Gender and Age Stratified Analyses of Nutrient and Dietary Pattern Associations with Circulating Lipid Levels Identify Novel Gender and Age-Specific Correlations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measures
2.3. Statistical Analyses
3. Results
3.1. Population Characteristics
3.2. Dietary Pattern/Principal Component Analysis
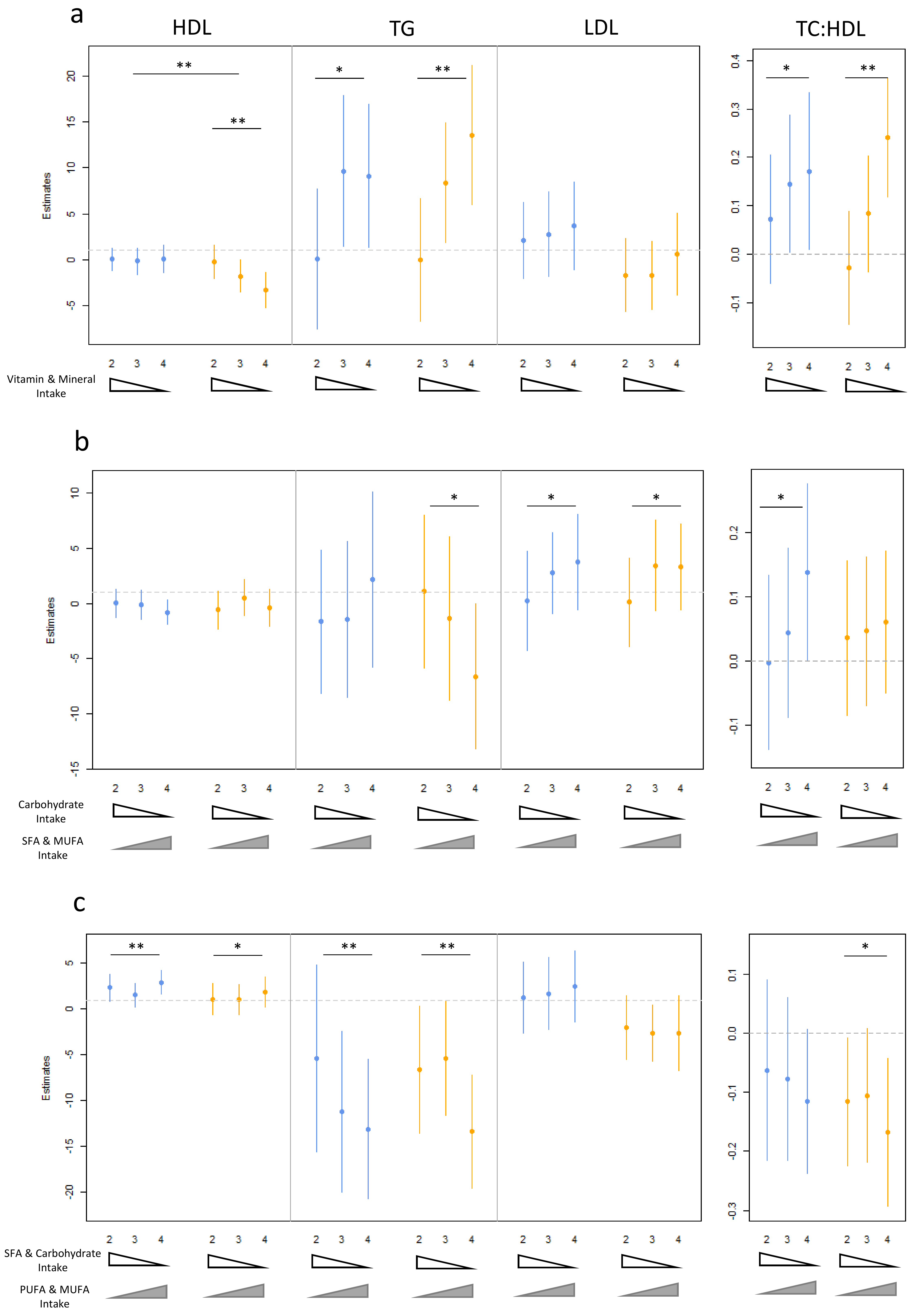
3.3. Dietary Pattern Associations with Lipid Levels
3.4. Age, Gender and Dietary Pattern Associations with Lipid Levels
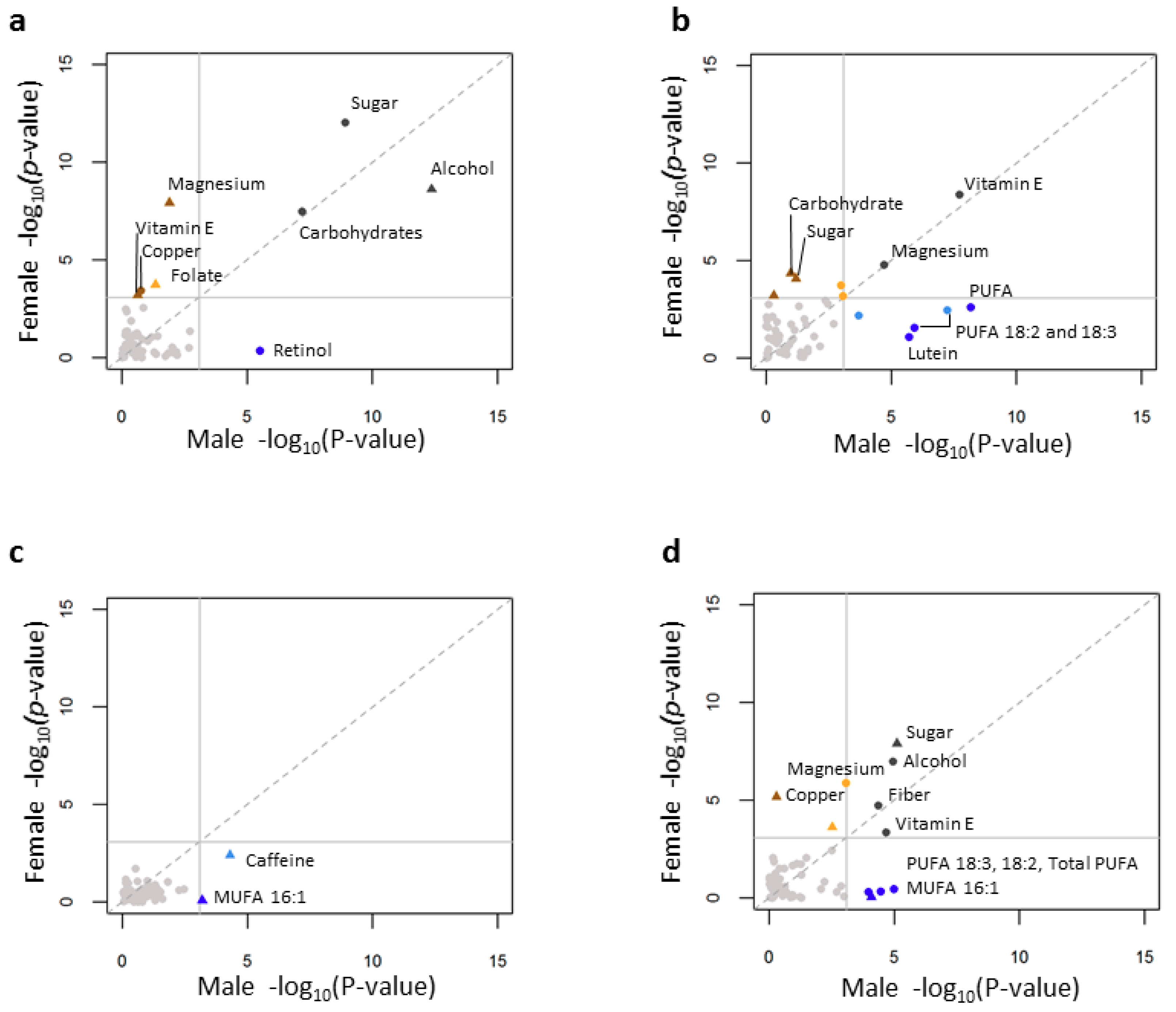
3.5. Individual Nutrient Consumption Associations with Lipid Levels
3.6. Age, Gender and Individual Nutrient Associations with Lipid Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laslett, L.J.; Alagona, P., Jr.; Clark, B.A., 3rd; Drozda, J.P., Jr.; Saldivar, F.; Wilson, S.R.; Poe, C.; Hart, M. The worldwide environment of cardiovascular disease: Prevalence, diagnosis, therapy, and policy issues: A report from the American College of Cardiology. J. Am. Coll. Cardiol. 2012, 60, S1–S49. [Google Scholar] [CrossRef] [PubMed]
- Yu-Poth, S.; Zhao, G.; Etherton, T.; Naglak, M.; Jonnalagadda, S.; Kris-Etherton, P.M. Effects of the National Cholesterol Education Program’s Step I and Step II dietary intervention programs on cardiovascular disease risk factors: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Van Horn, L.; McCoin, M.; Kris-Etherton, P.M.; Burke, F.; Carson, J.A.; Champagne, C.M.; Karmally, W.; Sikand, G. The evidence for dietary prevention and treatment of cardiovascular disease. J. Am. Diet. Assoc. 2008, 108, 287–331. [Google Scholar] [CrossRef] [PubMed]
- Howell, W.H.; McNamara, D.J.; Tosca, M.A.; Smith, B.T.; Gaines, J.A. Plasma lipid and lipoprotein responses to dietary fat and cholesterol: A meta-analysis. Am. J. Clin. Nutr. 1997, 65, 1747–1764. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Hu, F.B. Cardiovascular benefits of dietary fiber. Curr. Atheroscler. Rep. 2012, 14, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Katan, M.B. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler. Thromb. 1992, 12, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Krauss, R.M. Diet, lipids, and cardiovascular disease. Curr. Opin. Lipidol. 2016, 27, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Carreras, E.T.; Polk, D.M. Dyslipidemia: Current therapies and guidelines for treatment. US Cardiol. Rev. 2017, 11, 10–15. [Google Scholar] [CrossRef]
- Wadhera, R.K.; Steen, D.L.; Khan, I.; Giugliano, R.P.; Foody, J.M. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. J. Clin. Lipidol. 2016, 10, 472–489. [Google Scholar] [CrossRef] [PubMed]
- Knopp, R.H.; Paramsothy, P.; Retzlaff, B.M.; Fish, B.; Walden, C.; Dowdy, A.; Tsunehara, C.; Aikawa, K.; Cheung, M.C. Gender differences in lipoprotein metabolism and dietary response: Basis in hormonal differences and implications for cardiovascular disease. Curr. Atheroscler. Rep. 2005, 7, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Otvos, J.D.; Lamon-Fava, S.; Carrasco, W.V.; Lichtenstein, A.H.; McNamara, J.R.; Ordovas, J.M.; Schaefer, E.J. Men and women differ in lipoprotein response to dietary saturated fat and cholesterol restriction. J. Nutr. 2003, 133, 3428–3433. [Google Scholar] [CrossRef] [PubMed]
- Weggemans, R.M.; Zock, P.L.; Urgert, R.; Katan, M.B. Differences between men and women in the response of serum cholesterol to dietary changes. Eur. J. Clin. Investig. 1999, 29, 827–834. [Google Scholar] [CrossRef]
- Lapointe, A.; Balk, E.M.; Lichtenstein, A.H. Gender differences in plasma lipid response to dietary fat. Nutr. Rev. 2006, 64, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Zhu, K.; Feng, R.N.; Sun, C.H. Effects of multivitamin and mineral supplementation on adiposity, energy expenditure and lipid profiles in obese Chinese women. Int. J. Obes. 2010, 34, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Mietus-Snyder, M.L.; Shigenaga, M.K.; Suh, J.H.; Shenvi, S.V.; Lal, A.; McHugh, T.; Olson, D.; Lilienstein, J.; Krauss, R.M.; Gildengoren, G.; et al. A nutrient-dense, high-fiber, fruit-based supplement bar increases HDL cholesterol, particularly large HDL, lowers homocysteine, and raises glutathione in a 2-week trial. FASEB J. 2012, 26, 3515–3527. [Google Scholar] [CrossRef] [PubMed]
- Morcos, N.C. Increase in serum high-density lipoprotein following multivitamin and multimineral supplementation in adults with cardiovascular risk factors. Med. Sci. Res. 1999, 27, 121–126. [Google Scholar]
- Mazidi, M.; Pennathur, S.; Afshinnia, F. Link of dietary patterns with metabolic syndrome: Analysis of the National Health and Nutrition Examination Survey. Nutr. Diabetes 2017, 7, e255. [Google Scholar] [CrossRef] [PubMed]
- Cartmel, B.; Moon, T.E.; Levine, N. Effects of long-term intake of retinol on selected clinical and laboratory indexes. Am. J. Clin. Nutr. 1999, 69, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Burkhead, J.L.; Lutsenko, S. The Role of Copper as a Modifier of Lipid Metabolism; Intech Open: Rijeka, Croatia, 2013. [Google Scholar]
- Jeon, S.M.; Park, Y.B.; Kwon, O.S.; Huh, T.L.; Lee, W.H.; Do, K.M.; Park, T.; Choi, M.S. Vitamin E supplementation alters HDL-cholesterol concentration and paraoxonase activity in rabbits fed high-cholesterol diet: Comparison with probucol. J. Biochem. Mol. Toxicol. 2005, 19, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Burt, A.A.; Ranchalis, J.E.; Jarvik, L.E.; Eintracht, J.F.; Furlong, C.E.; Jarvik, G.P. Effects of dietary components on high-density lipoprotein measures in a cohort of 1566 participants. Nutr. Metab. 2014, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- National Center for Health Statistics. National Health and Nutrition Examination Survey Data-CDC; National Center for Health Statistics: Hyattsville, MD, USA, 2003–2012.
- National Center for Health Statistics. Analytic and Reporting Guidelines. 2006. Available online: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx (accessed on 1 April 2018).
- Lumley, T. Analysis of complex survey samples. J. Stat. Softw. 2004, 9, 1–19. [Google Scholar] [CrossRef]
- Yang, L.; Toriola, A.T. Leisure-time physical activity and circulating 25-hydroxyvitamin D levels in cancer survivors: A cross-sectional analysis using data from the US National Health and Nutrition Examination Survey. BMJ Open 2017, 7, e016064. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Parks, E.J.; Hellerstein, M.K. Carbohydrate-induced hypertriacylglycerolemia: Historical perspective and review of biological mechanisms. Am. J. Clin. Nutr. 2000, 71, 412–433. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Havel, P.J. Fructose consumption: Considerations for future research on its effects on adipose distribution, lipid metabolism, and insulin sensitivity in humans. J. Nutr. 2009, 139, 1236S–1241S. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated fatty acids and risk of coronary heart disease: Modulation by replacement nutrients. Curr. Atheroscler. Rep. 2010, 12, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Katsuyama, H.; Hamasaki, H.; Abe, S.; Tada, N.; Sako, A. Effects of dietary fat intake on HDL metabolism. J. Clin. Med. Res. 2015, 7, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, Y.; Chiriboga, D.E.; Olendzki, B.C.; Hebert, J.R.; Li, W.; Leung, K.; Hafner, A.R.; Ockene, I.S. Association between carbohydrate intake and serum lipids. J. Am. Coll. Nutr. 2006, 25, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Sharma, A.; Abramson, J.L.; Vaccarino, V.; Gillespie, C.; Vos, M.B. Caloric sweetener consumption and dyslipidemia among US adults. JAMA 2010, 303, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, R.; Vuori, E.; Viikari, J. Serum zinc and copper: Associations with cholesterol and triglyceride levels in children and adolescents. Cardiovascular risk in young Finns. J. Am. Coll. Nutr. 1989, 8, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Paredes, S.; Matta-Coelho, C.; Monteiro, A.M.; Fernandes, V.; Marques, O.; Alves, M. Copper Levels, Calcium Levels and Metabolic Syndrome. Rev. Port. Diabetes 2016, 11, 99–105. [Google Scholar]
- Rainwater, D.L.; Mahaney, M.C.; VandeBerg, J.L.; Wang, X.L. Vitamin E dietary supplementation significantly affects multiple risk factors for cardiovascular disease in baboons. Am. J. Clin. Nutr. 2007, 86, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.W.; Joss, J.D.; Mustacich, D.J.; Blatt, D.H.; Lee, Y.S.; Traber, M.G. Effects of vitamin E on cholesterol levels of hypercholesterolemic patients receiving statins. Am. J. Health Syst. Pharm. 2007, 64, 2257–2266. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, L.A.; Soladoye, A.O. Effect of increased magnesium intake on plasma cholesterol, triglyceride and oxidative stress in alloxan-diabetic rats. Afr. J. Med. Med. Sci. 2007, 36, 155–161. [Google Scholar] [PubMed]
- Karasu, C.; Ozansoy, G.; Bozkurt, O.; Erdogan, D.; Omeroglu, S. Antioxidant and triglyceride-lowering effects of vitamin E associated with the prevention of abnormalities in the reactivity and morphology of aorta from streptozotocin-diabetic rats. Antioxidants in Diabetes-Induced Complications (ADIC) Study Group. Metabolism 1997, 46, 872–879. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Jaquez-Chairez, F.O.; Rodriguez-Moran, M. Magnesium in metabolic syndrome: A review based on randomized, double-blind clinical trials. Magnes. Res. 2016, 29, 146–153. [Google Scholar] [PubMed]
- Hall, D.C. Nutritional Influences on Estrogen Metabolism. Available online: http://www.oakwayhealthcenter.com/store/MET_Nutritional-Influence-on-Estrogen-Metabolism.pdf (accessed on 13 November 2018).
- Cheng, Y.H.; Yin, P.; Xue, Q.; Yilmaz, B.; Dawson, M.I.; Bulun, S.E. Retinoic acid (RA) regulates 17 beta-hydroxysteroid dehydrogenase type 2 expression in endometrium: Interaction of RA receptors with specificity protein (SP) 1/SP3 for estradiol metabolism. J. Clin. Endocrinol. Metab. 2008, 93, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Kato, N.; Ichimura, T.; Abe, S.; Tanaka, Y.; Taniguchi, H.; Hoshida, Y.; Moriyama, M.; Wang, Y.; Shao, R.X.; et al. Vitamin K2 binds 17 beta-hydroxysteroid dehydrogenase 4 and modulates estrogen metabolism. Life Sci. 2005, 76, 2473–2482. [Google Scholar] [CrossRef] [PubMed]
- Vihtamaki, T.; Parantainen, J.; Koivisto, A.M.; Metsa-Ketela, T.; Tuimala, R. Oral ascorbic acid increases plasma oestradiol during postmenopausal hormone replacement therapy. Maturitas 2002, 42, 129–135. [Google Scholar] [CrossRef]
- Allgood, V.E.; Cidlowski, J.A. Vitamin B6 modulates transcriptional activation by multiple members of the steroid hormone receptor superfamily. J. Biol. Chem. 1992, 267, 3819–3824. [Google Scholar] [PubMed]
- Davidson, M.H.; Maki, K.C.; Marx, P.; Maki, A.C.; Cyrowski, M.S.; Nanavati, N.; Arce, J.C. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausal women. Arch. Intern. Med. 2000, 160, 3315–3325. [Google Scholar] [CrossRef] [PubMed]
- Lamon-Fava, S.; Posfai, B.; Asztalos, B.F.; Horvath, K.V.; Dallal, G.E.; Schaefer, E.J. Effects of estrogen and medroxyprogesterone acetate on subpopulations of triglyceride-rich lipoproteins and high-density lipoproteins. Metabolism 2003, 52, 1330–1336. [Google Scholar] [CrossRef]
- Surampudi, P.; Enkhmaa, B.; Anuurad, E.; Berglund, L. Lipid Lowering with Soluble Dietary Fiber. Curr. Atheroscler. Rep. 2016, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Mumford, S.L.; Schisterman, E.F.; Siega-Riz, A.M.; Gaskins, A.J.; Wactawski-Wende, J.; VanderWeele, T.J. Effect of dietary fiber intake on lipoprotein cholesterol levels independent of estradiol in healthy premenopausal women. Am. J. Epidemiol. 2011, 173, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Morrell, A.; Tallino, S.; Yu, L.; Burkhead, J.L. The role of insufficient copper in lipid synthesis and fatty-liver disease. IUBMB Life 2017, 69, 263–270. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, E.S.E.R.; Foster, D.; McGee Harper, M.; Seidman, C.E.; Smith, J.D.; Breslow, J.L.; Brinton, E.A. Alcohol consumption raises HDL cholesterol levels by increasing the transport rate of apolipoproteins A-I and A-II. Circulation 2000, 102, 2347–2352. [Google Scholar] [CrossRef]
- Van der Gaag, M.S.; van Tol, A.; Vermunt, S.H.; Scheek, L.M.; Schaafsma, G.; Hendriks, H.F. Alcohol consumption stimulates early steps in reverse cholesterol transport. J. Lipid Res. 2001, 42, 2077–2083. [Google Scholar] [PubMed]
- Parhofer, K.G. Interaction between Glucose and Lipid Metabolism: More than Diabetic Dyslipidemia. Diabetes Metab. J. 2015, 35, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Masson, L.F.; McNeill, G.; Avenell, A. Genetic variation and the lipid response to dietary intervention: A systematic review. Am. J. Clin. Nutr. 2003, 77, 1098–1111. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Esfandiar, Z.; Hosseini-Esfahani, F.; Koochakpoor, G.; Daneshpour, M.S.; Sedaghati-Khayat, B.; Azizi, F. Genetic variations of cholesteryl ester transfer protein and diet interactions in relation to lipid profiles and coronary heart disease: A systematic review. Nutr. Metab. 2017, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Pollin, T.I.; Quartuccio, M. What we know about diet, genes, and dyslipidemia: Is there potential for translation? Curr. Nutr. Rep. 2013, 2, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, V.; Daimiel, L.; Ordovas, J.M. Personalized nutrition and cardiovascular disease prevention: From framingham to PREDIMED. Adv. Nutr. 2014, 5, 368S–371S. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Rohatgi, A. The role of advanced lipid testing in the prediction of cardiovascular disease. Curr. Atheroscler. Rep. 2014, 16, 394. [Google Scholar] [CrossRef] [PubMed]
| Male | Female | Mean Difference | p-Value | |
|---|---|---|---|---|
| Participants (N) | 6127 | 6157 | - | - |
| Estimated Population | 34,634,163 | 36,631,640 | - | - |
| Age (mean ± SE) | 45.93 ± 0.36 | 47.61 ± 0.33 | - | - |
| Race (proportion) | ||||
| Non-Hispanic White | 0.76 | 0.75 | - | - |
| Non-Hispanic Black | 0.1 | 0.12 | - | - |
| Mexican American | 0.087 | 0.078 | - | - |
| Other Hispanic | 0.05 | 0.05 | - | - |
| HDL cholesterol(mg/dL) (mean ± SE) | 48.61 ± 0.24 | 59.08 ± 0.33 | −10.47 | <0.001 |
| 18–35 | 47.64 ± 0.36 | 55.93 ± 0.50 | −8.29 | <0.001 |
| 36–64 | 48.65 ± 0.38 | 59.80 ± 0.47 | −11.15 | <0.001 |
| 65–90 | 50.31 ± 0.58 | 61.75 ± 0.58 | −11.44 | <0.001 |
| Triglycerides (mg/dL) (mean ± SE) | 129.47 ± 1.53 | 116.57 ± 1.25 | 12.9 | <0.001 |
| 18–35 | 115.35 ± 2.52 | 97.96 ± 1.91 | 17.39 | <0.001 |
| 36–64 | 138.0 ± 2.08 | 119.56 ± 1.74 | 18.44 | <0.001 |
| 65–90 | 129.92 ± 2.19 | 135.95 ± 2.59 | −6.03 | <0.001 |
| LDL cholesterol (mg/dL) (mean ± SE) | 116.27 ± 0.67 | 115.79 ± 0.63 | 0.48 | 0.647 |
| 18–35 | 110.25 ± 1.30 | 102.99 ± 0.95 | 7.26 | <0.001 |
| 36–64 | 123.18 ± 0.82 | 121.87 ± 0.99 | 1.31 | 0.83 |
| 65–90 | 106.47 ± 1.19 | 118.08 ± 1.20 | −11.61 | <0.001 |
| TC:HDL cholesterol ratio (mean ± SE) | 4.15 ± 0.024 | 3.55 ± 0.020 | 0.6 | <0.001 |
| 18–35 | 4.01 ± 0.046 | 3.38 ± 0.034 | 0.63 | <0.001 |
| 36–64 | 4.34 ± 0.032 | 3.65 ± 0.029 | 0.69 | <0.001 |
| 65–90 | 3.84 ± 0.044 | 3.53 ± 0.036 | 0.31 | <0.001 |
| Vitamin and Mineral Consumption (PC1) | |||||||
| 18–35 | 36–64 | 65–90 | |||||
| padditive | Estimate (CI) | padditive | Estimate (CI) | padditive | Estimate (CI) | ||
| HDL cholesterol | Male | 0.059 | −0.60 (−1.21, 0.015) | 0.176 | 0.54 (−0.23, 1.31) | 0.495 | −0.39 (−1.42, 0.69) |
| Female | 1.94 × 10−4 | −1.93 (−2.90, −0.95) | 0.027 | −0.95 (−1.79, −0.12) | 0.817 | −0.16 (−1.49, 1.17) | |
| TG | Combined | 3.02× 10−3 | 4.15 (1.48, 6.83) | 2.03 × 10−3 | 4.19 (1.60, 6.78) | 7.02 × 10−4 | 6.36 (2.80, 9.92) |
| TC:HDL cholesterol | Male | 0.035 | 0.082 (0.00704, 0.16) | 0.445 | 0.028 (−0.044, 0.10) | 0.029 | 0.091 (0.019, 0.12) |
| Female | 3.26 × 10−4 | 0.124 (0.059, 0.190) | 9.44 × 10−3 | 0.071 (0.019, 0.12) | 0.428 | 0.038 (−0.056, 0.13) | |
| MUFA/PUFA vs. SFA Intake (PC3) | |||||||
| 18–35 | 36–64 | 65–90 | |||||
| padditive | Estimate (CI) | padditive | Estimate (CI) | padditive | Estimate (CI) | ||
| HDL cholesterol | Male | 9.03 × 10−3 | 1.00 (0.26, 1.74) | 7.19 × 10−2 | 0.52 (−0.04, 1.08) | 3.74 × 10−3 | 1.45 (0.60, 2.41) |
| Female | 0.246 | 0.45 (−0.31, 1.23) | 8.93 × 10−2 | 0.68 (−0.10, 1.45) | 0.319 | 0.48 (−0.46, 1.43) | |
| TG | Combined | 5.06 × 10−3 | −4.24 (−7.13, −1.34) | 9.32 × 10−5 | −4.72 (−6.98, −2.45) | 1.29 × 10−3 | −4.51 (−7.17, −1.84) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Nicodemus-Johnson, J. Gender and Age Stratified Analyses of Nutrient and Dietary Pattern Associations with Circulating Lipid Levels Identify Novel Gender and Age-Specific Correlations. Nutrients 2018, 10, 1760. https://doi.org/10.3390/nu10111760
Jin H, Nicodemus-Johnson J. Gender and Age Stratified Analyses of Nutrient and Dietary Pattern Associations with Circulating Lipid Levels Identify Novel Gender and Age-Specific Correlations. Nutrients. 2018; 10(11):1760. https://doi.org/10.3390/nu10111760
Chicago/Turabian StyleJin, Huifeng, and Jessie Nicodemus-Johnson. 2018. "Gender and Age Stratified Analyses of Nutrient and Dietary Pattern Associations with Circulating Lipid Levels Identify Novel Gender and Age-Specific Correlations" Nutrients 10, no. 11: 1760. https://doi.org/10.3390/nu10111760
APA StyleJin, H., & Nicodemus-Johnson, J. (2018). Gender and Age Stratified Analyses of Nutrient and Dietary Pattern Associations with Circulating Lipid Levels Identify Novel Gender and Age-Specific Correlations. Nutrients, 10(11), 1760. https://doi.org/10.3390/nu10111760




