Carnosic Acid Modulates Increased Hepatic Lipogenesis and Adipocytes Differentiation in Ovariectomized Mice Fed Normal or High-Fat Diets
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Preparation of Blood and Tissue Samples
2.3. Ovariectomy Procedure
2.4. Histological Tissue Analysis
2.5. Serum Measurements
2.6. Real-Time Quantitative PCR Analysis
2.7. Protein Extraction and Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. Body and Fat Tissue Weights
3.2. Serum Measurements
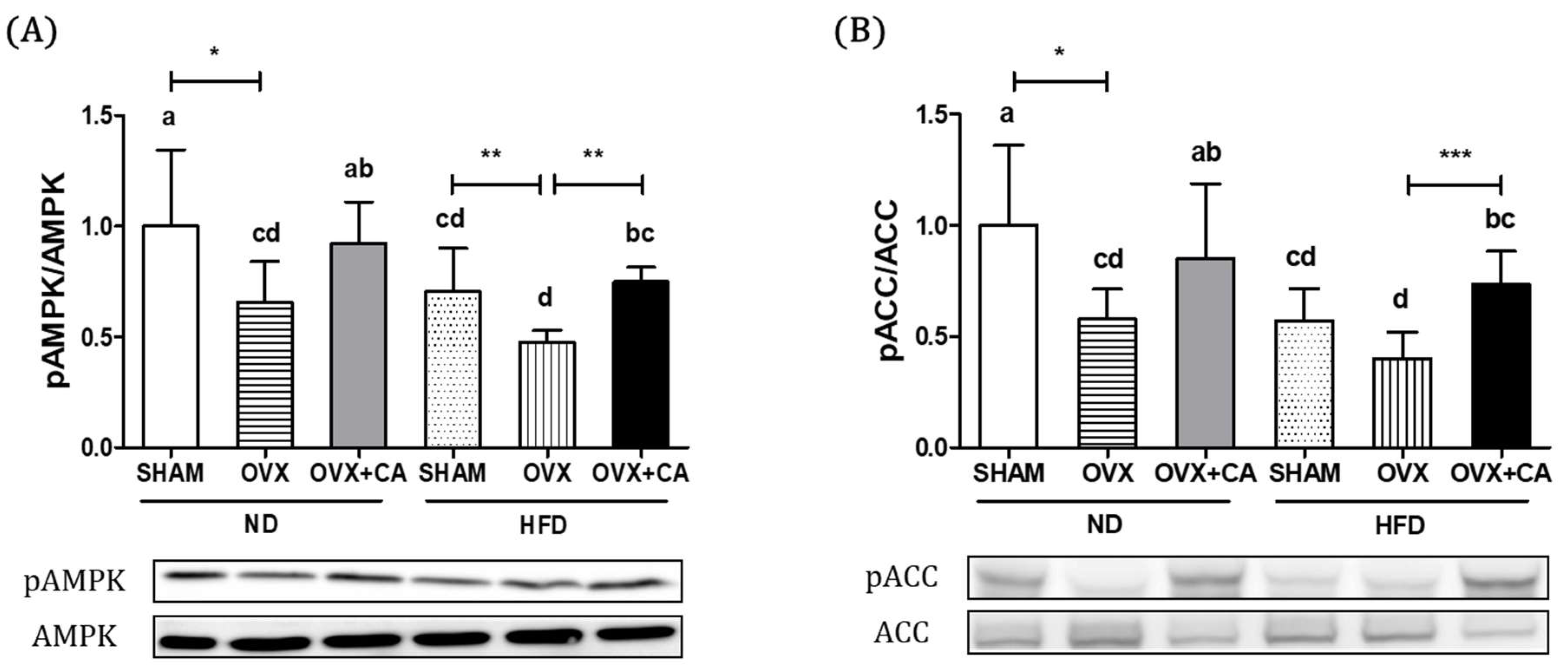
3.3. Expression of Liver AMPK and ACC Protein
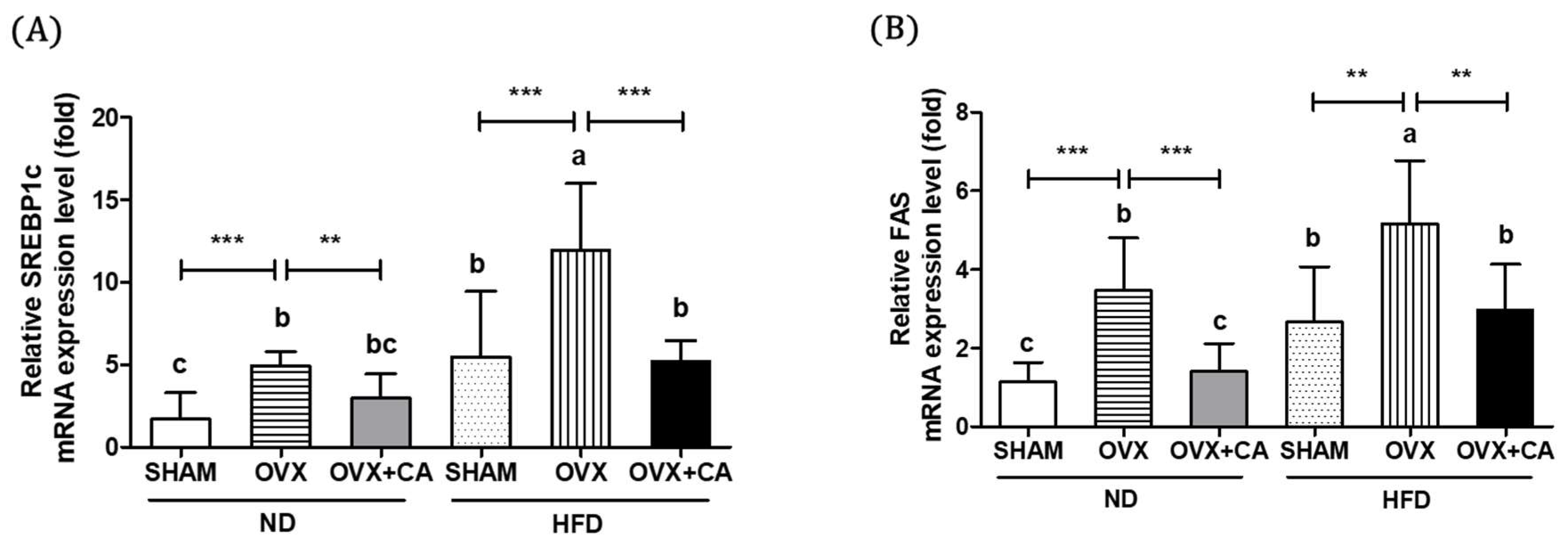
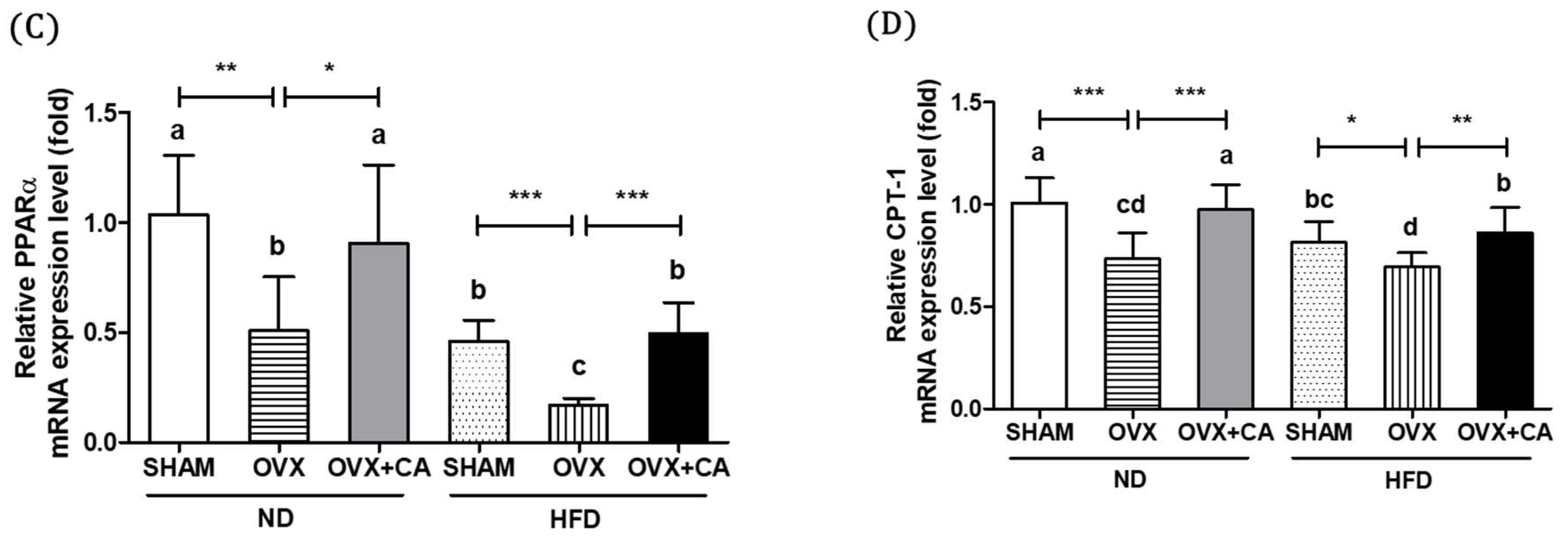
3.4. Expression of Liver Lipogenesis-Related Genes
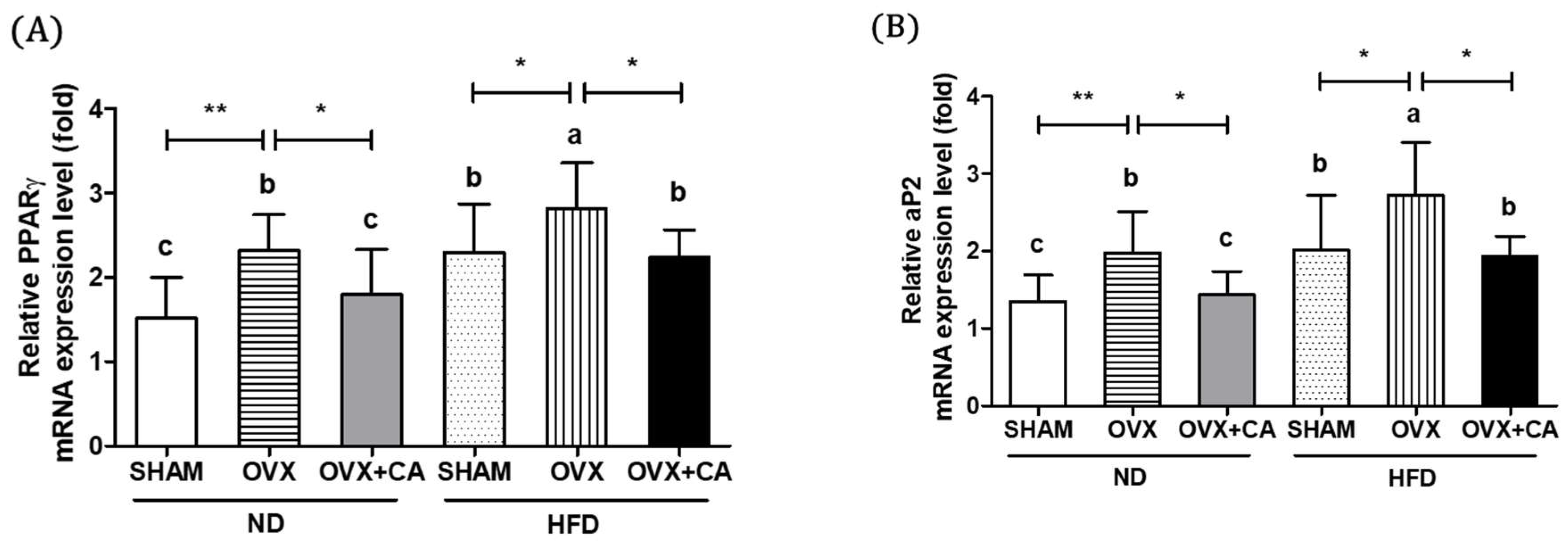
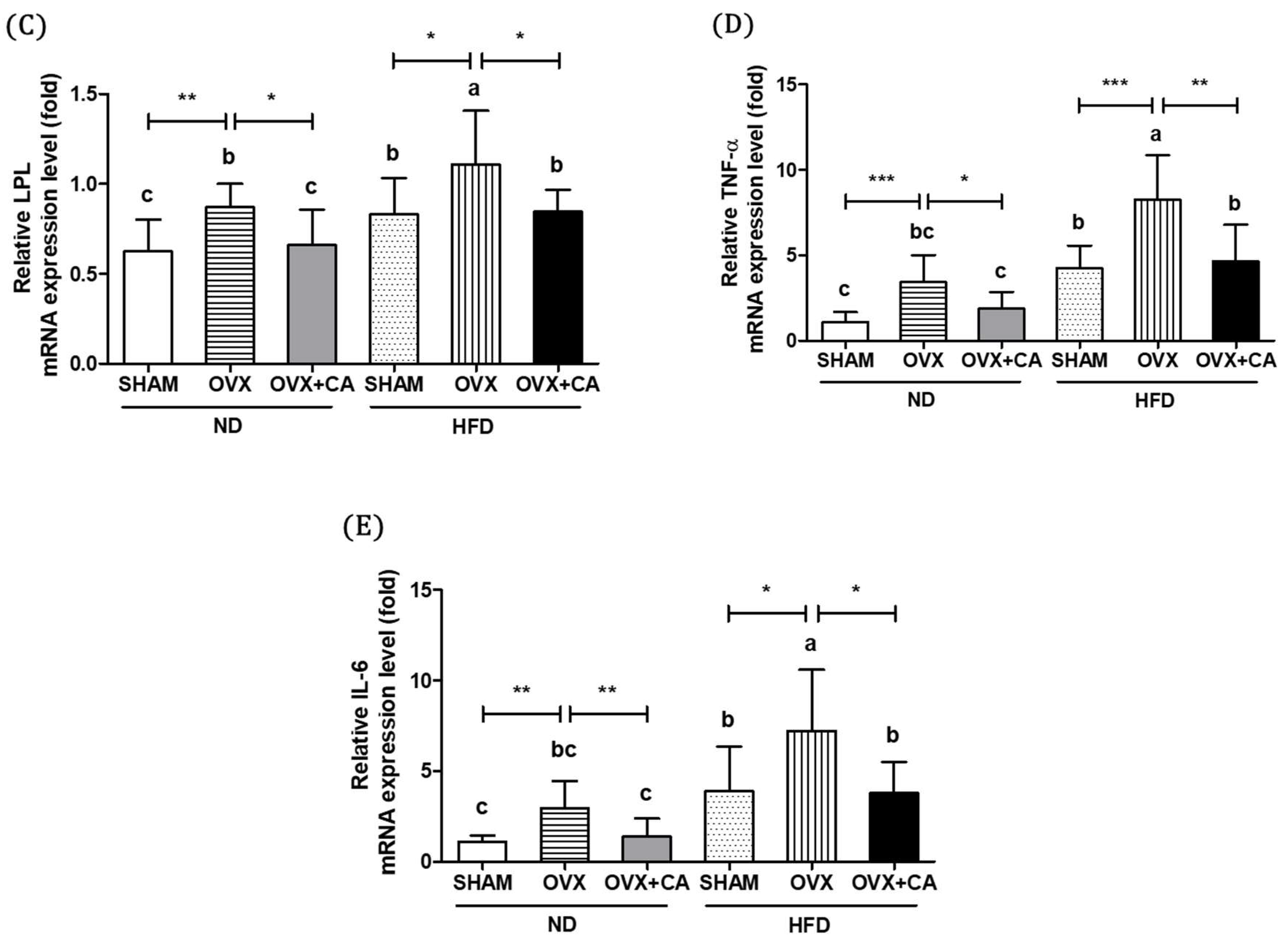
3.5. Expression of Adipocyte Differentiation- and Fat Accumulation-Related Genes in WAT
3.6. Expression of WAT Pro-Inflammatory Genes
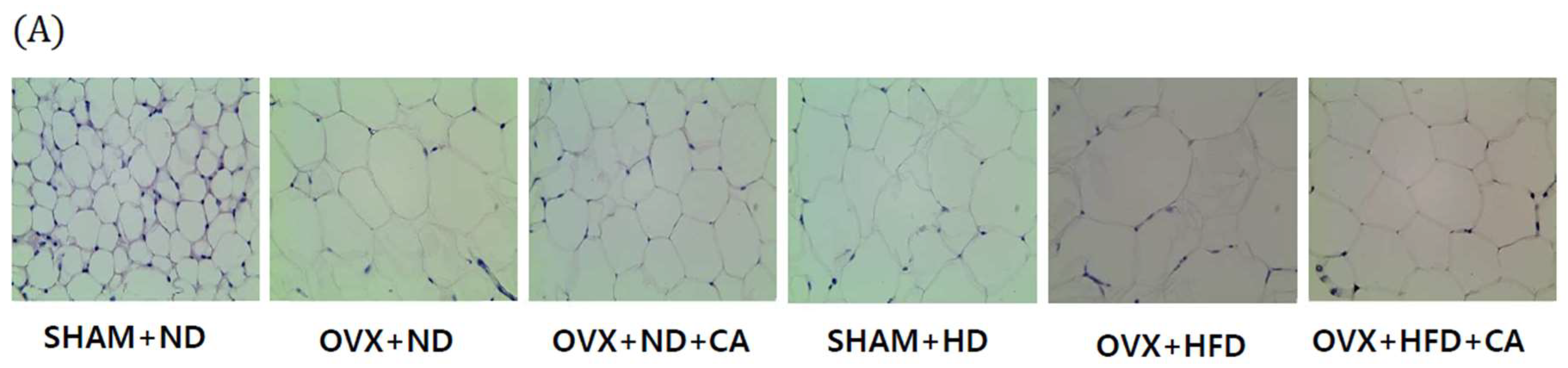
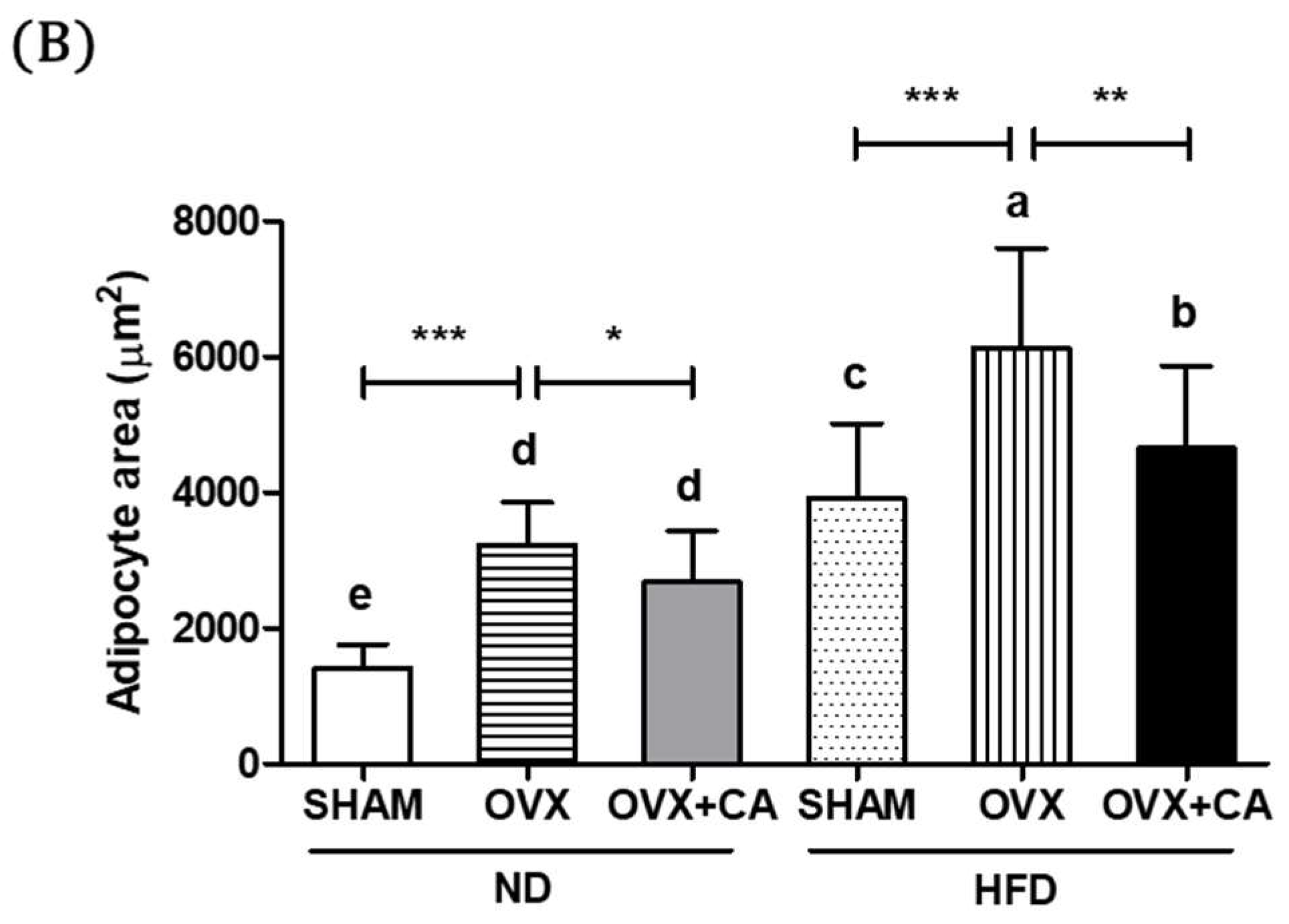
3.7. Adipocyte Size: Histological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- You, T.; Ryan, A.S.; Nicklas, B.J. The metabolic syndrome in obese postmenopausal women: Relationship to body composition, visceral fat, and inflammation. J. Clin. Endocrinol. Metab. 2004, 89, 5517–5522. [Google Scholar] [CrossRef] [PubMed]
- Stubbins, R.E.; Holcomb, V.B.; Hong, J.; Nunez, N.P. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur. J. Nutr. 2012, 51, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, J.R.; Dennerstein, L.; Dudley, E.C. Weight gain and the menopause: A 5-year prospective study. Climacteric 1999, 2, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.J.; Tchernof, A.; Sites, C.K.; Poehlman, E.T. Effect of menopausal status on body composition and abdominal fat distribution. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.A. Metabolic syndrome after menopause and the role of hormones. Maturitas 2008, 60, 10–18. [Google Scholar] [CrossRef] [PubMed]
- D’Eon, T.M.; Souza, S.C.; Aronovitz, M.; Obin, M.S.; Fried, S.K.; Greenberg, A.S. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J. Biol. Chem. 2005, 280, 35983–35991. [Google Scholar] [CrossRef] [PubMed]
- Bryzgalova, G.; Lundholm, L.; Portwood, N.; Gustafsson, J.A.; Khan, A.; Efendic, S.; Dahlman-Wright, K. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E904–E912. [Google Scholar] [CrossRef] [PubMed]
- Mosca, L.; Barrett-Connor, E.; Wenger, N.K. Sex/gender differences in cardiovascular disease prevention: What a difference a decade makes. Circulation 2011, 124, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.A. Diet and breast cancer: understanding risks and benefits. Nutr. Clin. Pract. 2012, 27, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Riant, E.; Waget, A.; Cogo, H.; Arnal, J.F.; Burcelin, R.; Gourdy, P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology 2009, 150, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.D.; Humphrey, L.L.; Nygren, P.; Teutsch, S.M.; Allan, J.D. Postmenopausal hormone replacement therapy: Scientific review. JAMA 2002, 288, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Cobin, R.H.; Goodman, N.F. American association of clinical endocrinologists and American college of endocrinology position statement on menopause-2017 update. Endocr. Pract. 2017, 23, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Harach, T.; Aprikian, O.; Monnard, I.; Moulin, J.; Membrez, M.; Beolor, J.C.; Raab, T.; Mace, K.; Darimont, C. Rosemary (Rosmarinus officinalis L.) leaf extract limits weight gain and liver steatosis in mice fed a high-fat diet. Planta. Med. 2010, 76, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Matsuda, H.; Shimoda, H.; Nishida, N.; Kasajima, N.; Yoshino, T.; Morikawa, T.; Yoshikawa, M. Carnosic acid, a new class of lipid absorption inhibitor from sage. Bioorg. Med. Chem. Lett. 2004, 14, 1943–1946. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Takikawa, Y.; Satoh, T.; Yoshioka, Y.; Kosaka, K.; Tatemichi, Y.; Suzuki, K. Carnosic acid prevents obesity and hepatic steatosis in ob/ob mice. Hepatol. Res. 2011, 41, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Mun, S.T. Dietary carnosic acid suppresses hepatic steatosis formation via regulation of hepatic fatty acid metabolism in high-fat diet-fed mice. Nutr. Res. Pract. 2013, 7, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Grady, D.; Rubin, S.M.; Petitti, D.B.; Fox, C.S.; Black, D.; Ettinger, B.; Ernster, V.L.; Cummings, S.R. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann. Intern. Med. 1992, 117, 1016–1037. [Google Scholar] [CrossRef] [PubMed]
- Psaty, B.M.; Heckbert, S.R.; Atkins, D.; Lemaitre, R.; Koepsell, T.D.; Wahl, P.W.; Siscovick, D.S.; Wagner, E.H. The risk of myocardial infarction associated with the combined use of estrogens and progestins in postmenopausal women. Arch. Intern. Med. 1994, 154, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Sidney, S.; Petitti, D.B.; Quesenberry, C.P., Jr. Myocardial infarction and the use of estrogen and estrogen-progestogen in postmenopausal women. Ann. Intern. Med. 1997, 127, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Hulley, S.; Grady, D.; Bush, T.; Furberg, C.; Herrington, D.; Riggs, B.; Vittinghoff, E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998, 280, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Hsia, J.; Johnson, K.C.; Rossouw, J.E.; Assaf, A.R.; Lasser, N.L.; Trevisan, M.; Black, H.R.; Heckbert, S.R.; Detrano, R.; et al. Estrogen plus progestin and the risk of coronary heart disease. N. Engl. J. Med. 2003, 349, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.V.; Van Horn, L.; Hsia, J.; Manson, J.E.; Stefanick, M.L.; Wassertheil-Smoller, S.; Kuller, L.H.; LaCroix, A.Z.; Langer, R.D.; Lasser, N.L.; et al. Low-fat dietary pattern and risk of cardiovascular disease: The women’s health initiative randomized controlled dietary modification trial. JAMA 2006, 295, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Harp, J.B. New insights into inhibitors of adipogenesis. Curr. Opin. Lipidol. 2004, 15, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Perez-Llamas, F.; Baraza, J.C.; Garcia-Prieto, M.D.; Fardy, P.S.; Tebar, F.J.; Zamora, S. Body fat distribution in pre-and post-menopausal women: metabolic and anthropometric variables. J. Nutr. Health Aging 2002, 6, 123–126. [Google Scholar] [PubMed]
- Zamboni, M.; Armellini, F.; Milani, M.P.; De Marchi, M.; Todesco, T.; Robbi, R.; Bergamo-Andreis, I.A.; Bosello, O. Body fat distribution in pre- and post-menopausal women: Metabolic and anthropometric variables and their inter-relationships. Int. J. Obes. Relat. Metab. Disord. 1992, 16, 495–504. [Google Scholar] [PubMed]
- Yamatani, H.; Takahashi, K.; Yoshida, T.; Soga, T.; Kurachi, H. Differences in the fatty acid metabolism of visceral adipose tissue in postmenopausal women. Menopause 2014, 21, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Despres, J.P.; Moorjani, S.; Lupien, P.J.; Tremblay, A.; Nadeau, A.; Bouchard, C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis 1990, 10, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Strissel, K.J.; Stancheva, Z.; Miyoshi, H.; Perfield, J.W., 2nd; DeFuria, J.; Jick, Z.; Greenberg, A.S.; Obin, M.S. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 2007, 56, 2910–2918. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, I.S.; Choue, R. Obesity, inflammation and diet. Pediatr. Gastroenterol. Hepatol. Nutr. 2013, 16, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Mattiasson, I.; Rendell, M.; Tornquist, C.; Jeppsson, S.; Hulthen, U.L. Effects of estrogen replacement therapy on abdominal fat compartments as related to glucose and lipid metabolism in early postmenopausal women. Horm. Metab. Res. 2002, 34, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Sung, M.K. Carnosic acid attenuates obesity-induced glucose intolerance and hepatic fat accumulation by modulating genes of lipid metabolism in C57BL/6J-ob/ob mice. J. Sci. Food Agric. 2015, 95, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Sung, M.K. Carnosic acid inhibits lipid accumulation in 3T3-L1 adipocytes through attenuation of fatty acid desaturation. J. Cancer Prev. 2015, 20, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. Sensing of energy and nutrients by AMP-activated protein kinase. Am. J. Clin. Nutr. 2011, 93, 891S–896S. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Andreelli, F.; Jorgensen, S.B.; Perrin, C.; Geloen, A.; Flamez, D.; Mu, J.; Lenzner, C.; Baud, O.; Bennoun, M.; et al. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J. Clin. Investig. 2003, 111, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, S.B.; Wojtaszewski, J.F.; Viollet, B.; Andreelli, F.; Birk, J.B.; Hellsten, Y.; Schjerling, P.; Vaulont, S.; Neufer, P.D.; Richter, E.A.; et al. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 2005, 19, 1146–1148. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Flores, L.M.; Lopez-Briones, S.; Macias-Cervantes, M.H.; Ramirez-Emiliano, J.; Perez-Vazquez, V. A PPARgamma, NF-kappaB and AMPK-dependent mechanism may be involved in the beneficial effects of curcumin in the diabetic db/db mice liver. Molecules 2014, 19, 8289–8302. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Xu, H.; Li, H.; Zhao, Y.; Hu, X.; Zhao, J.; Guo, X.; Guo, T.; Botchlett, R.; Qi, T.; et al. Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity. PLoS ONE 2014, 9, e91111. [Google Scholar] [CrossRef] [PubMed]
- Wohlers, L.M.; Sweeney, S.M.; Ward, C.W.; Lovering, R.M.; Spangenburg, E.E. Changes in contraction-induced phosphorylation of AMP-activated protein kinase and mitogen-activated protein kinases in skeletal muscle after ovariectomy. J. Cell. Biochem. 2009, 107, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Lee, Y.M.; Lam, K.K.; Wu, Y.C.; Yen, M.H.; Cheng, P.Y. The role of hypothalamic AMP-activated protein kinase in ovariectomy-induced obesity in rats. Menopause 2010, 17, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Guigas, B.; Leclerc, J.; Hebrard, S.; Lantier, L.; Mounier, R.; Andreelli, F.; Foretz, M. AMP-activated protein kinase in the regulation of hepatic energy metabolism: From physiology to therapeutic perspectives. Acta Physiol. 2009, 196, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.A.; Lamos, E.M.; Davis, S.N. A review of the efficacy and safety of oral antidiabetic drugs. Expert Opin. Drug. Saf. 2013, 12, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.G.; Park, C.Y. Anti-Obesity Drugs: A review about their effects and safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Li, H.; Li, X.X.; Cui, C.Y.; Wang, R.; Yu, N.X.; Chen, L.X. Acute and 30-day oral toxicity studies of administered carnosic acid. Food Chem. Toxicol. 2012, 50, 4348–4355. [Google Scholar] [CrossRef] [PubMed]
- Pettinelli, P.; Del Pozo, T.; Araya, J.; Rodrigo, R.; Araya, A.V.; Smok, G.; Csendes, A.; Gutierrez, L.; Rojas, J.; Korn, O.; et al. Enhancement in liver SREBP-1c/PPAR-alpha ratio and steatosis in obese patients: correlations with insulin resistance and n-3 long-chain polyunsaturated fatty acid depletion. Biochim. Biophys. Acta 2009, 1792, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.I.; Lee, J.; Choi, R.Y.; Lee, H.I.; Lee, J.H.; Jeong, Y.K.; Kim, M.J.; Lee, M.K. Anti-obesity and anti-insulin resistance effects of tomato vinegar beverage in diet-induced obese mice. Food Funct. 2014, 5, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Yoon, M. 17beta-Estradiol inhibition of PPARgamma-induced adipogenesis and adipocyte-specific gene expression. Acta Pharmacol. Sin. 2011, 32, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Tiyerili, V.; Muller, C.F.; Fung, S.; Panek, D.; Nickenig, G.; Becher, U.M. Estrogen improves vascular function via peroxisome-proliferator-activated-receptor-gamma. J. Mol. Cell. Cardiol. 2012, 53, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Widenmaier, S.B.; Schlein, C.; Johann, K.; Goncalves, R.L.S.; Eguchi, K.; Fischer, A.W.; Parlakgül, G.; Snyder, N.A.; Nguyen, T.B.; et al. Brown adipose tissue termogenic adaptation requires Nrf1-mediated proteasomal activity. Nat. Med. 2018, 24, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Romo Vaquero, M.I.; Yáñez-Gascón, M.J.; García Villalba, R.; Larrosa, M.; Fromentin, E.; Ibarra, A.; Roller, M.; Tomás-Barberán, F.; Espín de Gea, J.C.; García-Conesa, M.T. Inhibition of gastric lipase as a mechanism for body weight and plasma lipids reduction in Zucker rats fed a rosemary extract rich in carnosic acid. PLoS ONE 2012, 7, e3977. [Google Scholar] [CrossRef] [PubMed]
| Group | ||||||
|---|---|---|---|---|---|---|
| SHAM and ND | OVX and ND | OVX and ND and CA | SHAM and HFD | OVX and HFD | OVX and HFD and CA | |
| Body weight(g) | 22.82 ± 1.84 c | 26.70 ± 1.36 b | 24.45 ± 1.12 c | 28.11 ± 3.45 b | 32.32 ± 4.79 a | 28.06 ± 3.15 b |
| Uterus weight (g) | 0.46 ± 0.10 a | 0.11 ± 0.10 c | 0.11 ± 0.12 c | 0.36 ± 0.09 b | 0.08 ± 0.09 c | 0.11 ± 0.14 c |
| Total fat (% of BW 1) | 3.18 ± 1.24 d | 7.02 ± 1.92 b,c | 5.25 ± 2.02 c,d | 8.61 ± 3.71 b | 13.27 ± 6.88 a | 9.49 ± 4.09 b |
| Retroperitoneal fat | 0.77 ± 0.44 d | 2.04 ± 0.59 b,c | 1.50 ± 0.76 c,d | 2.44 ± 1.13 b,c | 3.88 ± 2.08 a | 2.95 ± 1.52 a,b |
| Gonadal fat | 0.60 ± 0.37 d | 1.23 ± 0.56 b,c,d | 0.99 ± 0.50 c,d | 1.69 ± 0.66 b | 2.52 ± 1.49 a | 1.58 ± 0.76 b,c |
| Perirenal fat | 0.40 ± 0.12 d | 1.03 ± 0.33 c | 0.70 ± 0.27 c,d | 1.17 ± 0.48 c,b | 2.15 ± 1.10 a | 1.54 ± 0.72 b |
| Mesenteric fat | 0.57 ± 0.35 d | 1.08 ± 0.34 b,c | 0.78 ± 0.32 c,d | 1.34 ± 0.40 b | 1.78 ± 0.86 a | 1.15 ± 0.55 c,b |
| Mammary fat pad | 0.83 ± 0.21 d | 1.63 ± 0.49 b,c | 1.28 ± 0.42 c,d | 1.97 ± 0.68 b,c | 2.95 ± 1.75 a | 2.07 ± 0.79 b |
| Parameter | Group | |||||
|---|---|---|---|---|---|---|
| SHAM and ND | OVX and ND | OVX and ND and CA | SHAM and HFD | OVX and HFD | OVX and HFD and CA | |
| Estradiol (pg/mL) | 18.92 ± 2.37 a | 5.97 ± 1.93 b | 7.01 ± 2.06 b | 18.99 ± 2.21 a | 6.52 ± 1.56 b | 7.43 ± 1.78 b |
| Insulin (ng/mL) | 1.33 ± 0.29 c | 1.82 ± 0.49 c | 1.55 ± 0.34 c | 3.24 ± 0.59 b | 4.54 ± 1.42 a | 3.40 ± 0.43 b |
| Leptin (ng/mL) | 0.59 ± 0.38 c | 1.61 ± 0.81 b | 1.05 ± 0.58 b,c | 1.62 ± 0.72 b | 2.32 ± 0.90 a | 1.40 ± 0.73 b |
| TG (mg/dL) | 56.97 ± 22.37 d | 133.51 ± 74.22 c | 102.61 ± 63.99 c,d | 214.89 ± 78.12 b | 273.43 ± 72.97 a | 189.71 ± 82.33 b |
| FFA (mEq/L) | 0.81 ± 0.18 c | 1.06 ± 0.18 b,c | 0.82 ± 0.06 c | 1.10 ± 0.20 b | 1.39 ± 0.08 a | 0.98 ± 0.27 b,c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-H.; Lim, W.; Sung, M.-K. Carnosic Acid Modulates Increased Hepatic Lipogenesis and Adipocytes Differentiation in Ovariectomized Mice Fed Normal or High-Fat Diets. Nutrients 2018, 10, 1984. https://doi.org/10.3390/nu10121984
Lee Y-H, Lim W, Sung M-K. Carnosic Acid Modulates Increased Hepatic Lipogenesis and Adipocytes Differentiation in Ovariectomized Mice Fed Normal or High-Fat Diets. Nutrients. 2018; 10(12):1984. https://doi.org/10.3390/nu10121984
Chicago/Turabian StyleLee, Yoon-Hee, Whasun Lim, and Mi-Kyung Sung. 2018. "Carnosic Acid Modulates Increased Hepatic Lipogenesis and Adipocytes Differentiation in Ovariectomized Mice Fed Normal or High-Fat Diets" Nutrients 10, no. 12: 1984. https://doi.org/10.3390/nu10121984
APA StyleLee, Y.-H., Lim, W., & Sung, M.-K. (2018). Carnosic Acid Modulates Increased Hepatic Lipogenesis and Adipocytes Differentiation in Ovariectomized Mice Fed Normal or High-Fat Diets. Nutrients, 10(12), 1984. https://doi.org/10.3390/nu10121984




