Iron Treatment May Be Difficult in Inflammatory Diseases: Inflammatory Bowel Disease as a Paradigm
Abstract
1. Introduction
2. Iron Homeostatic Hormone Hepcidin and Its Inflammatory Regulation
3. Prevalence of Anaemia in IBD
4. Iron Deficiency in IBD
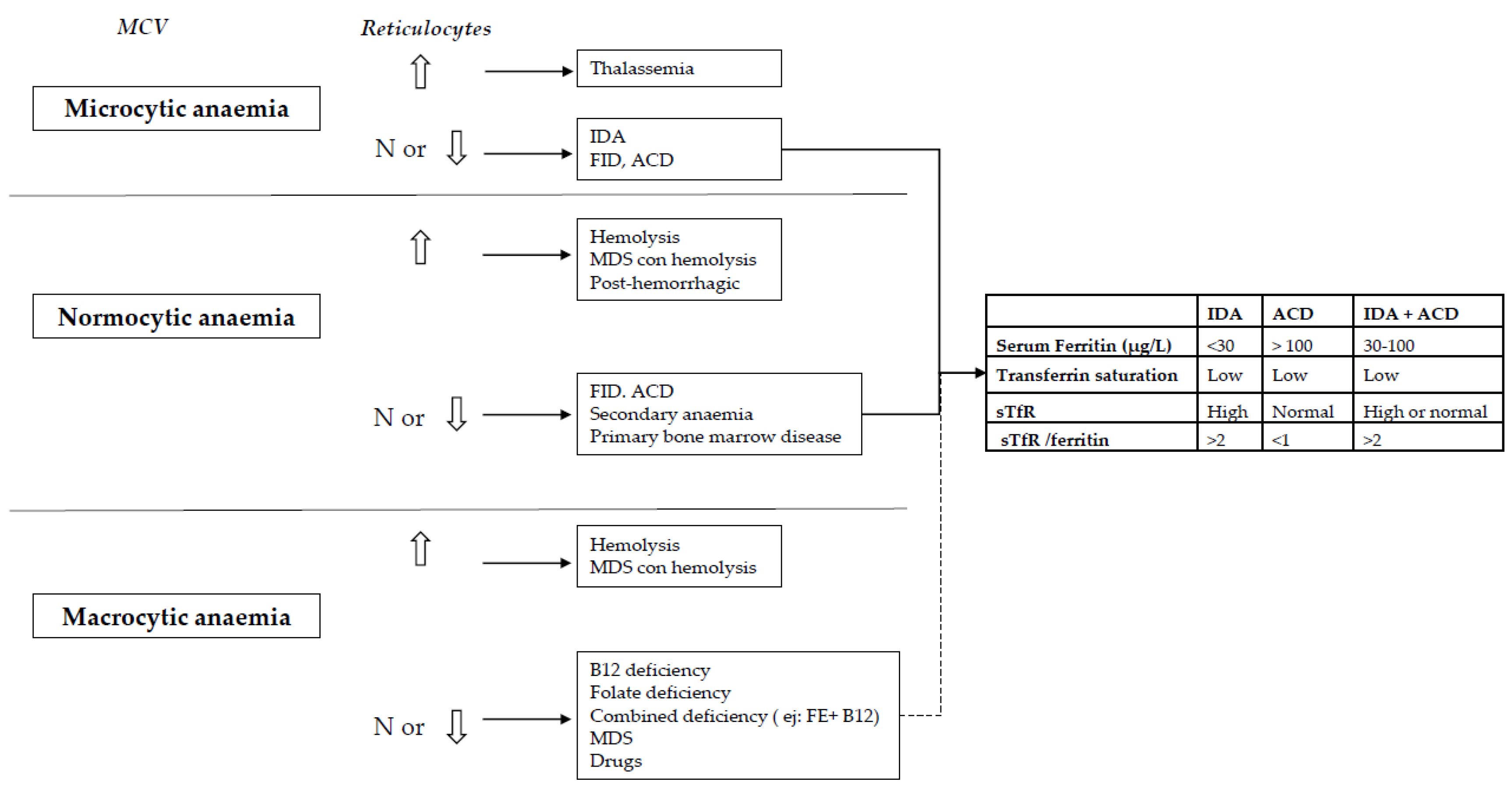
5. Diagnosis of Anaemia and Iron Deficiency in IBD
6. Treatment
6.1. Is it Necessary to Achieve IBD Remission for the Treatment of IDA?
6.2. When to Start Iron Supplementation in IBD Anaemic Patients and What Is the Goal?
6.3. What Is the Best Method of Iron Supplementation for IBD Patients? Oral or Intravenous Route?
6.3.1. Oral Iron in IBD
- (1)
- (2)
- Oral iron is often poorly tolerated by patients. The intolerance rate (mainly nausea, abdominal pain, or diarrhoea) is a frequent finding leading to discontinuation in up to 50% of patients [59,60]. Moreover, it is important to note that most IBD patients are receiving several drugs, and these side effects of oral iron could negatively affect the overall compliance [61]. In point of fact, it is plausible that bias partially explains oral iron efficacy in clinical trials, since patients with poor oral iron tolerance are probably under-represented in this setting.
- (3)
- Oral iron may be slow in filling iron stores and recovering anaemia. It can require months. A more rapid response is advisable in severe-moderate cases, particularly to make easy the fast return of the patient to an active way of life. Furthermore, in some patients, persistent intestinal blood loss is greater than the intestinal absorption of iron [18].
- (4)
- Finally, despite its crucial role in cellular processes, free colonic iron can generate toxic free radicals and reactive oxygen species, which can directly affect gut epithelial integrity via the promotion of redox stress [62]. Experimental evidence suggests that the excess of luminal iron can be harmful for the gut mucosa. In vitro studies with Caco-2 cells exposed to iron have shown an impaired epithelial integrity [63,64]. In several animal studies, free luminal iron has been shown to be directly harmful, pro-inflammatory, and even to favour carcinogenesis [19,65,66]. Wermer et al. show that free luminal iron favoured inflammation in the terminal ileum due to disturbances in the intestinal microbiota in a murine experimental model of IBD. On the contrary, intravenous iron did not result in intestinal mucosa lesions in the murine experimental model [67]. There is only one open-label clinical trial that has compared the effects of oral and intravenous iron replacement therapy on the gut microbiome and metabolome in patients with IBD. In this study, both oral and intravenous iron improved iron deficiency, but higher serum ferritin levels were reached with intravenous iron. Noticeable shifts in gut bacterial diversity were described in patients with IBD and iron deficiency following oral iron supplementation (however, other changes were also observed following intravenous treatment) and the gut microbiome in turn might have an effect on disease activity in IBD [68].
6.3.2. Intravenous Iron in IBD
- There is little experience with LMWID; moreover, a test dose is necessary, and in most cases it requires several hours to infuse the total dose, compared with shorter times for other preparations. In consequence, at the moment, this preparation cannot be recommended in IBD patients.
- IS has a dose limitation: a maximum dose of 200 mg per infusion can be given to prevent the release of potentially toxic free iron. As the iron deficit in IBD is frequently in the 1000–2500 mg range, usually 3 to 10 one-hour infusions are needed. By contrast, a dose of 1000 mg of FCM can be given in a single 15-min infusion. In 2011, Evstatiev and colleagues published a randomized, controlled non-inferiority trial to compare the use of FCM versus IS in out-patients with IBD and anaemia [94]. Both formulations were effective, well-tolerated, and demonstrated similar improvements in quality of life and in physical and mental components or more-specific IBD-related indexes. However, FCM was markedly better in correcting anaemia than IS; more patients on FCM had their haemoglobin levels increase by ≥20 g/L or achieved normalization than with IS. Moreover, FCM was much more suitable for patients. Of course, on a weight-by-weight basis, FCM formulation is more expensive than IS, but from an entire cycle of care perspective, FCM was cheaper.
- FXT is not approved in Europe for treatment of IDA in IBD patients, because it can interfere in the realization of magnetic resonance, a very useful imaging technique in our patients [38].
6.4. How Do We Estimate the Necessary Amount of Iron?
6.5. When to Use Erythropoiesis-Stimulating Agents and/or Blood Transfusion?
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Kulnigg, S.; Gasche, C. Systematic review: Managing anaemia in Crohn’s disease. Aliment. Pharmacol. Ther. 2006, 24, 1507–1523. [Google Scholar] [CrossRef] [PubMed]
- Gasche, C.; Berstad, A.; Befrits, R.; Beglinger, C.; Dignass, A.; Erichsen, K.; Gomollon, F.; Hjortswang, H.; Koutroubakis, I.; Kulnigg, S.; et al. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm. Bowel Dis. 2007, 13, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Gomollon, F. Common Misconceptions in the diagnosis and management of anemia in inflammatory bowel disease. Am. J. Gastroenterol. 2008, 103, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, L.T.; Weston, C.M.; Goldfarb, N.I.; Moretti, D.; Cobb, N.; Howell, J.B.; Infantolino, A.; Dimarino, A.J.; Cohen, S. Impact of chronic conditions on quality of life in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.W.; Lewis, S.; Barton, J.R.; Corbett, S. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm. Bowel Dis. 2006, 12, 123–130. [Google Scholar] [CrossRef]
- Cucino, C.; Sonnenberg, A. Cause of death in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2001, 7, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Duron, J.J.; Duron, E.; Dugue, T.; Pujol, J.; Muscari, F.; Collet, D.; Pessaux, P.; Hay, J.M. Risk factors for mortality in major digestive surgery in the elderly. Ann. Surg. 2011, 254, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.S.; Yu, C.S.; Yoon, Y.S.; Yoon, S.N.; Lim, S.B.; Kim, J.C. Risk factors for complications after bowel surgery in Korean patients with Crohn’s disease. J. Korean Surg. Soc. 2012, 83, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Systemic iron homeostasis. Physiol. Rev. 2013, 93, 1721–1741. [Google Scholar] [CrossRef] [PubMed]
- Sangkhae, V.; Nemeth, E. Regulation of the Iron Homeostatic Hormone Hepcidin. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Wessling-Resnick, M. Iron homeostasis and the inflammatory response. Annu. Rev. Nutr. 2010, 30, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Enculescu, M.; Metzendorf, C.; Sparia, R.; Hahnei, M.; Bode, J.; Muckenthaler, M.U.; Legewie, S. Modelling Systemic Iron Regulation during Dietary Iron Overload and Acute Inflammation: Role of Hepcidin-Independent Mechanisms. PLoS Comput. Biol. 2017, 13, e1005322. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.; Chiu, E.Y.; Hajjar, A.M.; Abkowitz, J.L. TLR stimulation dynamically regulates heme and iron export gene expression in macrophages. J. Immunol. Res. 2016, 2016, 4039038. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Schett, G. Anaemia in inflammatory rheumatic diseases. Nat. Rev. Rheumatol. 2013, 9, 205–215. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; United Nations Children’s Fund; United Nations University. Iron Deficiency Anemia: Assessment, Prevention and Control; Report of a Joint WHO/UNICEF/UNU Consultation; World Health Organization: Geneva, Switserland, 1998. [Google Scholar]
- Voetglin, M.; Vavricka, S.R.; Schoepfer, A.M.; Straumann, A.; Voetglin, J.; Rogler, G.; Ballabeni, P.; Pittel, V.; Buser, A.; Fried, M.; et al. Prevalence of anemia in inflammatory bowel disease in Switzerland a cross-sectional study in patients from private practice and university hospitals. J. Crohn’s Colitis 2010, 4, 642–648. [Google Scholar] [CrossRef]
- Bergamaschi, G.; Di Sabatino, A.; Albertini, R.; Ardizzone, S.; Biancheri, P.; Bonetti, E.; Cassinotti, A.; Cazzola, P.; Markopoulos, K.; Massari, A.; et al. Prevalence and pathogenesis of anemia in inflammatory bowel disease: Influence of anti-tumor necrosis factor-treatment. Haematologica 2010, 95, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Kaye, P.; Abdulla, K.; Wood, J.; James, P.; Foley, S.; Ragunath, K.; Atherton, J. Iron-induced mucosal pathology of the upper gastrointestinal tract: A common finding in patients on oral iron therapy. Histopathology 2008, 53, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Parsi, M.A.; Yerian, L.M. Iron ulcers. Clin. Gastroenterol. Hepatol. 2009, 7, A22. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, G.; Bukholm, G.; Jahnsen, J.; Moum, B.; Vatn, M.H.; IBSEN Study Group. The association between water supply and inflammatory bowel disease based on a 1990–1993 cohort study in Southeastern Norway. Am. J. Epidemiol. 2008, 168, 1065–1072. [Google Scholar] [CrossRef]
- Gomollón, F.; Gisbert, J.P. Intravenous iron in inflammatory bowel diseases. Curr. Opin. Gastroenterol. 2013, 29, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Begum, J.; Eksteen, B.; Elagib, A.; Brookes, M.; Cooper, B.T.; Tselepis, C.; Iqbal, T.H. Differential ferritin expression is associated with iron deficiency in coeliac disease. Eur. J. Gastroenterol. Hepatol. 2009, 21, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Bayele, H.; Johnston, K.; Tennant, J.; Srai, S.K.; Sharp, P. Tumour necrosis factor alpha regulates iron transport and transporter expression in human intestinal epithelial cells. FEBS Lett. 2004, 573, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.; Hartmann, F.; Dignass, A.U. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Bruner, A.B.; Joffe, A.; Duggan, A.K.; Casella, J.F.; Brandt, J. Randomised study of cognitive effects of iron supplementation in nonanaemic iron-deficient adolescent girls. Lancet 1996, 348, 992–996. [Google Scholar] [CrossRef]
- Lozoff, B.; Jimenez, E.; Smith, J.B. Double burden of iron deficiency in infancy and low socioeconomic status: A longitudinal analysis of cognitive test scores to age 19 years. Arch. Pediatr. Adolesc. Med. 2006, 160, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Verdon, F. Iron supplementation for unexplained fatigue in nonanaemic women: Double blind randomised placebo controlled trial. BMJ 2003, 326, 1124–1130. [Google Scholar] [CrossRef]
- Krayenbuehl, P.A.; Battegay, E.; Breymann, C.; Furrer, J.; Schulthess, G. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood 2011, 118, 3222–3227. [Google Scholar] [CrossRef]
- Vaucher, P.; Druais, P.-L.; Waldvogel, S.; Favrat, B. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: A randomized controlled trial. CMAJ 2012, 184, 1247–1254. [Google Scholar] [CrossRef]
- Hinton, P.S.; Sinclair, L.M. Iron supplementation maintains ventilatory threshold and improves energetic efficiency in iron-deficient nonanemic athletes. Eur. J. Clin. Nutr. 2006, 61, 30–39. [Google Scholar] [CrossRef]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Trinder, D. Athletic induced iron deficiency: New insights into the role of inflammation, cytokines and hormones. Eur. J. Appl. Physiol. 2008, 103, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Simanek, R.; Vormittag, R.; Ay, C.; Alguel, G.; Dunkler, D.; Schwarzinger, I.; Steger, G.; Jaeger, U.; Zielinski, C.; Pabinger, I. High platelet count associated with venous thromboembolism in cancer patients: Results from the Vienna Cancer and Thrombosis Study (CATS). J. Thromb. Haemost. 2010, 8, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Novacek, G.; Weltermann, A.; Sobala, A.; Tilg, H.; Petritsch, W.; Reinisch, W.; Mayer, A.; Haas, T.; Kaser, A.; Feichtenschlager, T.; et al. Inflammatory bowel disease is a risk factor for recurrent venous thromboembolism. Gastroenterology 2010, 139, 779–787. [Google Scholar] [CrossRef]
- Evstatiev, R.; Bukaty, A.; Jimenez, K.; Kulnigg-Dabsch, S.; Surman, L.; Schmid, W.; Eferl, R.; Lippert, K.; Scheiber-Mojdehkar, B.; Kvasnicka, H.M.; et al. Iron deficiency alters megakaryopoiesis and platelet phenotype independent of thrombopoietin. Am. J. Hematol. 2014, 89, 524–529. [Google Scholar] [CrossRef]
- Jimenez, K.; Khare, V.; Evstatiev, R.; Kulnigg-Dabsch, S.; Jambrich, M.; Strobl, H.; Gasche, C. Increased expression of HIF2α during iron deficiency-associated megakaryocytic differentiation. J. Thromb. Haemost. 2015, 13, 1113–1127. [Google Scholar] [CrossRef] [PubMed]
- Kulnigg-Dabsch, S.; Schmid, W.; Howaldt, S.; Stein, J.; Mickisch, O.; Waldhör, T.; Evstatiev, R.; Kamali, H.; Volf, I.; Gasche, C. Iron deficiency generates secondary thrombocytosis and platelet activation in IBD: The randomized, controlled thromboVIT trial. Inflamm. Bowel Dis. 2013, 19, 1609–1616. [Google Scholar] [CrossRef]
- Dignass, A.U.; Gasche, C.; Bettenworth, D.; Birgegård, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, K.; Koutroubakis, I.; et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J. Crohn’s Colitis 2015, 9, 211–222. [Google Scholar] [CrossRef]
- Taher, A.T.; Weatherall, D.J.; Cappellini, M.D. Thalassaemia. Lancet 2018, 391, 155–167. [Google Scholar] [CrossRef]
- Urrechaga, E.; Borque, L.; Escanero, J.F. Percentage of hypochromic erythrocytes as a potential marker of iron availability. Clin. Chem. Lab. Med. 2012, 50, 685–687. [Google Scholar] [CrossRef]
- Parodi, E.; Giraudo, M.T.; Davitto, M.; Ansaldi, G.; Mondino, A.; Garbarini, L.; Franzil, A.; Mazzone, R.; Russo, G.; Ramenghi, U. Reticulocyte parameters: Markers of early response to oral treatment in children with severe iron-deficiency anemia. J. Pediatr. Hematol. Oncol. 2012, 34, e249–e252. [Google Scholar] [CrossRef]
- Oldenburg, B.; Koningsberger, J.C.; Van Berge Henegouwen, G.P.; Koningsberger, J.C. Iron and inflammatory bowel disease. Aliment. Pharmacol. Ther. 2001, 15, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Tsiolakidou, G.; Koutroubakis, I.E. Stimulating erythropoiesis in inflammatory bowel disease associated anemia. World J. Gastroenterol. 2007, 13, 4798–4806. [Google Scholar] [CrossRef] [PubMed]
- Baillie, F.J.; Morrison, A.E.; Fergus, I. Soluble transferrin receptor: A discriminating assay for iron deficiency. Clin. Lab. Haematol. 2003, 25, 353–357. [Google Scholar] [CrossRef] [PubMed]
- WHO. Serum Transferrin Receptor Levels for the Assessment of Iron Status and Iron Deficiency in Populations. Vitamin and Mineral Nutrition Information System; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Punnonen, K.; Irjala, K.; Rajamaki, A. Serum transferrin receptor, ferritin and TfR-F index in identification of latent iron deficiency. Eur. J. Haematol. 1998, 60, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Harms, K.; Kaiser, T. Beyond soluble transferrin receptor: Old challenges and new horizons. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 799–810. [Google Scholar] [CrossRef]
- Junca, J.; Fernandez-Aviles, F.; Oriol, A.; Navarro, J.T.; Millá, F.; Sancho, J.M.; Feliu, E. The usefulness of the serum transferrin receptor in detecting iron deficiency in the anemia of chronic disorders. Haematologica 1998, 83, 676–680. [Google Scholar]
- Skikne, B.S.; Punnonen, K.; Caldron, P.H.; Bennett, M.T.; Rehu, M.; Gasior, G.H.; Chamberlin, J.S.; Sullivan, L.A.; Bray, K.R.; Southwick, P.C. Improved differential diagnosis of anemia of chronic disease and iron deficiency anemia: A prospective multicenter evaluation of soluble transferring receptor and the sTfR/log ferritin index. Am. J. Hematol. 2011, 86, 923–927. [Google Scholar] [CrossRef]
- Andrews, N.C. Forging a field: The golden age of iron biology. Blood 2008, 112, 219–230. [Google Scholar] [CrossRef]
- Mei, Z.; Cogswell, M.E.; Parvanta, I. Hemoglobin and ferritin are currently the most efficient indicators of population response to iron interventions: An analysis of nine randomized controlled trials. J. Nutr. 2005, 135, 1974–1980. [Google Scholar] [CrossRef]
- Gomollón, F.; Gisbert, J.P. Anemia and digestive diseases: An update for the clinician. World J. Gastroenterol. 2009, 15, 4615–4616. [Google Scholar] [CrossRef]
- Sharma, R.; Stanek, J.R.; Koch, T.L.; Grooms, L.; O´Brien, S.H. Intravenous iron therapy in non-anemic iron-deficient menstruating adolescent females with fatigue. Am. J. Hematol. 2016, 91, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Burden, R.J.; Morton, K.; Richards, T.; Whyte, G.P.; Pedlar, C.R. Is iron treatment beneficial in, iron-deficient but non-anaemic (IDNA) endurance athletes? A systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, T., IV; Utermohlen, V.; Hinton, P.S.; Giordano, C.; Haas, J.D. Marginal iron deficiency without anemia impairs aerobic adaptation among previously untrained women. Am. J. Clin. Nutr. 2002, 75, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Gasche, C.; Waldhoer, T.; Feichtenschlager, T.; Male, C.; Mayer, A.; Mittermaier, C.; Petritsch, W. Prediction of response to iron sucrose in inflammatory bowel disease-associated anemia. Am. J. Gastroenterol. 2001, 96, 2382–2387. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, N.D. Iron deficiency anemia in patients with inflammatory bowel disease. Clin. Exp. Gastroenterol. 2013, 6, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Semrin, G.; Fishman, D.S.; Bousvaros, A.; Zholudev, A.; Saunders, A.C.; Correia, C.E.; Nemeth, E.; Grand, R.J.; Weinstein, D.A. Impaired intestinal iron absorption in Crohn’s disease correlates with Treatment of Iron Deficiency in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2006, 12, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.; Powell, J.J. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0117383. [Google Scholar] [CrossRef]
- Rampton, D.S.; Goodhand, J.R.; Joshi, N.M.; Karim, A.B.; Koodun, Y.; Barakat, F.M.; Macken, L.; Ward, D.G.; Iqbal, T.H.; Epstein, J.; et al. Oral Iron Treatment Response and Predictors in Anaemic Adolescents and Adults with IBD: A Prospective Controlled Open-Label Trial. J. Crohn’s Colitis 2017, 11, 706–715. [Google Scholar] [CrossRef]
- Osterberg, L.; Blaschke, T. Adherence to medication. N. Engl. J. Med. 2005, 353, 487–497. [Google Scholar] [CrossRef]
- Lund, E.K.; Wharf, S.G.; Fairweather-Tait, S.J.; Johnson, I.T. Oral ferrous sulfate supplements increase the free radical-generating capacity of feces from healthy volunteers. Am. J. Clin. Nutr. 1999, 69, 250–255. [Google Scholar] [CrossRef]
- Ferruzza, S.; Scarino, M.L.; Gambling, L.; Natella, F.; Sambuy, Y. Biphasic effect of iron on human intestinal Caco-2 cells: Early effect on tight junction permeability with delayed onset of oxidative cytotoxic damage. Cell. Mol. Biol. 2003, 49, 89–99. [Google Scholar] [PubMed]
- Natoli, M.; Felsani, A.; Ferruzza, S.; Sambuy, Y.; Canali RScarino, M.L. Mechanisms of defence from Fe(II) toxicity in human intestinal Caco-2 cells. Toxicol. In Vitro 2009, 23, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82–83, 969–974. [Google Scholar] [CrossRef]
- Carrier, J.; Aghdassi, E.; Cullen, J.; Allard, J.P. Iron supplementation increases disease activity and vitamin E ameliorates the effect in rats with dextran sulfate sodium-induced colitis. J. Nutr. 2002, 132, 3146–3150. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.; Wagner, S.J.; Martinez, I.; Walter, J.; Chang, J.S.; Clavel, T.; Kisling, S.; Schuemann, K.; Haller, D. Depletion of luminal iron alters the gut microbiota and prevents Crohn’s disease-like ileitis. Gut 2011, 60, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Clavel, T.; Smirnov, K.; Schmidt, A.; Lagkouvardos, I.; Walker, A.; Lucio, M.; Michalke, B.; Schmitt-Kopplin, P.; Fedorak, R.; et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut 2017, 66, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.N. Oral and intravenous iron therapy in the anaemia of rheumatoid arthritis. Ann. Rheum. Dis. 1950, 9, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, S.; Bhandari, S. Oral Iron properties and current place in the treatment of anaemia. Prescriber 2012, 6, 12–18. [Google Scholar] [CrossRef]
- de Silva, A.D.; Mylonaki, M.; Ramptom, D.S. Oral iron therapy in inflammatory bowel disease: Usage, tolerance and effectivity. Inflamm. Bowel Dis. 2003, 9, 316–320. [Google Scholar] [CrossRef]
- Reifen, R.; Matas, Z.; Zeidel, L.; Berkovitch, Z.; Bujanover, Y. Iron supplementation may aggravate inflammatory status of colitis in a rat model. Dig. Dis. Sci. 2000, 45, 394–397. [Google Scholar] [CrossRef]
- Carrier, J.; Aghdassi, E.; Platt, I.; Cullen, J.; Allard, J.P. Effect of oral iron supplementation on oxidative stress and colonic inflammation in rats with induced colitis. Aliment. Pharmacol. Ther. 2001, 15, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Aghdassi, E.; Carrier, J.; Cullen, J.; Tischler, M.; Allard, J.P. Effect of iron supplementation on oxidative stress and intestinal inflammation in rats with acute colitis. Dig. Dis. Sci. 2001, 46, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, K.; Hausken, T.; Ulvik, R.J.; Svardal, A.; Berstad, A.; Berge, R.K. Ferrous fumarate deteriorated plasma antioxidant status in patients with Crohn disease. Scand. J. Gastroenterol. 2003, 38, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, K.; Ulvik, R.J.; Grimstad, T.; Berstad, A.; Berge, R.K.; Hausken, T. Effects of ferrous sulphate and non-ionic ironpolymaltose complex on markers of oxidative tissue damage in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2005, 22, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Rimon, E.; Kagansky, N.; Kagansky, M.; Mechnick, L.; Mashiah, T.; Namir, M.; Levy, S. Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am. J. Med. 2005, 118, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Bermejo, F.; Pajares, R.; Pérez-Calle, J.L.; Rodríguez, M.; Algaba, A.; Mancenido, N.; de la Morena, F.; Carneros, J.A.; McNicholl, A.G.; et al. Oral and intravenous iron treatment in inflammatory bowel disease: Haematological response and quality of life improvement. Inflamm. Bowel Dis. 2009, 15, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Moretti, D.; Goede, J.S.; Zeder, C.; Jiskra, M.; Chatzinakou, V.; Tjalsma, H.; Melse-Boonstra, A.; Brittenham, G.; Swinkels, D.W.; Zimmermann, M.B. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 2015, 126, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.J.; Cook, W.B.; Hutchinson, C.; Tolkien, Z.; Chatfield, M.; Pereira DIa Lomer, M.C. Dietary fortificant iron intake is negatively associated with quality of life in patients with mildly active inflammatory bowel disease. Nutr. Metab. 2013, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Okam, M.M.; Koch, T.A.; Tran, M.H. Iron Supplementation, Response in Iron-Deficiency Anemia: Analysis of Five Trials. Am. J. Med. 2017, 130, 991. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Ballard, H. Clinical use of intravenous iron: Administration, efficacy, and safety. Hematology 2011, 2010, 338–347. [Google Scholar] [CrossRef]
- Nissim, J.A. Toxic reactions after intravenous saccharated iron oxide in man. BMJ 1954, 1, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Coyne, D.; Ballard, H. Intravenous iron: From anathema to standard of care. Am. J. Hematol. 2008, 83, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Mason, P.D.; Vaage-Nilsen, O.; Ahlmén, J. Update on adverse drug events associated with parenteral iron. Nephrol. Dial. Transplant. 2006, 21, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Graham, D.J.; Kane, R.C.; Xie, D.; Wernecke, M.; Levenson, M.; MaCurdy, T.E.; Houstoun, M.; Ryan, Q.; Wong, S.; et al. Comparative Risk of Anaphylactic Reactions Associated with Intravenous Iron Products. JAMA 2015, 314, 2062–2068. [Google Scholar] [CrossRef]
- Hussain, I.; Bhoyroo, J.; Butcher, A.; Koch, T.A.; He, A.; Bregman, D.B. Direct Comparison of the Safety and Efficacy of Ferric Carboxymaltose versus Iron Dextran in Patients with Iron Deficiency Anemia. Anemia 2013, 2013, 169107. [Google Scholar] [CrossRef] [PubMed]
- Geisser, P.; Burckhardt, S. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics 2011, 3, 12–33. [Google Scholar] [CrossRef]
- Gomollón, F.; Gisbert, J.P. Current management of iron deficiency anemia in inflammatory bowel diseases: A practical guide. Drugs 2013, 73, 1761–1770. [Google Scholar] [CrossRef]
- Avni, T.; Bieber, A.; Steinmetz, T.; Leibovici, L.; Gafter-Gvili, A. Treatment of anemia in inflammatory bowel disease—Systematic review and meta-analysis. PLoS ONE 2013, 8, e75540. [Google Scholar] [CrossRef]
- Bonovas, S.; Fiorino, G.; Allocca, M.; Lytras, T.; Tsantes, A.; Peyrin-Biroulet, L.; Danese, S. Intravenous Versus Oral Iron for the Treatment of Anemia in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine 2016, 95, e2308. [Google Scholar] [CrossRef]
- Lee, T.W.; Kolber, M.R.; Fedorak, R.N.; van Zanten, S.V. Iron replacement therapy in inflammatory bowel disease patients with iron deficiency anemia: A systematic review and meta-analysis. J. Crohn’s Colitis 2012, 6, 267–275. [Google Scholar] [CrossRef]
- Aksan, A.; Isik, H.; Radeke, H.H.; Dignass, A.; Stein, J. Systematic review with network meta-analysis: Comparative efficacy and tolerability of different intravenous iron formulations for the treatment of iron deficiency anaemia in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 45, 1303–1318. [Google Scholar] [CrossRef] [PubMed]
- Evstatiev, R.; Marteau, P.; Iqbal, T.; Khalif, I.L.; Stein, J.; Bokemeyer, B.; Chopey, I.V.; Gutzwiller, F.S.; Riopel, L.; Gasche, C.; et al. FERGIcor: A randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology 2011, 141, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Gómez-Ramírez, S.; Besser, M.; Pavía, J.; Gomollón, F.; Liumbruno, G.M.; Bhandari, S.; Cladellas, M.; Shander, A.; Auerbach, M. Current misconceptions in diagnosis and management of iron deficiency. Blood Transfus. 2017, 15, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Kulnigg, S.; Teischinger, L.; Dejaco, C.; Waldhör, T.; Gasche, C. Rapid recurrence of IBD-associated anemia and iron deficiency after intravenous iron sucrose and erythropoietin treatment. Am. J. Gastroenterol. 2009, 104, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Evstatiev, R.; Alexeeva, O.; Bokemeyer, B.; Chopey, I.; Felder, M.; Gudehus, M.; Iqbal, T.; Khalif, I.; Marteau, P.; Stein, J.; et al. Ferric carboxymaltose prevents recurrence of anemia in patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2013, 11, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, W.; Altorjay, I.; Zsigmond, F.; Primas, C.; Vogelsang, H.; Novacek, G.; Reinisch, S.; Thomsen, L.L. A 1-year trial of repeated high-dose intravenous iron isomaltoside 1000 to maintain stable hemoglobin levels in inflammatory bowel disease. Scand. J. Gastroenterol. 2015, 50, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Goodnough, L.T.; Nemeth, E.; Ganz, T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood 2010, 116, 4754–4761. [Google Scholar] [CrossRef]
- Moreno López, R.; Sicilia Aladrén, B.; Gomollón, F. Use of agents stimulating erythropoiesis in digestive diseases. World J. Gastroenterol. 2009, 15, 4675–4685. [Google Scholar] [CrossRef]
| Body Weight ≥70 kg | Body Weight <70 kg | |
|---|---|---|
| Anaemia with Hb > 10 g/dL | 1500 mg | 1000 mg |
| Anaemia with Hb 7–10 g/dL | 2000 mg | 15000 mg |
| Anaemia with Hb < 7 g/dL | Probably 2500 mg | Probably 2000 mg |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gargallo-Puyuelo, C.J.; Alfambra, E.; García-Erce, J.A.; Gomollon, F. Iron Treatment May Be Difficult in Inflammatory Diseases: Inflammatory Bowel Disease as a Paradigm. Nutrients 2018, 10, 1959. https://doi.org/10.3390/nu10121959
Gargallo-Puyuelo CJ, Alfambra E, García-Erce JA, Gomollon F. Iron Treatment May Be Difficult in Inflammatory Diseases: Inflammatory Bowel Disease as a Paradigm. Nutrients. 2018; 10(12):1959. https://doi.org/10.3390/nu10121959
Chicago/Turabian StyleGargallo-Puyuelo, Carla J., Erika Alfambra, Jose Antonio García-Erce, and Fernando Gomollon. 2018. "Iron Treatment May Be Difficult in Inflammatory Diseases: Inflammatory Bowel Disease as a Paradigm" Nutrients 10, no. 12: 1959. https://doi.org/10.3390/nu10121959
APA StyleGargallo-Puyuelo, C. J., Alfambra, E., García-Erce, J. A., & Gomollon, F. (2018). Iron Treatment May Be Difficult in Inflammatory Diseases: Inflammatory Bowel Disease as a Paradigm. Nutrients, 10(12), 1959. https://doi.org/10.3390/nu10121959





