Copper Metabolism of Newborns Is Adapted to Milk Ceruloplasmin as a Nutritive Source of Copper: Overview of the Current Data
Abstract
:1. Introduction
2. Biological Roles of Copper
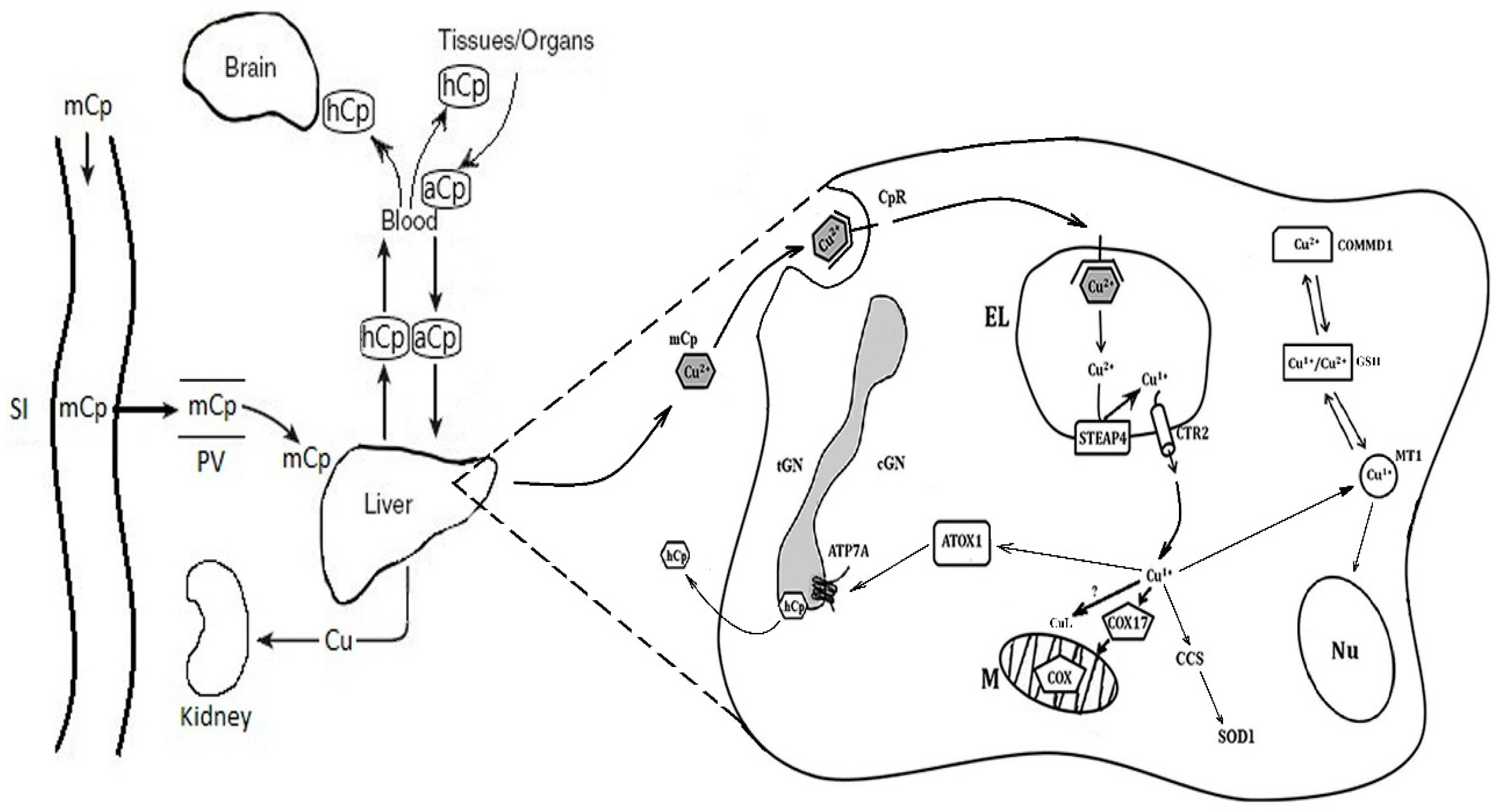
3. Transport of Copper to the Places of Cuproenzyme Formation in Adult Mammals
4. Copper Turnover in the Body of Adult Mammals
5. Ontogenetic Changes in Copper Metabolism in Mammals
6. Copper Metabolism in the Mammary Gland through Milk Ceruloplasmin Production
7. Milk Ceruloplasmin is a Source of Copper, Which Adapts to ETCM of Newborns
8. Specific Features of Copper Metabolism in Newborns Fed Infant Formulas
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dunn, J.T. Iodine supplementation and the prevention of cretinism. Ann. N. Y. Acad. Sci. 1993, 678, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.N.; Lazarus, J.H.; Moreno-Reyes, R.; Zimmermann, M.B. Consequences of iodine deficiency and excess in pregnant women: An overview of current knowns and unknowns. Am. J. Clin. Nutr. 2016, 104 (Suppl. 3), 918S–923S. [Google Scholar] [CrossRef] [PubMed]
- Steegers-Theunissen, R.P. Folate metabolism and neural tube defects: A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 61, 39–48. [Google Scholar] [CrossRef]
- Ebara, S. Nutritional role of folate. Congenit. Anom. (Kyoto) 2017, 57, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.M.; Fealy, S.M.; Bisquera, A.; Smith, R.; Collins, C.E.; Evans, T.J.; Hure, A.J. Effects of nutritional interventions during pregnancy on infant and child cognitive outcomes: A systematic review and meta-analysis. Nutrients 2017, 9, 1265. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.R.; Jack, B.W. Pregnancy; StatPearls Publishing: Treasure Island, FL, USA, 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK448166/ (accessed on 8 October 2017).
- Beard, J.L. Why iron deficiency is important in infant development. J. Nutr. 2008, 138, 2534–2536. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Xu, G.; Zhou, M.; Jiang, Y.; Richards, B.; Clark, K.M.; Kaciroti, N.; Georgieff, M.K.; Zhang, Z.; Tardif, T.; et al. Prenatal iron supplementation reduces maternal anemia, iron deficiency, and iron deficiency anemia in a randomized clinical trial in rural China, but iron deficiency remains widespread in mothers and neonates. J. Nutr. 2015, 145, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Darnton-Hill, I.; Mkparu, U.C. Micronutrients in pregnancy in low- and middle-income countries. Nutrients 2015, 7, 1744–1768. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Ridge, P.G.; Zhang, Y.; Gladyshev, V.N. Comparative genomic analyses of copper transporters and cuproproteomes reveal evolutionary dynamics of copper utilization and its link to oxygen. PLoS ONE 2008, 3, e1378. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, C.M.; Chang, C.J. Copper signaling in the brain and beyond. J. Biol. Chem. 2018, 293, 4628–4635. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J.H. Trace element deficiency in sheep in East Gippsland, Victoria. Aust. Vet. J. 1992, 69, 292–293. [Google Scholar] [CrossRef] [PubMed]
- Ellison, R.S. Major trace elements limiting livestock performance in New Zealand. N. Z. Vet. J. 2002, 50, 35–40. [Google Scholar] [CrossRef]
- Via, M.A.; Mechanick, J.I. Nutritional and Micronutrient Care of Bariatric Surgery Patients: Current Evidence Update. Curr. Obes. Rep. 2017, 6, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Lorincz, M.T. Wilson disease and related copper disorders. Handb. Clin. Neurol. 2018, 147, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Myint, Z.W.; Oo, T.H.; Thein, K.Z.; Tun, A.M.; Saeed, H. Copper deficiency anemia: Review article. Ann. Hematol. 2018, 97, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, H.; Kolkowska, P.; Watly, J.; Krzywoszynska, K.; Potocki, S. General aspects of metal toxicity. Curr. Med. Chem. 2014, 21, 3721–3740. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.E. A conspectus of research on copper metabolism and requirements of man. J. Nutr. 1979, 109, 1979–2066. [Google Scholar] [CrossRef] [PubMed]
- Hurley, L.S.; Keen, C.L.; Lönnerdal, B. Copper in fetal and neonatal development. Ciba Found. Symp. 1980, 79, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Shavlovski, M.M.; Chebotar, N.A.; Konopistseva, L.A.; Zakharova, E.T.; Kachourin, A.M.; Vassiliev, V.B.; Gaitskhoki, V.S. Embryotoxicity of silver ions is diminished by ceruloplasmin—Further evidence for its role in the transport of copper. Biometals 1995, 8, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Prohaska, J.R. Long-term functional consequences of malnutrition during brain development: Copper. Nutrition 2000, 16, 502–504. [Google Scholar] [CrossRef]
- Naveh, Y.; Hazani, A.; Berant, M. Copper deficiency with cow’s milk diet. Pediatrics 1981, 68, 397–400. [Google Scholar] [PubMed]
- Muller, T.; Muller, W.; Feichtinger, H. Idiopathic copper toxicosis. Am. J. Clin. Nutr. 1998, 67, 1082S–1086S. [Google Scholar] [CrossRef] [PubMed]
- Betard, C.; Rasquin-Weber, A.; Brewer, A.; Drouin, E.; Clark, S.; Verner, A.; Darmond-Zwaig, A.; Fortin, J.; Mercier, J.; Chagnon, P.; et al. Localization of a recessive gene for North American Indian childhood cirrhosis to chromosome region 16q22 and identification of a shared haplotype. Am. J. Hum. Genet. 2000, 67, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.S. Role of copper in Indian childhood cirrhosis. Am. J. Clin. Nutr. 1998, 67, 1074S–1081S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi Fru, E.; Rodríguez, N.P.; Partin, C.A.; Lalonde, S.V.; Andersson, P.; Weiss, D.J.; El Albani, A.; Rodushkin, I.; Konhauser, K.O. Cu isotopes in marine black shales record the Great Oxidation Event. Proc. Natl. Acad. Sci. USA 2016, 113, 4941–4946. [Google Scholar] [CrossRef] [PubMed]
- Rubino, J.T.; Franz, K.J. Coordination chemistry of copper proteins: How nature handles a toxic cargo for essential function. J. Inorg. Biochem. 2012, 107, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Timón-Gómez, A.; Nývltová, E.; Abriata, L.A.; Vila, A.J.; Hosler, J.; Barrientos, A. Mitochondrial cytochrome c oxidase biogenesis: Recent developments. Semin. Cell Dev. Biol. 2017, 76, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Panda, T.K.; Pradhan, T. Lysyl oxidase: Its diversity in health and diseases. Indian J. Clin. Biochem. 2017, 32, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Mains, R.E.; Eipper, B.A. 60 YEARS OF POMC: From POMC and αMSH to PAM, molecular oxygen, copper and vitamin C. J. Mol. Endocrinol. 2016, 56, T63–T76. [Google Scholar] [CrossRef] [PubMed]
- Brazeau, B.J.; Johnson, B.J.; Wilmot, C.M. Copper-containing amine oxidases. Biogenesis and catalysis; a structural perspective. Arch. Biochem. Biophys. 2004, 428, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Finney, J.; Moon, H.J.; Ronnebaum, T.; Lantz, M.; Mure, M. Human copper-dependent amine oxidases. Arch. Biochem. Biophys. 2014, 546, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. On the Metal Cofactor in the Tyrosinase Family. Int. J. Mol. Sci. 2018, 19, 633. [Google Scholar] [CrossRef] [PubMed]
- Bielli, P.; Calabrese, L. Structure to function relationships in ceruloplasmin: A ‘moonlighting’ protein. Cell. Mol. Life Sci. 2002, 59, 1413–1427. [Google Scholar] [CrossRef] [PubMed]
- Vashchenko, G.; MacGillivray, R.T.A. Multi-copper oxidases and human iron metabolism. Nutrients 2013, 5, 2289–2313. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; David, S. Glycosylphosphatidylinositol-anchored ceruloplasmin is required for iron efflux from cells in the central nervous system. J. Biol. Chem. 2003, 278, 27144–27148. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; Frazer, D.M. Recent advances in intestinal iron transport. Curr. Gastroenterol. Rep. 2005, 7, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Attieh, Z.K.; Syed, B.A.; Kuo, Y.M.; Stevens, V.; Fuqua, B.K.; Andersen, H.S.; Naylor, C.E.; Evans, R.W.; Gambling, L.; et al. Identification of zyklopen, a new member of the vertebrate multicopper ferroxidase family, and characterization in rodents and human cells. J. Nutr. 2010, 140, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Palm-Espling, M.E.; Niemiec, M.S.; Wittung-Stafshede, P. Role of metal in folding and stability of copper proteins in vitro. Biochim. Biophys. Acta 2012, 1823, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Raju, K.S.; Alessandri, G.; Ziche, M.; Gullino, M.N. Ceruloplasmin, copper ions, and angiogenesis. J. Natl. Cancer Inst. 1982, 69, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Bartuzi, P.; Hofker, M.H.; van de Sluis, B. Tuning NF-κB activity: A touch of COMMD proteins. Biochim. Biophys. Acta 2013, 1832, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, J.; Yang, H.; Wu, C.; Dang, X.; Liu, Y. Copper depletion inhibits CoCl2-induced aggressive phenotype of MCF-7 cells via downregulation of HIF-1 and inhibition of Snail/Twist-mediated epithelial-mesenchymal transition. Sci. Rep. 2015, 5, 12410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mufti, A.R.; Burstein, E.; Duckett, C.S. XIAP: Cell death regulation meets copper homeostasis. Arch. Biochem. Biophys. 2007, 463, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, M.M.; Polykretis, P.; Luchinat, E.; Wang, X.; Chen, S.N.; Zuo, H.H.; Yang, Y.; Chen, J.L.; Ye, Y.; Li, C.; et al. Solution structure and interaction with copper in vitro and in living cells of the first BIR domain of XIAP. Sci. Rep. 2017, 7, 16630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turski, M.L.; Brady, D.C.; Kim, H.J.; Kim, B.E.; Nose, Y.; Counter, C.M.; Winge, D.R.; Thiele, D.J. A novel role for copper in Ras/mitogen-activated protein kinase signaling. Mol. Cell. Biol. 2012, 32, 1284–1295. [Google Scholar] [CrossRef] [PubMed]
- Brady, D.C.; Crowe, M.S.; Turski, M.L.; Hobbs, G.A.; Yao, X.; Chaikuad, A.; Knapp, S.; Xiao, K.; Campbell, S.L.; Thiele, D.J.; et al. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature 2014, 509, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Finney, L.; Mandava, S.; Ursos, L.; Zhang, W.; Rodi, D.; Vogt, S.; Legnini, D.; Maser, J.; Ikpatt, F.; Olopade, O.I.; et al. X-ray fluorescence microscopy reveals large-scale relocalization and extracellular translocation of cellular copper during angiogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 2247–2252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grasso, G.; Santoro, A.M.; Magrì, A.; La Mendola, D.; Tomasello, M.F.; Zimbone, S.; Rizzarelli, E. The inorganic perspective of VEGF: Interactions of Cu(2+) with peptides encompassing a recognition domain of the VEGF receptor. J. Inorg. Biochem. 2016, 159, 149–158. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosi, N.; Rossi, L. Copper at synapse: Release, binding and modulation of neurotransmission. Neurochem. Int. 2015, 90, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.C.; Welch, N.C.; Brideau, C.; Stys, P.K. Functional ionotropic glutamate receptors on peripheral axons and myelin. Muscle Nerve 2016, 54, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, L.; Cotruvo, J.A., Jr.; Chan, J.; Kaluarachchi, H.; Muchenditsi, A.; Pendyala, V.S.; Jia, S.; Aron, A.T.; Ackerman, C.M.; Wal, M.N.; et al. Copper regulates cyclic-AMP-dependent lipolysis. Nat. Chem. Biol. 2016, 12, 586–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prudovsky, I. Nonclassically secreted regulators of angiogenesis. Angiol. Open Access 2013, 1, 1000101. [Google Scholar] [CrossRef] [PubMed]
- Haremaki, T.; Fraser, S.T.; Kuo, Y.M.; Baron, M.H.; Weinstein, D.C. Vertebrate Ctr1 coordinates morphogenesis and progenitor cell fate and regulates embryonic stem cell differentiation. Proc. Natl. Acad. Sci. USA 2007, 104, 12029–12034. [Google Scholar] [CrossRef] [PubMed]
- Vašák, M.; Meloni, G. Chemistry and biology of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J. Metallothionein redox cycle and function. Exp. Biol. Med. (Maywood) 2006, 231, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Maine, G.N.; Burstein, E. COMMD proteins: COMMing to the scene. Cell. Mol. Life Sci. 2007, 64, 1997–2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leary, S.C.; Cobine, P.A.; Kaufman, B.A.; Guercin, G.-H.; Mattman, A.; Palaty, J.; Lockitch, G.; Winge, D.R.; Rustin, P.; Horvath, R.; et al. The human cytochrome c oxidase assembly factors SCO1 and SCO2 have regulatory roles in the maintenance of cellular copper homeostasis. Cell Metab. 2007, 5, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Leary, S.C. Redox regulation of SCO protein function: Controlling copper at a mitochondrial crossroad. Antioxid. Redox. Signal. 2010, 13, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.W.; Peng, F.; Molloy, S.A.; Pendyala, V.S.; Muchenditsi, A.; Muzik, O.; Lee, J.; Kaplan, J.H.; Lutsenko, S. Urinary copper elevation in a mouse model of Wilson’s disease is a regulated process to specifically decrease the hepatic copper load. PLoS ONE 2012, 7, e38327. [Google Scholar] [CrossRef] [PubMed]
- Cobine, P.A.; Pierrel, F.; Bestwick, M.L.; Winge, D.R. Mitochondrial matrix copper complex used in metallation of cytochrome oxidase and superoxide dismutase. J. Biol. Chem. 2006, 281, 36552–36559. [Google Scholar] [CrossRef] [PubMed]
- Boulet, A.; Vest, K.E.; Maynard, M.K.; Gammon, M.G.; Russell, A.C.; Mathews, A.T.; Cole, S.E.; Zhu, X.; Phillips, C.B.; Kwong, J.Q.; et al. The mammalian phosphate carrier SLC25A3 is a mitochondrial copper transporter required for cytochrome c oxidase biogenesis. J. Biol. Chem. 2018, 293, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, T.; Eustace, A.; West, C. Lysyl oxidase: From basic science to future cancer treatment. Cell Struct. Funct. 2012, 37, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.L.; Hendrix, M.J.; Kirschmann, D.A. Paradoxical roles for lysyl oxidases in cancer—A prospect. J. Cell. Biochem. 2007, 101, 1338–1354. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.D.; Tsai, W.B.; Lee, M.Y.; Savaraj, N.; Kuo, M.T. Specificity protein 1 (Sp1) oscillation is involved in copper homeostasis maintenance by regulating human high-affinity copper transporter 1 expression. Mol. Pharmacol. 2012, 81, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chen, S.; Xi, Z.; Liu, Y. Copper-finger protein of Sp1: The molecular basis of copper sensing. Metallomics 2017, 9, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Wegner, S.V.; Sun, F.; Hernandez, N.; He, C. The tightly regulated copper window in yeast. Chem. Commun. (Camb.) 2011, 47, 2571–2573. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Chakraborty, K.; Shukla, A. Cellular copper homeostasis: Current concepts on its interplay with glutathione homeostasis and its implication in physiology and human diseases. Metallomics 2017, 9, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Baker, Z.N.; Cobine, P.A.; Leary, S.C. The mitochondrion: A central architect of copper homeostasis. Metallomics 2017, 9, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; McRae, R.; Henary, M.M.; Patel, R.; Lai, B.; Vogt, S.; Fahrni, C.J. Imaging of the intracellular topography of copper with a fluorescent sensor and by synchrotron X-ray fluorescence microscopy. Proc. Natl. Acad. Sci. USA 2005, 102, 11179–11184. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.A. Ctr1 and its role in body copper homeostasis. Int. J. Biochem. Cell Biol. 2003, 35, 288–291. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Liebig, J.K.; Tsigelny, I.F.; Howell, S.B. The copper transporter 1 (CTR1) is required to maintain the stability of copper transporter 2 (CTR2). Metallomics 2015, 7, 1477–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öhrvik, H.; Thiele, D.J. The role of Ctr1 and Ctr2 in mammalian copper homeostasis and platinum-based chemotherapy. J. Trace Elem. Med. Biol. 2015, 31, 178–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öhrvik, H.; Logeman, B.; Noguchi, G.; Eriksson, I.; Kjellén, L.; Thiele, D.J.; Pejler, G. Ctr2 regulates mast cell maturation by affecting the storage and expression of tryptase and proteoglycans. J. Immunol. 2015, 195, 3654–3664. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, H.; Manfredi, G. Import, maturation, and function of SOD1 and its copper chaperone CCS in the mitochondrial intermembrane space. Antioxid. Redox Signal. 2010, 13, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Kako, K.; Ohmura, K.; Tsumori, K.; Ohmasa, Y.; Kashiwabara, S.; Baba, T.; Munekatat, E. Genomic structure of mouse copper chaperone, COX17. DNA Seq. 2001, 12, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Yang, N.; Bothe, J.; Tonelli, M.; Nokhrin, S.; Dolgova, N.V.; Braiterman, L.; Lutsenko, S.; Dmitriev, O.Y. The metal chaperone Atox1 regulates the activity of the human copper transporter ATP7B by modulating domain dynamics. J. Biol. Chem. 2017, 292, 18169–18177. [Google Scholar] [CrossRef] [PubMed]
- Hatori, Y.; Lutsenko, S. The role of copper chaperone ATOX1 in coupling redox homeostasis to intracellular copper distribution. Antioxidants 2016, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.; Klomp, L.W. ATOX1: A novel copper-responsive transcription factor in mammals? Int. J. Biochem. Cell Biol. 2009, 41, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Lutsenko, S.; Barnes, N.L.; Bartee, M.Y.; Dmitriev, O.Y. Function and regulation of human copper-transporting ATPases. Physiol. Rev. 2007, 87, 1011–1046. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Song, Y.; Li, J.; Wang, C.; Li, F. Structure and metal ion binding of the first transmembrane domain of DMT1. Biochim. Biophys. Acta 2011, 1808, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, M.; Munoz, P.; Mura, C.V.; Nunez, M.T. DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am. J. Physiol. Cell Physiol. 2003, 284, C1525–C1530. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhang, Z.; Wang, T.; Chen, C.; Kang, Y.J. Copper uptake by DMT1: A compensatory mechanism for CTR1 deficiency in human umbilical vein endothelial cells. Metallomics 2015, 7, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Zimnicka, A.M.; Ivy, K.; Kaplan, J.H. Acquisition of dietary copper: A role for anion transporters in intestinal apical copper uptake. Am. J. Physiol. Cell Physiol. 2011, 300, C588–C599. [Google Scholar] [CrossRef] [PubMed]
- Shawki, A.; Anthony, S.R.; Nose, Y.; Engevik, M.A.; Niespodzany, E.J.; Barrientos, T.; Öhrvik, H.; Worrell, R.T.; Thiele, D.J.; Mackenzie, B. Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G635–G647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nose, Y.; Kim, B.E.; Thiele, D.J. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006, 4, 235–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nose, Y.; Wood, L.K.; Kim, B.E.; Prohaska, J.R.; Fry, R.S.; Spears, J.W.; Thiele, D.J. Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J. Biol. Chem. 2010, 285, 32385–32392. [Google Scholar] [CrossRef] [PubMed]
- Wyman, S.; Simpson, R.J.; McKie, A.T.; Sharp, P.A. Dcytb (Cybrd1) functions as both a ferric and a cupric reductase in vitro. FEBS Lett. 2008, 11, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, R.S.; Campagna, D.R.; McDonald, A.; Fleming, M.D. The Steap proteins are metalloreductases. Blood 2006, 108, 1388–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Mitra, S.; Wu, G.; Berka, V.; Song, J.; Yu, Y.; Poget, S.; Wang, D.N.; Tsai, A.L.; Zhou, M. Six-transmembrane epithelial antigen of prostate 1 (STEAP1) has a single b heme and is capable of reducing metal ion complexes and oxygen. Biochemistry 2016, 55, 6673–6684. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, A.; Alonzo, E.; Sauble, E.; Chu, Y.L.; Nguyen, D.; Linder, M.C.; Sato, D.S.; Mason, A.Z. Copper binding components of blood plasma and organs, and their responses to influx of large doses of (65)Cu, in the mouse. Biometals 2008, 21, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Zatulovskiy, E.; Samsonov, S.; Skvortsov, A. Doking study on mammalian CTR1 copper importer motifs. BMC Syst. Biol. 2007, 1, 54. [Google Scholar] [CrossRef]
- Ramos, D.; Mar, D.; Ishida, M.; Vargas, R.; Gaite, M.; Montgomery, A.; Linder, M.C. Mechanism of copper uptake from blood plasma ceruloplasmin by mammalian cells. PLoS ONE 2016, 11, e0149516. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.C. Ceruloplasmin and other copper binding components of blood plasma and their functions: An update. Metallomics 2016, 8, 887–905. [Google Scholar] [CrossRef] [PubMed]
- Field, L.S.; Luk, E.; Culotta, V.C. Copper chaperones: Personal escorts for metal ions. J. Bioenerg. Biomembr. 2002, 34, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Knutson, M.D. Steap proteins: Implications for iron and copper metabolism. Nutr. Rev. 2007, 65, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Rae, T.D.; Schmidt, P.J.; Pufahl, R.A.; Culotta, V.C.; O’Halloran, T.V. Undetectable intracellular free copper: The requirement of a copper chaperone for superoxide dismutase. Science 1999, 284, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.C. The relationship of copper to DNA damage and damage prevention in humans. Mutat. Res. 2012, 733, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Shimberg, G.D.; Ok, K.; Neu, H.M.; Splan, K.E.; Michel, S.L.J. Cu(I) disrupts the structure and function of the nonclassical zinc finger protein tristetraprolin (TTP). Inorg. Chem. 2017, 56, 6838–6848. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, D.; Gallo, A.; Piccioli, M.; Novellino, E.; Ciofi-Baffoni, S.; Banci, L. [4Fe-4S] cluster assembly in mitochondria and its impairment by copper. J. Am. Chem. Soc. 2017, 139, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Gnandt, E.; Dörner, K.; Strampraad, M.F.J.; de Vries, S.; Friedrich, T. The multitude of iron-sulfur clusters in respiratory complex I. Biochim. Biophys. Acta 2016, 1857, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Fuss, J.O.; Tsai, S.L.; Ishida, J.P.; Tainer, G.A. Emerging critical roles of Fe-S clusters in DNA replication and repair. Biochim. Biophys. Acta 2015, 1853, 1253–1271. [Google Scholar] [CrossRef] [PubMed]
- Pain, D.; Dancis, A. Roles of Fe-S proteins: From cofactor synthesis to iron homeostasis to protein synthesis. Curr. Opin. Genet. Dev. 2016, 38, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Bernevic, B.; El-Khatib, A.H.; Jakubowski, N.; Weller, M.G. Online immunocapture ICP-MS for the determination of the metalloprotein ceruloplasmin in human serum. BMC Res. Notes 2018, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Waldrop, G.L.; Ettinger, M.J. Effects of albumin and histidine on kinetics of copper transport by fibroblasts. Am. J. Physiol. 1990, 259, G212–G218. [Google Scholar] [CrossRef] [PubMed]
- Moriya, M.; Ho, Y.H.; Grana, A.; Nguyen, L.; Alvarez, A.; Jamil, R.; Ackland, M.L.; Michalczyk, A.; Hamer, P.; Ramos, D.; et al. Copper is taken up efficiently from albumin and alpha2-macroglobulin by cultured human cells by more than one mechanism. Am. J. Physiol. Cell Physiol. 2008, 295, C708–C721. [Google Scholar] [CrossRef] [PubMed]
- Cousin, R.J. Absorption, transport, and hepatic metabolism of copper and zinc: Special reference to metallothionein and ceruloplasmin. Physiol. Rev. 1985, 65, 238–309. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.D. Copper transport: An overview. Proc. Soc. Exp. Biol. Med. 1991, 196, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.E.; Nevitt, T.; Thiele, D.J. Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol. 2008, 4, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, V.N.; Zaitseva, I.; Papiz, M.; Lindley, P.F. An X-ray crystallographic study of the binding sites of the azide inhibitor and organic substrates to ceruloplasmin, a multi-copper oxidase in the plasma. J. Biol. Inorg. Chem. 1999, 4, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Samygina, V.R.; Sokolov, A.V.; Bourenkov, G.; Schneider, T.R.; Anashkin, V.A.; Kozlov, S.O.; Kolmakov, N.N.; Vasilyev, V.B. Rat ceruloplasmin: A new labile copper binding site and zinc/copper mosaic. Metallomics 2017, 9, 1828–1838. [Google Scholar] [CrossRef] [PubMed]
- Golenkina, E.A.; Viryasova, G.M.; Galkina, S.I.; Gaponova, T.V.; Sud’ina, G.F.; Sokolov, A.V. Fine regulation of neutrophil oxidative status and apoptosis by ceruloplasmin and its derivatives. Cells 2018, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.V.; Frieden, E. Partial purification of the rat erythrocyte ceruloplasmin receptor monitored by an electrophoresis mobility shift assay. Anal. Biochem. 1993, 212, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Puchkova, L.V.; Verbina, I.A.; Denezhkina, V.V.; Vakharlovskii, V.G.; Voitsekhovskii, B.L.; Gaitskhoki, V.S.; Neifakh, S.A. Molecular forms of ceruloplasmin in hepatolenticular degeneration and their interaction with human erythrocyte ceruloplasmin receptor. Biomed. Sci. 1990, 1, 460–466. [Google Scholar] [PubMed]
- Hilton, M.; Spenser, D.C.; Ross, P.; Ramsey, A.; McArdle, H.J. Characterisation of the copper uptake mechanism and isolation of the ceruloplasmin receptor/copper transporter in human placental vesicles. Biochim. Biophys. Acta 1995, 1245, 153–160. [Google Scholar] [CrossRef]
- Puchkova, L.V.; Sasina, L.K.; Aleinikova, T.D.; Zakharova, E.T.; Gaitskhoki, V.S. Reconstitution of the intercellular transfer pathway of the peptide moiety of ceruloplasmin in mammals. Biochemistry 1997, 62, 697–703. [Google Scholar] [PubMed]
- Gregoriadis, G.; Morell, A.G.; Sternlieb, I.; Scheinberg, I.H. Catabolism of desialylated ceruloplasmin in the liver. J. Biol. Chem. 1970, 245, 5833–5837. [Google Scholar] [PubMed]
- Verbina, I.A.; Puchkova, L.V.; Gaitskhoki, V.S.; Neifakh, S.A. Isolation and partial characterization of molecular forms of ceruloplasmin from human bile. FEBS Lett. 1992, 298, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Bingle, C.D.; Epstein, O.; Srai, S.K.; Gitlin, J.D. Hepatic ceruloplasmin-gene expression during development in the guinea-pig. Correlation with changes in hepatic copper metabolism. Biochem. J. 1991, 276, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Zatulovskaia, Y.A.; Ilyechova, E.Y.; Puchkova, L.V. The features of copper metabolism in the rat liver during development. PLoS ONE 2015, 10, e0140797. [Google Scholar] [CrossRef] [PubMed]
- Luza, S.C.; Speisky, H.C. Liver copper storage and transport during development: Implications for cytotoxicity. Am. J. Clin. Nutr. 1996, 63, 812S–820S. [Google Scholar] [CrossRef] [PubMed]
- Lenartowicz, M.; Kennedy, C.; Hayes, H.; McArdle, H.J. Transcriptional regulation of copper metabolism genes in the liver of fetal and neonatal control and iron-deficient rats. Biometals 2015, 28, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Gyulikhandanova, N.E.; Tsymbalenko, N.V.; Platonova, N.A.; Babich, V.S.; Puchkova, L.V. Regulation of ceruloplasmin gene in mammals. Bull. Exp. Biol. Med. 2004, 137, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Intestinal regulation of copper homeostasis: A developmental perspective. Am. J. Clin. Nutr. 2008, 88, 846S–850S. [Google Scholar] [CrossRef] [PubMed]
- Van den Berghe, P.V.; Klomp, L.W. New developments in the regulation of intestinal copper absorption. Nutr. Rev. 2009, 67, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Platonova, N.A.; Barabanova, S.V.; Povalikhin, R.G.; Tsymbalenko, N.V.; Danilovskii, M.A.; Voronina, O.V.; Dorokhova, I.I.; Puchkova, L.V. In Vivo expression of copper-transporting proteins in rat brain regions. Biol. Bull. 2005, 32, 108–120. [Google Scholar] [CrossRef]
- Babich, P.S.; Ilyechova, E.Y.; Skomorokhova, E.A.; Puchkova, L.V. The changes of copper concentration and ceruloplasmin gene expression level in the brain departments during development of the rats. 2018; in press. [Google Scholar]
- Hatori, Y.; Yan, Y.; Schmidt, K.; Furukawa, E.; Hasan, N.M.; Yang, N.; Liu, C.N.; Sockanathan, S.; Lutsenko, S. Neuronal differentiation is associated with a redox-regulated increase of copper flow to the secretory pathway. Nat. Commun. 2016, 7, 10640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogra, Y.; Tejima, A.; Hatakeyama, N.; Shiraiwa, M.; Wu, S.; Ishikawa, T.; Yawata, A.; Anan, Y.; Suzuki, N. Changes in intracellular copper concentration and copper-regulating gene expression after PC12 differentiation into neurons. Sci. Rep. 2016, 6, 33007. [Google Scholar] [CrossRef] [PubMed]
- Platonova, N.A.; Zhiguleva, E.A.; Tsymbalenko, N.V.; Mishchenko, B.S.; Vasin, A.V.; Zhivul’ko, T.V.; Puchkova, L.V. Age-related features of ceruloplasmin biosynthesis and distribution in rats. Ontogenez 2004, 35, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.N.; Dunn, R.J.; David, S. Alternative RNA splicing generates a glycosylphosphatidylinositol-anchored form of ceruloplasmin in mammalian brain. J. Biol. Chem. 2000, 275, 4305–4310. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, R.C.; Kosman, D.J. Activation of C6 glioblastoma cell ceruloplasmin expression by neighboring human brain endothelia-derived interleukins in an in vitro blood-brain barrier model system. Cell Commun. Signal. 2014, 12, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conley, L.; Geurs, T.L.; Levin, L.A. Transcriptional regulation of ceruloplasmin by an IL-6 response element pathway. Brain Res. Mol. Brain Res. 2005, 139, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Ho, K.; Du, J.; Zhu, L.; Xu, Y.; Wang, Q.; Wang, C.Y.; Li, L.; Ge, X.; Chang, Y.; et al. Role of soluble ceruloplasmin in iron uptake by midbrain and hippocampus neurons. J. Cell. Biochem. 2006, 98, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Gitlin, J.D. Mechanisms of copper incorporation during the biosynthesis of human ceruloplasmin. J. Biol. Chem. 1991, 266, 5128–5134. [Google Scholar]
- Ilyechova, E.; Skvortsov, A.; Zatulovsky, E.; Tsymbalenko, N.; Shavlovsky, M.; Broggini, M.; Puchkova, L. Experimental switching of copper status in laboratory rodents. J. Trace Elem. Med. Biol. 2011, 25, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Donley, S.A.; Ilagan, B.J.; Rim, H.; Linder, M.C. Copper transport to mammary gland and milk during lactation in rats. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E667–E675. [Google Scholar] [CrossRef] [PubMed]
- McArdle, H.J.; Danks, D.M. Secretion of copper 64 into breast milk following intravenous injection in a human subject. J. Trace Elements Exp. Med. 1991, 4, 81–84. [Google Scholar]
- Puchkova, L.V.; Aleinikova, T.D.; Tsymbalenko, N.V.; Zakharova, E.T.; Konopistseva, L.A.; Chebotar’, N.A.; Gaitskhoki, V.S. Biosynthesis and secretion of ceruloplasmin by rat mammary cells during lactation. Biokhimiia 1994, 59, 296–303. [Google Scholar] [PubMed]
- Jaeger, J.L.; Shimizu, N.; Gitlin, J.D. Tissue-specific ceruloplasmin gene expression in the mammary gland. Biochem. J. 1991, 280, 671–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyosawa, I.; Matsuyama, J.; Nyui, S.; Fukuda, A. Ceruloplasmin concentration in human colostrum and mature milk. Biosci. Biotechnol. Biochem. 1995, 59, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Wooten, L.; Shulze, R.A.; Lancey, R.W.; Leitsow, M.; Linder, M.C. Ceruloplasmin is found in milk and amniotic fluid and may have a nutritional role. J. Nutr. Biochem. 1996, 7, 632–639. [Google Scholar] [CrossRef]
- Puchkova, L.V.; Zakharova, E.T.; Aleinikova, T.D.; Mokshina, S.V.; Tsymbalenko, N.V.; Sasina, L.K.; Shirmanova, M.R.; Rogacheva, N.P.; Gaitskhoki, V.S. Comparative analysis of the molecular heterogeneity of ceruloplasmin from human blood and breast milk. Biochemistry 1997, 62, 928–930. [Google Scholar] [PubMed]
- Platonova, N.A.; Orlov, I.A.; Klotchenko, S.A.; Babich, V.S.; Ilyechova, E.Y.; Babich, P.S.; Garmai, Y.P.; Vasin, A.V.; Tsymbalenko, N.V.; Puchkova, L.V. Ceruloplasmin gene expression profile changes in the rat mammary gland during pregnancy, lactation and involution. J. Trace Elem. Med. Biol. 2017, 43, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Freestone, D.; Denoyer, D.; Jakab, M.; Leigh Ackland, M.; Cater, M.A.; Michalczyk, A. Ceruloplasmin is regulated by copper and lactational hormones in PMC42-LA mammary epithelial cell culture models. Metallomics 2016, 8, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Kim, J.V.; Lee, J.T.; Cho, M.S.; Kang, B.S.; Choi, H.; Kim, Y. Association of maternal diet with zinc, copper, and iron concentrations in transitional human milk produced by Korean mothers. Clin. Nutr. Res. 2016, 5, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta, N.; Lanata, C.; Butron, B.; Peerson, J.M.; Brown, K.H.; Lönnerdal, B. Effect of acute maternal infection on quantity and composition of breast milk. Am. J. Clin. Nutr. 1995, 62, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Platonova, N.; Guolikhandanova, N.; Tsymbalenko, N.; Zhiguleva, E.; Zhivulko, T.; Vasin, A.; Evsukova, I.; Puchkova, L. Milk ceruloplasmin is a valuable source of nutrient copper ions for mammalian newborns. J. Trace Elem. Med. Biol. 2007, 21, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Tsymbalenko, N.V.; Giulikhandanova, N.E.; Platononova, N.A.; Babich, V.S.; Evsiukova, I.I.; Puchkova, L.V. Regulation of ceruloplasmin gene activity in mammary gland cells. Genetika 2009, 45, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Kılıç Altun, S.; Dinç, H.; Temamoğulları, F.K.; Paksoy, N. Analyses of essential elements and heavy metals by using ICP-MS in maternal breast milk from Şanlıurfa, Turkey. Int. J. Anal. Chem. 2018, 2018, 1784073. [Google Scholar] [CrossRef] [PubMed]
- Adkins, Y.; Lepine, A.J.; Lönnerdal, B. Changes in protein and nutrient composition of milk throughout lactation in dogs. Am. J. Vet. Res. 2001, 62, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Cerveza, P.J.; Mehrbod, F.; Cotton, S.J.; Lomeli, N.; Linder, M.C.; Fonda, E.G.; Wickler, S.J. Milk ceruloplasmin and its expression by mammary gland and liver in pigs. Arch. Biochem. Biophys. 2000, 373, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Grace, N.D.; Pearce, S.G.; Firth, E.C.; Fennessy, P.F. Concentrations of macro- and micro-elements in the milk of pasture-fed thoroughbred mares. Aust. Vet. J. 1999, 77, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Keen, C.L.; Lonnerdal, B.; Clegg, M.S.; Hurley, L.S.; Morris, J.G.; Rogers, Q.R.; Rucker, R.B. Developmental changes in composition of cats milk: Trace elements, minerals, protein, carbohydrate and fat. J. Nutr. 1982, 112, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.L.; Sauble, E.N.; Cabrera, A.; Roth, A.; Ackland, M.L.; Mercer, J.F.; Linder, M.C. Lack of ceruloplasmin expression alters aspects of copper transport to the fetus and newborn, as determined in mice. Biometals 2012, 25, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Theophilos, M.B.; Cox, D.W.; Mercer, J.F. The toxic milk mouse is a murine model of Wilson disease. Hum. Mol. Genet. 1996, 5, 1619–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coronado, V.; Nanji, M.; Cox, D.W. The Jackson toxic milk mouse as a model for copper loading. Mamm. Genome 2001, 12, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Puchkova, L.V.; Aleinikova, T.D.; Bichevaia, N.K.; Mokshina, S.A.; Platonova, N.A.; Sasina, L.K.; Skvortsova, N.N.; Tsymbalenko, N.V.; Chebotar’, N.A.; Gaitskhoki, V.S. A comparative study of the transport dynamics of the peptide moiety of the milk ceruloplasmin molecule in the body of rats with embryonic and adult types of copper metabolism. Ontogenez 1999, 30, 31–39. [Google Scholar] [PubMed]
- Tavassoli, M.; Kishimoto, T.; Kataoka, M. Liver endothelium mediates the hepatocyte’s uptake of ceruloplasmin. J. Cell Biol. 1986, 102, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Bauerly, K.A.; Kelleher, S.L.; Lönnerdal, B. Effects of copper supplementation on copper absorption, tissue distribution, and copper transporter expression in an infant rat model. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G1007–G1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, C.R.; Gahl, W.A. Disorders of metal metabolism. Transl. Sci. Rare Dis. 2017, 18, 101–139. [Google Scholar] [CrossRef] [PubMed]
- Haschke, F.; Haiden, N.; Thakkar, S.K. Nutritive and Bioactive Proteins in Breastmilk. Ann. Nutr. Metab. 2016, 69 (Suppl. 2), 17–26. [Google Scholar] [CrossRef]
- Lonnerdal, B.; Erdmann, P.; Thakkar, S.K.; Sauser, J.; Destaillats, F. Longitudinal evolution of true protein, amino acids and bioactive proteins in breast milk: A developmental perspective. J. Nutr. Biochem. 2016, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Lopez, V.; Shafizadeh, T.B.; Halsted, C.H.; Lönnerdal, B. Cloning of a pig homologue of the human lactoferrin receptor: Expression and localization during intestinal maturation in piglets. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 584–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheah, D.M.; Deal, Y.J.; Wright, P.F.; Buck, N.E.; Chow, C.W.; Mercer, J.F.; Allen, K.J. Heterozygous tx mice have an increased sensitivity to copper loading: Implications for Wilson’s disease carriers. Biometals 2007, 20, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Celauro, E.; Muka, A.; Fierro-González, J.C.; Wittung-Stafshed, P. Copper chaperone ATOX1 regulates pluripotency factor OCT4 in preimplantation mouse embryos. Biochem. Biophys. Res. Commun. 2017, 491, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Ozumi, K.; Sudhahar, V.; Kim, H.W.; Chen, G.F.; Kohno, T.; Finney, L.; Vogt, S.; McKinney, R.D.; Ushio-Fukai, M.; Fukai, T. Role of copper transport protein antioxidant 1 in angiotensin II-induced hypertension: A key regulator of extracellular superoxide dismutase. Hypertension 2012, 60, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Fang, C.; Terao, C.; Giannopoulou, E.G.; Lee, Y.J.; Lee, M.J.; Mun, S.H.; Bae, S.; Qiao, Y.; Yuan, R.; et al. Hypoxia-sensitive COMMD1 integrates signaling and cellular metabolism in human macrophages and suppresses osteoclastogenesis. Immunity 2017, 57, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Mu, P.; Akashi, T.; Lu, F.; Kishida, S.; Kadomatsu, K. A novel nuclear complex of DRR1, F-actin and COMMD1 involved in NF-κB degradation and cell growth suppression in neuroblastoma. Oncogene 2017, 36, 5745–5756. [Google Scholar] [CrossRef] [PubMed]
- Riera-Romo, M. COMMD1: A multifunctional regulatory protein. J. Cell. Biochem. 2018, 119, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.R.; Hediger, M.L.; Levine, R.J.; Naficy, A.B.; Vik, T. Effect of breastfeeding on cognitive development of infants born small for gestational age. Acta Pediatr. 2002, 91, 267–274. [Google Scholar] [CrossRef]
- Zhou, G.; Ji, X.; Cui, N.; Cao, S.; Liu, C.; Liu, J. Association between serum copper status and working memory in schoolchildren. Nutrients 2015, 7, 7185–7196. [Google Scholar] [CrossRef] [PubMed]
| Cuproenzyme | Localization (The Main Place) | The Main Functions |
|---|---|---|
| SOD1 (Cu(II)/Zn(II)-superoxide dismutase) | Cytosol, nuclear matrix, lysosomes, peroxisomes, mitochondria | Disproportionation of superoxide anions to oxygen and hydrogen peroxide [29] |
| SOD3 (Cu(II)/Zn(II)-superoxide dismutase) | Extracellular liquids (blood serum, lymph, sclera, etc.) | Antioxidant functions, signaling, stimulation of cell proliferation, decrease of apoptosis, and inflammation [29] |
| COX (cytochrome-c-oxidase) | Mitochondrial inner membrane | Transfer of electrons from the respiration chain to molecular oxygen [30] |
| Protein-lysine 6-oxidase (lysyl oxidase) | Extracellular matrix | Oxidation of lysine residues to aldehydes in collagen and elastin precursors [31] |
| PAM (peptidylglycine α-hydroxylating monooxygenase) | Vesicles, membrane-bound, and soluble forms | Pro-neuropeptide processing by converting to the corresponding amide [32] |
| DBH (dopamine-β-hydroxylase) | Vesicles, membrane-bound, and soluble forms | Conversion of dihydroxyphenylalanine (DOPA) to noradrenaline [33,34] |
| Tyrosinase (phenol oxidase) | Melanosomes | Synthesis of melanin from tyrosine [35] |
| Soluble ceruloplasmin (Cp) | Blood serum, milk, cerebrospinal fluid, and other extracellular liquids | Oxidation of Fe(II) to Fe(III), oxidation of aromatic amines, copper transporter [36,37] |
| GPI-Cp 1 (splice isoform Cp) | Plasma membrane, brain | Oxidation of Fe(II) to Fe(III) [38] |
| Hephaestin | Plasma membrane, enterocytes | Oxidation of Fe(II) to Fe(III) [39] |
| Zyklopen | Plasma membranes, placenta | Oxidation of Fe(II) to Fe(III) [40] |
| Protein/Substance | Localization | Function |
|---|---|---|
| Metallothionein (a large set of isoforms) | Cytosol, mitochondrial matrix, nucleoplasm, blood serum | Detoxification of heavy metals, maintenance of copper and zinc balance, control of apoptosis, and cell protection from death and neoplasia [56,57] |
| COMMD1 (Copper Metabolism gene MURR Domain 1; previously named MURR1) | Cytosol, nucleoplasm | Excretion of Cu(II) through bile, stabilization of the ATP7B 1 structure, and participation in copper-dependent signaling with NF-kB 2 [58] |
| XIAP (X-linked inhibitor of apoptosis protein) | Cytosol | The inhibitor of caspase 3, ubiquitin-ligase related to COMMD1, and a copper level regulator in the cell [45,46] |
| SCO1/SCO2 (assembly of cytochrome c oxidase 1/2) | Inner mitochondrial membrane | Incorporation of copper ions into COX 3, assembly of the COX complex, and control of copper balance in the cells [59,60] |
| SCC (small copper carrier) | Circulation, urine | Removal of copper from the liver into the bloodstream [61] |
| CuL (Copper ligand) | Cytosol, mitochondrial matrix | Transfer of copper between the mitochondrial matrix, mitochondrial intermembrane space, and cytosol [62,63] |
| LOXL (1-4) (lysyl-oxidase-like proteins) transcription factors | Cytosol, nucleus | Copper-dependent suppressors and activators of tumor growth and metastasis [64,65] |
| Sp1 (specificity protein 1) | Cytosol, nucleus | Multifunctional transcription factor; regulator of CTR1 gene activity [66,67] |
| MAC1 * (Copper-sensing transcription factor) | Cytosol, nucleus | Transcription factor involved in the regulation of the CTR1 gene [68] |
| ACE1 * (transcription factor) | Cytosol, nucleus | Regulation of the expression of the metallothionein gene [68] |
| Protein | Localization | Function |
|---|---|---|
| CTR1 (high affinity copper importer 1) | Plasma membrane homotrimeric integral protein, universal copper importer | Transfer of Cu(I) from the extracellular space to the cytosol (ATP-independent) [72]; Cu1+/K1+-exchanger; control of morphogenesis [72]; stabilization of the CTR2 structure [73,74,75] |
| CCS (Cu(I)-chaperon for SOD1) | Cytosol | Transportation of Cu(I) from CTR1 to apo-SOD1 [76] |
| COX17 (Cu(I)-chaperon for COX) | Mitochondrial intermembrane space | Transportation of Cu(I) from CTR1 to SCO1/SCO2 [77] |
| SCO1/SCO2 | Inner mitochondrial membrane | Insertion of copper to COX; control of copper balance in the cell, implementation of the Cu(II)→Cu(I) redox cycle [59,60] |
| ATOX1 (Antioxidant 1, Cu(I)-chaperon for ATP7A/B) | Cytosol | Transportation of Cu(I) from CTR1 to the copper-binding motifs of the ATP7A/B [78]; a component of the cytosolic antioxidant system [79], transcription factor [80] |
| Menkes ATPase (ATP7A, Cu(I)/Cu(II)-transporting ATPase P1 type) | Membranes of the trans-network Golgi complex (except for hepatocytes of adult mammals) | Acceptance of Cu(I) from ATOX1 and its ATP-dependent transfer to the lumen of the Golgi complex; oxidation of Cu(I) to Cu(II) and insertion of copper into extracellular cuproenzymes [81] |
| Wilson ATPase (ATP7B, Cu(I)/Cu(II)-transporting ATPase P1 type) | Membranes of the trans-network Golgi complex and plasma membrane of the liver, mammary gland, and brain cells | Acceptance of Cu(I) from ATOX1 and its ATP-dependent transfer to the lumen of the Golgi complex; oxidation of Cu(I) to Cu(II) and insertion of copper into ceruloplasmin, copper excretion through bile [81] |
| CTR2 (low affinity copper transporter 2) | Membranes of the endolysosomes, plasma membrane | Transfer of Cu(I) from lysosomes to the cytosol; regulation of copper import [74,75] |
| DMT1 (divalent metal transporter 1) | Apical domain of the plasma membrane of the enterocytes and other cells | Transfer of Cu(II)/Cu(I) from GIT 1 into enterocytes [82,83]; compensation of CTR1 deficiency [84] |
| Characteristics | Control | Experiment |
|---|---|---|
| Body weight, g | 11.8 ± 1.3 (11) | 9.4 ± 1.2 (11) |
| Serum Cp oxidase activity, mg/100 mL | 14.1 ± 2.13 (6) | 38.47 ± 4.46 (6) § |
| Serum Cp antigen activity, mg/100 mL | 14.3 ± 1.2 (6) | 43.2 ± 2.1 (7) § |
| Serum Cu concentration, μg/L | 300 ± 50 (6) | 940 ± 71 (7) |
| Cu atoms per 1 serum Cp molecule * | 4.5 | 4.5 |
| Cu content in liver, μg/g dry weight | 4.58 ± 0.04 (5) | 1.91 ± 1.20 (5) § |
| Cu content in brain, μg | 1.71 ± 0.40 (5) | 1.72 ± 0.61 (5) |
| 1 CSF Cp antigen activity, mg/100 mL & | 0.3 | 2.3 |
| Cu concentration in CSF, μg/L | 15 | 85 |
| Liver total RNA, mg/g tissue | 1.7 | 1.8 |
| Cp-mRNA, μg/mg of total RNA | 0.11 | 0.23 |
| Carbonyl group concentration, A370/mg protein: Cytoplasm Ψ of brain | 0.131 ± 0.02 (5) | 0.101 ± 0.013 (5) |
| Cytoplasm of liver | 0.093 ± 0.01 | 0.086 ± 0.07 |
| Serum | 0.155 ± 0.01 | 0.127 ± 0.014 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puchkova, L.V.; Babich, P.S.; Zatulovskaia, Y.A.; Ilyechova, E.Y.; Di Sole, F. Copper Metabolism of Newborns Is Adapted to Milk Ceruloplasmin as a Nutritive Source of Copper: Overview of the Current Data. Nutrients 2018, 10, 1591. https://doi.org/10.3390/nu10111591
Puchkova LV, Babich PS, Zatulovskaia YA, Ilyechova EY, Di Sole F. Copper Metabolism of Newborns Is Adapted to Milk Ceruloplasmin as a Nutritive Source of Copper: Overview of the Current Data. Nutrients. 2018; 10(11):1591. https://doi.org/10.3390/nu10111591
Chicago/Turabian StylePuchkova, Ludmila V., Polina S. Babich, Yulia A. Zatulovskaia, Ekaterina Y. Ilyechova, and Francesca Di Sole. 2018. "Copper Metabolism of Newborns Is Adapted to Milk Ceruloplasmin as a Nutritive Source of Copper: Overview of the Current Data" Nutrients 10, no. 11: 1591. https://doi.org/10.3390/nu10111591
APA StylePuchkova, L. V., Babich, P. S., Zatulovskaia, Y. A., Ilyechova, E. Y., & Di Sole, F. (2018). Copper Metabolism of Newborns Is Adapted to Milk Ceruloplasmin as a Nutritive Source of Copper: Overview of the Current Data. Nutrients, 10(11), 1591. https://doi.org/10.3390/nu10111591






