Potential Application of Ixeris dentata in the Prevention and Treatment of Aging-Induced Dry Mouth
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Preparation
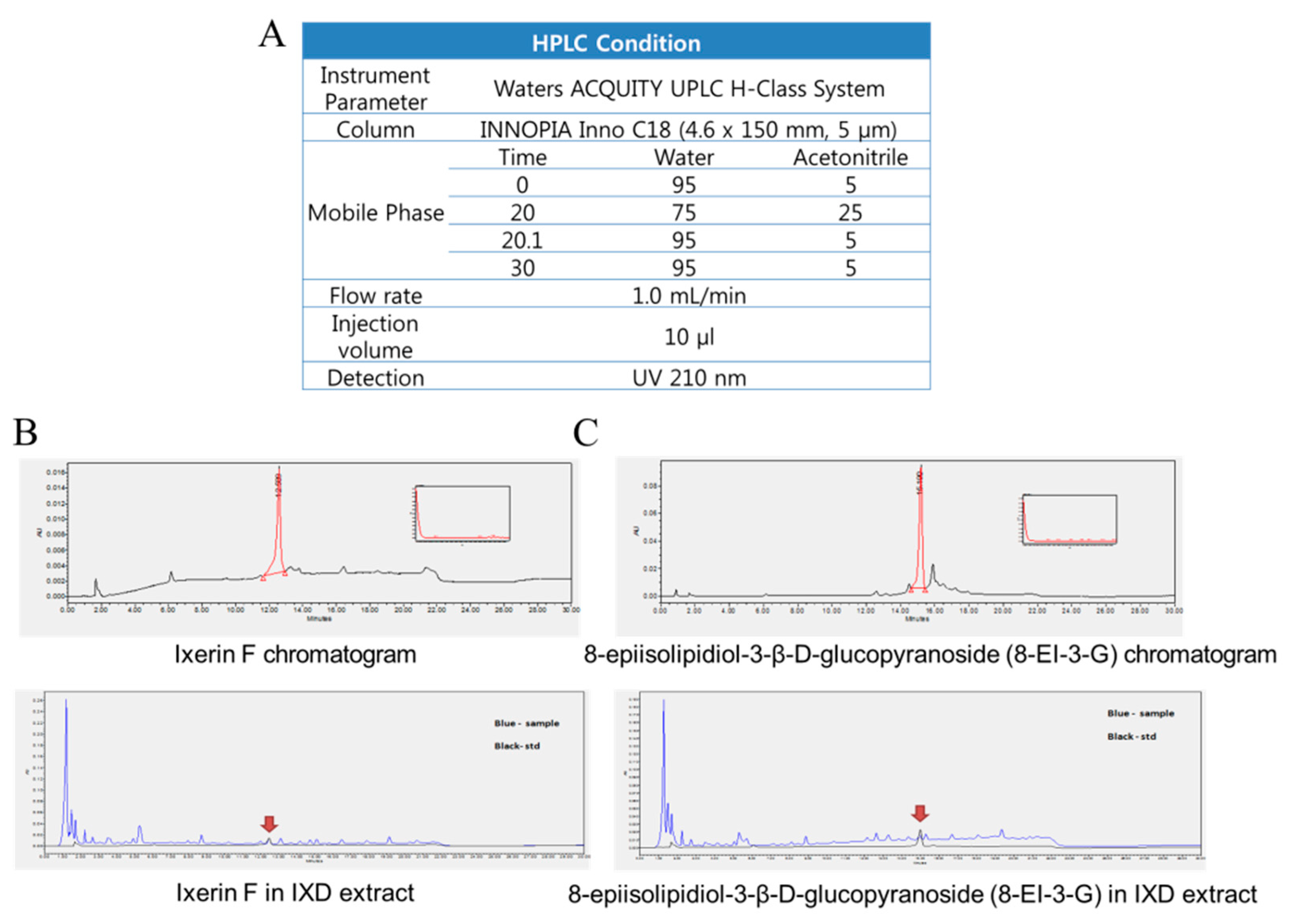
2.2. Quantitation of Pure Compounds Using HPLC-DAD
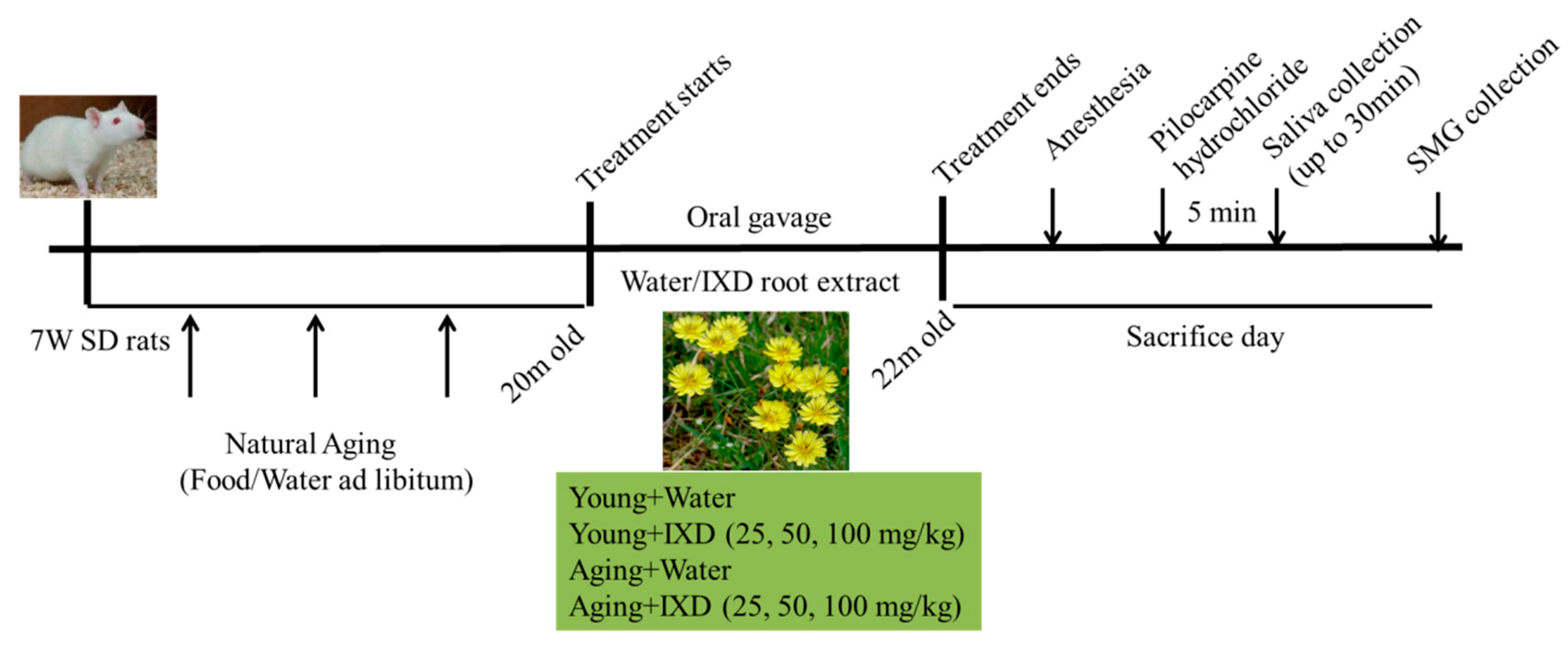
2.3. Animals and Experimental Designs
2.4. Dosage Regimen
2.5. Collection of Saliva and Measurement of Salivary Flow Rate
2.6. Amylase Activity Measurement
2.7. Histological Analysis
2.8. ROS Detection
2.9. Immunoblotting
2.10. Immunohistochemistry and Immunofluorescence
2.11. Detection of Carbonylated PDI
2.12. Real-time PCR
2.13. Statistical Analysis
3. Results
3.1. Ixeris dentata Improves Aging-Induced Salivary Dysfunction
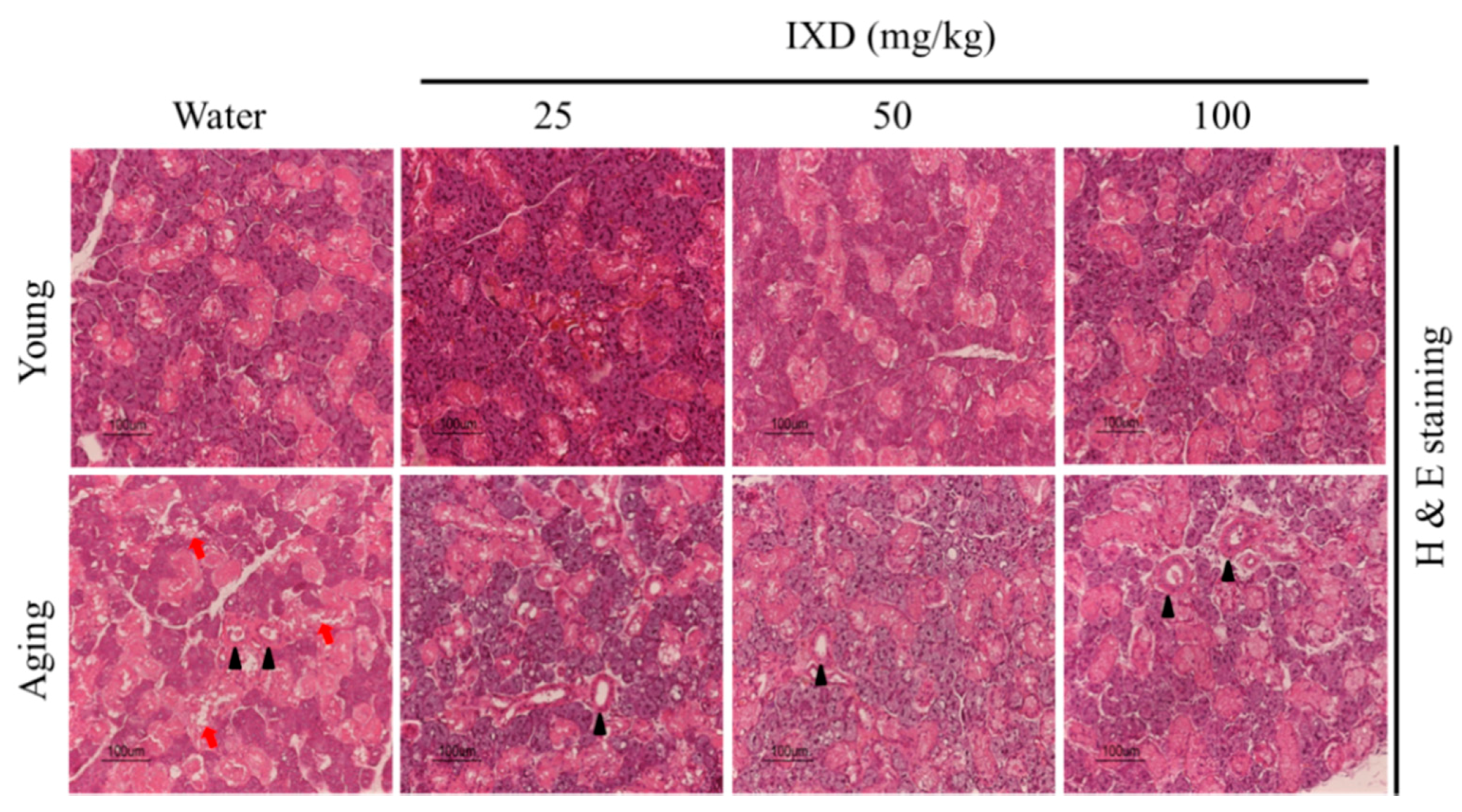
3.2. Morphological Analysis of Young and Aged Submandibular Gland Treated with Water or IXD Extract
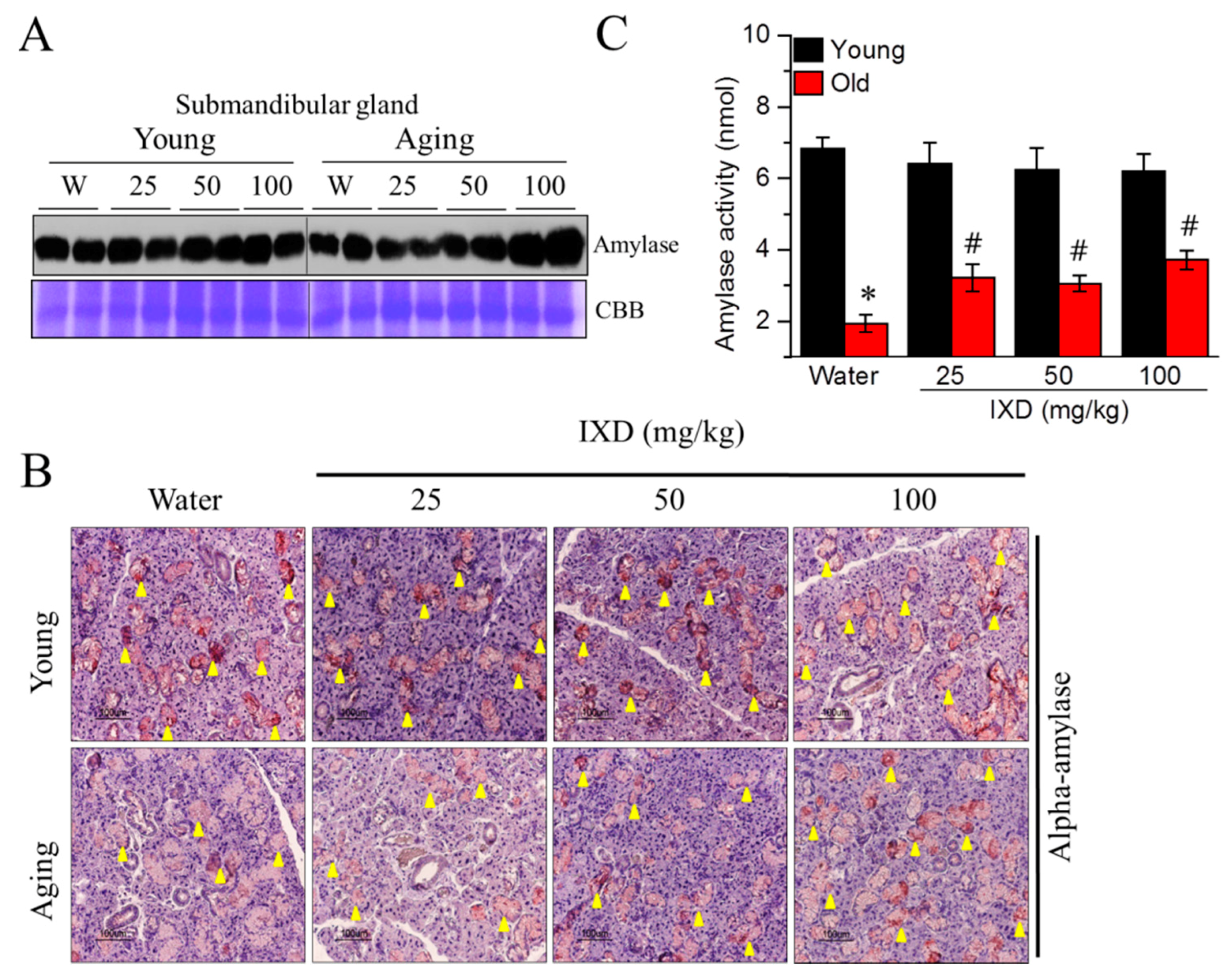
3.3. Ixeris dentata Extract Increased the Alpha-Amylase Protein Expression and Its Activity in the Aged Submandibular Glands
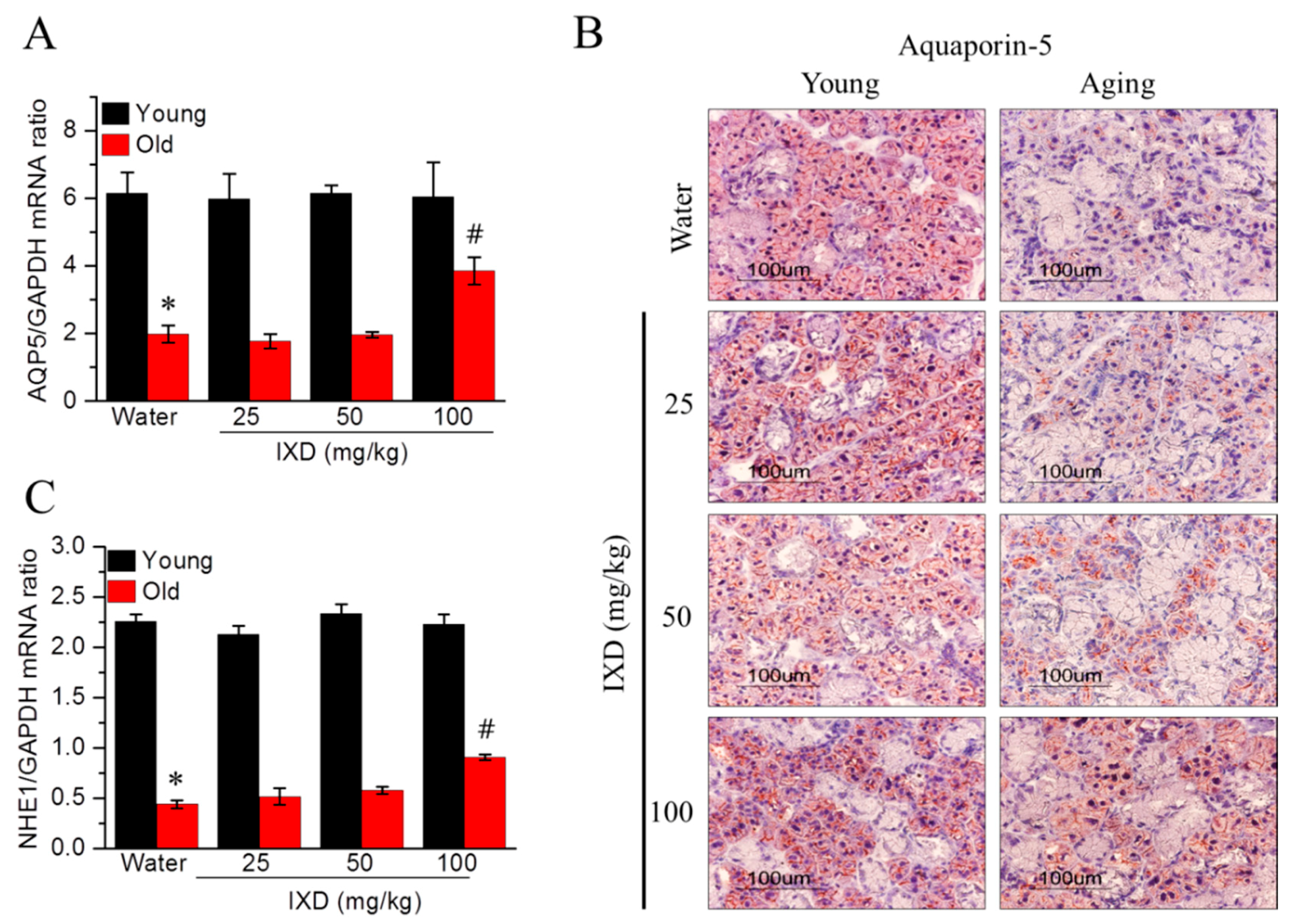
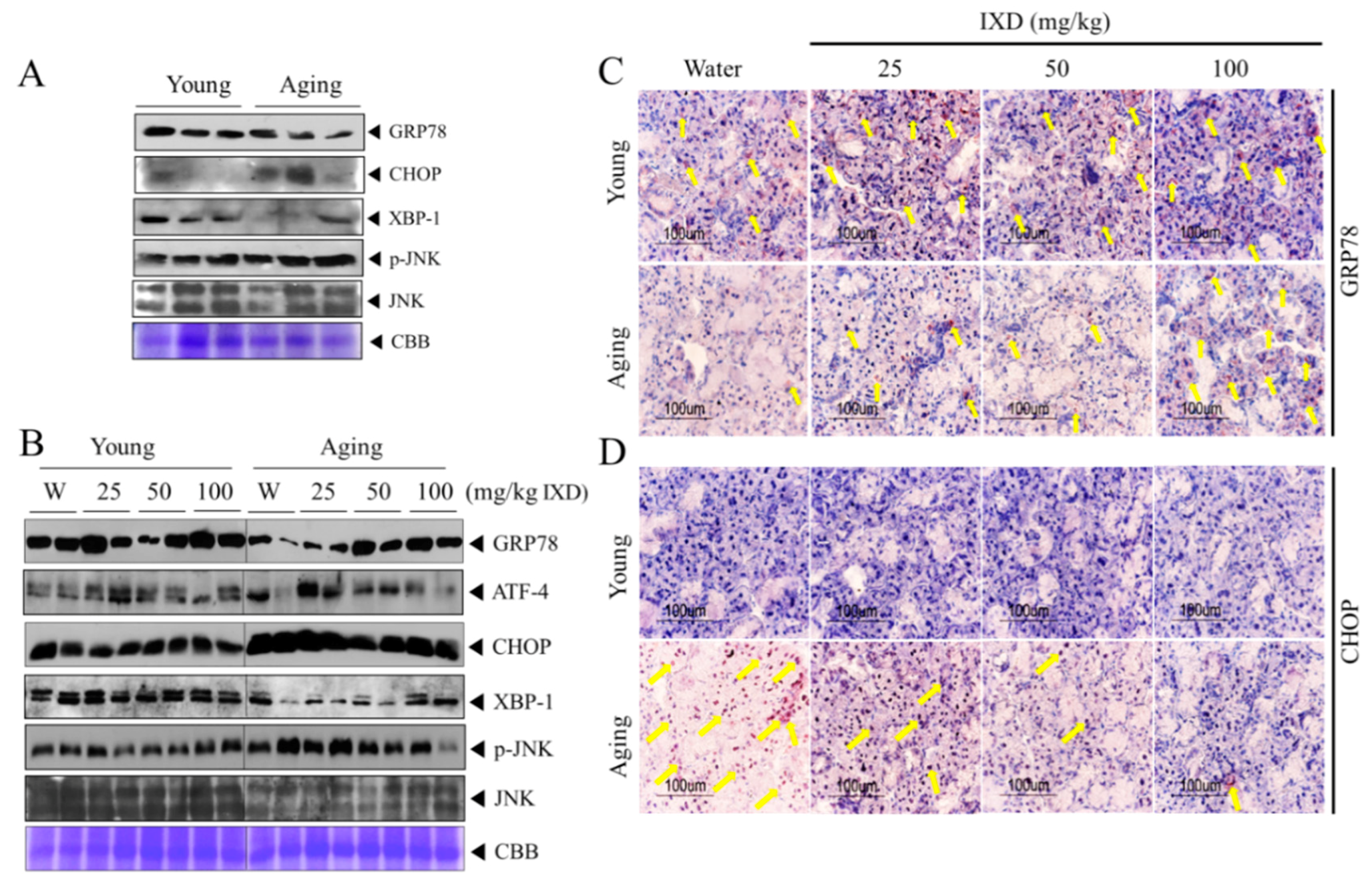
3.4. Ixeris dentata Extract Increased the Expression of Water Channel Protein from Aged Submandibular Glands
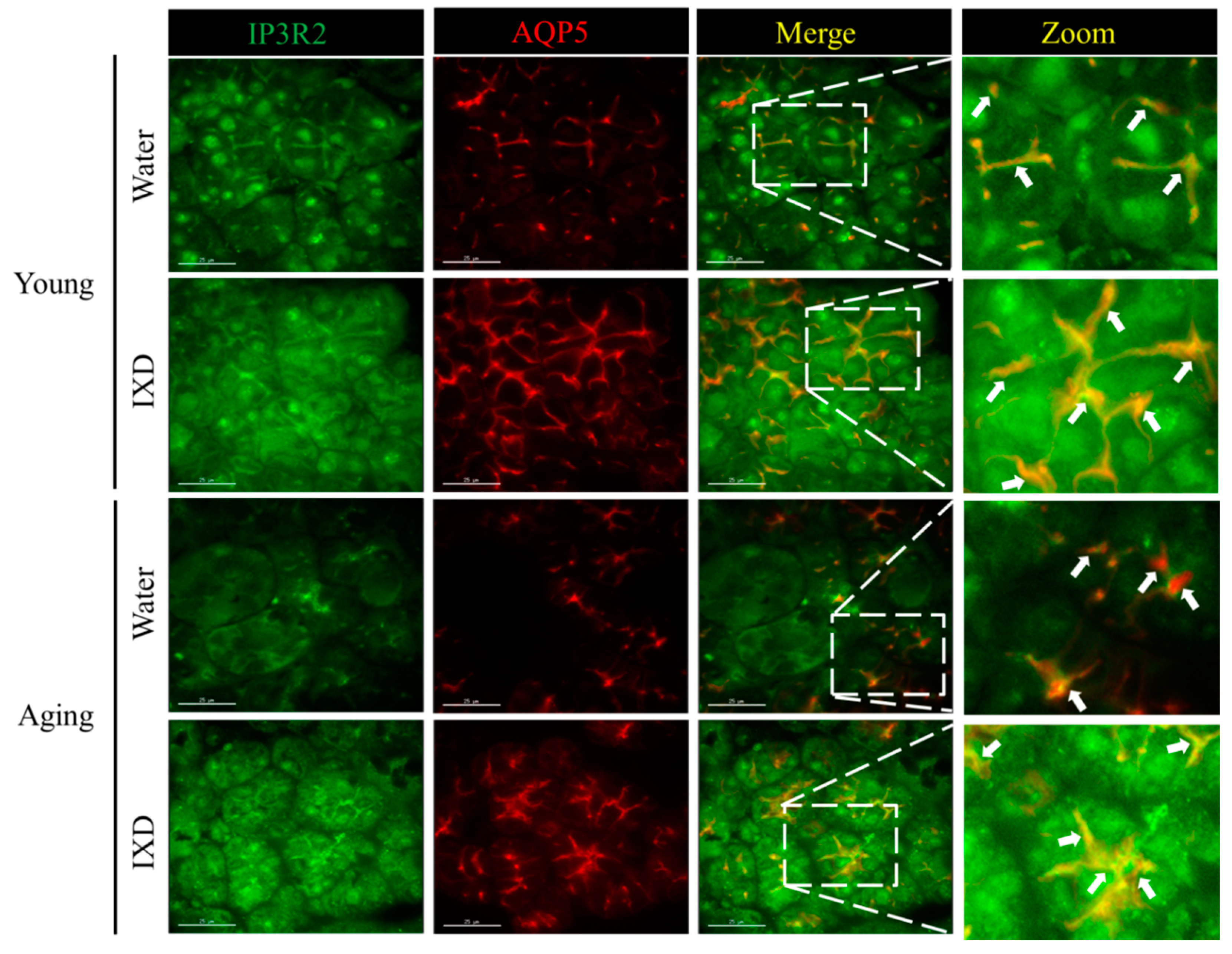
3.5. Acinar Cell-Specific Localization and Expression of IP3R2 and AQP5 Was Increased by the Treatment with IXD in Aging Submandibular Glands
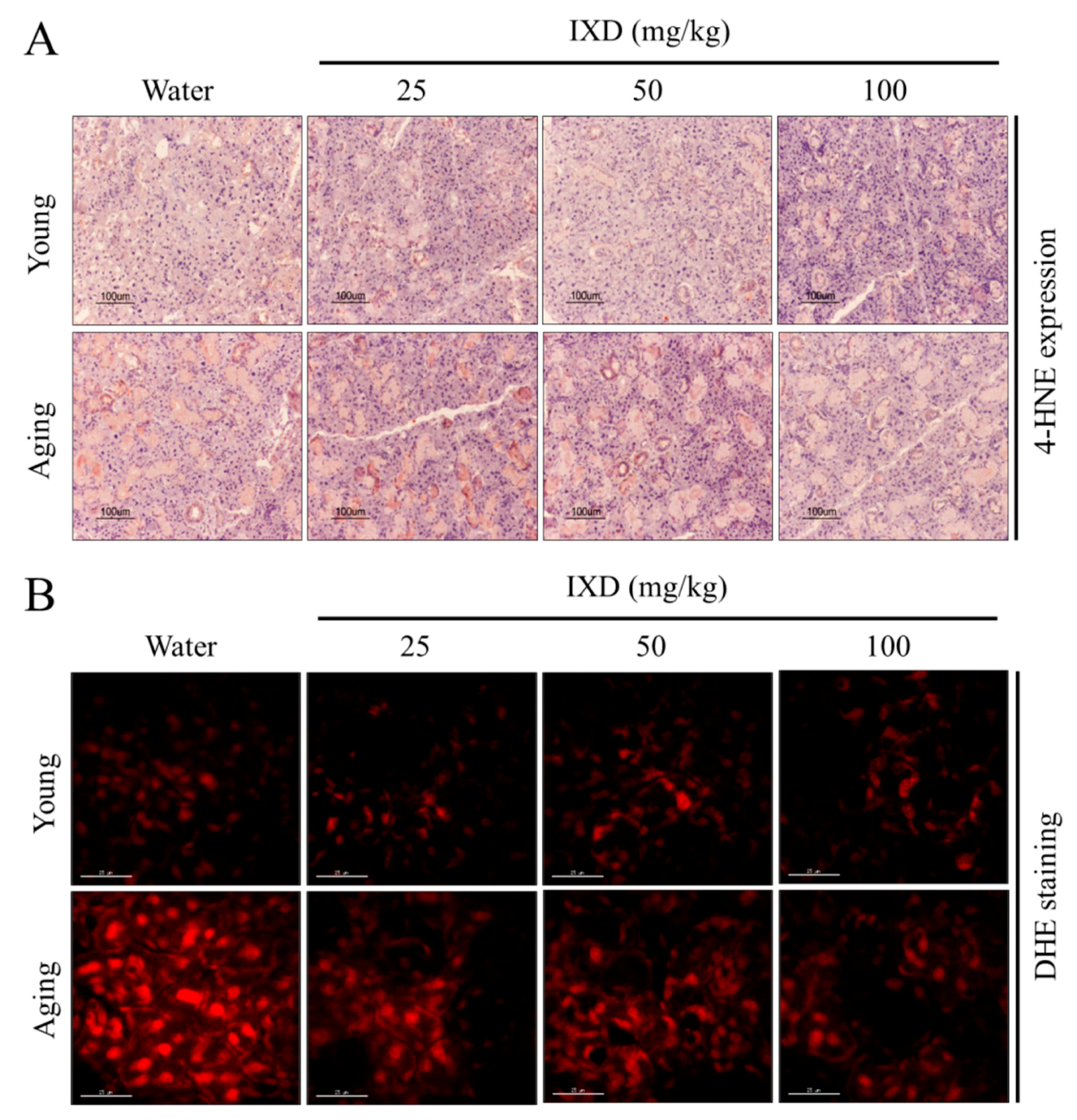
3.6. Aging-Induced Free Radical Generation Was Attenuated by Ixeris dentata Extract
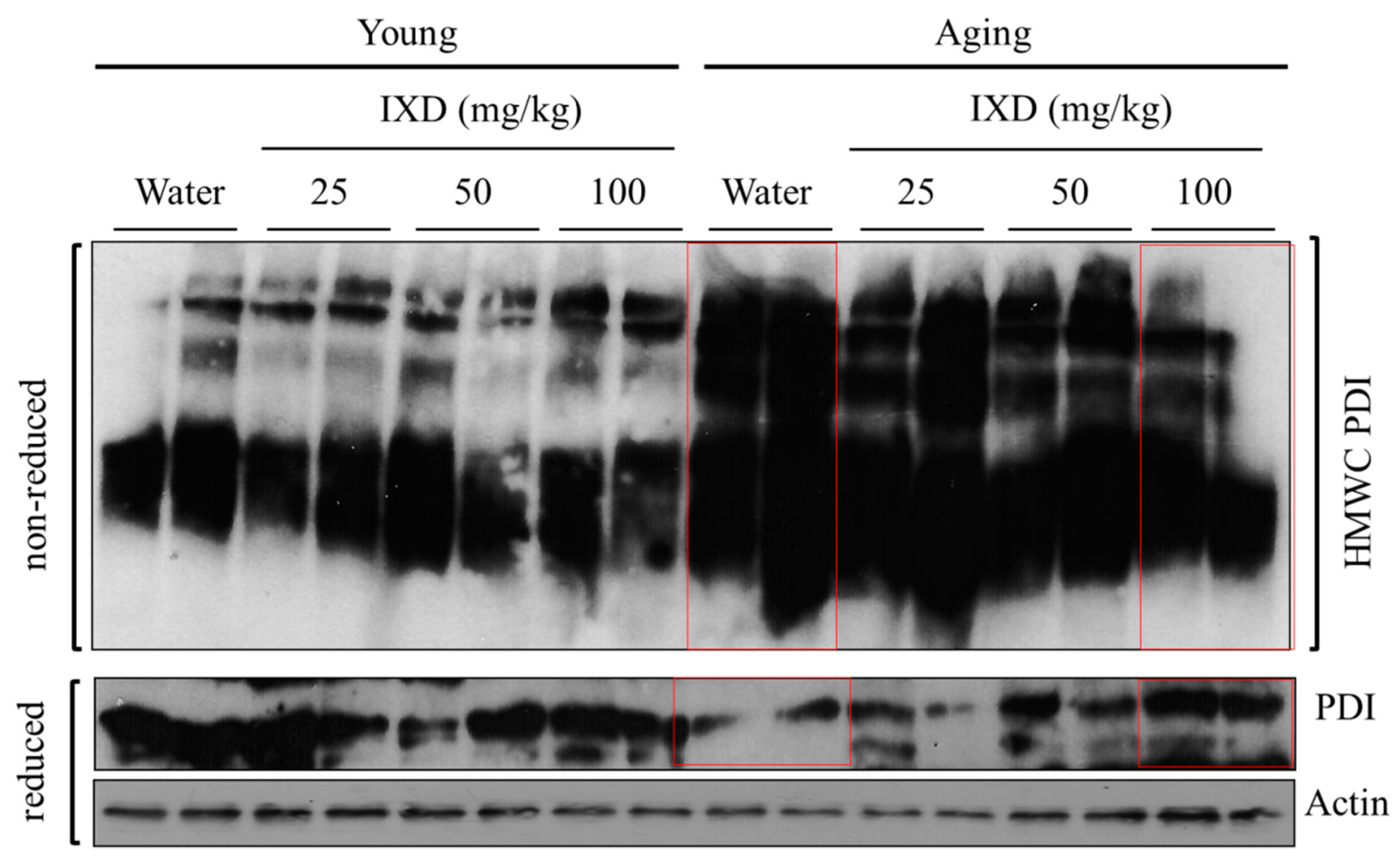
3.7. IXD Extract Regulates Disulfide Bond Formation during Oxidative Protein Folding
3.8. IXD Extract Regulates Aging-Induced UPR Impairment in the Submandibular Glands
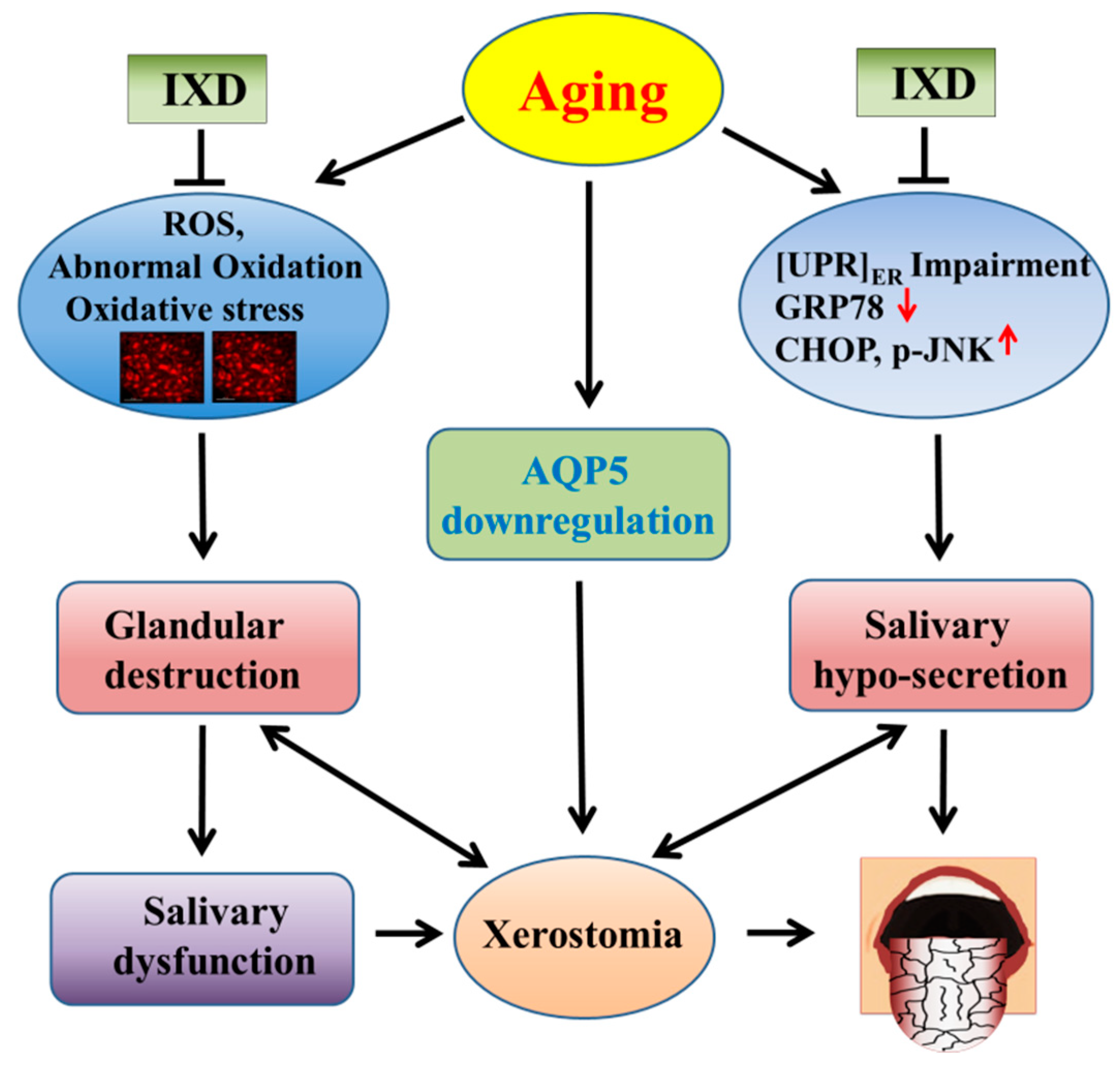
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Hahnel, S.; Schwarz, S.; Zeman, F.; Schafer, L.; Behr, M. Prevalence of xerostomia and hyposalivation and their association with quality of life in elderly patients in dependence on dental status and prosthetic rehabilitation: A pilot study. J. Dent. 2014, 42, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Ship, J.A.; Pillemer, S.R.; Baum, B.J. Xerostomia and the geriatric patient. J. Am. Geriatr. Soc. 2002, 50, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.; Hiraishi, N.; Islam, M.S.; Otsuki, M.; Tagami, J. Age-related changes in salivary biomarkers. J. Dent. Sci. 2014, 9, 85–90. [Google Scholar] [CrossRef]
- Erden-Inal, M.; Sunal, E.; Kanbak, G. Age-related changes in the glutathione redox system. Cell Biochem. Funct. 2002, 20, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Ying Joanna, N.D.; Thomson, W.M. Dry mouth—An overview. Singap. Dent. J. 2015, 36, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, K.R.; Junjappa, R.; Handigund, M.; Kim, H.R.; Chae, H.J. The imprint of salivary secretion in autoimmune disorders and related pathological conditions. Autoimmun. Rev. 2018, 17, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Suarez-Durall, P.; Mulligan, R. Dry mouth: A critical topic for older adult patients. J. Prosthodont. Res. 2015, 59, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Bulthuis, M.S.; Jan Jager, D.H.; Brand, H.S. Relationship among perceived stress, xerostomia, and salivary flow rate in patients visiting a saliva clinic. Clin. Oral Investig. 2018, 22, 3121–3127. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Epstein, J.B.; Sroussi, H. Hyposalivation in elderly patients. J. Can. Dent. Assoc. 2006, 72, 841–846. [Google Scholar]
- Ambatipudi, K.S.; Lu, B.; Hagen, F.K.; Melvin, J.E.; Yates, J.R. Quantitative analysis of age specific variation in the abundance of human female parotid salivary proteins. J. Proteome Res. 2009, 8, 5093–5102. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, K.R.; Kim, H.R.; Chae, H.J. Compliance with Saliva Collection Protocol in Healthy Volunteers: Strategies for Managing Risk and Errors. Int. J. Med. Sci. 2018, 15, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Raisanen, I.T.; Heikkinen, A.M.; Siren, E.; Tervahartiala, T.; Gieselmann, D.R.; van der Schoor, G.J.; van der Schoor, P.; Sorsa, T. Point-of-Care/Chairside aMMP-8 Analytics of Periodontal Diseases’ Activity and Episodic Progression. Diagnostics 2018, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Hershkovich, O.; Shafat, I.; Nagler, R.M. Age-related changes in salivary antioxidant profile: Possible implications for oral cancer. J. Gerontol. Ser. A Biol. Sci. Méd. Sci. 2007, 62, 361–366. [Google Scholar] [CrossRef]
- Perri, E.R.; Thomas, C.J.; Parakh, S.; Spencer, D.M.; Atkin, J.D. The Unfolded Protein Response and the Role of Protein Disulfide Isomerase in Neurodegeneration. Front. Cell Dev. Biol. 2015, 3, 80. [Google Scholar] [CrossRef] [PubMed]
- Cuanalo-Contreras, K.; Mukherjee, A.; Soto, C. Role of protein misfolding and proteostasis deficiency in protein misfolding diseases and aging. Int. J. Cell Biol. 2013, 2013, 638083. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, N. ER and aging-Protein folding and the ER stress response. Res. Rev. 2009, 8, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Lumb, R.A.; Bulleid, N.J. Is protein disulfide isomerase a redox-dependent molecular chaperone? EMBO J. 2002, 21, 6763–6770. [Google Scholar] [CrossRef]
- Ellgaard, L.; Helenius, A. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 2003, 4, 181–191. [Google Scholar] [CrossRef]
- Brown, M.K.; Naidoo, N. The endoplasmic reticulum stress response in aging and age-related diseases. Front. Physiol. 2012, 3, 263. [Google Scholar] [CrossRef]
- Naidoo, N. The endoplasmic reticulum stress response and aging. Rev. Neurosci. 2009, 20, 23–37. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Lee, H.Y.; Kim, S.H.; Kim, H.R.; Chae, H.J. Ixeris dentata Extract Increases Salivary Secretion through the Regulation of Endoplasmic Reticulum Stress in a Diabetes-Induced Xerostomia Rat Model. Int. J. Mol. Sci. 2018, 19, 1059. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Jeong, D.; Kim, K.; Hwang, E. The composition of the root of Ixeris dentata var. albiflora Nakai. and cell viability and DPPH radical scavenging activities of its extract. Korean J. Nutr. 2010, 43, 105–113. [Google Scholar] [CrossRef]
- Jeon, Y.D.; Kee, J.Y.; Kim, D.S.; Han, Y.H.; Kim, S.H.; Kim, S.J.; Um, J.Y.; Hong, S.H. Effects of Ixeris dentata water extract and caffeic acid on allergic inflammation in vivo and in vitro. BMC Complement. Altern. Med. 2015, 15, 196. [Google Scholar] [CrossRef] [PubMed]
- Sassaki, K.T.; Delbem, A.C.; dos Santos, O.A.; Shimabucoro, C.E.; Nakamune, A.C.; Bedran-de-Castro, J.C.; Oliveira-Filho, R.M. Neuroendocrine alterations impair enamel mineralization, tooth eruption and saliva in rats. Pesqui. Odontol. Bras. = Braz. Oral Res. 2003, 17, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, K.R.; Lee, S.W.; Kim, S.H.; Kim, H.R.; Chae, H.J. Ixeris dentata extract regulates salivary secretion through the activation of aquaporin-5 and prevents diabetes-induced xerostomia. J. Exp. Pharmacol. 2017, 9, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Lee, G.H.; Bhattarai, K.R.; Park, B.H.; Koo, S.H.; Kim, H.R.; Chae, H.J. Bax Inhibitor-1 regulates hepatic lipid accumulation via ApoB secretion. Sci. Rep. 2016, 6, 27799. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, G.H.; Bhattarai, K.R.; Junjappa, R.P.; Lee, H.Y.; Handigund, M.; Marahatta, A.; Bhandary, B.; Baek, I.H.; Pyo, J.S.; et al. PI3Kdelta contributes to ER stress-associated asthma through ER-redox disturbances: The involvement of the RIDD-RIG-I-NF-kappaB axis. Exp. Mol. Med. 2018, 50, e444. [Google Scholar] [CrossRef] [PubMed]
- Teos, L.Y.; Zhang, Y.; Cotrim, A.P.; Swaim, W.; Won, J.H.; Ambrus, J.; Shen, L.; Bebris, L.; Grisius, M.; Jang, S.I.; et al. IP3R deficit underlies loss of salivary fluid secretion in Sjogren’s Syndrome. Sci. Rep. 2015, 5, 13953. [Google Scholar] [CrossRef]
- Ambudkar, I.S. Ca2+ signaling and regulation of fluid secretion in salivary gland acinar cells. Cell Calcium 2014, 55, 297–305. [Google Scholar] [CrossRef]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef]
- Hamilton, M.L.; Van Remmen, H.; Drake, J.A.; Yang, H.; Guo, Z.M.; Kewitt, K.; Walter, C.A.; Richardson, A. Does oxidative damage to DNA increase with age? Proc. Natl. Acad. Sci. USA 2001, 98, 10469–10474. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Laguna, L.; Sarkar, A. Aging-related changes in quantity and quality of saliva: Where do we stand in our understanding? J. Texture Stud. 2018. [Google Scholar] [CrossRef] [PubMed]
- An, J.Y.; Darveau, R.; Kaeberlein, M. Oral health in geroscience: Animal models and the aging oral cavity. GeroScience 2018, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barbe, A.G.; Heinzler, A.; Derman, S.; Hellmich, M.; Timmermann, L.; Noack, M.J. Hyposalivation and xerostomia among Parkinson’s disease patients and its impact on quality of life. Oral Dis. 2017, 23, 464–470. [Google Scholar] [CrossRef] [PubMed]
- An, J.Y.; Quarles, E.K.; Mekvanich, S.; Kang, A.; Liu, A.; Santos, D.; Miller, R.A.; Rabinovitch, P.S.; Cox, T.C.; Kaeberlein, M. Rapamycin treatment attenuates age-associated periodontitis in mice. GeroScience 2017, 39, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. mTOR is a key modulator of ageing and age-related disease. Nature 2013, 493, 338–345. [Google Scholar] [CrossRef]
- Bitto, A.; Ito, T.K.; Pineda, V.V.; LeTexier, N.J.; Huang, H.Z.; Sutlief, E.; Tung, H.; Vizzini, N.; Chen, B.; Smith, K.; et al. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. eLife 2016, 5, e16351. [Google Scholar] [CrossRef]
- Zhu, Z.; Pang, B.; Iglesias-Bartolome, R.; Wu, X.; Hu, L.; Zhang, C.; Wang, J.; Gutkind, J.S.; Wang, S. Prevention of irradiation-induced salivary hypofunction by rapamycin in swine parotid glands. Oncotarget 2016, 7, 20271–20281. [Google Scholar] [CrossRef]
- Villa, A.; Connell, C.L.; Abati, S. Diagnosis and management of xerostomia and hyposalivation. Ther. Clin. Risk Manag. 2015, 11, 45–51. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, G.; Williams, R.C.; Siciliano, V.I.; Alibrandi, A.; Cordasco, G.; Ramaglia, L. The effects of a desiccant agent in the treatment of chronic periodontitis: A randomized, controlled clinical trial. Clin. Oral Investig. 2018, 22, 791–800. [Google Scholar] [CrossRef]
- Matarese, G.; Ramaglia, L.; Cicciu, M.; Cordasco, G.; Isola, G. The Effects of Diode Laser Therapy as an Adjunct to Scaling and Root Planing in the Treatment of Aggressive Periodontitis: A 1-Year Randomized Controlled Clinical Trial. Photomed. Laser Surg. 2017, 35, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Mardani, H.; Ghannadi, A.; Rashnavadi, B.; Kamali, R. The Effect of ginger herbal spray on reducing xerostomia in patients with type II diabetes. Avicenna J. Phytomed. 2017, 7, 308–316. [Google Scholar]
- Luo, H.; Li, X.; Liu, J.; Andrew, F.; George, L. Chinese Herbal Medicine in Treating Primary Sjogren’s Syndrome: A Systematic Review of Randomized Trials. Evid.-Based Complement. Altern. Med. 2012, 2012, 640658. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Lee, B.-J.; Bu, Y.-M.; Yeo, I.-K.; Kim, J.-S.; Ryu, B.-H. Effects of Korean red ginseng on dry mouth: A randomized, double-blind, placebo-controlled trial. J. Ginseng Res. 2010, 34, 183–191. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, I.S.; Kim, S.K.; Lim, J.Y.; Kim, Y.M. Analysis of age-related changes in the functional morphologies of salivary glands in mice. Arch. Oral Biol. 2013, 58, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Moritsuka, M.; Kitasako, Y.; Burrow, M.F.; Ikeda, M.; Tagami, J.; Nomura, S. Quantitative assessment for stimulated saliva flow rate and buffering capacity in relation to different ages. J. Dent. 2006, 34, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Matsuno, T.; Omata, K.; Satoh, T. Relationship between hyposalivation and oxidative stress in aging mice. J. Clin. Biochem. Nutr. 2017, 61, 40–46. [Google Scholar] [CrossRef]
- Lasisi, T.J.; Shittu, S.T.; Oguntokun, M.M.; Tiamiyu, N.A. Aging affects morphology but not stimulated secretion of saliva in rats. Ann. Ib. Postgrad. Med. 2014, 12, 109–114. [Google Scholar]
- Park, K.; Evans, R.L.; Watson, G.E.; Nehrke, K.; Richardson, L.; Bell, S.M.; Schultheis, P.J.; Hand, A.R.; Shull, G.E.; Melvin, J.E. Defective fluid secretion and NaCl absorption in the parotid glands of Na+/H+ exchanger-deficient mice. J. Biol. Chem. 2001, 276, 27042–27050. [Google Scholar] [CrossRef]
- Inaba, T.; Hisatsune, C.; Sasaki, Y.; Ogawa, Y.; Ebisui, E.; Ogawa, N.; Matsui, M.; Takeuchi, T.; Mikoshiba, K.; Tsubota, K. Mice lacking inositol 1,4,5-trisphosphate receptors exhibit dry eye. PLoS ONE 2014, 9, e99205. [Google Scholar] [CrossRef]
- Okabayashi, K.; Narita, T.; Takahashi, Y.; Sugiya, H. Effect of Oxidative Stress on Secretory Function in Salivary Gland Cells. In Oxidative Stress-Environmental Induction and Dietary Antioxidants; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Li, J.; Holbrook, N.J. Elevated gadd153/chop expression and enhanced c-Jun N-terminal protein kinase activation sensitizes aged cells to ER stress. Exp. Gerontol. 2004, 39, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, N.J.; Ikeyama, S. Age-related decline in cellular response to oxidative stress: Links to growth factor signaling pathways with common defects. Biochem. Pharmacol. 2002, 64, 999–1005. [Google Scholar] [CrossRef]
- Hussain, S.G.; Ramaiah, K.V. Reduced eIF2alpha phosphorylation and increased proapoptotic proteins in aging. Biochem. Biophys. Res. Commun. 2007, 355, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.; Duran-Aniotz, C.; Cabral-Miranda, F.; Vivar, J.P.; Hetz, C. Endoplasmic reticulum proteostasis impairment in aging. Aging Cell 2017, 16, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Paz Gavilan, M.; Vela, J.; Castano, A.; Ramos, B.; del Rio, J.C.; Vitorica, J.; Ruano, D. Cellular environment facilitates protein accumulation in aged rat hippocampus. Neurobiol. Aging. 2006, 27, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Nuss, J.E.; Choksi, K.B.; DeFord, J.H.; Papaconstantinou, J. Decreased enzyme activities of chaperones PDI and BiP in aged mouse livers. Biochem. Biophys. Res. Commun. 2008, 365, 355–361. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattarai, K.R.; Lee, H.-Y.; Kim, S.-H.; Park, J.-S.; Kim, H.-R.; Chae, H.-J. Potential Application of Ixeris dentata in the Prevention and Treatment of Aging-Induced Dry Mouth. Nutrients 2018, 10, 1989. https://doi.org/10.3390/nu10121989
Bhattarai KR, Lee H-Y, Kim S-H, Park J-S, Kim H-R, Chae H-J. Potential Application of Ixeris dentata in the Prevention and Treatment of Aging-Induced Dry Mouth. Nutrients. 2018; 10(12):1989. https://doi.org/10.3390/nu10121989
Chicago/Turabian StyleBhattarai, Kashi Raj, Hwa-Young Lee, Seung-Hyun Kim, Jong-Sug Park, Hyung-Ryong Kim, and Han-Jung Chae. 2018. "Potential Application of Ixeris dentata in the Prevention and Treatment of Aging-Induced Dry Mouth" Nutrients 10, no. 12: 1989. https://doi.org/10.3390/nu10121989
APA StyleBhattarai, K. R., Lee, H.-Y., Kim, S.-H., Park, J.-S., Kim, H.-R., & Chae, H.-J. (2018). Potential Application of Ixeris dentata in the Prevention and Treatment of Aging-Induced Dry Mouth. Nutrients, 10(12), 1989. https://doi.org/10.3390/nu10121989





