The Effects of Adjuvant Fermented Wheat Germ Extract on Cancer Cell Lines: A Systematic Review
Abstract
:1. Introduction
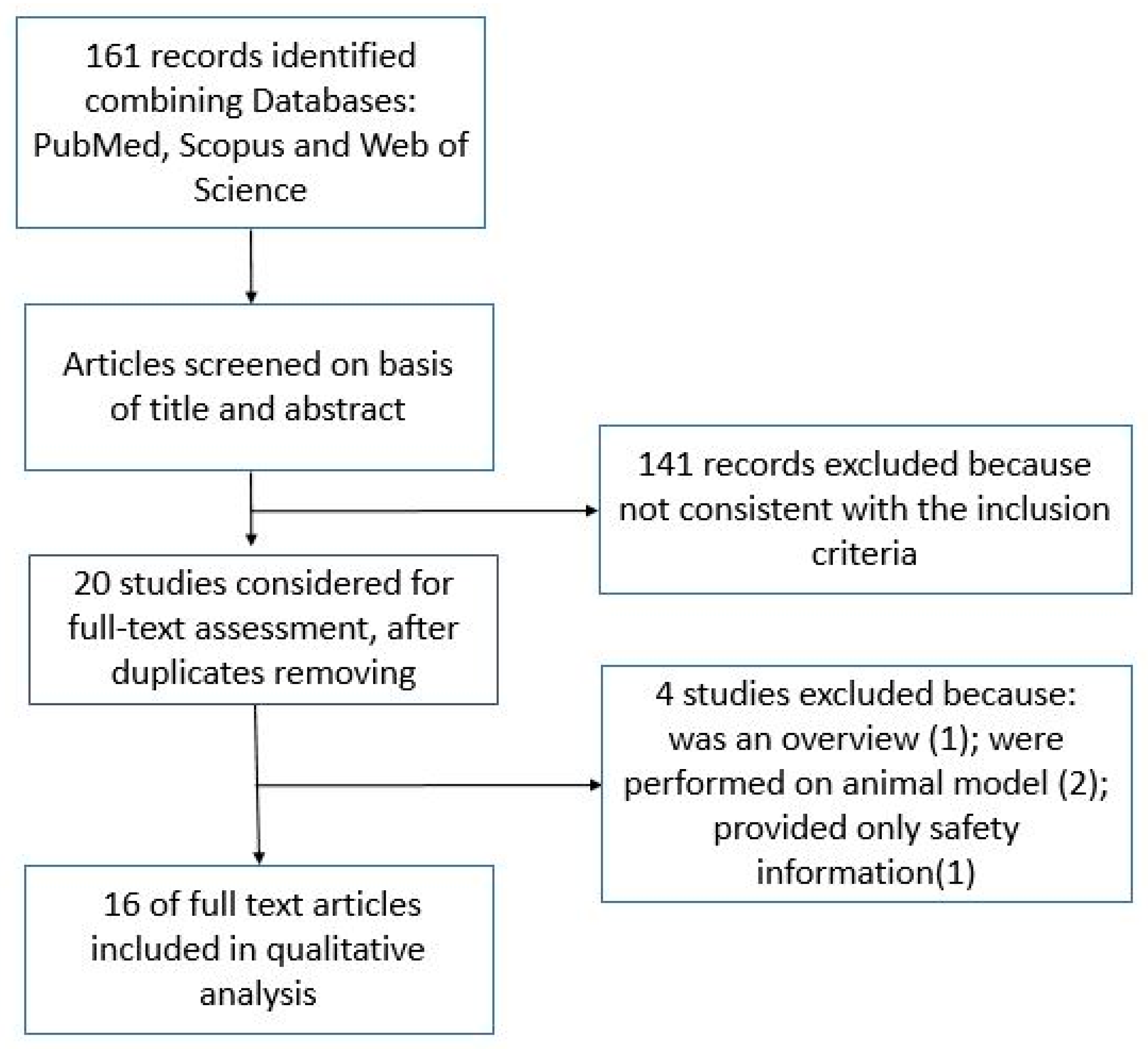
2. Materials and Methods
3. Results
3.1. Characteristics of Included Studies and Primary Outcomes
- (1)
- (2)
- (3)
- (4)
- (5)
- (6)
- (7)
- (8)
- Other types of cell lines investigated in the included studies were prostate cancer cells, endocervical adenocarcinoma [22], cervical epidermoid carcinoma cells [25], testicular cancer cell lines [25], head and neck cancer [25], thyroid and pancreatic cancer cells [13], melanoma, hepatoma, glioblastoma, neuroblastoma [22], and oral squamous carcinoma cells [30]. In all cases, the effects of AVEMAR treatment provided results similar to those previously mentioned.
3.2. Secondary Outcomes
- (1)
- Enzyme activities evaluation. In particular, FWGE was found to inhibit Glucose-6-phosphate dehydrogenase (G6PDH), Lactate dehydrogenase (LDH) and Hexokinase (HK) activity in Jurkat T-progeny leukemia cells [18]. The inhibition of ribonucleotide reductase (RR) activity in promyelocytic leukemia cells was established by Saiko et al. [19]. A suppression of the expression of matrix metalloproteinase-2 (MMP-2) and urokinase plasminogen activator (u-PA) was revealed in oral cancer cells (SCC-4) treated with Avemar [30];
- (2)
- Presumable anti-angiogenic effects of FWGE on human cervical carcinoma (HeLa) and human lung adenocarcinoma (A549) cells through the inhibition of vascular endothelial growth factor (VEGF) and cyclooxygenase-2 (Cox-2) levels [22];
- (3)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmad, A.; Ginnebaugh, K.R.; Li, Y.; Padhye, S.B.; Sarkar, F.H. Molecular targets of naturopathy in cancer research: Bridge to modern medicine. Nutrients 2015, 7, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Ardito, F.; Pellegrino, M.R.; Perrone, D.; Troiano, G.; Cocco, A.; Lo Muzio, L. In vitro study on anti-cancer properties of genistein in tongue cancer. Onco Targets Ther. 2017, 10, 5405–5415. [Google Scholar] [CrossRef] [PubMed]
- Perrone, D.; Ardito, F.; Giannatempo, G.; Dioguardi, M.; Troiano, G.; Lo Russo, L.; DE Lillo, A.; Laino, L.; Lo Muzio, L. Biological and therapeutic activities, and anticancer properties of curcumin. Exp. Ther. Med. 2015, 10, 1615–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvie, M. Nutritional supplements and cancer: Potential benefits and proven harms. Am. Soc. Clin. Oncol. Educ. Book 2014, e478–e486. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Liu, T.; Shi, J.; Qiao, L. Curcumin: A promising agent targeting cancer stem cells. Anticancer Agents Med. Chem. 2014, 14, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.G.; D’Orazio, J.A.; Pearson, K.J. Resveratrol and cancer: Focus on in vivo evidence. Endocr. Relat. Cancer 2014, 21, R209–R225. [Google Scholar] [CrossRef] [PubMed]
- Megna, B.W.; Carney, P.R.; Nukaya, M.; Geiger, P.; Kennedy, G.D. Indole-3-carbinol induces tumor cell death: Function follows form. J. Surg. Res. 2016, 204, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Hidvegi, M.; Raso, E.; Tomoskozi-Farkas, R.; Paku, S.; Lapis, K.; Szende, B. Effect of avemar and avemar + vitamin c on tumor growth and metastasis in experimental animals. Anticancer Res. 1998, 18, 2353–2358. [Google Scholar] [PubMed]
- Telekes, A.; Hegedus, M.; Chae, C.H.; Vekey, K. Avemar (wheat germ extract) in cancer prevention and treatment. Nutr. Cancer 2009, 61, 891–899. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.J. Molecular mechanisms of quinone cytotoxicity. Chem. Biol. Interact. 1991, 80, 1–41. [Google Scholar] [CrossRef]
- Piccart-Gebhart, M.J. Anthracyclines and the tailoring of treatment for early breast cancer. N. Engl. J. Med. 2006, 354, 2177–2179. [Google Scholar] [CrossRef] [PubMed]
- Johanning, G.L.; Wang-Johanning, F. Efficacy of a medical nutriment in the treatment of cancer. Altern. Ther. Health Med. 2007, 13, 56–63. [Google Scholar] [PubMed]
- Boros, L.G.; Nichelatti, M.; Shoenfeld, Y. Fermented wheat germ extract (avemar) in the treatment of cancer and autoimmune diseases. Ann. N. Y. Acad. Sci. 2005, 1051, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Brown, L. Fermented wheat germ extract (avemar) in the treatment of cardiac remodeling and metabolic symptoms in rats. Evid. Based Complement. Altern. Med. 2011, 2011, 508957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illmer, C.; Madlener, S.; Horvath, Z.; Saiko, P.; Losert, A.; Herbacek, I.; Grusch, M.; Krupitza, G.; Fritzer-Szekeres, M.; Szekeres, T. Immunologic and biochemical effects of the fermented wheat germ extract avemar. Exp. Biol. Med. 2005, 230, 144–149. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Hidvegi, M.; Raso, E.; Tomoskozi-Farkas, R.; Szende, B.; Paku, S.; Pronai, L.; Bocsi, J.; Lapis, K. Msc, a new benzoquinone-containing natural product with antimetastatic effect. Cancer Biother. Radiopharm. 1999, 14, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Comin-Anduix, B.; Boros, L.G.; Marin, S.; Boren, J.; Callol-Massot, C.; Centelles, J.J.; Torres, J.L.; Agell, N.; Bassilian, S.; Cascante, M. Fermented wheat germ extract inhibits glycolysis/pentose cycle enzymes and induces apoptosis through poly(ADP-ribose) polymerase activation in jurkat T-cell leukemia tumor cells. J. Biol. Chem. 2002, 277, 46408–46414. [Google Scholar] [CrossRef] [PubMed]
- Saiko, P.; Ozsvar-Kozma, M.; Madlener, S.; Bernhaus, A.; Lackner, A.; Grusch, M.; Horvath, Z.; Krupitza, G.; Jaeger, W.; Ammer, K.; et al. Avemar, a nontoxic fermented wheat germ extract, induces apoptosis and inhibits ribonucleotide reductase in human HL-60 promyelocytic leukemia cells. Cancer Lett. 2007, 250, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Fajka-Boja, R.; Hidvegi, M.; Shoenfeld, Y.; Ion, G.; Demydenko, D.; Tomoskozi-Farkas, R.; Vizler, C.; Telekes, A.; Resetar, A.; Monostori, E. Fermented wheat germ extract induces apoptosis and downregulation of major histocompatibility complex class i proteins in tumor t and b cell lines. Int. J. Oncol. 2002, 20, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Imir, N.G.; Aydemir, E.; Simsek, E. Mechanism of the anti-angiogenic effect of avemar on tumor cells. Oncol. Lett. 2018, 15, 2673–2678. [Google Scholar] [CrossRef] [PubMed]
- Judson, P.L.; Al Sawah, E.; Marchion, D.C.; Xiong, Y.; Bicaku, E.; Bou Zgheib, N.; Chon, H.S.; Stickles, X.B.; Hakam, A.; Wenham, R.M.; et al. Characterizing the efficacy of fermented wheat germ extract against ovarian cancer and defining the genomic basis of its activity. Int. J. Gynecol. Cancer 2012, 22, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Marcsek, Z.; Kocsis, Z.; Jakab, M.; Szende, B.; Tompa, A. The efficacy of tamoxifen in estrogen receptor-positive breast cancer cells is enhanced by a medical nutriment. Cancer Biother. Radiopharm. 2004, 19, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Jordan, K.; Voigt, W. Promising cytotoxic activity profile of fermented wheat germ extract (Avemar®) in human cancer cell lines. J. Exp. Clin. Cancer Res. 2011, 30, 42. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.; Hahlbrock, T.; Eich, K.; Karaaslan, F.; Jurgens, C.; Germer, C.T.; Wiegering, A.; Kammerer, U. Antiproliferative and antimetabolic effects behind the anticancer property of fermented wheat germ extract. BMC Complement. Altern. Med. 2016, 16, 160. [Google Scholar] [CrossRef] [PubMed]
- Saiko, P.; Ozsvar-Kozma, M.; Graser, G.; Lackner, A.; Grusch, M.; Madlener, S.; Krupitza, G.; Jaeger, W.; Hidvegi, M.; Agarwal, R.P.; et al. Avemar, a nontoxic fermented wheat germ extract, attenuates the growth of sensitive and 5-FdUrd/Ara-c cross-resistant H9 human lymphoma cells through induction of apoptosis. Oncol. Rep. 2009, 21, 787–791. [Google Scholar] [PubMed]
- Tai, C.J.; Wang, W.C.; Wang, C.K.; Wu, C.H.; Yang, M.D.; Chang, Y.J.; Jian, J.Y.; Tai, C.J. Fermented wheat germ extract induced cell death and enhanced cytotoxicity of cisplatin and 5-fluorouracil on human hepatocellular carcinoma cells. Evid. Based Complement. Altern. Med. 2013, 2013, 121725. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Wang, C.K.; Chang, Y.J.; Choong, C.Y.; Lin, C.S.; Tai, C.J.; Tai, C.J. Preclinical evaluation on the tumor suppression efficiency and combination drug effects of fermented wheat germ extract in human ovarian carcinoma cells. Evid. Based Complement. Altern. Med. 2015, 2015, 570785. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.D.; Chang, W.S.; Tsai, C.W.; Wang, M.F.; Chan, Y.C.; Chan, K.C.; Lu, M.C.; Kao, A.W.; Hsu, C.M.; Bau, D.T. Inhibitory effects of avemar on proliferation and metastasis of oral cancer cells. Nutr. Cancer 2016, 68, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Xiao, X.; Dong, Y.; Wu, J.; Yao, F.; Zhou, X.H. Effect of fermented wheat germ extract with lactobacillus plantarum dy-1 on ht-29 cell proliferation and apoptosis. J. Agric. Food Chem. 2015, 63, 2449–2457. [Google Scholar] [CrossRef] [PubMed]
- Barisone, G.A.; O’Donnell, R.T.; Ma, Y.; Abuhay, M.W.; Lundeberg, K.; Gowda, S.; Tuscano, J.M. A purified, fermented, extract of triticum aestivum has lymphomacidal activity mediated via natural killer cell activation. PLoS ONE 2018, 13, e0190860. [Google Scholar] [CrossRef] [PubMed]
- Szende, B.; Marcsek, Z.; Kocsis, Z.; Tompa, A. Effect of simultaneous administration of avemar® and cytostatic drugs on viability of cell cultures, growth of experimental tumors, and survival of tumor-bearing mice. Cancer Biother. Radiopharm. 2004, 19, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Boros, L.G.; Lapis, K.; Szende, B.; Tömösközi-Farkas, R.; Balogh, A.; Boren, J.; Marin, S.; Cascante, M.; Hidvégi, M. Wheat germ extract decreases glucose uptake and rna ribose formation but increases fatty acid synthesis in mia pancreatic adenocarcinoma cells. Pancreas 2001, 23, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.T.; Sebestyen, G.; Semjen, G.; Kennepohl, E. Safety studies regarding a standardized extract of fermented wheat germ. Int. J. Toxicol. 2007, 26, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Inoue, K.; Tanaka, G.; Akimoto, K.; Kubota, K. Augmented pentose phosphate pathway plays critical roles in colorectal carcinomas. Oncology 2015, 88, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Kitajjma, S.; Sato, S.; Miyauchi, M.; Ogawa, I.; Takata, T. Establishment of an oral squamous cell carcinoma cell line with high invasive and p27 degradation activities from a lymph node metastasis. Oral Oncol. 2003, 39, 515–520. [Google Scholar] [CrossRef]
- Lo Muzio, L.; Pannone, G.; Santarelli, A.; Bambini, F.; Mascitti, M.; Rubini, C.; Testa, N.F.; Dioguardi, M.; Leuci, S.; Bascones, A.; et al. Is expression of p120ctn in oral squamous cell carcinomas a prognostic factor? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; De Caterina, R.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through mmp-9 and cox-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Hidvegi, M.; Raso, E.; Tomoskozi Farkas, R.; Lapis, K.; Szende, B. Effect of msc on the immune response of mice. Immunopharmacology 1999, 41, 183–186. [Google Scholar] [CrossRef]
- Jakab, F.; Shoenfeld, Y.; Balogh, A.; Nichelatti, M.; Hoffmann, A.; Kahan, Z.; Lapis, K.; Mayer, A.; Sapy, P.; Szentpetery, F.; et al. A medical nutriment has supportive value in the treatment of colorectal cancer. Br. J. Cancer 2003, 89, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Yeend, T.; Robinson, K.; Lockwood, C.; McArthur, A. The effectiveness of fermented wheat germ extract as an adjunct therapy in the treatment of cancer: A systematic review. JBI Libr. Syst. Rev. 2012, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Title | Cell Type | Investigations | Main Results | Secondary Outcomes |
|---|---|---|---|---|---|---|
| Comin-Anduix et al. [18] | 2002 | Fermented Wheat Germ Extract Inhibits Glycolysis/Pentose Cycle Enzymes and Induces Apoptosis through Poly (ADP-ribose) Polymerase Activation in Jurkat T-cell Leukemia Tumor Cells. | Jurkat T-cell Leukemia Tumor Cells. | Cell cycle analysis, cell viability assay, assessment of apoptosis. | Cytotoxic effects, alteration of the cell cycle, induction of apoptosis. | Cleavage of PARP, Transketolase, G6PDH, HK, LDH inhibition. |
| Fajka-boja et al. [21] | 2002 | Fermented wheat germ extract induces apoptosis and downregulation of major histocompatibility complex class I proteins in tumor T and B cell lines. | Jurkat leukemic T cells, Burkitt lymphoma B cell lines, myelo-monocytic cell line. | Detection of apoptotic cells, measurement of cell proliferation. | Induction of apoptosis, antiproliferative effect. | Elevation of intracellular Ca2+ concentration. |
| Imir et al. [22] | 2018 | Mechanism of the anti-angiogenic effect of AVEMAR on tumor cells. | NCI-N87 (gastric tubular adenocarcinoma), PC3 (prostate carcinoma), HeLa (adenocarcinoma) and A549 (lung adenocarcinoma) | Investigation of anti-angiogenic effects. | Inhibition of induced VEGF levels. | Inhibition of Cox-2 levels. |
| Judson et al. [23] | 2012 | Characterizing the efficacy of fermented wheat germ extract against ovarian cancer and defining the genomic basis of its activity. | Ovarian cancer cell lines. | Cell viability assays. | Cytotoxic effects, increase of cisplatin sensitivity. | |
| Marcsek et al. [24] | 2004 | The Efficacy of Tamoxifen in Estrogen Receptor–Positive Breast Cancer Cells Is Enhanced by a Medical Nutriment. | MCF-7 breast cancer cells. | Cytotoxic effects evaluation, detection of apoptosis and mitosis, evaluation of tamoxifen-combined treatment. | Cytotoxicity, induction of apoptosis. | |
| Mueller et al. [25] | 2011 | Promising cytotoxic activity profile of fermented wheat germ extract (Avemar®) in human cancer cell lines. | testicular cancer (H12.1, 2102EP, 1411HP, 1777NRpmet), colon cancer (HCT-8, HCT-15, HCT-116, HT-29, DLD-1, SW480, COLO205, COLO320DM), NSCLC (A549, A427, H322, H358), head and neck cancer (FADU, A253), cervical epidermoid carcinoma (A431), mammary adenocarcinoma (MCF-7, BT474), ovarian adenocarcinoma (A2780), gastric Cancer (M2), anaplastic thyroid cancer (8505C, SW1736), papillary thyroid cancer (BCPAP), follicular thyroid cancer (FTC133), melanoma, hepatoma (HepG2), glioblastoma (U87MG), neuroblastoma (SHSY5Y, SIMA). | Growth inhibition experiments, apoptosis evaluation. | Antiproliferative activity. | |
| Otto et al. [26] | 2016 | Antiproliferative and antimetabolic effects behind the anticancer property of fermented wheat germ extract. | Adenocarcinoma of the breast (MDA-MB-468) and (MDA-MB-231) and (BT-20), adenocarcinoma of the pancreas (ASPC-1) and (BxPC-3), adenocarcinoma of the stomach (23132/87), adenocarcinoma of the colon (HT-29) and (HRT-18), invasive breast ductal carcinoma (MCF-7). | Effects on cell growth, Cell cycle analysis. | Cytotoxic, antiproliferative and growth delay effects. | Depletion in cellular ATP and decrease in the NADH/NAD+ ratio. Impaired glucose consumption and significantly reduced production of lactic acid. Induction of autophagy in HRT-18 cells. |
| Saiko et al. [19] | 2007 | Avemar, a nontoxic fermented wheat germ extract, induces apoptosis and inhibits ribonucleotide reductase in human HL-60 promyelocytic leukemia cells. | Human HL-60 promyelocytic leukemia cells. | Apoptosis evaluation, cell cycle distribution analysis. | Induction of apoptosis, cell growth inhibition. | Decreasing of dNTPs, direct enzyme attenuation (ribonucleotide reductase; RR). |
| Saiko et al. [27] | 2009 | Avemar, a nontoxic fermented wheat germ extract, attenuates the growth of sensitive and 5-FdUrd/Ara-C cross-resistant H9 human lymphoma cells through induction of apoptosis. | Human lymphoma cells H9, 5-FdUrd/Ara-C cross-resistant H9 human lymphoma cell line. | Growth inhibition assay, apoptosis evaluation. | Growth inhibition, induction of apoptosis. | |
| Tai et al. [28] | 2013 | Fermented Wheat Germ Extract Induced Cell Death and Enhanced Cytotoxicity of Cisplatin and 5-Fluorouracil on Human Hepatocellular Carcinoma Cells. | Hepatocellular carcinoma (HCC) HepG2, Hep3B, and HepJ5 cells. | Cell viability Assay, evaluation of cisplatin and 5-fluorouracil combined treatment. | Antiproliferative activity, enhanced cytotoxicity of chemotherapeutic. | |
| Wang et al. [29] | 2015 | Preclinical Evaluation on the Tumor Suppression Efficiency and Combination Drug Effects of Fermented Wheat Germ Extract in Human Ovarian Carcinoma Cells. | SKOV-3 and ES-2 human ovarian carcinoma cells. | Cell viability evaluation, cell death markers analysis, evaluation of cisplatin- or docetaxel-combined treatment. | Suppression of cell proliferation, caspase-related apoptosis activation, increased cytotoxicity of cisplatin and docetaxel. | |
| Yang et al. [30] | 2016 | Inhibitory Effects of AVEMAR on Proliferation and Metastasis of Oral Cancer Cells. | Human oral squamous carcinoma SCC-4 cells. | Cell viability evaluation, cell apoptosis assay wound-healing migration assay, cell invasion assay. | Inhibition of cell viability, induction of cell apoptosis, suppression of migration and invasion capacity. | |
| Zhang et al. [31] | 2015 | Effect of Fermented Wheat Germ Extract with Lactobacillus plantarum dy-1 on HT-29 Cell Proliferation and Apoptosis. | Human HT-29 colon cancer cells. | Growth inhibition assay, assessment of apoptosis. | High antiproliferative effects, induction of cell apoptosis. | |
| Barisone et al. [32] | 2017 | A purified, fermented, extract of Triticum aestivum has lymphomacidal activity mediated via natural killer cell activation. | Lymphoma cells, T-cell leukemia (Jurkat), lung (H1650), breast (MCF-7) and hepatic (HepG2) cancer cell lines. | Cytotoxic activity assay, apoptosis and cell cycle. | Cytotoxic activity, apoptotic activity. | |
| Szende et al. [33] | 2004 | Effect of Simultaneous Administration of Avemar® and Cytostatic Drugs on Viability of Cell Cultures, Growth of Experimental Tumors, and Survival of Tumor-Bearing Mice. | Human breast adenocarcinoma cell line (MCF-7), hepatocyte carcinoma (HepG2). | Cytotoxicity testing of Avemar associated with various cytostatic drugs (5 FU, Dacarbazine, Adriblastina). | Did not increase nor decrease cell viability. | |
| Boros et al. [34] | 2001 | Wheat Germ Extract Decreases Glucose Uptake and RNA Ribose Formation but Increases Fatty Acid Synthesis in MIA Pancreatic Adenocarcinoma Cells. | MIA pancreatic adenocarcinoma cells. | Evaluation of glucose utilization rates and lactate production. | Regulation of tumor cell proliferation. | Inhibitory effect on glucose consumption, little effect on lactate production. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhurakivska, K.; Troiano, G.; Caponio, V.C.A.; Dioguardi, M.; Arena, C.; Lo Muzio, L. The Effects of Adjuvant Fermented Wheat Germ Extract on Cancer Cell Lines: A Systematic Review. Nutrients 2018, 10, 1546. https://doi.org/10.3390/nu10101546
Zhurakivska K, Troiano G, Caponio VCA, Dioguardi M, Arena C, Lo Muzio L. The Effects of Adjuvant Fermented Wheat Germ Extract on Cancer Cell Lines: A Systematic Review. Nutrients. 2018; 10(10):1546. https://doi.org/10.3390/nu10101546
Chicago/Turabian StyleZhurakivska, Khrystyna, Giuseppe Troiano, Vito Carlo Alberto Caponio, Mario Dioguardi, Claudia Arena, and Lorenzo Lo Muzio. 2018. "The Effects of Adjuvant Fermented Wheat Germ Extract on Cancer Cell Lines: A Systematic Review" Nutrients 10, no. 10: 1546. https://doi.org/10.3390/nu10101546
APA StyleZhurakivska, K., Troiano, G., Caponio, V. C. A., Dioguardi, M., Arena, C., & Lo Muzio, L. (2018). The Effects of Adjuvant Fermented Wheat Germ Extract on Cancer Cell Lines: A Systematic Review. Nutrients, 10(10), 1546. https://doi.org/10.3390/nu10101546










