Within-Day Amino Acid Intakes and Nitrogen Balance in Male Collegiate Swimmers during the General Preparation Phase
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Participants
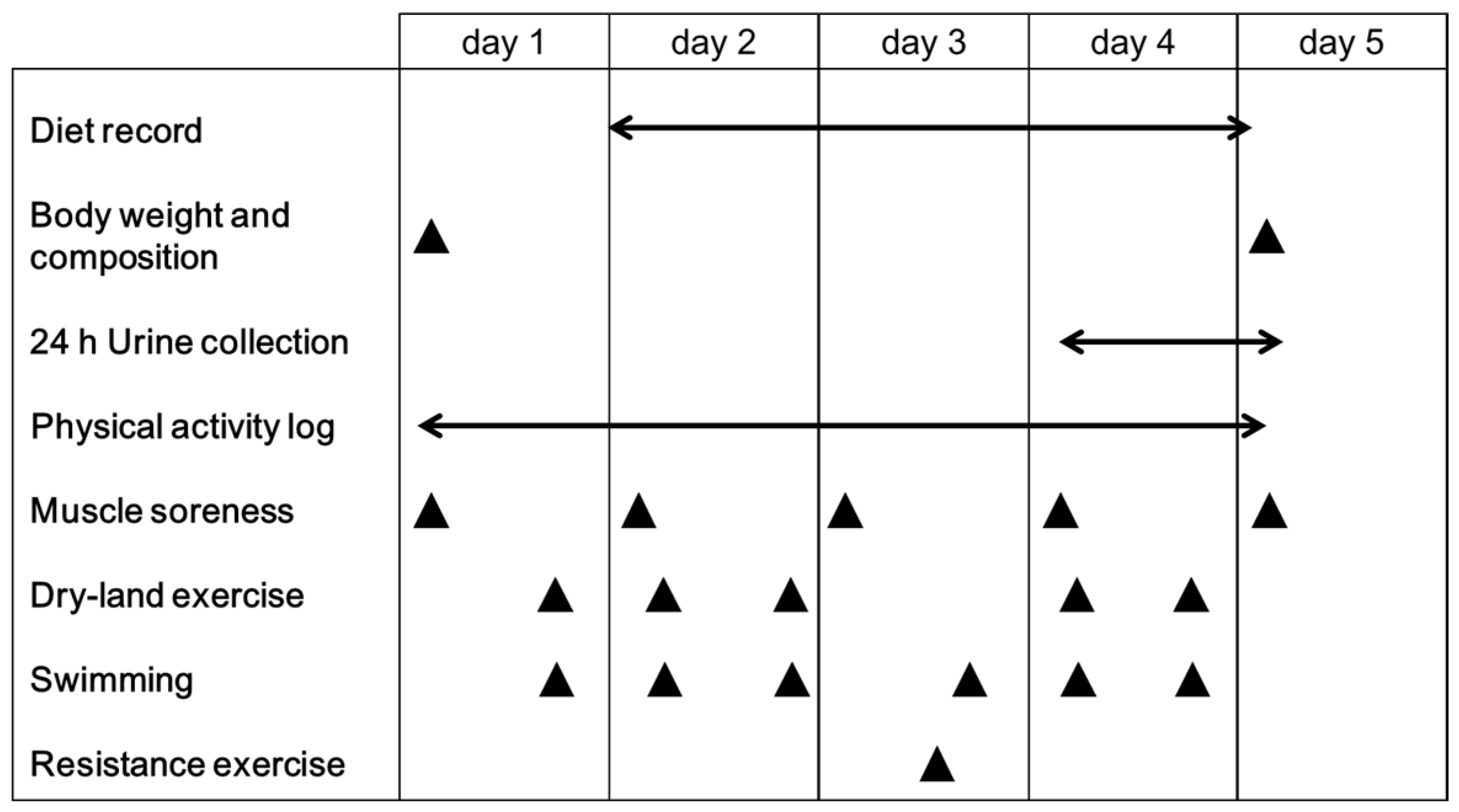
2.3. Experimental Design
2.4. Energy Expenditure
2.5. Dietary Intakes
2.6. Nitrogen Balance
2.7. Statistical Analysis
3. Results
3.1. Body Weight and Composition
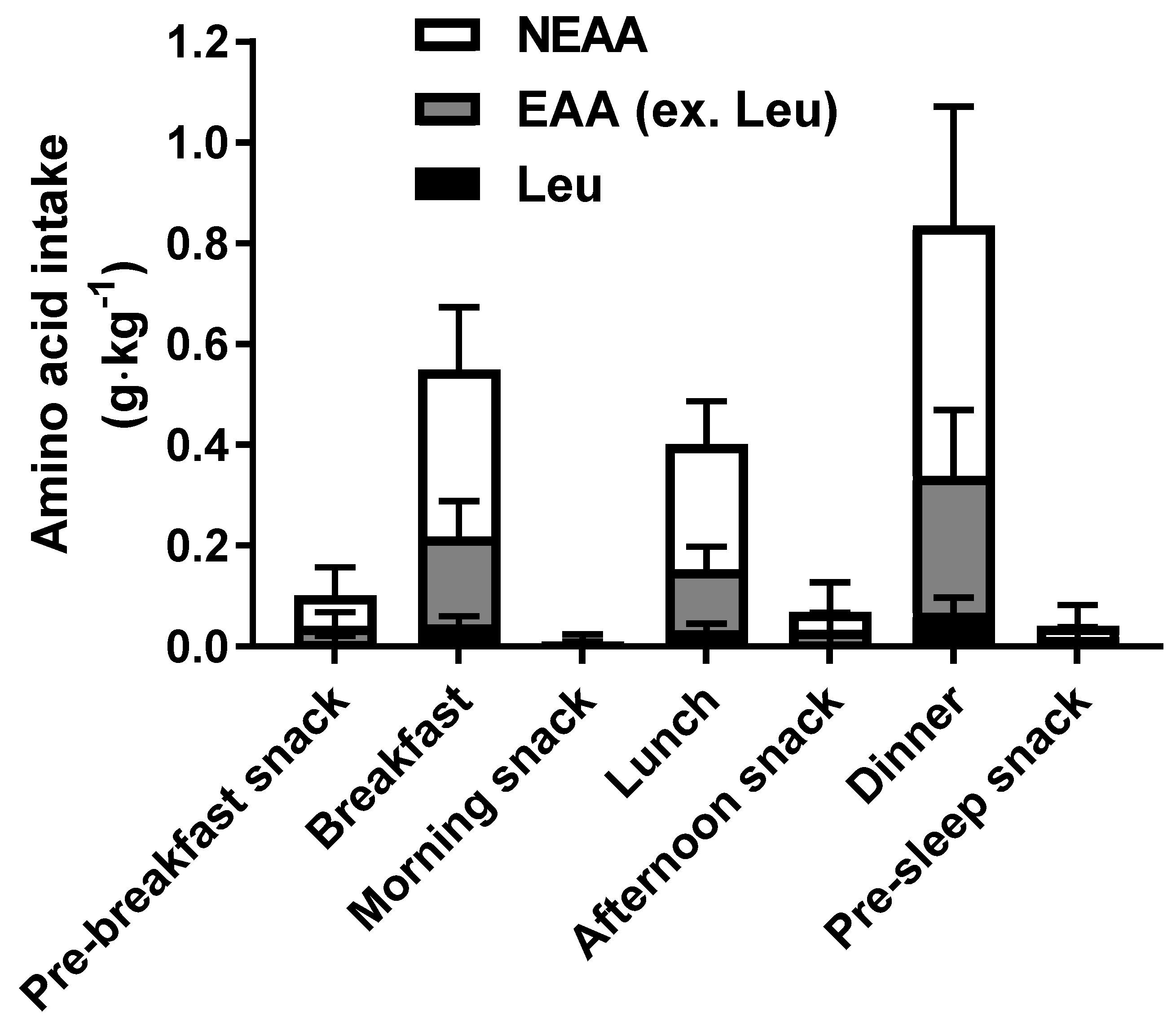
3.2. Daily Dietary Intakes
3.3. Energy Expenditure
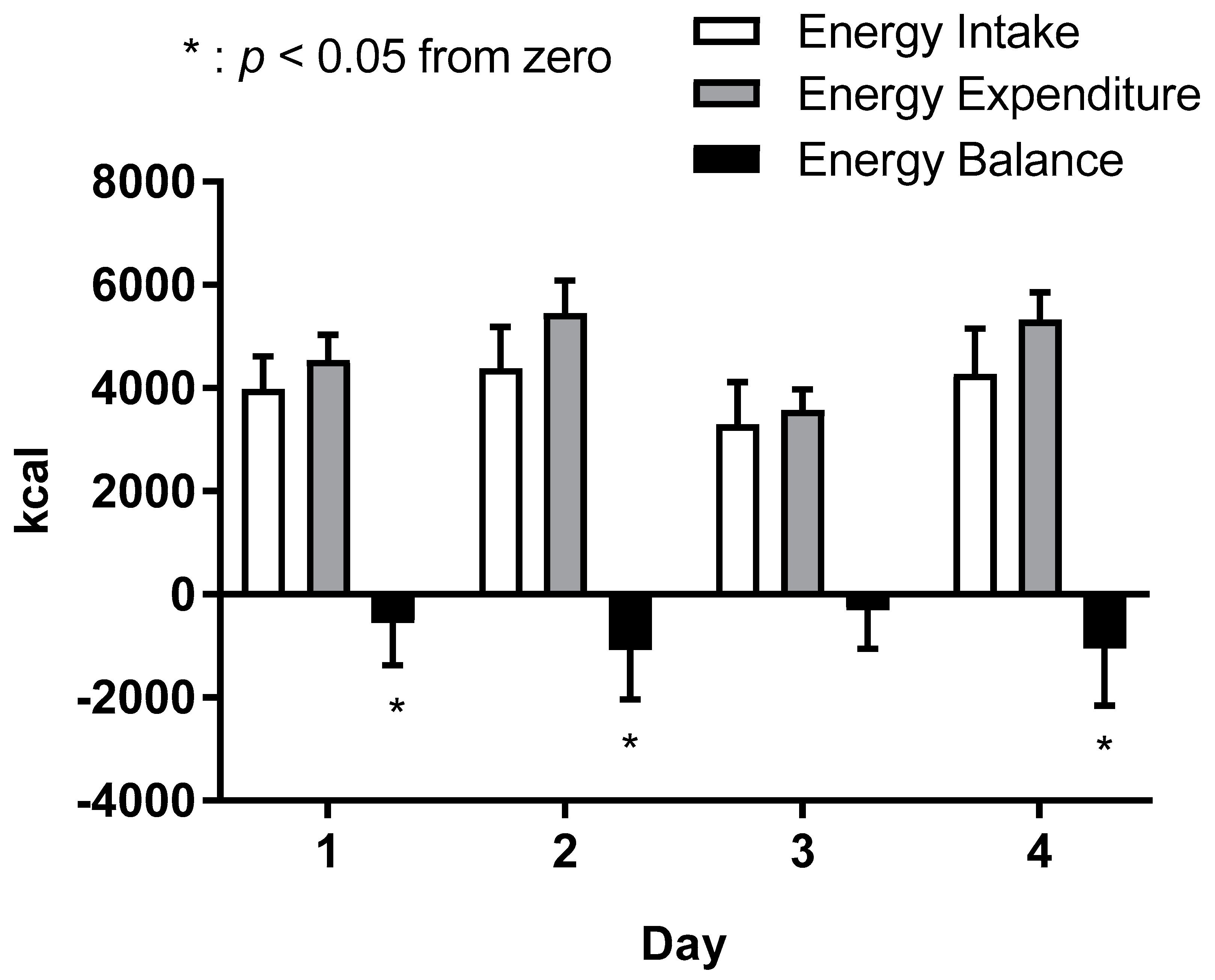
3.4. Energy Balance
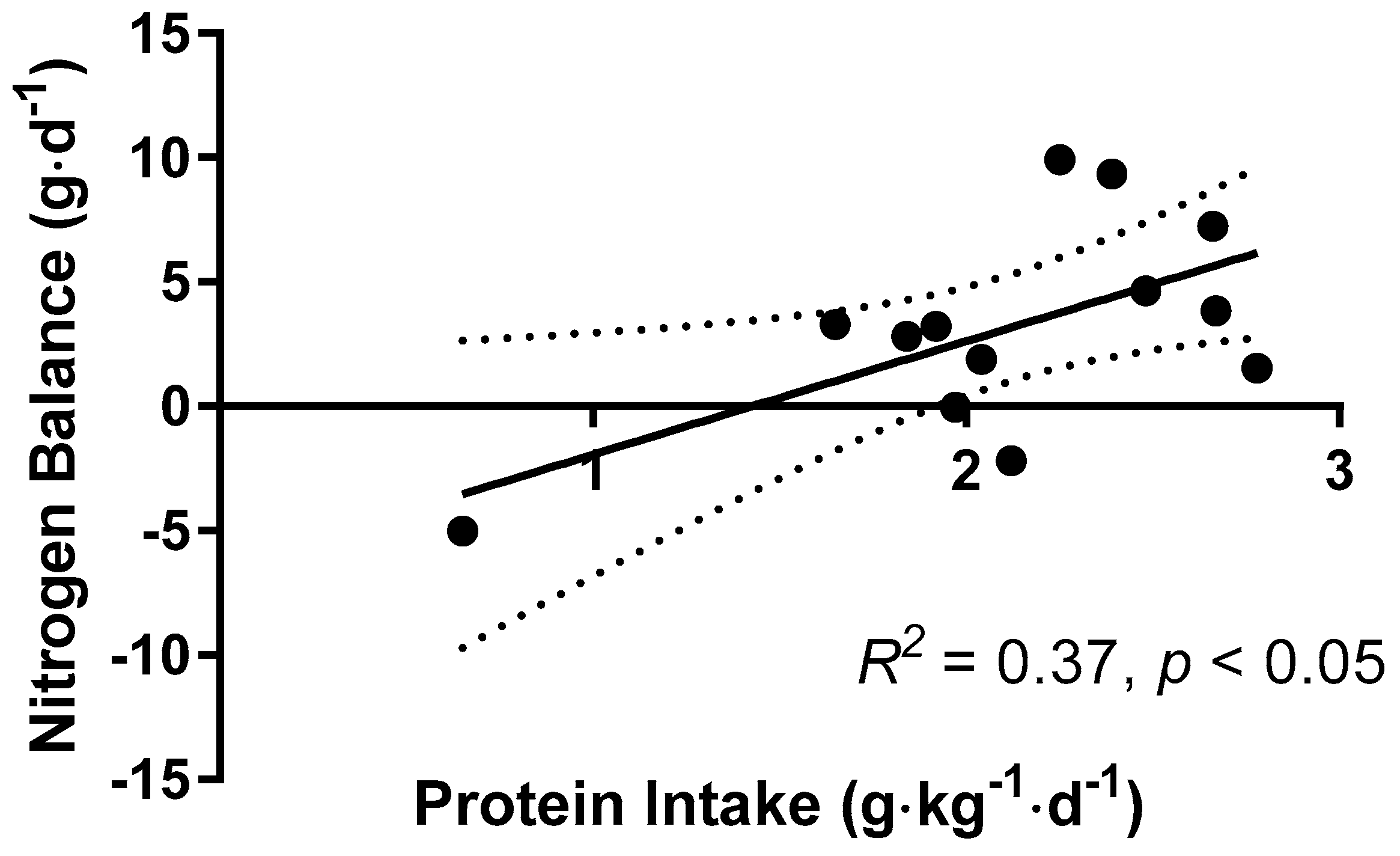
3.5. Nitrogen Balance
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mujika, I.; Stellingwerff, T.; Tipton, K. Nutrition and training adaptations in aquatic sports. Int. J. Sport. Nutr. Exerc. Metab. 2014, 24, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Costill, D.L.; Flynn, M.G.; Kirwan, J.P.; Houmard, J.A.; Mitchell, J.B.; Thomas, R.; Park, S.H. Effects of repeated days of intensified training on muscle glycogen and swimming performance. Med. Sci. Sports Exerc. 1988, 20, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Trappe, T.A.; Gastaldelli, A.; Jozsi, A.C.; Troup, J.P.; Wolfe, R.R. Energy expenditure of swimmers during high volume training. Med. Sci. Sports Exerc. 1997, 29, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Lemon, P.W.; Mullin, J.P. Effect of initial muscle glycogen levels on protein catabolism during exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980, 48, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Hassapidou, M.N.; Manstrantoni, A. Dietary intakes of elite female athletes in Greece. J. Hum. Nutr. Diet. 2001, 14, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Almeras, N.; Lemieux, S.; Bouchard, C.; Tremblay, A. Fat gain in female swimmers. Physiol. Behav. 1997, 61, 811–817. [Google Scholar] [CrossRef]
- Helms, E.R.; Zinn, C.; Rowlands, D.S.; Brown, S.R. A systematic review of dietary protein during caloric restriction in resistance trained lean athletes: A case for higher intakes. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Hector, A.J.; Phillips, S.M. Protein Recommendations for Weight Loss in Elite Athletes: A Focus on Body Composition and Performance. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Williams, M.M.; Vickovic, M.M.; Handcock, P.J. Muscle power predicts freestyle swimming performance. Br. J. Sports Med. 1992, 26, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R.L.; Troup, J.P.; Costill, D.L. Relationship between power and sprint freestyle swimming. Med. Sci. Sports Exerc. 1982, 14, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Tonnessen, E.; Sylta, O.; Haugen, T.A.; Hem, E.; Svendsen, I.S.; Seiler, S. The road to gold: Training and peaking characteristics in the year prior to a gold medal endurance performance. PLoS ONE 2014, 9, e101796. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D.; Ferrando, A.A.; Williams, B.D.; Wolfe, R.R. Muscle protein metabolism in female swimmers after a combination of resistance and endurance exercise. J. Appl. Physiol. 1996, 81, 2034–2038. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Tipton, K.D.; Aarsland, A.; Wolf, S.E.; Wolfe, R.R. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J. Physiol. 1997, 273, E99–E107. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.R.; DiMarco, N.M.; Langley, S.; American Dietetic, A.; Dietitians of, C.; American College of Sports Medicine, N.; Athletic, P. Position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J. Am. Diet Assoc. 2009, 109, 509–527. [Google Scholar] [PubMed]
- Tarnopolsky, M. Protein requirements for endurance athletes. Nutrition 2004, 20, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.A.; Elango, R.; Ball, R.O.; Pencharz, P.B. Reevaluation of the protein requirement in young men with the indicator amino acid oxidation technique. Am. J. Clin. Nutr. 2007, 86, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International society of sports nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2017, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Aragon, A.A. How much protein can the body use in a single meal for muscle-building? Implications for daily protein distribution. J. Int. Soc. Sports Nutr. 2018, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa-Takata, K.; Takimoto, H. Current protein and amino acid intakes among Japanese people: Analysis of the 2012 National Health and Nutrition Survey. Geriatr. Gerontol. Int. 2018, 18, 723–731. [Google Scholar] [CrossRef] [PubMed]
- UMIN Clinical Trials Registry (UMIN-CTR). Available online: www.umin.ac.jp/ctr/index.htm (accessed on 17 November 2018).
- Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Weststrate, J.A.; Weys, P.J.; Poortvliet, E.J.; Deurenberg, P.; Hautvast, J.G. Diurnal variation in postabsorptive resting metabolic rate and diet-induced thermogenesis. Am. J. Clin. Nutr. 1989, 50, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Phillips, W.T.; Ziuraitis, J.R. Energy cost of the ACSM single-set resistance training protocol. J. Strength Cond. Res. 2003, 17, 350–355. [Google Scholar] [CrossRef]
- Yasui, T. Advances in Food Composition Tables of Japan—Amino Acid, Fatty Acid and Available Carbohydrate Tables. J. Nutr. Sci. Vitaminol. 2015, 61, S28–S30. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kawai, R. Advances in food composition tables in Japan-Standard Tables Of Food Composition in Japan—2015—(Seventh Revised Edition). Food Chem. 2018, 238, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Tarnopolsky, M.A.; MacDougall, J.D.; Atkinson, S.A. Influence of protein intake and training status on nitrogen balance and lean body mass. J. Appl. Physiol. 1988, 64, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients); The National Academy Press: Washington, DC, USA, 2005. [Google Scholar]
- Macnaughton, L.S.; Wardle, S.L.; Witard, O.C.; McGlory, C.; Hamilton, D.L.; Jeromson, S.; Lawrence, C.E.; Wallis, G.A.; Tipton, K.D. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiol. Rep. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Robinson, M.J.; Fry, J.L.; Tang, J.E.; Glover, E.I.; Wilkinson, S.B.; Prior, T.; Tarnopolsky, M.A.; Phillips, S.M. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2009, 89, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Witard, O.C.; Jackman, S.R.; Breen, L.; Smith, K.; Selby, A.; Tipton, K.D. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 2014, 99, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Areta, J.L.; Burke, L.M.; Ross, M.L.; Camera, D.M.; West, D.W.; Broad, E.M.; Jeacocke, N.A.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M.; et al. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J. Physiol. 2013, 591, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.B.; Tarnopolsky, M.A.; Macdonald, M.J.; Macdonald, J.R.; Armstrong, D.; Phillips, S.M. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am. J. Clin. Nutr. 2007, 85, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Crozier, S.J.; Kimball, S.R.; Emmert, S.W.; Anthony, J.C.; Jefferson, L.S. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J. Nutr. 2005, 135, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Suryawan, A.; Nguyen, H.V.; Almonaci, R.D.; Davis, T.A. Differential regulation of protein synthesis in skeletal muscle and liver of neonatal pigs by leucine through an mTORC1-dependent pathway. J. Anim. Sci. Biotechnol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Norton, L.E.; Wilson, G.J.; Layman, D.K.; Moulton, C.J.; Garlick, P.J. Leucine content of dietary proteins is a determinant of postprandial skeletal muscle protein synthesis in adult rats. Nutr. Metab. 2012, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Ispoglou, T.; King, R.F.; Polman, R.C.; Zanker, C. Daily L-leucine supplementation in novice trainees during a 12-week weight training program. Int. J. Sports. Physiol. Perform. 2011, 6, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Jackman, S.R.; Witard, O.C.; Philp, A.; Wallis, G.A.; Baar, K.; Tipton, K.D. Branched-Chain Amino Acid Ingestion Stimulates Muscle Myofibrillar Protein Synthesis following Resistance Exercise in Humans. Front. Physiol. 2017, 8, 390. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; McClung, H.L.; McClung, J.P.; Margolis, L.M.; Andersen, N.E.; Cloutier, G.J.; Pikosky, M.A.; Rood, J.C.; Fielding, R.A.; Young, A.J. Leucine-enriched essential amino acid supplementation during moderate steady state exercise enhances postexercise muscle protein synthesis. Am. J. Clin. Nutr. 2011, 94, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Suzuki, H.; Mimura, M.; Inoue, Y.; Sugita, M.; Suzuki, K.; Kobayashi, H. Leucine-enriched essential amino acids attenuate muscle soreness and improve muscle protein synthesis after eccentric contractions in rats. Amino Acids 2015, 47, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Suzuki, H.; Inoue, Y.; Suzuki, K.; Kobayashi, H. Leucine-Enriched Essential Amino Acids Augment Mixed Protein Synthesis, But Not Collagen Protein Synthesis, in Rat Skeletal Muscle after Downhill Running. Nutrients 2016, 8, 399. [Google Scholar] [CrossRef] [PubMed]
- Boisseau, N.; Vermorel, M.; Rance, M.; Duche, P.; Patureau-Mirand, P. Protein requirements in male adolescent soccer players. Eur. J. Appl. Physiol. 2007, 100, 27–33. [Google Scholar] [CrossRef] [PubMed]
- American Dietetic, A.; Dietitians of, C.; American College of Sports, M.; Rodriguez, N.R.; Di Marco, N.M.; Langley, S. American College of Sports Medicine position stand. Nutrition and athletic performance. Med. Sci. Sports Exerc. 2009, 41, 709–731. [Google Scholar] [CrossRef]
- Meredith, C.N.; Zackin, M.J.; Frontera, W.R.; Evans, W.J. Dietary protein requirements and body protein metabolism in endurance-trained men. J. Appl. Physiol. 1989, 66, 2850–2856. [Google Scholar] [CrossRef] [PubMed]
- Gaine, P.C.; Pikosky, M.A.; Martin, W.F.; Bolster, D.R.; Maresh, C.M.; Rodriguez, N.R. Level of dietary protein impacts whole body protein turnover in trained males at rest. Metabolism 2006, 55, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Camera, D.M.; Areta, J.L.; Hawley, J.A. Beyond muscle hypertrophy: Why dietary protein is important for endurance athletes. Appl. Physiol. Nutr. Metab. 2014, 39, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, L.J.; Forse, R.A. Protein metabolic effects of a prolonged fast and hypocaloric refeeding. Am. J. Physiol. 1990, 258, E832–E840. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.P.; Rumpler, W.V.; Leskiw, M.J.; Schluter, M.D.; Staples, R.; Bodwell, C.E. Effect of reduced dietary intake on energy expenditure, protein turnover, and glucose cycling in man. Metabolism 1991, 40, 478–483. [Google Scholar] [CrossRef]
- Areta, J.L.; Burke, L.M.; Camera, D.M.; West, D.W.; Crawshay, S.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M.; Hawley, J.A.; Coffey, V.G. Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E989–E997. [Google Scholar] [CrossRef] [PubMed]
- Hector, A.J.; Marcotte, G.R.; Churchward-Venne, T.A.; Murphy, C.H.; Breen, L.; von Allmen, M.; Baker, S.K.; Phillips, S.M. Whey protein supplementation preserves postprandial myofibrillar protein synthesis during short-term energy restriction in overweight and obese adults. J. Nutr. 2015, 145, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Vislocky, L.M.; Carbone, J.W.; Altieri, N.; Konopelski, K.; Freake, H.C.; Anderson, J.M.; Ferrando, A.A.; Wolfe, R.R.; Rodriguez, N.R. Acute energy deprivation affects skeletal muscle protein synthesis and associated intracellular signaling proteins in physically active adults. J. Nutr. 2010, 140, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Howarth, K.R.; Phillips, S.M.; MacDonald, M.J.; Richards, D.; Moreau, N.A.; Gibala, M.J. Effect of glycogen availability on human skeletal muscle protein turnover during exercise and recovery. J. Appl. Physiol. 2010, 109, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Pyne, D.B.; Sharp, R.L. Physical and energy requirements of competitive swimming events. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Tarnopolsky, M.A.; Atkinson, S.A.; MacDougall, J.D.; Chesley, A.; Phillips, S.; Schwarcz, H.P. Evaluation of protein requirements for trained strength athletes. J. Appl. Physiol. 1992, 73, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Boisseau, N.; Le Creff, C.; Loyens, M.; Poortmans, J.R. Protein intake and nitrogen balance in male non-active adolescents and soccer players. Eur. J. Appl. Physiol. 2002, 88, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Millward, D.J. Methodological considerations. Proc. Nutr. Soc. 2001, 60, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Forbes, G.B. Another source of error in the metabolic balance method. Nutr. Rev. 1973, 31, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Zello, G.A.; Wykes, L.J.; Ball, R.O.; Pencharz, P.B. Recent advances in methods of assessing dietary amino acid requirements for adult humans. J. Nutr. 1995, 125, 2907–2915. [Google Scholar] [PubMed]
- Elango, R.; Ball, R.O.; Pencharz, P.B. Indicator amino acid oxidation: Concept and application. J. Nutr. 2008, 138, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Suzuki, K.; Bannai, M.; Moore, D.R. Branched-Chain Amino Acids Are the Primary Limiting Amino Acids in the Diets of Endurance-Trained Men after a Bout of Prolonged Exercise. J. Nutr. 2018, 148, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Bandegan, A.; Courtney-Martin, G.; Rafii, M.; Pencharz, P.B.; Lemon, P.W. Indicator Amino Acid-Derived Estimate of Dietary Protein Requirement for Male Bodybuilders on a Nontraining Day Is Several-Fold Greater than the Current Recommended Dietary Allowance. J. Nutr. 2017, 147, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Suzuki, K.; Bannai, M.; Moore, D.R. Protein Requirements Are Elevated in Endurance Athletes after Exercise as Determined by the Indicator Amino Acid Oxidation Method. PLoS ONE 2016, 11, e0157406. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Volterman, K.A.; West, D.W.D.; Suzuki, K.; Moore, D.R. Nutritionally non-essential amino acids are dispensable for whole-body protein synthesis after exercise in endurance athletes with an adequate essential amino acid intake. Amino Acids 2018, 50, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.; Kato, H.; Volterman, K.A.; Suzuki, K.; Moore, D.R. The Effect of Dietary Protein on Protein Metabolism and Performance in Endurance-trained Males. Med. Sci. Sports Exerc. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
| Mean ± SD (n = 13) | |
|---|---|
| Age (year) | 19.7 ± 1.0 |
| Height (cm) | 172.8 ± 5.1 |
| Body weight (kg) | 67.4 ± 5.1 |
| FFM (kg) | 57.0 ± 3.5 |
| Body Fat (%) | 15.4 ± 3.3 |
| VO2max (mL·kg−1·min−1) | 63.9 ± 3.7 |
| Day 1 | Day 5 | p-Value | |
|---|---|---|---|
| Body weight, kg | 67.4 ± 5.1 | 67.7 ± 5.2 | >0.05 |
| Fat mass, % | 15.4 ± 3.3 | 14.1 ± 2.4 | <0.01 |
| Fat mass, kg | 10.5 ± 2.9 | 9.6 ± 2.3 | <0.01 |
| Fat-free mass, kg | 57.0 ± 3.5 | 58.0 ± 3.6 | <0.05 |
| Day 2 | Day 3 | Day 4 (Test Day) | Average | |
|---|---|---|---|---|
| Energy | ||||
| kcal·day−1 | 4385 ± 803 ** | 3279 ± 817 | 4279 ± 876 ** | 3981 ± 629 |
| Protein | ||||
| g·day−1 | 141.7 ± 27.7 * | 105.8 ± 37.5 | 142 ± 39.3 * | 129.8 ± 25.9 |
| g·kg−1·day−1 | 2.1 ± 0.4 * | 1.6 ± 0.6 | 2.1 ± 0.6 * | 1.9 ± 0.4 |
| Energy % | 13 ± 2 | 13 ± 2 | 13 ± 2 | 13 ± 1.4 |
| Fat | ||||
| g·day−1 | 153.2 ± 32.5 * | 110.8 ± 47.6 | 155.9 ± 47.7 | 140 ± 30 |
| g·kg−1·day−1 | 2.3 ± 0.6 * | 1.7 ± 0.8 | 2.4 ± 0.8 | 2.1 ± 0.5 |
| Energy% | 33 ± 4 | 31 ± 6 | 35 ± 6 | 31.5 ± 3.8 |
| Carbohydrate | ||||
| g·day−1 | 589.1 ± 123 ** | 449.3 ± 84.4 | 553.4 ± 111.1 ** | 530.6 ± 86.8 |
| g·kg−1·day−1 | 8.7 ± 1.8 ** | 6.7 ± 1.3 | 8.2 ± 1.7 ** | 7.9 ± 1.3 |
| Energy % | 54 ± 5 | 56 ± 7 | 52 ± 6 | 53 ± 4 |
| Morning Sessions | Evening Sessions | ||||
|---|---|---|---|---|---|
| Dry-Land | Swimming | Dry-Land | Swimming | ||
| day 1 | HR (bpm) | 112 ± 15 | 134 ± 6 | ||
| Time (mins) | 82 ± 5 | 117 ± 3 | |||
| Distance (m) | 5500 | ||||
| day 2 | HR (bpm) | 92 ± 8 | 124 ± 5 | 101 ± 14 | 129 ± 7 |
| Time (mins) | 55 ± 8 | 90 ± 6 | 76 ± 10 | 122 ± 1 | |
| Distance (m) | 4400 | 5500 | |||
| day 3 | HR (bpm) | 119 ± 7 | |||
| Time (mins) | 57 ± 9 | ||||
| Distance (m) | 2000 | ||||
| day 4 | HR (bpm) | 91 ± 8 | 118 ± 7 | 99 ± 11 | 127 ± 7 |
| Time (mins) | 55 ± 6 | 92 ± 5 | 80 ± 11 | 121 ± 2 | |
| Distance (m) | 4400 | 5500 | |||
| Kcal/day | Day 1 | Day 2 | Day 3 | Day 4 |
|---|---|---|---|---|
| Resting energy expenditure | 1975 ± 245 | |||
| Diet-induced thermogenesis | 283 ± 45 * | 312 ± 57 | 233 ± 58 | 304 ± 63 |
| Exercise-induced energy expenditure | ||||
| Habitual physical activity | 446 ± 181 | 481 ± 215 | 481 ± 215 | 456 ± 141 |
| Exercise sessions | 1838 ± 283 | 2691 ± 371 | 926 ± 98 | 2589 ± 355 |
| Total energy expenditure | 4541 ± 498 | 5457 ± 629 | 3579 ± 401 | 5323 ± 529 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuda, T.; Kato, H.; Suzuki, H.; Mizugaki, A.; Ezaki, T.; Ogita, F. Within-Day Amino Acid Intakes and Nitrogen Balance in Male Collegiate Swimmers during the General Preparation Phase. Nutrients 2018, 10, 1809. https://doi.org/10.3390/nu10111809
Matsuda T, Kato H, Suzuki H, Mizugaki A, Ezaki T, Ogita F. Within-Day Amino Acid Intakes and Nitrogen Balance in Male Collegiate Swimmers during the General Preparation Phase. Nutrients. 2018; 10(11):1809. https://doi.org/10.3390/nu10111809
Chicago/Turabian StyleMatsuda, Takeshi, Hiroyuki Kato, Haruka Suzuki, Ami Mizugaki, Takahiko Ezaki, and Futoshi Ogita. 2018. "Within-Day Amino Acid Intakes and Nitrogen Balance in Male Collegiate Swimmers during the General Preparation Phase" Nutrients 10, no. 11: 1809. https://doi.org/10.3390/nu10111809
APA StyleMatsuda, T., Kato, H., Suzuki, H., Mizugaki, A., Ezaki, T., & Ogita, F. (2018). Within-Day Amino Acid Intakes and Nitrogen Balance in Male Collegiate Swimmers during the General Preparation Phase. Nutrients, 10(11), 1809. https://doi.org/10.3390/nu10111809




