Complementary Biomarker Assessment of Components Absorbed from Diet and Creatinine Excretion Rate Reflecting Muscle Mass in Dialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Study Population
2.2. Dialysis Settings
2.3. Sample Collection
2.4. Laboratory Measurements
2.5. Dietary Intake Assessment
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Clinical and Laboratory Parameters Before and After Dialysis
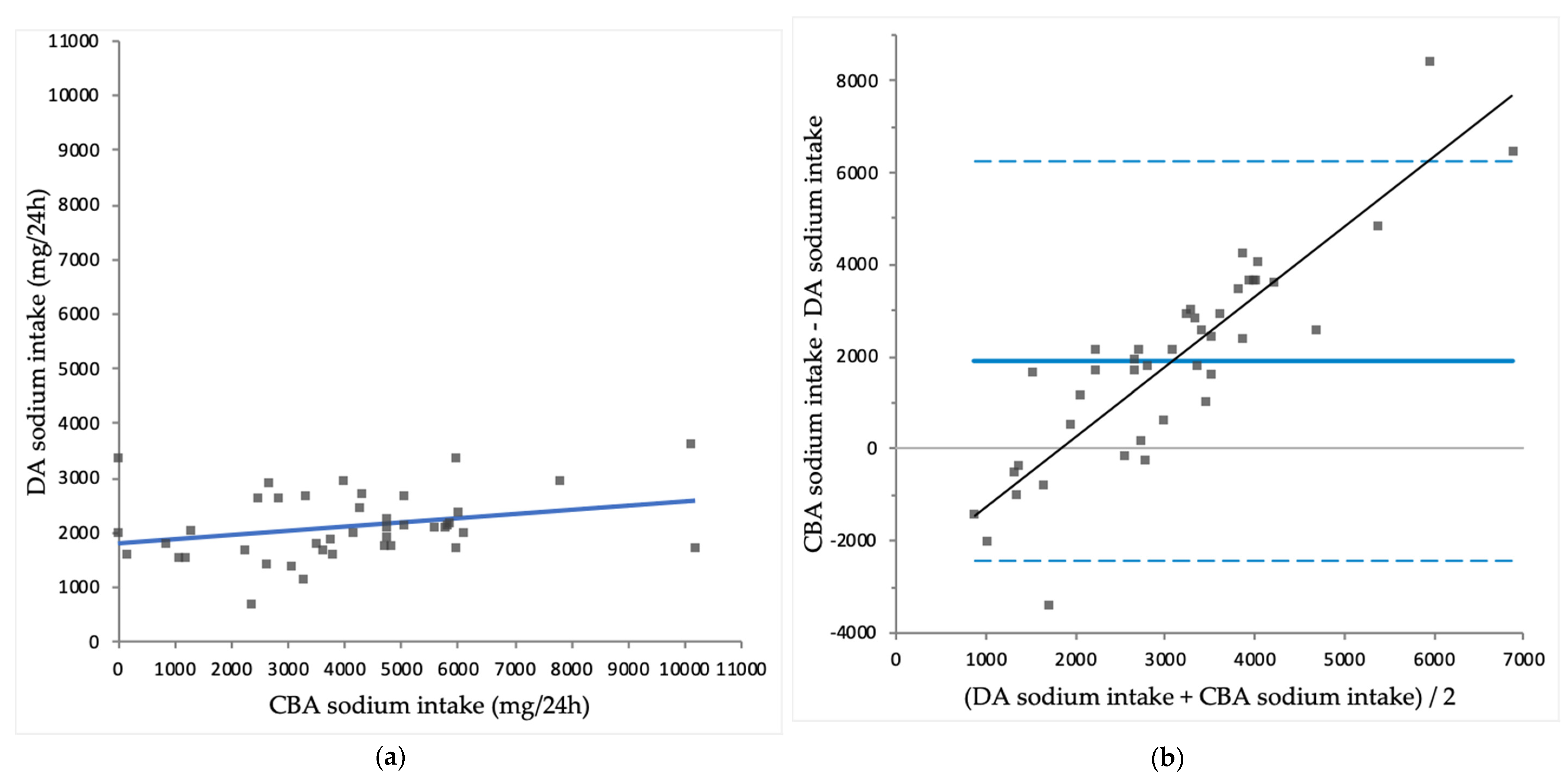
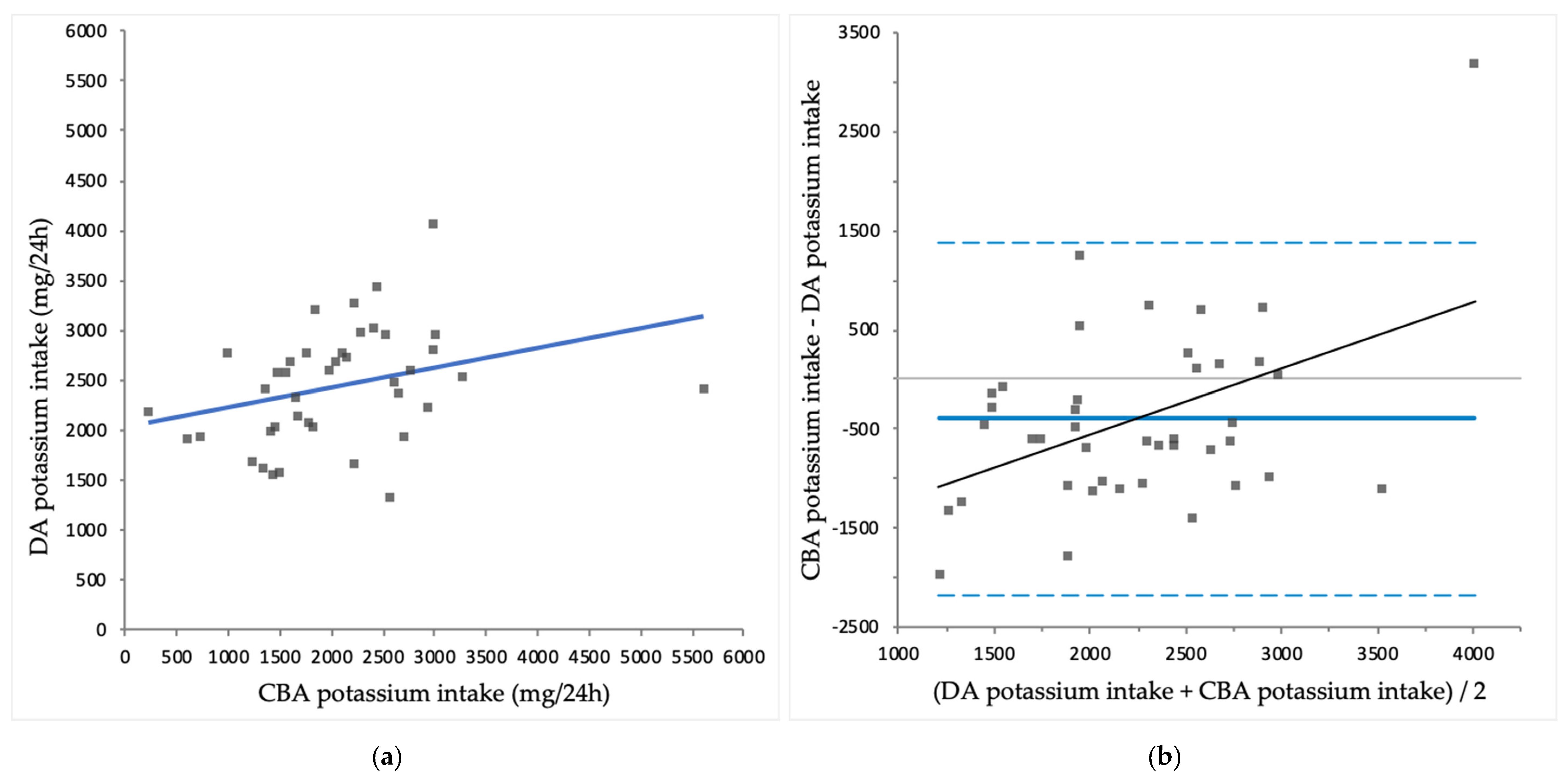
3.3. Comparison of CBA Intake with DA Intake
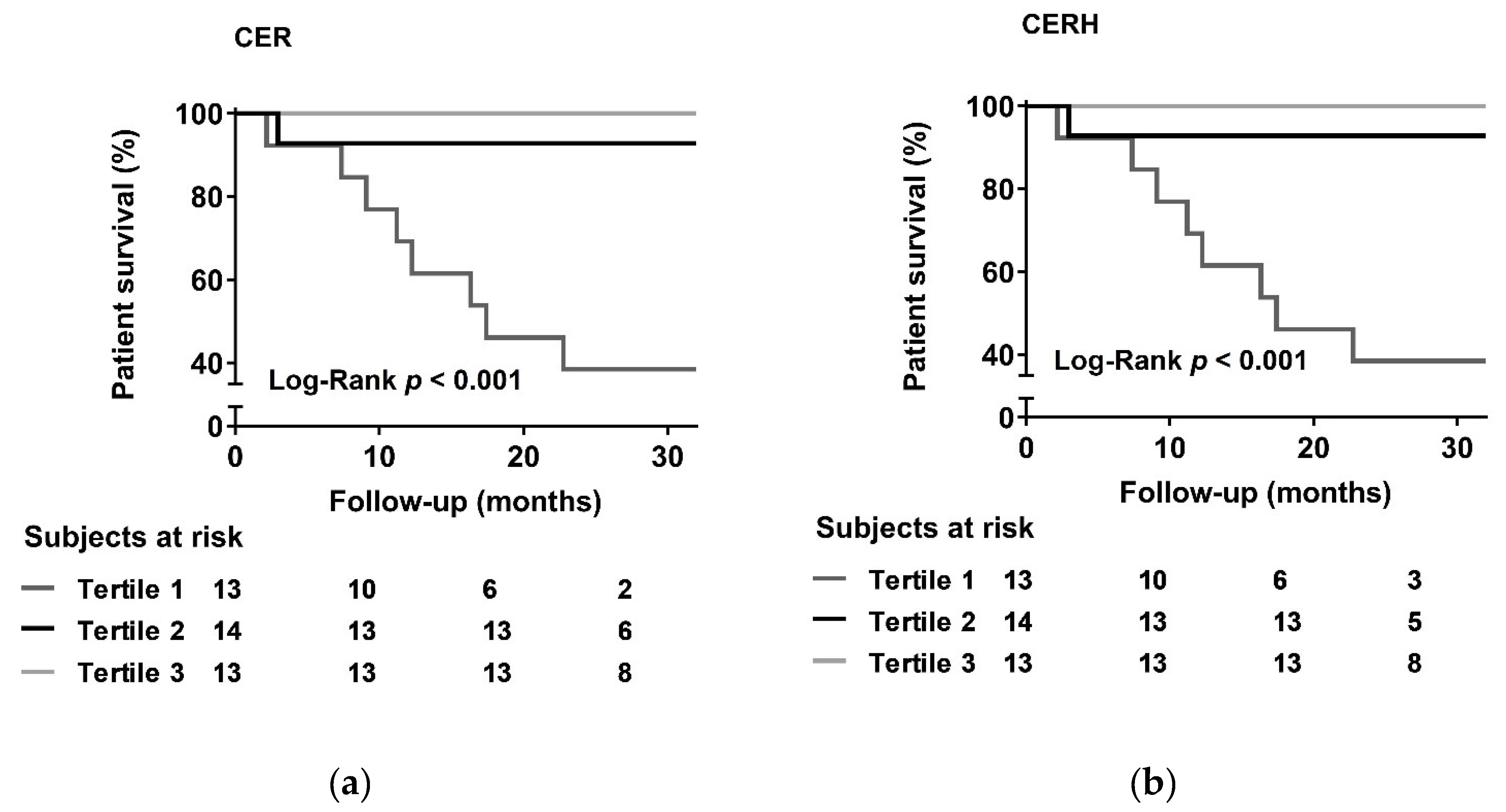
3.4. Prospective Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jadeja, Y.P.; Kher, V. Protein energy wasting in chronic kidney disease: An update with focus on nutritional interventions to improve outcomes. Indian J. Endocrinol. Metab. 2012, 16, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Bistrian, B.R.; McCowen, K.C.; Chan, S. Protein-energy malnutrition in dialysis patients. Am. J. Kidney Dis. 1999, 33, 172–175. [Google Scholar] [CrossRef]
- Kopple, J.D. Pathophysiology of protein-energy wasting in chronic renal failure. J. Nutr. 1999, 129, 247S–251S. [Google Scholar] [CrossRef] [PubMed]
- Mc Causland, F.R.; Waikar, S.S.; Brunelli, S.M. The relevance of dietary sodium in hemodialysis. Nephrol. Dial. Transplant 2013, 28, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Zager, P.G.; Nikolic, J.; Brown, R.H.; Campbell, M.A.; Hunt, W.C.; Peterson, D.; Van Stone, J.; Levey, A.; Meyer, K.B.; Klag, M.J.; et al. “U” curve association of blood pressure and mortality in hemodialysis patients. Kidney Int. 1998, 54, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.A.; Hu, Y.; Singh, B.; Menoyo, J.A.; Wetmore, J.B. Serum Potassium levels and mortality in hemodialysis patients: A retrospective cohort study. Am. J. Nephrol. 2016, 44, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Block, G.A.; Hulbert-Shearon, T.E.; Levin, N.W.; Port, F.K. Association of serum phosphorus and calcium X phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am. J. Kidney Dis. 1998, 31, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health. 2014, 36, e2014009. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee on Dietary Risk Assessment in the WIC Program. Dietary Risk Assessment in the WIC Program, 5th ed.; National Academies Press: Washington, DC, USA, 2002.
- Naska, A.; Lagiou, A.; Lagiou, P. Dietary assessment methods in epidemiological research: Current state of the art and future prospects. F1000Res. 2017, 6, 926. [Google Scholar] [CrossRef] [PubMed]
- Campino, C.; Hill, C.; Baudrand, R.; Martinez-Aguayo, A.; Aglony, M.; Carrasco, C.A.; Ferrada, C.; Loureiro, C.; Vecchiola, A.; Bancalari, R.; et al. Usefulness and pitfalls in sodium intake estimation: Comparison of dietary assessment and urinary excretion in Chilean children and adults. Am. J. Hypertens. 2016, 29, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, C.; Abreu, S.; Padrao, P.; Pinho, O.; Graca, P.; Breda, J.; Santos, R.; Moreira, P. Sodium and potassium urinary excretion and dietary intake: A cross-sectional analysis in adolescents. Food Nutr. Res. 2016, 60, 29442. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.M.; Farmer, V.L.; Nettleton, A.; Cameron, C.M.; Cook, N.R.; Campbell, N.R.C.; TRUE Consortium (International Consortium for Quality Research on Dietary Sodium/Salt). Assessment of dietary sodium intake using a food frequency questionnaire and 24-hour urinary sodium excretion: A systematic literature review. J. Clin. Hypertens. (Greenwich) 2017, 19, 1214–1230. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Sykes, L.; Paul, C.; Dary, O.; Legetic, B.; Campbell, N.R.; Cappuccio, F.P.; Sub-Group for Research and Surveillance of the PAHO-WHO Regional Expert Group for Cardiovascular Disease Prevention Through Population-wide Dietary Salt Reduction. Systematic review of studies comparing 24-hour and spot urine collections for estimating population salt intake. Rev. Panam. Salud Publica 2012, 32, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Potischman, N. Biologic and methodologic issues for nutritional biomarkers. J. Nutr. 2003, 133 (Suppl. 3), 875S–880S. [Google Scholar] [CrossRef]
- Morimoto, Y.; Sakuma, M.; Ohta, H.; Suzuki, A.; Matsushita, A.; Umeda, M.; Ishikawa, M.; Taketani, Y.; Takeda, E.; Arai, H. Estimate of dietary phosphorus intake using 24-h urine collection. J. Clin. Biochem. Nutr. 2014, 55, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Oterdoom, L.H.; van Ree, R.M.; de Vries, A.P.; Gansevoort, R.T.; Schouten, J.P.; van Son, W.J.; Homan van der Heide, J.J.; Navis, G.; de Jong, P.E.; Gans, R.O.; et al. Urinary creatinine excretion reflecting muscle mass is a predictor of mortality and graft loss in renal transplant recipients. Transplantation 2008, 86, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Oterdoom, L.H.; Gansevoort, R.T.; Schouten, J.P.; de Jong, P.E.; Gans, R.O.; Bakker, S.J. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis 2009, 207, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Stam, S.P.; Oste, M.C.J.; Eisenga, M.F.; Blokzijl, H.; van den Berg, A.P.; Bakker, S.J.L.; de Meijer, V.E. Posttransplant muscle mass measured by urinary creatinine excretion rate predicts long-term outcomes after liver transplantation. Am. J. Transplant. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sinkeler, S.J.; Kwakernaak, A.J.; Bakker, S.J.; Shahinfar, S.; Esmatjes, E.; de Zeeuw, D.; Navis, G.; Lambers Heerspink, H.J. Creatinine excretion rate and mortality in type 2 diabetes and nephropathy. Diabetes Care 2013, 36, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Hessels, L.; Koopmans, N.; Gomes Neto, A.W.; Volbeda, M.; Koeze, J.; Lansink-Hartgring, A.O.; Bakker, S.J.; Oudemans-van Straaten, H.M.; Nijsten, M.W. Urinary creatinine excretion is related to short-term and long-term mortality in critically ill patients. Intensive Care Med. 2018, 44, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Ter Maaten, J.M.; Damman, K.; Hillege, H.L.; Bakker, S.J.; Anker, S.D.; Navis, G.; Voors, A.A. Creatinine excretion rate, a marker of muscle mass, is related to clinical outcome in patients with chronic systolic heart failure. Clin. Res. Cardiol. 2014, 103, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989, 5, 303–311. [Google Scholar] [PubMed]
- Maroni, B.J.; Steinman, T.I.; Mitch, W.E. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985, 27, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Baumgartner, R.N.; Ross, R.; Rosenberg, I.H.; Roubenoff, R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am. J. Epidemiol. 2004, 159, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M.; Peters, K.W.; Shardell, M.D.; McLean, R.R.; Dam, T.T.; Kenny, A.M.; Fragala, M.S.; Harris, T.B.; Kiel, D.P.; Guralnik, J.M.; et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Jenab, M.; Slimani, N.; Bictash, M.; Ferrari, P.; Bingham, S.A. Biomarkers in nutritional epidemiology: Applications, needs and new horizons. Hum. Genet. 2009, 125, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A. Optimal nutrition in hemodialysis patients. Adv. Chronic Kidney Dis. 2013, 20, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Kopple, J.D. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am. J. Kidney Dis. 2001, 37, S66–S70. [Google Scholar] [CrossRef] [PubMed]
- Burrowes, J.D.; Larive, B.; Cockram, D.B.; Dwyer, J.; Kusek, J.W.; McLeroy, S.; Poole, D.; Rocco, M.V.; Hemodialysis (HEMO) Study Group. Effects of dietary intake, appetite, and eating habits on dialysis and non-dialysis treatment days in hemodialysis patients: Cross-sectional results from the HEMO study. J. Ren. Nutr. 2003, 13, 191–198. [Google Scholar] [CrossRef]
- Ovesen, L.; Boeing, H.; EFCOSUM Group. The use of biomarkers in multicentric studies with particular consideration of iodine, sodium, iron, folate and vitamin D. Eur. J. Clin. Nutr. 2002, 56 (Suppl. 2), S12–S17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Ho, S.C.; Tang, N.; Chan, R.; Chen, Y.M.; Woo, J. Urinary sodium excretion and dietary sources of sodium intake in Chinese postmenopausal women with prehypertension. PLoS ONE 2014, 9, e104018. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.M.; Day, N.E.; Welch, A.A.; Runswick, S.A.; Luben, R.N.; Mulligan, A.A.; McTaggart, A.; Bingham, S.A. Use of biological markers to validate self-reported dietary intake in a random sample of the European prospective investigation into cancer United Kingdom Norfolk cohort. Am. J. Clin. Nutr. 2001, 74, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.A.; Appel, L.J.; Okuda, N.; Brown, I.J.; Chan, Q.; Zhao, L.; Ueshima, H.; Kesteloot, H.; Miura, K.; Curb, J.D.; et al. Dietary sources of sodium in China, Japan, the United Kingdom, and the United States, women and men aged 40 to 59 years: The INTERMAP study. J. Am. Diet. Assoc. 2010, 110, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Espeland, M.A.; Kumanyika, S.; Wilson, A.C.; Reboussin, D.M.; Easter, L.; Self, M.; Robertson, J.; Brown, W.M.; McFarlane, M.; TONE Cooperative Research Group. Statistical issues in analyzing 24-hour dietary recall and 24-hour urine collection data for sodium and potassium intakes. Am. J. Epidemiol. 2001, 153, 996–1006. [Google Scholar] [CrossRef]
- Meneton, P.; Galan, P.; Bertrais, S.; Heudes, D.; Hercberg, S.; Menard, J. High plasma aldosterone and low renin predict blood pressure increase and hypertension in middle-aged Caucasian populations. J. Hum. Hypertens. 2008, 22, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Meneton, P.; Lafay, L.; Tard, A.; Dufour, A.; Ireland, J.; Menard, J.; Volatier, J.L. Dietary sources and correlates of sodium and potassium intakes in the French general population. Eur. J. Clin. Nutr. 2009, 63, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Pool, L.R.; Wolf, M. FGF23 and nutritional metabolism. Annu. Rev. Nutr. 2017, 37, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M. Mineral (Mal)adaptation to kidney disease—Young investigator award address: American society of Nephrology kidney week 2014. Clin. J. Am. Soc. Nephrol. 2015, 10, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; Gallieni, M. Urinary phosphorus excretion: Not what we have believed it to be? Clin. J. Am. Soc. Nephrol. 2018, 13, 973–974. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Bertrand, K.A.; Franke, A.A.; Rosner, B.; Curhan, G.C.; Willett, W.C. Reproducibility of urinary biomarkers in multiple 24-h urine samples. Am. J. Clin. Nutr. 2017, 105, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Trautvetter, U.; Ditscheid, B.; Jahreis, G.; Glei, M. Habitual intakes, food sources and excretions of phosphorus and calcium in three German study collectives. Nutrients 2018, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.M.; Leon, J.B.; Sehgal, A.R. Phosphorus-containing food additives and the accuracy of nutrient databases: Implications for renal patients. J. Ren. Nutr. 2007, 17, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Torres, R.; Ruiz-Lopez, M.D.; Artacho, R.; Oliva, P.; Baena, F.; Baro, L.; Lopez, C. Dietary intake of calcium, magnesium and phosphorus in an elderly population using duplicate diet sampling Vs food composition tables. J. Nutr. Health Aging 2001, 5, 253–255. [Google Scholar] [PubMed]
- Oenning, L.L.; Vogel, J.; Calvo, M.S. Accuracy of methods estimating calcium and phosphorus intake in daily diets. J. Am. Diet. Assoc. 1988, 88, 1076–1080. [Google Scholar] [PubMed]
- Sherman, R.A.; Mehta, O. Dietary phosphorus restriction in dialysis patients: Potential impact of processed meat, poultry, and fish products as protein sources. Am. J. Kidney Dis. 2009, 54, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Carrigan, A.; Klinger, A.; Choquette, S.S.; Luzuriaga-McPherson, A.; Bell, E.K.; Darnell, B.; Gutierrez, O.M. Contribution of food additives to sodium and phosphorus content of diets rich in processed foods. J. Ren. Nutr. 2014, 24, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Tosato, M.; Marzetti, E.; Cesari, M.; Savera, G.; Miller, R.R.; Bernabei, R.; Landi, F.; Calvani, R. Measurement of muscle mass in sarcopenia: from imaging to biochemical markers. Aging Clin. Exp. Res. 2017, 29, 19–27. [Google Scholar] [CrossRef] [PubMed]


| Baseline Characteristics | Average/Number | Range |
|---|---|---|
| Demographics | ||
| Age, years | 64 ± 13 | 25–86 |
| Gender, n male (%) | 22 (52) | |
| Race, n Caucasian (%) | 39 (93) | |
| Dialysis-related | ||
| Dialysis sessions, n (%) | ||
| 2 sessions per week | 1 (2) | |
| 3 sessions per week | 41 (98) | |
| Hours per dialysis, n (%) | ||
| 3 to 3.5 h | 6 (14) | |
| 4 h | 34 (81) | |
| 4.5 to 5 h | 2 (5) | |
| Residual diuresis, n (%) | 22 (52) | |
| Urinary volume, L | 0.84 ± 0.57 | 0.14–2.39 |
| Dialysis vintage, months | 14 (6–45) | 2–202 |
| Ultrafiltration volume, ml | 1926 ± 921 | 1425–2725 |
| Body composition | ||
| Target body weight, kg | 80.2 ± 15.6 | 72.5–89.9 |
| Interdialytic weight gain, kg | 1.17 ± 1.12 | −1.7–4.4 |
| Height, m | 1.75 ± 0.09 | 1.66–1.83 |
| BMI, kg/m2 | 25.6 ± 4.3 | 22.7–28.8 |
| BSA, m2 | 1.93 ± 0.21 | 1.82–2.06 |
| Pre-existing disease | ||
| Hypertension, n (%) | 28 (67) | |
| Diabetes, n (%) | 12 (29) | |
| Cardiovascular disease, n (%) | 18 (43) | |
| Medication usage | ||
| Use of phosphate binders, n (%) | 21 (50) | |
| Sevelamer | 15 (36) | |
| Calciumcarbonate or lanthanumcarbonate | 11 (26) | |
| Calciumacetate and magnesiumcarbonate (OsvaRen) | 6 (14) | |
| Use of potassium binders, n (%) * | 4 (10) |
| Variable | Before Dialysis | After Dialysis | p-Value |
|---|---|---|---|
| Clinical | |||
| Systolic blood pressure (mmHg) | 147 ± 21 | 138 ± 27 | 0.012 |
| Diastolic blood pressure, (mmHg) | 69 ± 12 | 67 ± 11 | 0.163 |
| Pulse, min−1 | 74 ± 14 | 73 ± 12 | 0.631 |
| Body weight, kg | 80.0 ± 16.0 | 78.7 ± 16.0 | < 0.001 |
| Laboratory | |||
| Hemoglobin (mmol/L) | 6.9 ± 0.7 | 7.4 ± 1.0 | 0.001 |
| Hematocrit | 0.34 ± 0.04 | 0.36 ± 0.04 | 0.012 |
| Sodium (mmol/L) | 138 ± 3 | 139 ± 2 | 0.023 |
| Potassium (mmol/L) | 4.9 ± 0.5 | 3.5 ± 0.4 | < 0.001 |
| Phosphate (mmol/L) | 1.6 ± 0.6 | 0.8 ± 0.2 | < 0.001 |
| Albumin (g/L) | 40 ± 5 | 43 ± 4 | < 0.001 |
| Urea (mmol/L) | 20 ± 5 | 6 ± 2 | < 0.001 |
| Creatinine (µmol/L) | 707 ± 196 | 265 ± 94 | < 0.001 |
| Variable | CBA Intake * | DA Intake | p-Value |
|---|---|---|---|
| Protein (g/24 h) | 63 ± 19 | 71 ± 19 | 0.003 |
| Sodium (mg/24 h) | 4035 ± 2316 | 2123 ± 616 | < 0.001 |
| Potassium (mg/24 h) | 2041 ± 907 | 2445 ± 568 | 0.008 |
| Phosphate (mg/24 h) | 1427 ± 637 | 1221 ± 276 | 0.029 |
| Model | CER | CERH | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| 1 | 0.59 (0.42–0.84) | 0.003 | 0.13 (0.04–0.45) | 0.001 |
| 2 | 0.50 (0.29–0.83) | 0.007 | 0.14 (0.03–0.61) | 0.009 |
| 3 | 0.47 (0.28–0.79) | 0.005 | 0.12 (0.03–0.56) | 0.007 |
| 4 | 0.49 (0.29–0.82) | 0.007 | 0.14 (0.03–0.62) | 0.010 |
| 5 | 0.50 (0.30–0.82) | 0.007 | 0.14 (0.03–0.61) | 0.009 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Post, A.; Ozyilmaz, A.; Westerhuis, R.; Ipema, K.J.R.; Bakker, S.J.L.; Franssen, C.F.M. Complementary Biomarker Assessment of Components Absorbed from Diet and Creatinine Excretion Rate Reflecting Muscle Mass in Dialysis Patients. Nutrients 2018, 10, 1827. https://doi.org/10.3390/nu10121827
Post A, Ozyilmaz A, Westerhuis R, Ipema KJR, Bakker SJL, Franssen CFM. Complementary Biomarker Assessment of Components Absorbed from Diet and Creatinine Excretion Rate Reflecting Muscle Mass in Dialysis Patients. Nutrients. 2018; 10(12):1827. https://doi.org/10.3390/nu10121827
Chicago/Turabian StylePost, Adrian, Akin Ozyilmaz, Ralf Westerhuis, Karin J. R. Ipema, Stephan J. L. Bakker, and Casper F. M. Franssen. 2018. "Complementary Biomarker Assessment of Components Absorbed from Diet and Creatinine Excretion Rate Reflecting Muscle Mass in Dialysis Patients" Nutrients 10, no. 12: 1827. https://doi.org/10.3390/nu10121827
APA StylePost, A., Ozyilmaz, A., Westerhuis, R., Ipema, K. J. R., Bakker, S. J. L., & Franssen, C. F. M. (2018). Complementary Biomarker Assessment of Components Absorbed from Diet and Creatinine Excretion Rate Reflecting Muscle Mass in Dialysis Patients. Nutrients, 10(12), 1827. https://doi.org/10.3390/nu10121827






