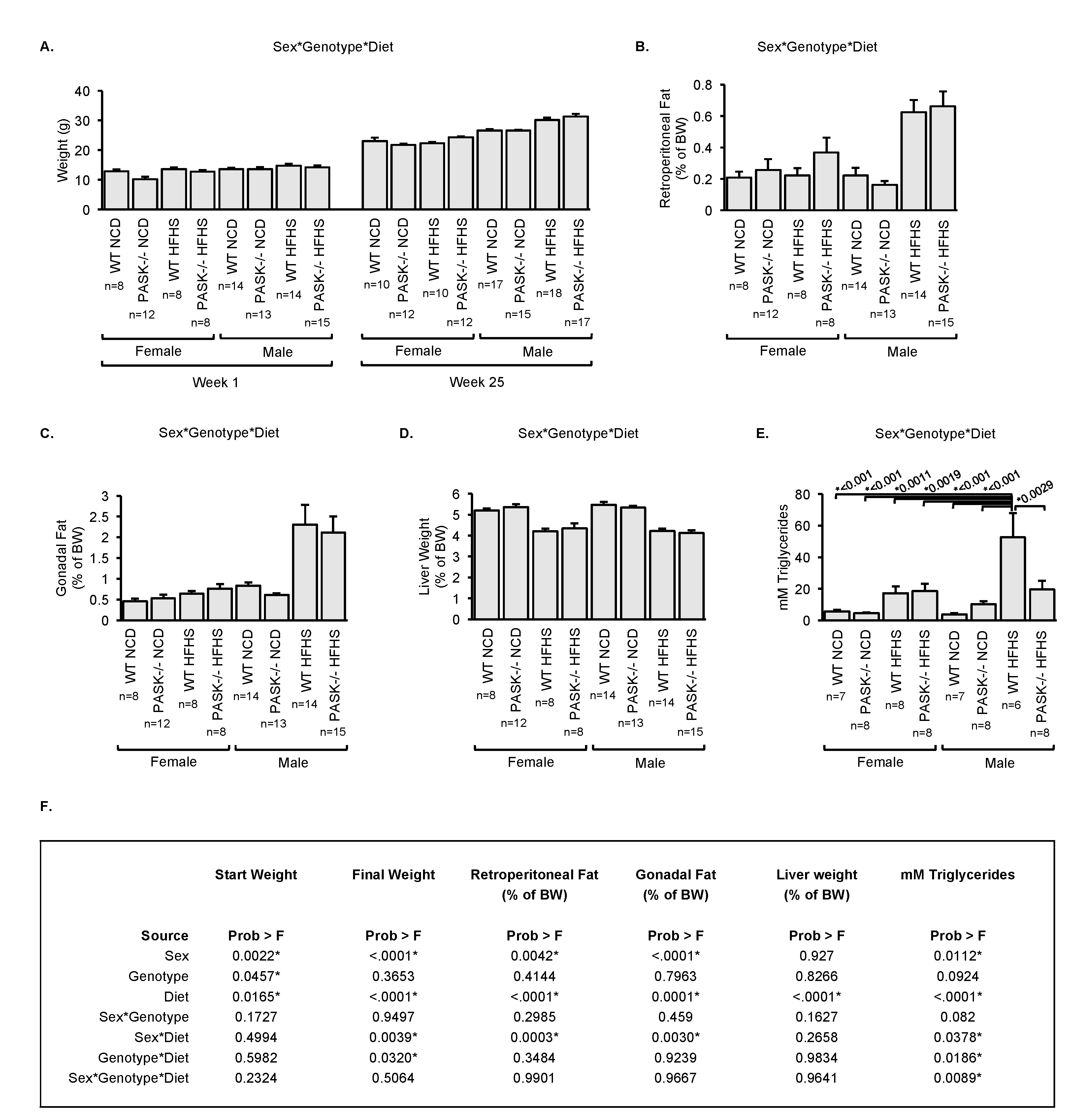
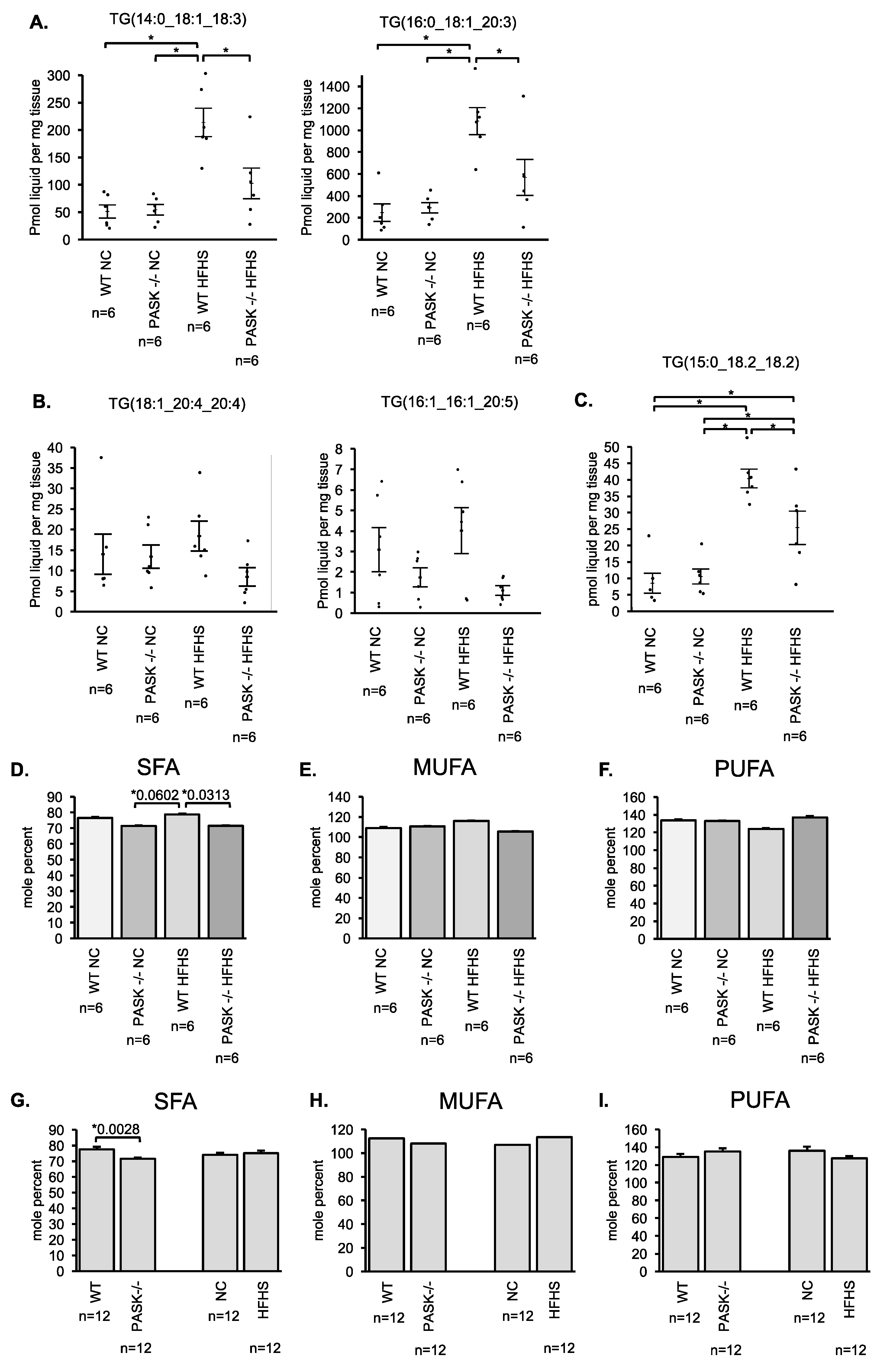
3.3. Male PASK−/− Mice Displayed Resistance to Accumulation of Hepatic Triglyceride When Placed on A HFHS Diet
3.4. Male PASK−/− Mice Resist Hepatic Triglyceride Accumulation in A Relatively Non-Specific Manner
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Ford, E.S.; Giles, W.H.; Dietz, W.H. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA 2002, 287, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Mokdad, A.H.; Serdula, M.K.; Dietz, W.H.; Bowman, B.A.; Marks, J.S.; Koplan, J.P. The spread of the obesity epidemic in the United States, 1991–1998. JAMA 1999, 282, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.A.; Drewnowski, A.; Christakis, D.A. Dietary energy density is associated with obesity and the metabolic syndrome in U.S. adults. Diabetes Care 2007, 30, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, J.E.; Rutter, J. Nutrient sensing and metabolic decisions. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 139, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Efeyan, A.; Comb, W.C.; Sabatini, D.M. Nutrient-sensing mechanisms and pathways. Nature 2015, 517, 302–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutter, J.; Michnoff, C.H.; Harper, S.M.; Gardner, K.H.; McKnight, S.L. PAS kinase: An evolutionarily conserved PAS domain-regulated serine/threonine kinase. Proc. Natl. Acad. Sci. USA 2001, 98, 8991–8996. [Google Scholar] [CrossRef] [Green Version]
- Cardon, C.M.; Rutter, J. PAS kinase: Integrating nutrient sensing with nutrient partitioning. Semin. Cell Dev. Biol. 2012, 23, 626–630. [Google Scholar] [CrossRef] [Green Version]
- DeMille, D.; Grose, J.H. PAS kinase: A nutrient sensing regulator of glucose homeostasis. IUBMB Life 2013, 65, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Sabatini, P.V.; Lynn, F.C. All-encomPASsing regulation of beta-cells: PAS domain proteins in beta-cell dysfunction and diabetes. Trends Endocrinol. Metab. 2015, 26, 49–57. [Google Scholar] [CrossRef]
- Schlafli, P.; Borter, E.; Spielmann, P.; Wenger, R.H. The PAS-domain kinase PASKIN: A new sensor in energy homeostasis. Cell Mol. Life Sci. 2009, 66, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Zhang, J.G.; Wang, Y.Z.; Liu, Y.; Liu, G.L.; Li, X.Y. Per-Arnt-Sim Kinase (PASK): An Emerging Regulator of Mammalian Glucose and Lipid Metabolism. Nutrients 2015, 7, 7437–7450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Romero, D.; Swiatek, W.I.; Dorweiler, I.; Kikani, C.K.; Sabic, H.; Zweifel, B.S.; McKearn, J.; Blitzer, J.T.; Nickols, G.A.; et al. PAS kinase drives lipogenesis through SREBP-1 maturation. Cell Rep. 2014, 8, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Xavier, G.; Farhan, H.; Kim, H.; Caxaria, S.; Johnson, P.; Hughes, S.; Bugliani, M.; Marselli, L.; Marchetti, P.; Birzele, F.; Sun, G.; et al. Per-arnt-sim (PAS) domain-containing protein kinase is downregulated in human islets in type 2 diabetes and regulates glucagon secretion. Diabetologia 2011, 54, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Semplici, F.; Vaxillaire, M.; Fogarty, S.; Semache, M.; Bonnefond, A.; Fontes, G.; Philippe, J.; Meur, G.; Diraison, F.; Sessions, R.B.; et al. Human mutation within Per-Arnt-Sim (PAS) domain-containing protein kinase (PASK) causes basal insulin hypersecretion. J. Biol. Chem. 2011, 286, 44005–44014. [Google Scholar] [CrossRef] [PubMed]
- Semplici, F.; Mondragon, A.; Macintyre, B.; Madeyski-Bengston, K.; Persson-Kry, A.; Barr, S.; Ramne, A.; Marley, A.; McGinty, J.; French, P.; et al. Cell type-specific deletion in mice reveals roles for PAS kinase in insulin and glucagon production. Diabetologia 2016, 59, 1938–1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, W.A.; Skurat, A.V.; Probst, B.; de Paoli-Roach, A.; Roach, P.J.; Rutter, J. Control of mammalian glycogen synthase by PAS kinase. Proc. Natl. Acad. Sci. USA 2005, 102, 16596–16601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, H.X.; Cardon, C.M.; Swiatek, W.; Cooksey, R.C.; Smith, T.L.; Wilde, J.; Boudina, S.; Abel, E.D.; McClain, D.A.; Rutter, J. PAS kinase is required for normal cellular energy balance. Proc. Natl. Acad. Sci. USA 2007, 104, 15466–15471. [Google Scholar] [CrossRef] [Green Version]
- An, R.; da Silva Xavier, G.; Hao, H.X.; Semplici, F.; Rutter, J.; Rutter, G.A. Regulation by Per-Arnt-Sim (PAS) kinase of pancreatic duodenal homeobox-1 nuclear import in pancreatic beta-cells. Biochem. Soc. Trans. 2006, 34, 791–793. [Google Scholar] [CrossRef]
- Fontes, G.; Semache, M.; Hagman, D.K.; Tremblay, C.; Shah, R.; Rhodes, C.J.; Rutter, J.; Poitout, V. Involvement of Per-Arnt-Sim Kinase and extracellular-regulated kinases-1/2 in palmitate inhibition of insulin gene expression in pancreatic beta-cells. Diabetes 2009, 58, 2048–2058. [Google Scholar] [CrossRef]
- Hurtado-Carneiro, V.; Roncero, I.; Blazquez, E.; Alvarez, E.; Sanz, C. PAS kinase as a nutrient sensor in neuroblastoma and hypothalamic cells required for the normal expression and activity of other cellular nutrient and energy sensors. Mol. Neurobiol. 2013, 48, 904–920. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keef, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.S.; Hancock, C.R.; Ray, J.D.; Kener, K.B.; Draney, C.; Garland, K.; Hardman, J.; Bikman, B.T.; Tessem, J.S. B-Cell deletion of Nr4a1 and Nr4a3 nuclear receptors impedes mitochondrial respiration and insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E186–E201. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeMille, D.; Bikman, B.T.; Mathis, A.D.; Prince, J.T.; Mackay, J.T.; Sowa, S.W.; Hall, T.D.; Grose, J.H. A comprehensive protein-protein interactome for yeast PAS kinase 1 reveals direct inhibition of respiration through the phosphorylation of Cbf1. Mol. Biol. Cell 2014, 25, 2199–2215. [Google Scholar] [CrossRef] [PubMed]
- DeMille, D.; Pape, J.A.; Bikman, B.T.; Ghassemian, M.; Grose, J.H. The regulation of Cbf1 by PAS kinase is a pivotal control point for lipogenesis versus respiration in Saccharomyces cerevisiae. G3 2018. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Nascimbeni, F.; Baldelli, E.; Marrazzo, A.; Romagnoli, D.; Lonardo, A. NAFLD as a Sexual Dimorphic Disease: Role of Gender and Reproductive Status in the Development and Progression of Nonalcoholic Fatty Liver Disease and Inherent Cardiovascular Risk. Adv. Ther. 2017, 34, 1291–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grose, J.H.; Smith, T.L.; Sabic, H.; Rutter, J. Yeast PAS kinase coordinates glucose partitioning in response to metabolic and cell integrity signaling. EMBO J. 2007, 26, 4824–4830. [Google Scholar] [CrossRef] [Green Version]
- Morley, B.J.; Dolan, D.F.; Ohlemiller, K.K.; Simmons, D.D. Generation and Characterization of α9 and α10 Nicotinic Acetylcholine Receptor Subunit Knockout Mice on a C57BL/6J Background. Front. Neurosci. 2017, 11, 516. [Google Scholar] [CrossRef]
- Ji, H.; Pai, A.V.; West, C.A.; Wu, X.; Speth, R.C.; Sandberg, K. Loss of Resistance to Angiotensin II-Induced Hypertension in the Jackson Laboratory Recombination-Activating Gene Null Mouse on the C57BL/6J Background. Hypertension 2017, 69, 1121–1127. [Google Scholar] [CrossRef]
- Zurita, E.; Chagoyen, M.; Cantero, M.; Alonso, R.; González-Neira, A.; López-Jiménez, A.; López-Moreno, J.A.; Landel, C.P.; Benítez, J.; Pazos, F.; et al. Genetic polymorphisms among C57BL/6 mouse inbred strains. Transgenic Res. 2011. [Google Scholar] [CrossRef]
- Shimano, H.; Sato, R. SREBP-regulated lipid metabolism: Convergent physiology-divergent pathophysiology. Nat. Rev. Endocrinol. 2017, 13, 710–730. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000, 14, 1293–1307. [Google Scholar] [PubMed]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002, 109, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Auer, S.; Hahne, P.; Soyal, S.M.; Felder, T.; Miller, K.; Paulmichl, M.; Krempler, F.; Oberkofler, H.; Patsch, W. Potential role of upstream stimulatory factor 1 gene variant in familial combined hyperlipidemia and related disorders. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1535–1544. [Google Scholar] [CrossRef]
- Naukkarinen, J.; Ehnholm, C.; Peltonen, L. Genetics of familial combined hyperlipidemia. Curr. Opin. Lipidol. 2006, 17, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Coon, H.; Xin, Y.; Hopkins, P.N.; Cawthon, R.M.; Hasstedt, S.J.; Hunt, S.C. Upstream stimulatory factor 1 associated with familial combined hyperlipidemia, LDL cholesterol, and triglycerides. Hum. Genet. 2005, 117, 444–451. [Google Scholar] [CrossRef]
- Holzapfel, C.; Baumert, J.; Grallert, H.; Muller, A.M.; Thorand, B.; Khuseyinova, N.; Herder, C.; Meisinger, C.; Hauner, H.; Wichmann, H.E.; et al. Genetic variants in the USF1 gene are associated with low-density lipoprotein cholesterol levels and incident type 2 diabetes mellitus in women: Results from the MONICA/KORA Augsburg case-cohort study, 1984–2002. Eur. J. Endocrinol. 2008, 159, 407–416. [Google Scholar] [CrossRef]
- Huertas-Vazquez, A.; Aguilar-Salinas, C.; Lusis, A.J.; Cantor, R.M.; Canizales-Quinteros, S.; Lee, J.C.; Mariana-Nuñez, L.; Riba-Ramirez, R.M.; Jokiaho, A.; Tusie-Luna, T.; et al. Familial combined hyperlipidemia in Mexicans: Association with upstream transcription factor 1 and linkage on chromosome 16q24.1. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1985–1991. [Google Scholar] [CrossRef]
- Komulainen, K.; Alanne, M.; Auro, K.; Kilpikari, R.; Pajukanta, P.; Saarela, J.; Ellonen, P.; Salminen, K.; Kulathinal, S.; Kuulasmaa, K.; et al. Risk alleles of USF1 gene predict cardiovascular disease of women in two prospective studies. PLoS Genet. 2006, 2, e69. [Google Scholar] [CrossRef]
- Lee, J.C.; Weissglas-Volkov, D.; Kyttala, M.; Sinsheimer, J.S.; Jokiaho, A.; de Bruin, T.W.; Lusis, A.J.; Brennan, M.L.; van Greevenbroek, M.M.; van der Kallen, C.J.; et al. USF1 contributes to high serum lipid levels in Dutch FCHL families and U.S. whites with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2222–2227. [Google Scholar] [CrossRef] [PubMed]
- Naukkarinen, J.; Gentile, M.; Soro-Paavonen, A.; Saarela, J.; Koistinen, H.A.; Pajukanta, P.; Taskinen, M.R.; Peltonen, L. USF1 and dyslipidemias: Converging evidence for a functional intronic variant. Hum. Mol. Genet. 2005, 14, 2595–2605. [Google Scholar] [CrossRef] [PubMed]
- Naukkarinen, J.; Nilsson, E.; Koistinen, H.A.; Soderlund, S.; Lyssenko, V.; Vaag, A.; Poulsen, P.; Groop, L.; Taskinen, M.R.; Peltonen, L. Functional variant disrupts insulin induction of USF1: Mechanism for USF1-associated dyslipidemias. Circ. Cardiovasc. Genet. 2009, 2, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.C.; Miyake, K.; So, W.Y.; Poon, E.W.; Lam, V.K.; Li, J.K.; Cox, N.J.; Bell, G.I.; Chan, J.C. The linkage and association of the gene encoding upstream stimulatory factor 1 with type 2 diabetes and metabolic syndrome in the Chinese population. Diabetologia 2005, 48, 2018–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plaisier, C.L.; Horvath, S.; Huertas-Vazquez, A.; Cruz-Bautista, I.; Herrera, M.F.; Tusie-Luna, T.; Aguilar-Salinas, C.; Pajukanta, P. A systems genetics approach implicates USF1, FADS3, and other causal candidate genes for familial combined hyperlipidemia. PLoS Genet. 2009, 5, e1000642. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.P.; Carlson, C.S.; Jenny, N.S.; Durda, J.P.; Siscovick, D.S.; Nickerson, D.A.; Tracy, R.P. USF1 gene variants, cardiovascular risk, and mortality in European Americans: Analysis of two US cohort studies. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2736–2742. [Google Scholar] [CrossRef] [PubMed]
- Van der Vleuten, G.M.; Isaacs, A.; Hijmans, A.; van Duijn, C.M.; Stalenhoef, A.F.; de Graaf, J. The involvement of upstream stimulatory factor 1 in Dutch patients with familial combined hyperlipidemia. J. Lipid Res. 2007, 48, 193–200. [Google Scholar] [CrossRef]
- Laurila, P.P.; Soronen, J.; Kooijman, S.; Forsstrom, S.; Boon, M.R.; Surakka, I.; Kaiharju, E.; Coomans, C.P.; Van Den Berg, S.A.; Autio, A.; et al. USF1 deficiency activates brown adipose tissue and improves cardiometabolic health. Sci. Transl. Med. 2016, 8, 323ra13. [Google Scholar] [CrossRef]
- Wang, D.; Sul, H.S. Upstream stimulatory factors bind to insulin response sequence of the fatty acid synthase promoter. USF1 is regulated. J. Biol. Chem. 1995, 270, 28716–28722. [Google Scholar] [CrossRef]
- Casado, M.; Vallet, V.S.; Kahn, A.; Vaulont, S. Essential role in vivo of upstream stimulatory factors for a normal dietary response of the fatty acid synthase gene in the liver. J. Biol. Chem. 1999, 274, 2009–2013. [Google Scholar] [CrossRef]
- Wang, D.; Sul, H.S. Upstream stimulatory factor binding to the E-box at -65 is required for insulin regulation of the fatty acid synthase promoter. J. Biol. Chem. 1997, 272, 26367–26374. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.J.; Wong, R.H.; Pandya, N.; Sul, H.S. Direct interaction between USF and SREBP-1c mediates synergistic activation of the fatty-acid synthase promoter. J. Biol. Chem. 2007, 282, 5453–5467. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.J.; Sul, H.S. Insulin regulation of fatty acid synthase gene transcription: Roles of USF and SREBP-1c. IUBMB Life 2004, 56, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Latasa, M.J.; Griffin, M.J.; Moon, Y.S.; Kang, C.; Sul, H.S. Occupancy and function of the -150 sterol regulatory element and -65 E-box in nutritional regulation of the fatty acid synthase gene in living animals. Mol. Cell. Biol. 2003, 23, 5896–5907. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pape, J.A.; Newey, C.R.; Burrell, H.R.; Workman, A.; Perry, K.; Bikman, B.T.; Bridgewater, L.C.; Grose, J.H. Per-Arnt-Sim Kinase (PASK) Deficiency Increases Cellular Respiration on a Standard Diet and Decreases Liver Triglyceride Accumulation on a Western High-Fat High-Sugar Diet. Nutrients 2018, 10, 1990. https://doi.org/10.3390/nu10121990
Pape JA, Newey CR, Burrell HR, Workman A, Perry K, Bikman BT, Bridgewater LC, Grose JH. Per-Arnt-Sim Kinase (PASK) Deficiency Increases Cellular Respiration on a Standard Diet and Decreases Liver Triglyceride Accumulation on a Western High-Fat High-Sugar Diet. Nutrients. 2018; 10(12):1990. https://doi.org/10.3390/nu10121990
Chicago/Turabian StylePape, Jenny A., Colleen R. Newey, Haley R. Burrell, Audrey Workman, Katelyn Perry, Benjamin T. Bikman, Laura C. Bridgewater, and Julianne H. Grose. 2018. "Per-Arnt-Sim Kinase (PASK) Deficiency Increases Cellular Respiration on a Standard Diet and Decreases Liver Triglyceride Accumulation on a Western High-Fat High-Sugar Diet" Nutrients 10, no. 12: 1990. https://doi.org/10.3390/nu10121990
APA StylePape, J. A., Newey, C. R., Burrell, H. R., Workman, A., Perry, K., Bikman, B. T., Bridgewater, L. C., & Grose, J. H. (2018). Per-Arnt-Sim Kinase (PASK) Deficiency Increases Cellular Respiration on a Standard Diet and Decreases Liver Triglyceride Accumulation on a Western High-Fat High-Sugar Diet. Nutrients, 10(12), 1990. https://doi.org/10.3390/nu10121990