Maternal Pre-Pregnancy Obesity Attenuates Response to Omega-3 Fatty Acids Supplementation During Pregnancy
Abstract
1. Introduction
2. Materials and Methods
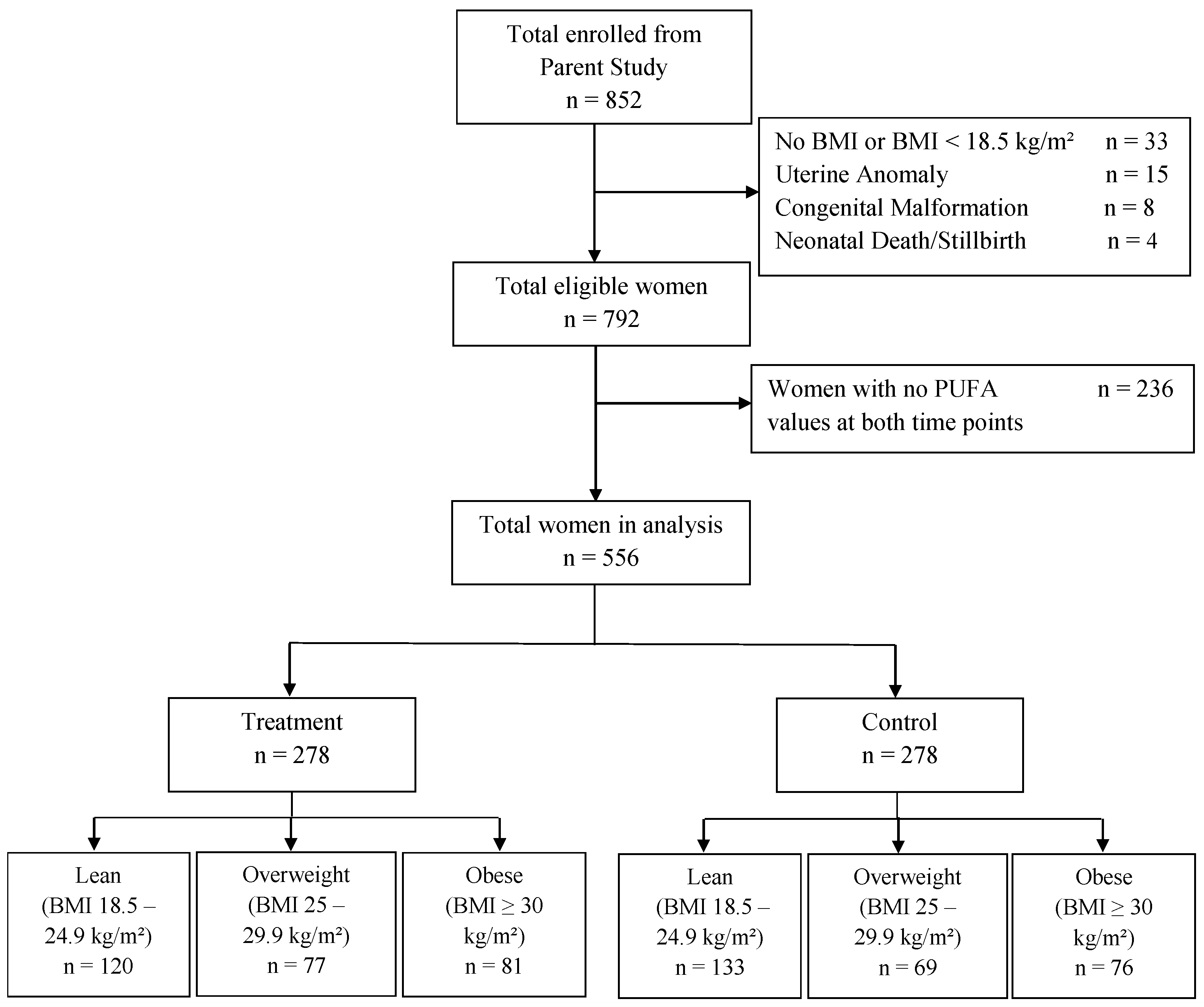
2.1. Study Design and Participants
2.2. Exposure
2.3. Outcomes
2.4. Confounders
2.5. Statistics
3. Results
3.1. Participant Characteristics
3.2. Baseline Plasma PUFA Concentrations before n-3 Supplementation by BMI
3.2.1. N-3 PUFA
3.2.2. N-6 PUFA
3.2.3. N-6/N-3 PUFA Ratio
3.3. Change in Plasma PUFA Concentrations Following n-3 PUFA Supplementation by BMI
3.3.1. N-3 PUFA
3.3.2. N-6 PUFA
3.3.3. N-6/N-3 PUFA Ratio
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flegal, K.M.; Kruszon-Moran, D.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Trends in Obesity among Adults in the United States, 2005 to 2014. JAMA 2016, 315, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Schieve, L.A.; Sharma, A.J.; Hinkle, S.N.; Li, R.; Lind, J.N. Maternal prepregnancy body mass index and child psychosocial development at 6 years of age. Pediatrics 2015, 135, e1198–e1209. [Google Scholar] [CrossRef] [PubMed]
- Segovia, S.A.; Vickers, M.H.; Gray, C.; Reynolds, C.M. Maternal obesity, inflammation, and developmental programming. BioMed Res. Int. 2014, 418975. [Google Scholar] [CrossRef] [PubMed]
- van der Burg, J.W.; Sen, S.; Chomitz, V.R.; Seidell, J.C.; Leviton, A.; Dammann, O. The role of systemic inflammation linking maternal BMI to neurodevelopment in children. Pediatr. Res. 2015, 79, 3–12. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, K.; Ponsonby, A.-L.; Collier, F.; Allen, K.; Tang, M.L.; Carlin, J.B.; Saffery, R.; Skilton, M.R.; Cheung, M.; Ranganathan, S.; et al. The association between higher maternal pre-pregnancy body mass index and increased birth weight, adiposity and inflammation in the newborn. Pediatr. Obes. 2016, 13, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Bilbo, S.D.; Tsang, V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010, 24, 2104–2115. [Google Scholar] [CrossRef]
- Gaillard, R.; Rifas-Shiman, S.L.; Perng, W.; Oken, E.; Gillman, M.W. Maternal inflammation during pregnancy and childhood adiposity. Obesity 2016, 24, 1320–1327. [Google Scholar] [CrossRef]
- Rifas-Shiman, S.L.; Rich-Edwards, J.W.; Kleinman, K.P.; Oken, E.; Gillman, M.W. Dietary quality during pregnancy varies by maternal characteristics in project Viva: A US cohort. J. Am. Diet. Assoc. 2009, 109, 1004–1011. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 Polyunsaturated Fatty Acids, Inflammation, and Inflammatory Diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Can Early Omega-3 Fatty Acid Exposure Reduce Risk of Childhood Allergic Disease? Nutrients 2017, 9, 784. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Yacoubian, S.; Yang, R. Anti-inflammatory and pro-resolving lipid mediators. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 279–312. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Q.; Qiu, Y.; Mu, Y.; Zhang, X.-J.; Liu, L.; Hou, X.-H.; Zhang, L.; Xu, X.-N.; Ji, A.-L.; Cao, R.; et al. A high ratio of dietary n-3/n-6 polyunsaturated fatty acids improves obesity-linked inflammation and insulin resistance through suppressing activation of TLR4 in SD rats. Nutr. Res. 2013, 33, 849–858. [Google Scholar] [CrossRef]
- Heerwagen, M.J.R.; Stewart, M.S.; De La Houssaye, B.A.; Janssen, R.C.; Friedman, J.E. Transgenic increase in n-3/n-6 Fatty Acid ratio reduces maternal obesity-associated inflammation and limits adverse developmental programming in mice. PLoS ONE 2013, 8, e67791. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.G.; Song, Z.X.; Yin, H.; Wang, Y.Y.; Shu, G.F.; Lu, H.X.; Wang, S.K.; Sun, G.J. Low n-6/n-3 PUFA Ratio Improves Lipid Metabolism, Inflammation, Oxidative Stress and Endothelial Function in Rats Using Plant Oils as n-3 Fatty Acid Source. Lipids 2015, 51, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Lager, S.; Ramirez, V.I.; Acosta, O.; Meireles, C.; Miller, E.; Gaccioli, F.; Rosario, F.J.; Gelfond, J.A.L.; Hakala, K.; Weintraub, S.T.; et al. Docosahexaenoic Acid Supplementation in Pregnancy Modulates Placental Cellular Signaling and Nutrient Transport Capacity in Obese Women. J. Clin. Endocrinol. Metab. 2017, 102, 4557–4567. [Google Scholar] [CrossRef] [PubMed]
- Calabuig-Navarro, V.; Puchowicz, M.; Glazebrook, P.; Haghiac, M.; Minium, J.; Catalano, P.; Demouzon, S.H.; O’Tierney-Ginn, P. Effect of ω-3 supplementation on placental lipid metabolism in overweight and obese women. Am. J. Clin. Nutr. 2016, 103, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, K.P.; Olsen, S.F.; Oken, E.; Rich-Edwards, J.W.; Gillman, M.W. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: Results from a US pregnancy cohort. Am. J. Epidemiol. 2004, 160, 774–783. [Google Scholar] [CrossRef]
- Drouillet, P.; Forhan, A.; De Lauzon-Guillain, B.; Thiebaugeorges, O.; Goua, V.; Magnin, G.; Schweitzer, M.; Kaminski, M.; Ducimetiere, P.; Charles, M.-A.; et al. Maternal fatty acid intake and fetal growth: Evidence for an association in overweight women. The ‘EDEN mother–child’ cohort (study of pre- and early postnatal determinants of the child’s development and health). Br. J. Nutr. 2008, 101, 575. [Google Scholar] [CrossRef] [PubMed]
- Donahue, S.M.; Rifas-Shiman, S.L.; Gold, D.R.; Jouni, Z.E.; Gillman, M.W.; Oken, E. Prenatal fatty acid status and child adiposity at age 3 y: Results from a US pregnancy cohort. Am. J. Clin. Nutr. 2011, 93, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Vidakovic, A.J.; Gishti, O.; Voortman, T.; Felix, J.F.; Williams, M.A.; Hofman, A.; Demmelmair, H.; Koletzko, B.; Tiemeier, H.; Jaddoe, V.W.; et al. Maternal plasma PUFA concentrations during pregnancy and childhood adiposity: The Generation R Study. Am. J. Clin. Nutr. 2016, 103, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, H.; Stokholm, J.; Chawes, B.L.; Vissing, N.H.; Bjarnadóttir, E.; Schoos, A.-M.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Følsgaard, N.V.; et al. Fish oil–derived fatty acids in pregnancy and wheeze and asthma in offspring. N. Engl. J. Med. 2016, 375, 2530–2539. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.Y.; De Agostini, M.; Forhan, A.; De Lauzon-Guillain, B.; Heude, B.; Charles, M.-A. The dietary n6:n3 fatty acid ratio during pregnancy is inversely associated with child neurodevelopment in the EDEN mother-child cohort. J. Nutr. 2013, 143, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R.; Davis, J.M.; Steer, C.; Emmett, P.; Rogers, I.; Williams, C.; Golding, J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): An observational cohort study. Lancet 2007, 369, 578–585. [Google Scholar] [CrossRef]
- Larqué, E.; Demmelmair, H.; Gil-Sánchez, A.; Prieto-Sánchez, M.T.; Blanco, J.E.; Pagán, A.; Faber, F.L.; Zamora, S.; Parrilla, J.J.; Koletzko, B. Placental transfer of fatty acids and fetal implications. Am. J. Clin. Nutr. 2011, 94, 1908–1913. [Google Scholar] [CrossRef]
- ACOG Practice Advisory: Update on Seafood Consumption During Pregnancy [Internet]: ACOG. 2017. Available online: https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/ACOG-Practice-Advisory-Seafood-Consumption-During-Pregnancy (accessed on 12 May 2018).
- Harper, M.; Thom, E.; Klebanoff, M.A.; Thorp, J.; Sorokin, Y.; Varner, M.W.; Wapner, R.J.; Caritis, S.N.; Iams, J.D.; Carpenter, M.W.; et al. Omega-3 fatty acid supplementation to prevent recurrent preterm birth. Obstet. Gynecol. 2010, 115, 234–242. [Google Scholar] [CrossRef]
- Reece, M.S.; McGregor, J.A.; Allen, K.G.; Harris, M.A. Maternal and perinatal long-chain fatty acids: Possible roles in preterm birth. Am. J. Obstet. Gynecol. 1997, 176, 907–914. [Google Scholar] [CrossRef]
- Willett, W.C.; Sampson, L.; Stampfer, M.J.; Rosner, B.; Bain, C.; Witschi, J.; Hennekens, C.H.; Speizer, F.E. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am. J. Epidemiol. 1985, 122, 51–65. [Google Scholar] [CrossRef]
- Wijendran, V.; Bendel, R.B.; Couch, S.C.; Philipson, E.H.; Thomsen, K.; Zhang, X.; Lammi-Keefe, C.J. Maternal plasma phospholipid polyunsaturated fatty acids in pregnancy with and without gestational diabetes mellitus: Relations with maternal factors. Am. J. Clin. Nutr. 1999, 70, 53–61. [Google Scholar] [CrossRef]
- Tomedi, L.E.; Chang, C.-C.H.; Newby, P.; Evans, R.W.; Luther, J.F.; Wisner, K.L.; Bodnar, L.M. Pre-pregnancy obesity and maternal nutritional biomarker status during pregnancy: A factor analysis. Public Health Nutr. 2013, 16, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Vidakovic, A.J.; Jaddoe, V.W.V.; Gishti, O.; Felix, J.F.; Williams, M.A.; Hofman, A.; Demmelmair, H.; Koletzko, B.; Tiemeier, H.; Gaillard, R.; et al. Body mass index, gestational weight gain and fatty acid concentrations during pregnancy: The Generation R Study. Eur. J. Epidemiol. 2015, 30, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Sibbons, C.M.; Brenna, J.T.; Lawrence, P.; Hoile, S.P.; Clarke-Harris, R.; Lillycrop, K.A.; Burdge, G.C. Effect of sex hormones on n-3 polyunsaturated fatty acid biosynthesis in HepG2 cells and in human primary hepatocytes. Prostaglandins Leukot. Essent. Fat. Acids (Plefa) 2014, 90, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Gómez Candela, C.; Bermejo López, L.M.; Loria Kohen, V. Importance of a balanced omega 6/omega 3 ratio for the maintenance of health: Nutritional recommendations. Nutr. Hosp. 2011, 26. [Google Scholar] [CrossRef]
- Kang, J.X. The importance of omega-6/omega-3 fatty acid ratio in cell function. The gene transfer of omega-3 fatty acid desaturase. World Rev. Nutr. Diet. 2003, 92, 23–36. [Google Scholar] [PubMed]
- Simopoulos, A. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Khaire, A.A.; Kale, A.A.; Joshi, S.R. Maternal omega-3 fatty acids and micronutrients modulate fetal lipid metabolism: A review. Prostaglandins Leukot. Essent. Fat. Acids (Plefa) 2015, 98, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Aye, I.L.; Lager, S.; Ramirez, V.I.; Gaccioli, F.; Dudley, D.J.; Jansson, T.; Powell, T. Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways. Boil. Reprod. 2014, 90, 129. [Google Scholar] [CrossRef] [PubMed]
- Panagos, P.G.; Vishwanathan, R.; Penfield-Cyr, A.; Matthan, N.R.; Shivappa, N.; Wirth, M.D.; Hebert, J.R.; Sen, S. Breastmilk from obese mothers has pro-inflammatory properties and decreased neuroprotective factors. J. Perinatol. 2016, 36, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, H.; Cheema, S.K. Breastmilk with a high omega-6 to omega-3 fatty acid ratio induced cellular events similar to insulin resistance and obesity in 3T3-L1 adipocytes. Pediatr. Obes. 2017, 13, 285–291. [Google Scholar] [CrossRef]
- Vaidya, H.; Cheema, S.K. Arachidonic acid has a dominant effect to regulate lipogenic genes in 3T3-L1 adipocytes compared to omega-3 fatty acids. Food Nutr. Res. 2015, 59, 25866. [Google Scholar] [CrossRef] [PubMed]
- Christian, L.M.; Young, A.S.; Mitchell, A.M.; Belury, M.A.; Gracious, B.L.; Arnold, L.E.; Fristad, M.A. Body weight affects ω-3 polyunsaturated fatty acid (PUFA) accumulation in youth following supplementation in post-hoc analyses of a randomized controlled trial. PLoS ONE 2017, 12, e0173087. [Google Scholar] [CrossRef]
- Yee, L.D.; Lester, J.L.; Cole, R.M.; Richardson, J.R.; Hsu, J.C.; Li, Y.; Lehman, A.; Belury, M.A.; Clinton, S.K. ω-3 Fatty acid supplements in women at high risk of breast cancer have dose-dependent effects on breast adipose tissue fatty acid composition. Am. J. Clin. Nutr. 2010, 91, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Wang, Y.; Xu, J.Y.; Stejskal, D.; Tam, S.; Zhang, J.; Wat, N.M.; Wong, W.K.; Lam, K.S. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin. Chem. 2006, 52, 405–413. [Google Scholar] [CrossRef]
- Krauss-Etschmann, S.; Shadid, R.; Campoy, C.; Hoster, E.; Demmelmair, H.; Jiménez, M.; Gil, A.; Rivero, M.; Veszprémi, B.; Decsi, T.; et al. Effects of fish-oil and folate supplementation of pregnant women on maternal and fetal plasma concentrations of docosahexaenoic acid and eicosapentaenoic acid: A European randomized multicenter trial. Am. J. Clin. Nutr. 2007, 85, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Pottala, J.V.; Lacey, S.M.; Vasan, R.S.; Larson, M.G.; Robins, S.J. Clinical correlates and heritability of erythrocyte eicosapentaenoic and docosahexaenoic acid content in the framingham heart study. Atherosclerosis 2012, 225, 425–431. [Google Scholar] [CrossRef]
- Block, R.C.; Harris, W.S.; Pottala, J.V. Determinants of blood cell omega-3 fatty acid content. Open Biomark J. 2008, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, R.; Rondanelli, M.; Russo-Volpe, S.; Ferrari, E.; Cestaro, B. Decreased membrane fluidity and altered susceptibility to peroxidation and lipid composition in overweight and obese female erythrocytes. J. Lipid Res. 2004, 45, 1846–1851. [Google Scholar] [CrossRef]
- Stark, K.D.; Van Elswyk, M.E.; Higgins, M.R.; Weatherford, C.A.; Salem, N., Jr. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog. Lipid Res. 2016, 63, 132–152. [Google Scholar] [CrossRef]
| Characteristics | Total n = 556 | Pre-Pregnancy BMI (kg/m2) Category | p-Value a | ||
|---|---|---|---|---|---|
| Lean n = 253 (46%) | Overweight n = 146 (26%) | Obese n = 157 (28%) | |||
| Age at enrollment (years) | 27 (23, 32) | 27 (24, 32) | 28 (23, 33) | 27 (23, 32) | 0.79 |
| BMI (kg/m2) | 26.5 (22, 30) | 22 (21, 23) | 27 (25, 28) | 34 (32, 38) | <0.001 b,c,d |
| Gestational age at randomization (days) | 136 (125, 146) | 135 (124, 146) | 138 (126, 147) | 135 (125, 145) | 0.43 |
| Education (years) | 13 (12, 16) | 14 (12, 16) | 12 (11, 15) | 12 (12, 14) | <0.001 c,d |
| Race/Ethnicity e | |||||
| White | 254 (46) | 142 (56) | 57 (39) | 55 (35) | <0.001 |
| Black, non-Hispanic | 204 (37) | 63 (25) | 58 (40) | 83 (53) | |
| Hispanic | 81 (14) | 36 (14) | 28 (19) | 17 (11) | |
| Other | 16 (3) | 11 (4) | 3 (2) | 2 (1) | |
| Smoking in pregnancy (Yes, %) | 89 (16) | 32 (13) | 24 (16) | 33 (21) | 0.08 |
| Marital Status | |||||
| Married/Living with Partner | 371 (67) | 183 (72) | 95 (65) | 93 (59) | 0.08 |
| Divorced/Widowed/Separated | 29 (5) | 9 (4) | 9 (6) | 11 (7) | |
| Never Married | 156 (28) | 61 (24) | 42 (29) | 53 (34) | |
| Length of supplementation (days) | 56 (42, 63) | 55 (41, 64) | 56 (40, 63) | 55 (42, 63) | 0.84 |
| Fish intake (servings/week) | |||||
| <1 | 269 (48) | 136 (54) | 71 (49) | 62 (40) | 0.006 f |
| 1 | 183 (33) | 76 (30) | 50 (34) | 57 (36) | |
| 2 | 41 (8) | 13 (5) | 10 (7) | 18 (11) | |
| ≥3 | 63 (11) | 28 (11) | 15 (10) | 20 (13) | |
| Treatment Arm | |||||
| Treatment | 278 (50) | 120 (47) | 77 (53) | 81 (52) | 0.53 |
| Placebo | 278 (50) | 133 (53) | 69 (47) | 76 (48) | |
| Study Compliance (%) | 92 (81, 99) | 93 (83, 99) | 93 (82, 99) | 89 (78, 99) | 0.15 |
| n | Total | Pre-Pregnancy BMI (kg/m2) Category | p-Value a | |||
|---|---|---|---|---|---|---|
| Lean | Overweight | Obese | ||||
| n-3 PUFA | ||||||
| Total n-3 PUFA | 532 | 4.1 (3.3, 5.1) | 4.1 (3.4, 5.2) | 3.9 (3.1, 5.0) | 4.1 (3.4, 5.0) | 0.16 |
| DHA + EPA | 532 | 3.9 (3.1, 4.7) | 3.9 (3.1, 4.7) | 3.8 (2.7, 4.6) | 4.0 (3.2, 4.6) | 0.33 |
| n-6 PUFA | ||||||
| Total n-6 PUFA | 532 | 38.0 (33.6, 40.1) | 37.9 (33.2, 40.0) | 38.3 (34.1, 40.2) | 37.9 (33.7, 40.1) | 0.73 |
| AA | 532 | 11.7 (9.7, 13.4) | 11.5 (9.3, 13.0) | 11.5 (9.4, 13.0) | 12.6 (10.6, 14.6) | 0.001 b,c |
| n-6/n-3 PUFA ratio | ||||||
| Total n-6/n-3 PUFA | 477 | 8.9 (6.9, 10.7) | 9.0 (6.7, 10.6) | 8.8 (6.7, 10.9) | 8.8 (7.1, 10.5) | 0.83 |
| AA/DHA + EPA | 461 | 3.0 (2.4, 3.4) | 2.9 (2.3, 3.3) | 3.0 (2.3, 3.5) | 3.1 (2.7, 3.6) | 0.006 c |
| n | Model 0 | Model 1 | Model 2 | |
|---|---|---|---|---|
| Total n-3 PUFA | 531 | |||
| Lean | Ref | Ref | Ref | |
| Overweight | −1.97 (−3.51, −0.43) a | −1.80 (−3.39, −0.21) a | −1.80 (−3.39, −0.21) a | |
| Obese | −0.96 (−2.46, 0.55) | −0.58 (−2.15, 1.00) | −0.57 (−2.15, 1.00) | |
| DHA+EPA | 531 | |||
| Lean | Ref | Ref | Ref | |
| Overweight | −0.25 (−0.61, 0.11) | −0.25 (−0.61, 0.11) | −0.24 (−0.60, 0.11) | |
| Obese | −0.11 (−0.24, 0.46) | 0.16 (−0.20, 0.52) | 0.13 (−0.22, 0.49) | |
| Total n-6 PUFA | 531 | |||
| Lean | Ref | Ref | Ref | |
| Overweight | 0.68 (−1.14, 2.50) | 0.45 (−1.42, 2.31) | 0.47 (−1.39, 2.34) | |
| Obese | 0.48 (−1.29, 2.26) | 0.21 (−1.64, 2.06) | 0.15 (−1.70, 1.99) | |
| AA | 531 | |||
| Lean | Ref | Ref | Ref | |
| Overweight | −0.24 (−1.05, 0.57) | −0.47 (−1.31, 0.37) | −0.45 (−1.29, 0.39) | |
| Obese | 1.30 (0.51, 2.10) a | 1.00 (0.17, 1.83) a | 0.96 (0.13, 1.79) a | |
| Total n-6/n-3 PUFA | 476 | |||
| Lean | Ref | Ref | Ref | |
| Overweight | 0.24 (−0.62, 1.10) | 0.25 (−0.65, 1.14) | 0.24 (−0.65, 1.14) | |
| Obese | 0.21 (−0.61, 1.03) | 0.26 (−0.61, 1.13) | 0.27 (−0.60, 1.15) | |
| AA/DHA+EPA | 460 | |||
| Lean | Ref | Ref | Ref | |
| Overweight | 0.19 (−0.04, 0.41) | 0.12 (−0.10, 0.35) | 0.12 (−0.10, 0.35) | |
| Obese | 0.35 (0.14, 0.56) a | 0.26 (0.04, 0.47) a | 0.26 (0.05, 0.48) a |
| n | Treatment Group | p-Value a | ||
|---|---|---|---|---|
| Placebo | Treatment | |||
| Total n-3 PUFA | ||||
| All | 472 | −0.2 (−1.2, 0.6) b | 1.5 (−0.3, 5.1) b | <0.000 |
| Lean | −0.02 (−1.6, 0.6) | 3.4 (−0.2, 6.6) b | <0.000 | |
| Overweight | −0.2 (−1.6, 0.6) | 1.5 (0.0, 4.2) b | <0.000 | |
| Obese | −0.6 (−1.5, 0.3) b | 0.4 (−0.8, 2.5) | 0.002 | |
| DHA + EPA | ||||
| All | 472 | −0.2 (−0.9, 0.4) b | 2.3 (0.0, 5.3) b | <0.000 |
| Lean | −0.1 (−0.9, 0.5) | 4.5 (0.7, 6.7) b | <0.000 | |
| Overweight | −0.2 (−0.6, 0.4) | 1.9 (0.0, 4.2) b | <0.000 | |
| Obese | −0.5 (−1.2, 0.0) b | 1.9 (0.0, 4.2) b | <0.000 | |
| Total n-6 PUFA | ||||
| All | 472 | −0.3 (−3.5, 2.7) | −2.4 (−5.4, 2.1) b | 0.02 |
| Lean | 0.6 (−3.4, 2.2) | −3.7 (−6.5, 1.6) b | 0.01 | |
| Overweight | 0.1 (−4.6, 3.3) | −2.0 (−5.2, 2.7) | 0.32 | |
| Obese | −0.2 (−3.4, 2.2) | −0.5 (−4.2, 2.2) | 0.65 | |
| AA | ||||
| All | 472 | −0.9 (−2.3, 0.4) b | −1.5 (−3.1, −0.3) b | 0.001 |
| Lean | −0.7(−1.8, 0.4) b | −2.0 (−3.5, −0.8) b | <0.000 | |
| Overweight | −1.1 (−2.0, 0.4) b | −1.5 (−3.0, 0.0) b | <0.000 | |
| Obese | −1.0 (−3.1, 0.0) b | −1.0 (−2.2, −0.1) b | 0.54 | |
| Total n-6/n-3 | ||||
| All | 396 | 0.3 (−1.2, 1.6) | −2.9 (−6.1, 0.9) b | <0.000 |
| Lean | −0.1 (−1.8, 1.4) | −4.3 (−7.1, −0.1) b | <0.000 | |
| Overweight | 0.5 (−0.6, 1.6) | −2.8 (−5.3, 1.2) b | <0.000 | |
| Obese | 1.0 (−0.9, 2.0) | −1.1 (−3.8, 1.5) b | 0.016 | |
| AA/DHA + EPA | ||||
| All | 384 | −0.1 (−0.4, −0.2) | −1.4 (−2.0, −0.4) b | <0.000 |
| Lean | −0.1 (−0.3, 0.1) | −1.6 (−2.1, −0.8) b | <0.000 | |
| Overweight | 0.0 (−0.4, 0.3) | −1.4 (−2.2, −0.5) b | <0.000 | |
| Obese | −0.1 (−0.6, 0.2) | −0.5 (−1.5, −0.1) b | 0.002 | |
| n | Overall | pa | Pre-Pregnancy BMI Category | p for Interaction | |||
|---|---|---|---|---|---|---|---|
| Lean | Overweight | Obese | |||||
| n-3 PUFA | |||||||
| Total n-3 PUFA | 471 | 1.62 (0.03, 3.21) | 0.046 | 2.52 (−0.16, 5.20) | 2.33 (−0.16, 4.81) | 0.19 (−2.98, 3.22) | 0.230 |
| DHA + EPA | 471 | 3.08 (2.59, 3.56) | 0.000 | 4.03 (3.24, 4.82) b | 2.14 (1.17, 3.10) b | 2.12 (1.32, 2.92) b | 0.000 |
| n-6 PUFA | |||||||
| Total n-6 PUFA | 471 | −0.46 (−2.41, 1.49) | 0.640 | −1.70 (−4.59, 1.19) | 1.17 (−5.69, 3.36) | 0.21 (−3.17, 3.58) | 0.430 |
| AA | 471 | −0.42 (−1.12, 0.27) | 0.240 | −1.13 (−2.16, −0.11) b | −0.67 (−2.08, 0.74) | 0.53 (−0.87, 1.93) | 0.190 |
| n-6/n-3 PUFA ratio | |||||||
| Total n-6/n-3 PUFA | 395 | −2.71 (−3.70, −1.72) | 0.000 | −3.67 (−5.13, −2.21) b | −2.83 (−5.16, −0.51) b | −1.55 (−3.41, −0.30) b | 0.017 |
| AA/ DHA + EPA | 383 | −1.13 (−1.36, −0.91) | 0.000 | −1.48 (−1.78, −1.17) b | −1.21 (−1.80, −0.63) b | −0.52 (−0.94, −0.10) b | 0.046 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monthé-Drèze, C.; Penfield-Cyr, A.; Smid, M.C.; Sen, S. Maternal Pre-Pregnancy Obesity Attenuates Response to Omega-3 Fatty Acids Supplementation During Pregnancy. Nutrients 2018, 10, 1908. https://doi.org/10.3390/nu10121908
Monthé-Drèze C, Penfield-Cyr A, Smid MC, Sen S. Maternal Pre-Pregnancy Obesity Attenuates Response to Omega-3 Fatty Acids Supplementation During Pregnancy. Nutrients. 2018; 10(12):1908. https://doi.org/10.3390/nu10121908
Chicago/Turabian StyleMonthé-Drèze, Carmen, Annie Penfield-Cyr, Marcela C. Smid, and Sarbattama Sen. 2018. "Maternal Pre-Pregnancy Obesity Attenuates Response to Omega-3 Fatty Acids Supplementation During Pregnancy" Nutrients 10, no. 12: 1908. https://doi.org/10.3390/nu10121908
APA StyleMonthé-Drèze, C., Penfield-Cyr, A., Smid, M. C., & Sen, S. (2018). Maternal Pre-Pregnancy Obesity Attenuates Response to Omega-3 Fatty Acids Supplementation During Pregnancy. Nutrients, 10(12), 1908. https://doi.org/10.3390/nu10121908




