Botanical Formulation HX109 Ameliorates TP-Induced Benign Prostate Hyperplasia in Rat Model and Inhibits Androgen Receptor Signaling by Upregulating Ca2+/CaMKKβ and ATF3 in LNCaP Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of HX109
2.2. High-Performance Liquid Chromatography (HPLC) Analysis
2.3. Animals
2.4. TP-Induced Benign Prostate Hyperplasia Rat Model
2.5. H&E Staining
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Cell Culture and Reagents
2.8. RNA Isolation and qRT-PCR
2.9. Western Blot
2.10. Luciferase Reporter Plasmid Assay
2.11. Extraction of Nuclear and Cytoplasmic Fractions
2.12. siRNA Transfection
2.13. Calcium Assay
2.14. Statistical Analysis
3. Results
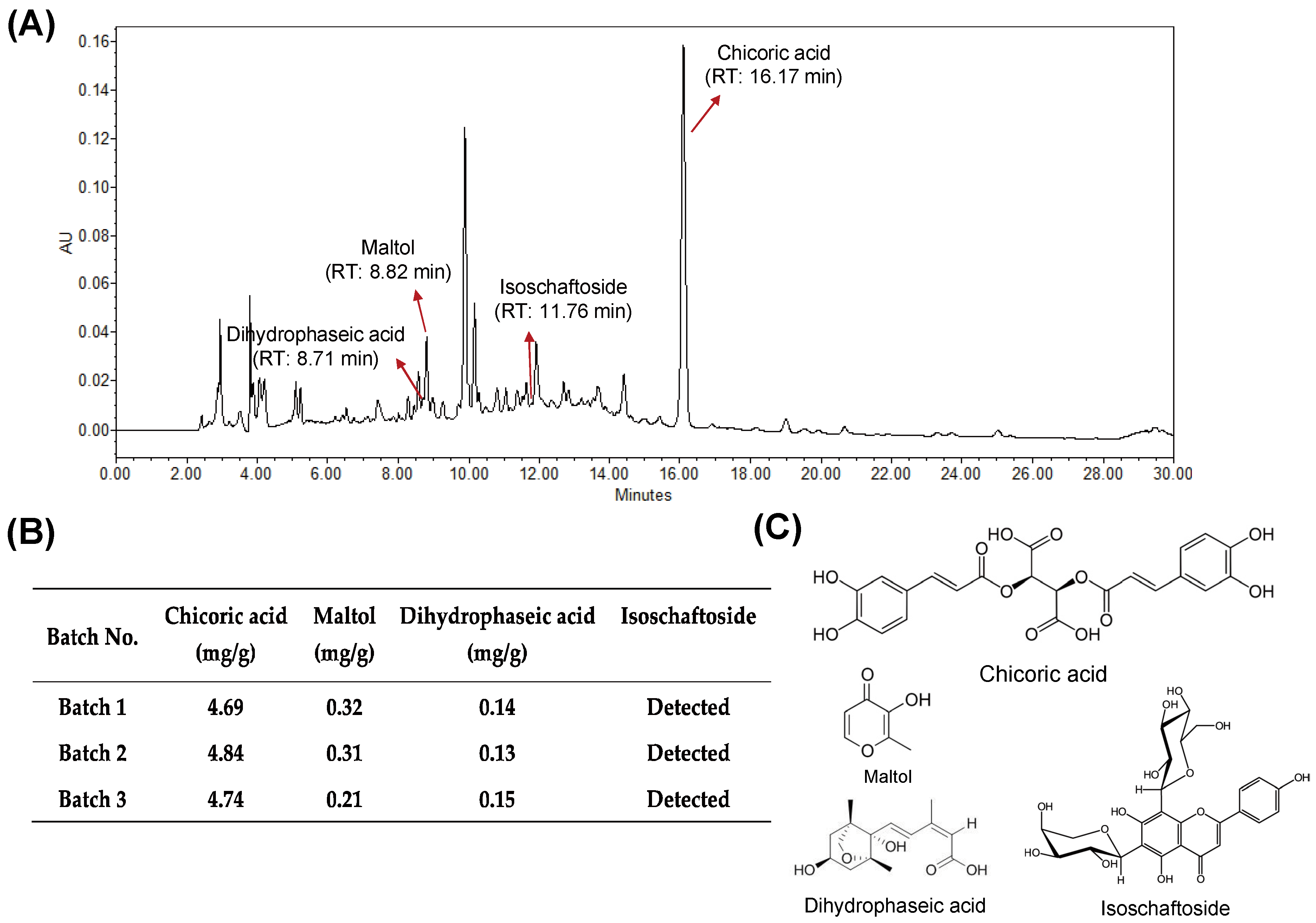
3.1. Quality of HX109 Is Monitored by HPLC Analysis and Cell-Based Bioassay
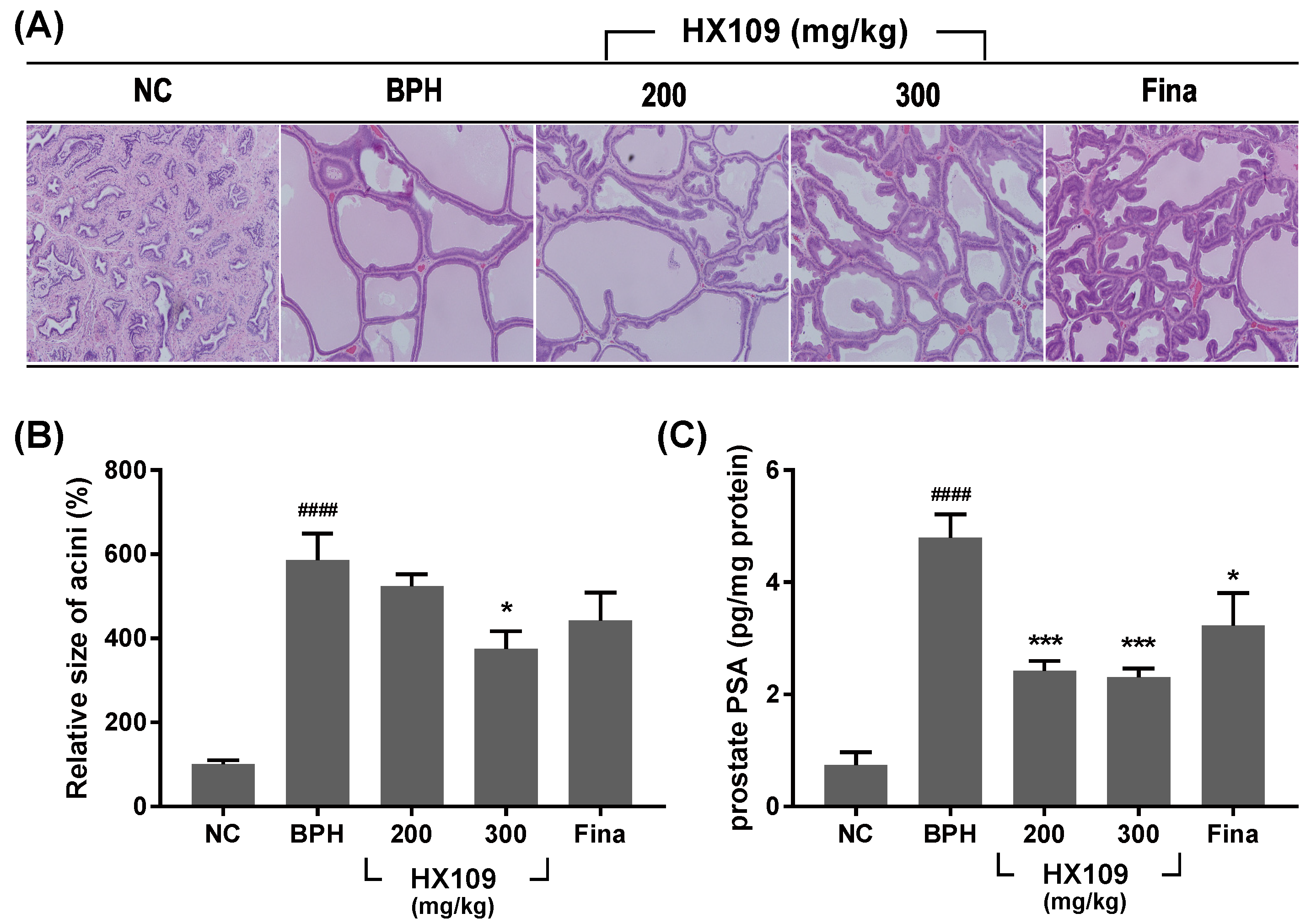
3.2. HX109 Ameliorates TP-Induced Benign Prostatic Hyperplasia in Castrated Sprague Dawley Rats
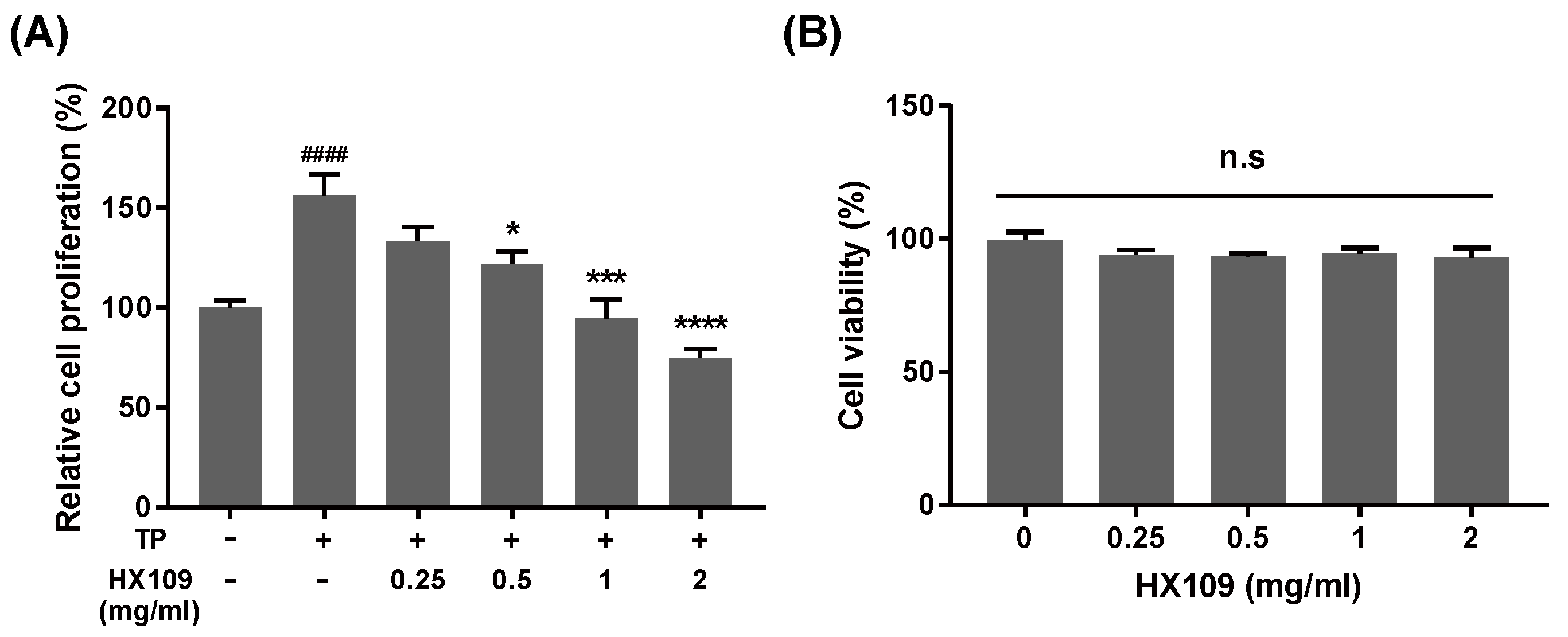
3.3. HX109 Suppresses Androgen-Dependent Proliferation of LNCaP Cells
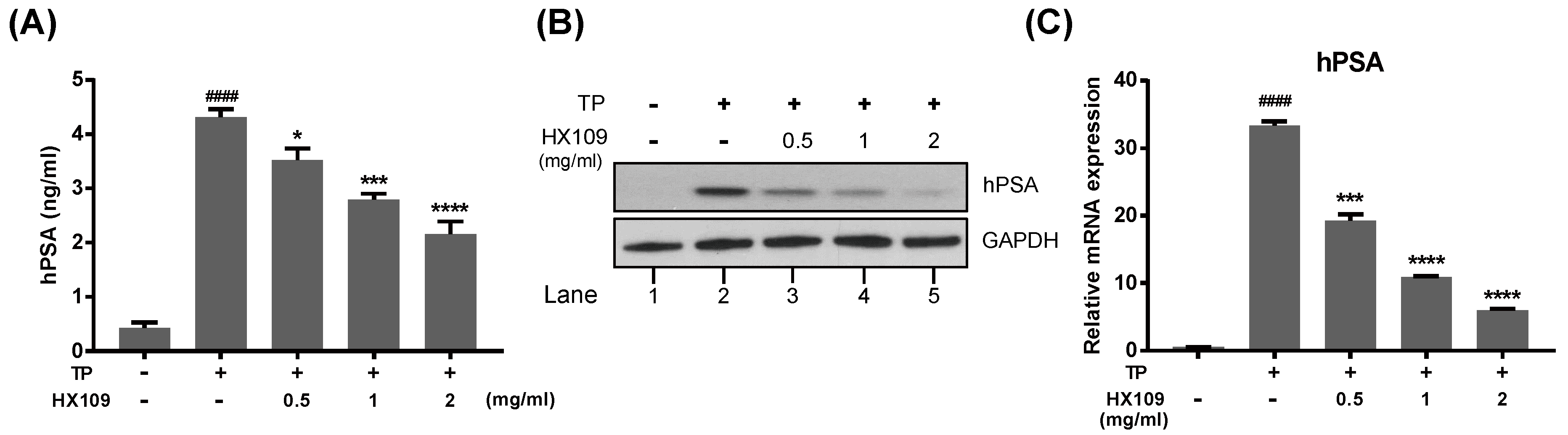
3.4. HX109 Inhibits Androgen-Induced PSA Expression
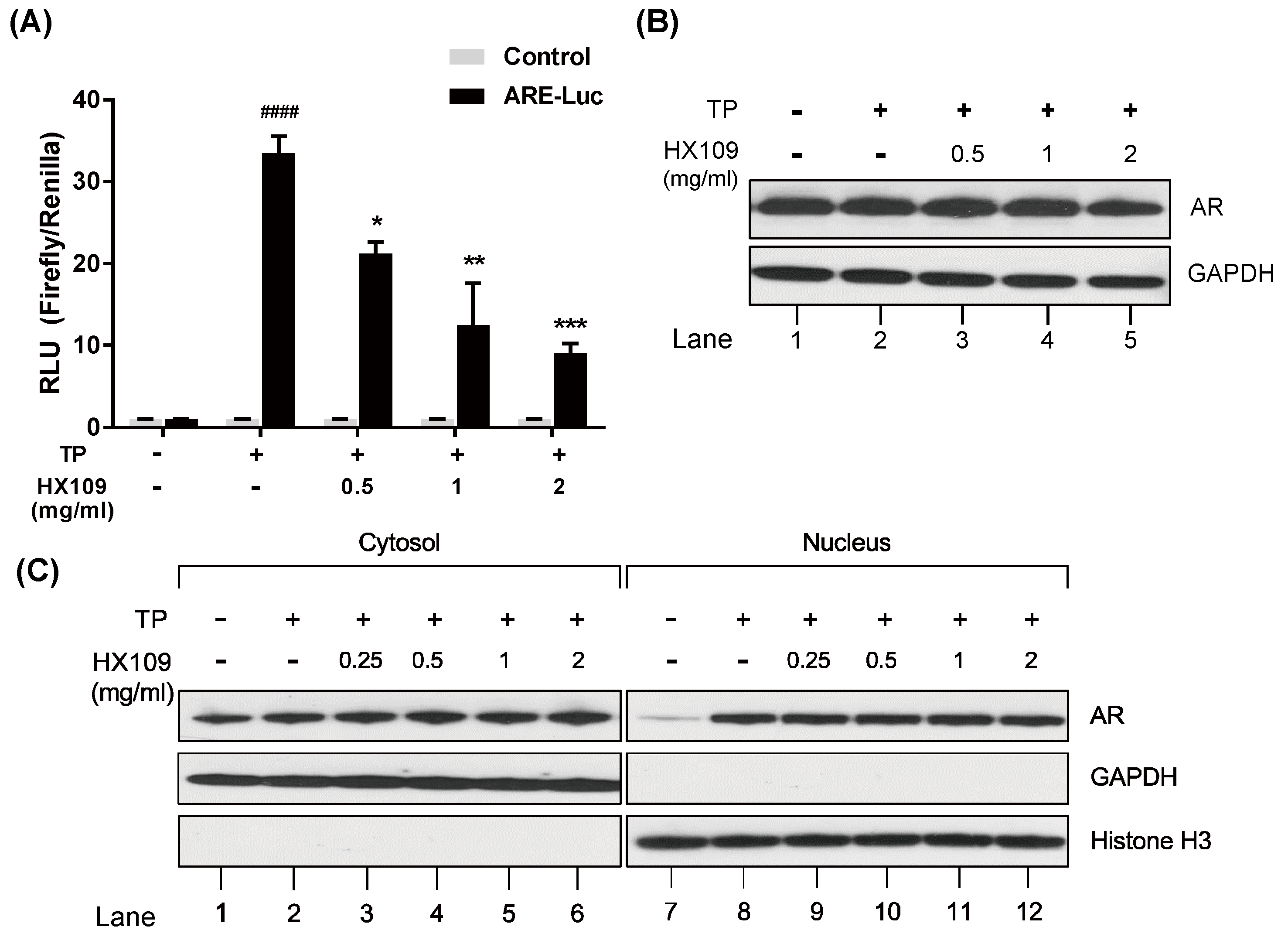
3.5. HX109 Inhibits AR Transcriptional Activity Without Affecting AR Expression and Translocation
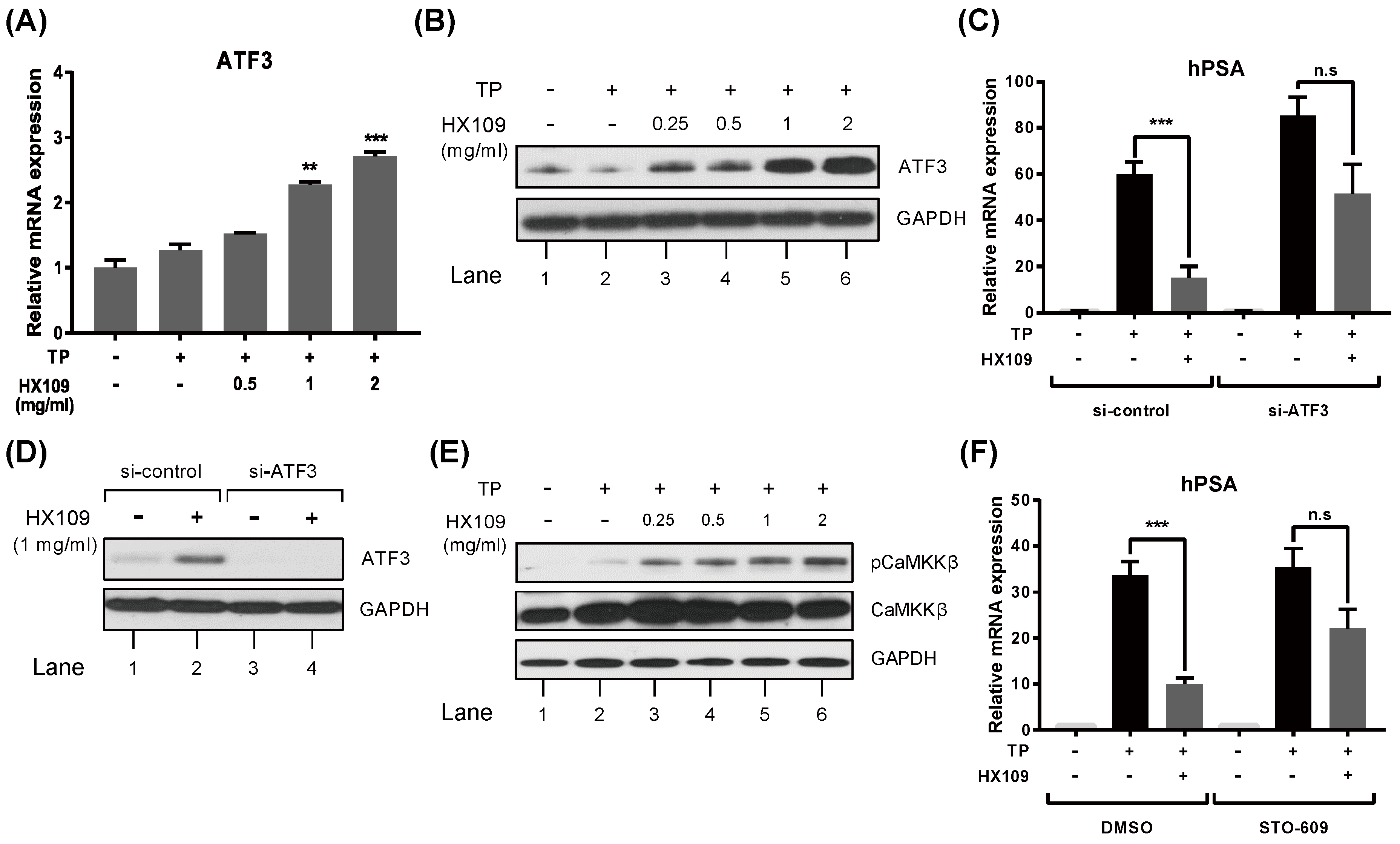
3.6. HX109 Regulates AR Transactivation Through Upregulation of CaMKKβ and ATF3
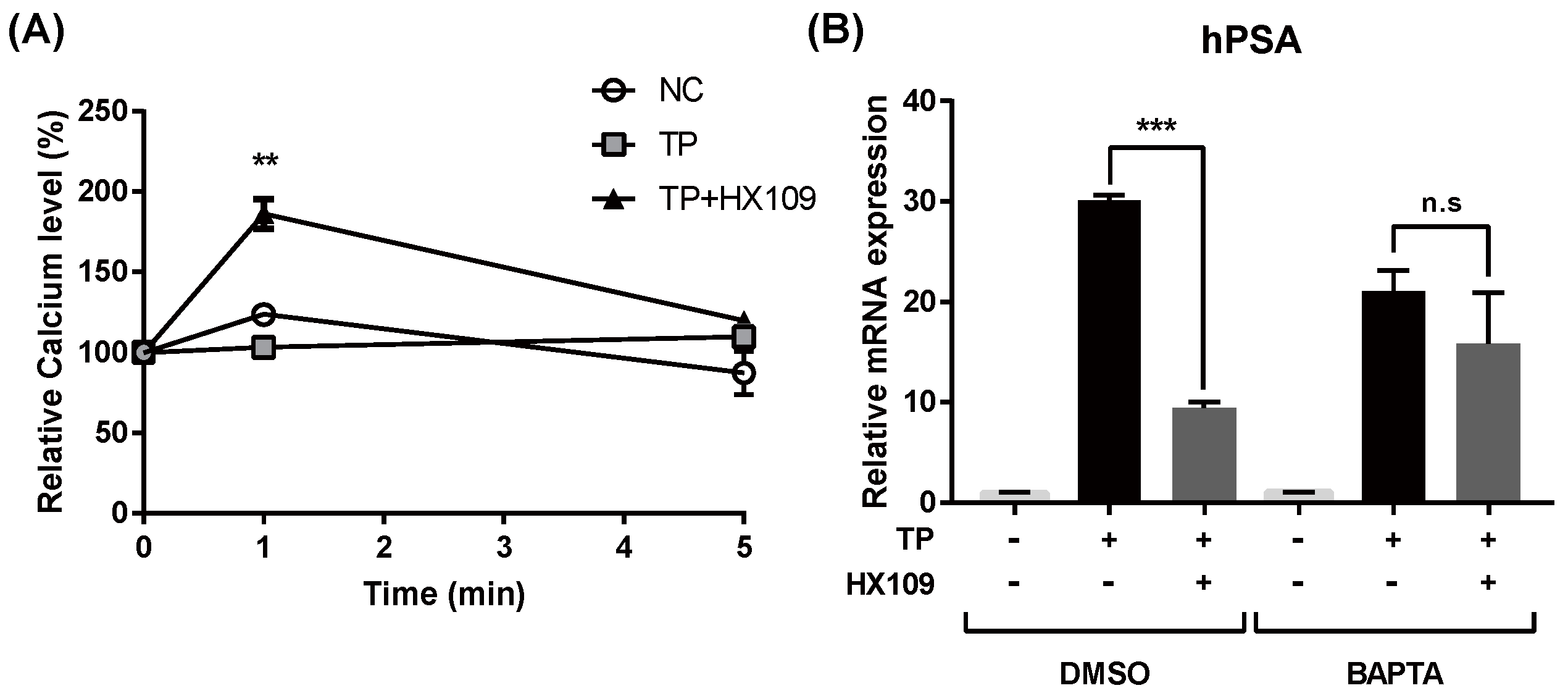
3.7. Calcium Increase by HX109 Plays A Crucial Role in the Regulation of AR Transactivation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- McVary, K.T. BPH: Epidemiology and comorbidities. Am. J. Manag. Care 2006, 12, S122–S128. [Google Scholar]
- Parsons, J.K.; Bergstrom, J.; Silberstein, J.; Barrett-Connor, E. Prevalence and characteristics of lower urinary tract symptoms in men aged > or = 80 years. Urology 2008, 72, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Sarma, A.V.; Wei, J.T. Clinical practice. Benign prostatic hyperplasia and lower urinary tract symptoms. N. Engl. J. Med. 2012, 367, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Roehrborn, C.G. Pathology of benign prostatic hyperplasia. Int. J. Impot. Res. 2008, 20, S11–S18. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, G.; Madersbacher, S.; Berger, P. Benign prostatic hyperplasia: Age-related tissue-remodeling. Exp. Gerontol. 2005, 40, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Briganti, A.; Capitanio, U.; Suardi, N.; Gallina, A.; Salonia, A.; Bianchi, M.; Tutolo, M.; Di Girolamo, V.; Guazzoni, G.; Rigatti, P. Benign prostatic hyperplasia and its aetiologies. Eur. Urol. Suppl. 2009, 8, 865–871. [Google Scholar] [CrossRef]
- Mizokami, A.; Koh, E.; Izumi, K.; Narimoto, K.; Takeda, M.; Honma, S.; Dai, J.; Keller, E.T.; Namiki, M. Prostate cancer stromal cells and LNCaP cells coordinately activate the androgen receptor through synthesis of testosterone and dihydrotestosterone from dehydroepiandrosterone. Endocr. Relat. Cancer 2009, 16, 1139–1155. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.K.; Lavrovsky, Y.; Song, C.S.; Chen, S.; Jung, M.H.; Velu, N.K.; Bi, B.Y.; Chatterjee, B. Regulation of androgen action. Vitam. Horm. 1999, 55, 309–352. [Google Scholar] [PubMed]
- Hai, T.; Wolfgang, C.D.; Marsee, D.K.; Allen, A.E.; Sivaprasad, U. ATF3 and stress responses. Gene Expr. 1999, 7, 321–335. [Google Scholar]
- Wang, H.; Jiang, M.; Cui, H.; Chen, M.; Buttyan, R.; Hayward, S.W.; Hai, T.; Wang, Z.; Yan, C. The stress response mediator ATF3 represses androgen signaling by binding the androgen receptor. Mol. Cell. Biol. 2012, 32, 3190–3202. [Google Scholar] [CrossRef]
- Shima, T.; Mizokami, A.; Miyagi, T.; Kawai, K.; Izumi, K.; Kumaki, M.; Ofude, M.; Zhang, J.; Keller, E.T.; Namiki, M. Down-regulation of calcium/calmodulin-dependent protein kinase kinase 2 by androgen deprivation induces castration-resistant prostate cancer. Prostate 2012, 72, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, A.; Farmer, R.; Verhamme, K.; Berges, R.; Navarrete, R.V. The efficacy of drugs for the treatment of LUTS/BPH, a study in 6 European countries. Eur. Urol. 2007, 51, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Irwig, M.S. Persistent sexual side effects of finasteride: Could they be permanent? J. Sex. Med. 2012, 9, 2927–2932. [Google Scholar] [CrossRef] [PubMed]
- Gormley, G.J.; Stoner, E.; Bruskewitz, R.C.; Imperato-McGinley, J.; Walsh, P.C.; McConnell, J.D.; Andriole, G.L.; Geller, J.; Bracken, B.R.; Tenover, J.S.; et al. The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group. N. Engl. J. Med. 1992, 327, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Boon, H.; Wong, J. Botanical medicine and cancer: A review of the safety and efficacy. Expert Opin. Pharmacother. 2004, 5, 2485–2501. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Ko, E.; No, S.; Park, Y.; Seo, B.; Seo, Y. Herbology; Younlimsa: Seoul, Korea, 2000. [Google Scholar]
- Sigstedt, S.C.; Hooten, C.J.; Callewaert, M.C.; Jenkins, A.R.; Romero, A.E.; Pullin, M.J.; Kornienko, A.; Lowrey, T.K.; Slambrouck, S.V.; Steelant, W.F. Evaluation of aqueous extracts of Taraxacum officinale on growth and invasion of breast and prostate cancer cells. Int. J. Oncol. 2008, 32, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.J.; Kang, H.J.; Jung, H.J.; Kang, Y.S.; Lim, C.J.; Kim, Y.M.; Park, E.H. Anti-inflammatory activity of Taraxacum officinale. J. Ethnopharmacol. 2008, 115, 82–88. [Google Scholar] [CrossRef]
- Park, J.; Lee, C. The Encyclopedia of Medicinal Plants; Shinilbooks: Seoul, Korea, 2000. [Google Scholar]
- Gupta, M.; Mazumder, U.; Mukhopadhyay, R.; Sarkar, S. Antisteroidogenic effect of the seed extract of Nelumbo nucifera in the testis and the ovary of the rat. Indian J. Pharm. Sci. 1996, 58, 236. [Google Scholar]
- Wethangkaboworn, Y.; Munglue, P. Effect of ethanolic seed extract of Nelumbo nucifera on male rat sexual behavior. KKU Res. J. 2014, 19, 156–161. [Google Scholar]
- Rai, S.; Wahile, A.; Mukherjee, K.; Saha, B.P.; Mukherjee, P.K. Antioxidant activity of Nelumbo nucifera (sacred lotus) seeds. J. Ethnopharmacol. 2006, 104, 322–327. [Google Scholar] [CrossRef]
- Jung, H.J.; Sung, H.M.; Kim, K.M.; Shin, Y.-R.; Wee, J.-H. Comparison of antioxidant activities of water extract from dandelion (Taraxacum officinale) aerial parts, roots, and their mixtures. J. Korean Soc. Food Sci. Nutr. 2015, 44, 1157–1164. [Google Scholar] [CrossRef]
- Rho, T.; Yoon, K.D. Application of off-line two-dimensional high-performance countercurrent chromatography on the chloroform-soluble extract of Cuscuta auralis seeds. J. Sep. Sci. 2018, 41, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Wu, J.; Chen, L.G.; Du, H.; Xu, Y.J.; Wang, L.J.; Zhang, H.J.; Zheng, X.C.; Wang, L.S. Biogenesis of C-glycosyl flavones and profiling of flavonoid glycosides in lotus (Nelumbo nucifera). PLoS ONE 2014, 9, e108860. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liu, T.; Guo, M. Current Advances in the Metabolomics Study on Lotus Seeds. Front. Plant Sci. 2016, 7, 891. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, G.; Rittmaster, R.S.; Klocker, H. Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia. Eur. Urol. 2000, 37, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Coetzee, G.A. Prostate specific antigen gene regulation by androgen receptor. J. Cell. Biochem. 2004, 93, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Uzawa, K.; Suzuki, H.; Tanzawa, H.; Ichikawa, T. Characteristic gene expression profiles of benign prostatic hypertrophy and prostate cancer. Int. J. Oncol. 2009, 35, 499–509. [Google Scholar] [PubMed]
- Erdemir, F.; Harbin, A.; Hellstrom, W.J. 5-alpha reductase inhibitors and erectile dysfunction: The connection. J. Sex. Med. 2008, 5, 2917–2924. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Gerbino, A.; Russo, D.; Colella, M.; Procino, G.; Svelto, M.; Milella, L.; Carmosino, M. Dandelion Root Extract Induces Intracellular Ca2+ Increases in HEK293 Cells. Int. J. Mol. Sci. 2018, 19, 1112. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Zhang, Z.; Ratnam, M.; Dou, Q.P. The interplay of AMP-activated protein kinase and androgen receptor in prostate cancer cells. J. Cell. Physiol. 2014, 229, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Huang, W.; Tao, R.; Ibaragi, S.; Lan, F.; Ido, Y.; Wu, X.; Alekseyev, Y.O.; Lenburg, M.E.; Hu, G.F.; et al. Inactivation of AMPK alters gene expression and promotes growth of prostate cancer cells. Oncogene 2009, 28, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, C. Emerging roles of ATF3 in the suppression of prostate cancer. Mol. Cell. Oncol. 2016, 3, e1010948. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, J. Study on Flavonoids of Cuscuta australis R. Br. Zhongguo Zhong Yao Za Zhi 1997, 22, 38–39. (In Chinese) [Google Scholar]
- Murota, K.; Terao, J. Antioxidative flavonoid quercetin: Implication of its intestinal absorption and metabolism. Arch. Biochem. Biophys. 2003, 417, 12–17. [Google Scholar] [CrossRef]
- Choi, J.; Kang, H.J.; Kim, S.Z.; Kwon, T.O.; Jeong, S.I.; Jang, S.I. Antioxidant effect of astragalin isolated from the leaves of Morus alba L. against free radical-induced oxidative hemolysis of human red blood cells. Arch. Pharm. Res. 2013, 36, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Kao, C.L.; Wu, H.M.; Li, W.J.; Huang, C.T.; Li, H.T.; Chen, C.Y. Antioxidant and anticancer aporphine alkaloids from the leaves of Nelumbo nucifera Gaertn. cv. Rosa-plena. Molecules 2014, 19, 17829–17838. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Zu, X.B.; Chen, M.F.; Qi, L. Dauricine can inhibit the activity of proliferation of urinary tract tumor cells. Asian Pac. J. Trop. Med. 2012, 5, 973–976. [Google Scholar] [CrossRef]
| Group | Body Weight (g) | Prostate Weight (g) | Prostate Index (mg/100 g Body Weight) | Growth Inhibition (%) | |
|---|---|---|---|---|---|
| Initial | Final | ||||
| NC | 356.93 ± 4.58 | 445.26 ± 5.42 | 0.0323 ± 0.0026 | 0.73 ± 0.063 | |
| BPH | 356.12 ± 5.79 | 436.29 ± 8.05 | 0.779 ± 0.025 #### | 17.88 ± 0.64 #### | |
| HX109 200 mg/kg | 356.56 ± 5.67 | 421.35 ± 10.15 | 0.686 ± 0.042 | 16.44 ± 1.28 | 8.4% |
| HX109 300 mg/kg | 356.44 ± 5.09 | 432.98 ± 8.60 | 0.528 ± 0.036 **** | 12.17 ± 0.72 *** | 33.29% |
| Fina | 536.05 ± 8.29 | 429.99 ± 11.26 | 0.454 ± 0.018 **** | 10.55 ± 0.15 **** | 42.74% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.; Lee, W.; Lee, D.S.; Nam, I.-J.; Yun, N.; Jeong, Y.; Rho, T.; Kim, S. Botanical Formulation HX109 Ameliorates TP-Induced Benign Prostate Hyperplasia in Rat Model and Inhibits Androgen Receptor Signaling by Upregulating Ca2+/CaMKKβ and ATF3 in LNCaP Cells. Nutrients 2018, 10, 1946. https://doi.org/10.3390/nu10121946
Lim S, Lee W, Lee DS, Nam I-J, Yun N, Jeong Y, Rho T, Kim S. Botanical Formulation HX109 Ameliorates TP-Induced Benign Prostate Hyperplasia in Rat Model and Inhibits Androgen Receptor Signaling by Upregulating Ca2+/CaMKKβ and ATF3 in LNCaP Cells. Nutrients. 2018; 10(12):1946. https://doi.org/10.3390/nu10121946
Chicago/Turabian StyleLim, Seonung, Wonwoo Lee, Doo Suk Lee, In-Jeong Nam, Nayoung Yun, Yoonseon Jeong, Taewoong Rho, and Sunyoung Kim. 2018. "Botanical Formulation HX109 Ameliorates TP-Induced Benign Prostate Hyperplasia in Rat Model and Inhibits Androgen Receptor Signaling by Upregulating Ca2+/CaMKKβ and ATF3 in LNCaP Cells" Nutrients 10, no. 12: 1946. https://doi.org/10.3390/nu10121946
APA StyleLim, S., Lee, W., Lee, D. S., Nam, I.-J., Yun, N., Jeong, Y., Rho, T., & Kim, S. (2018). Botanical Formulation HX109 Ameliorates TP-Induced Benign Prostate Hyperplasia in Rat Model and Inhibits Androgen Receptor Signaling by Upregulating Ca2+/CaMKKβ and ATF3 in LNCaP Cells. Nutrients, 10(12), 1946. https://doi.org/10.3390/nu10121946





