Evaluation of Sentinel-2A Satellite Imagery for Mapping Cotton Root Rot
Abstract
:1. Introduction
2. Materials and Methods
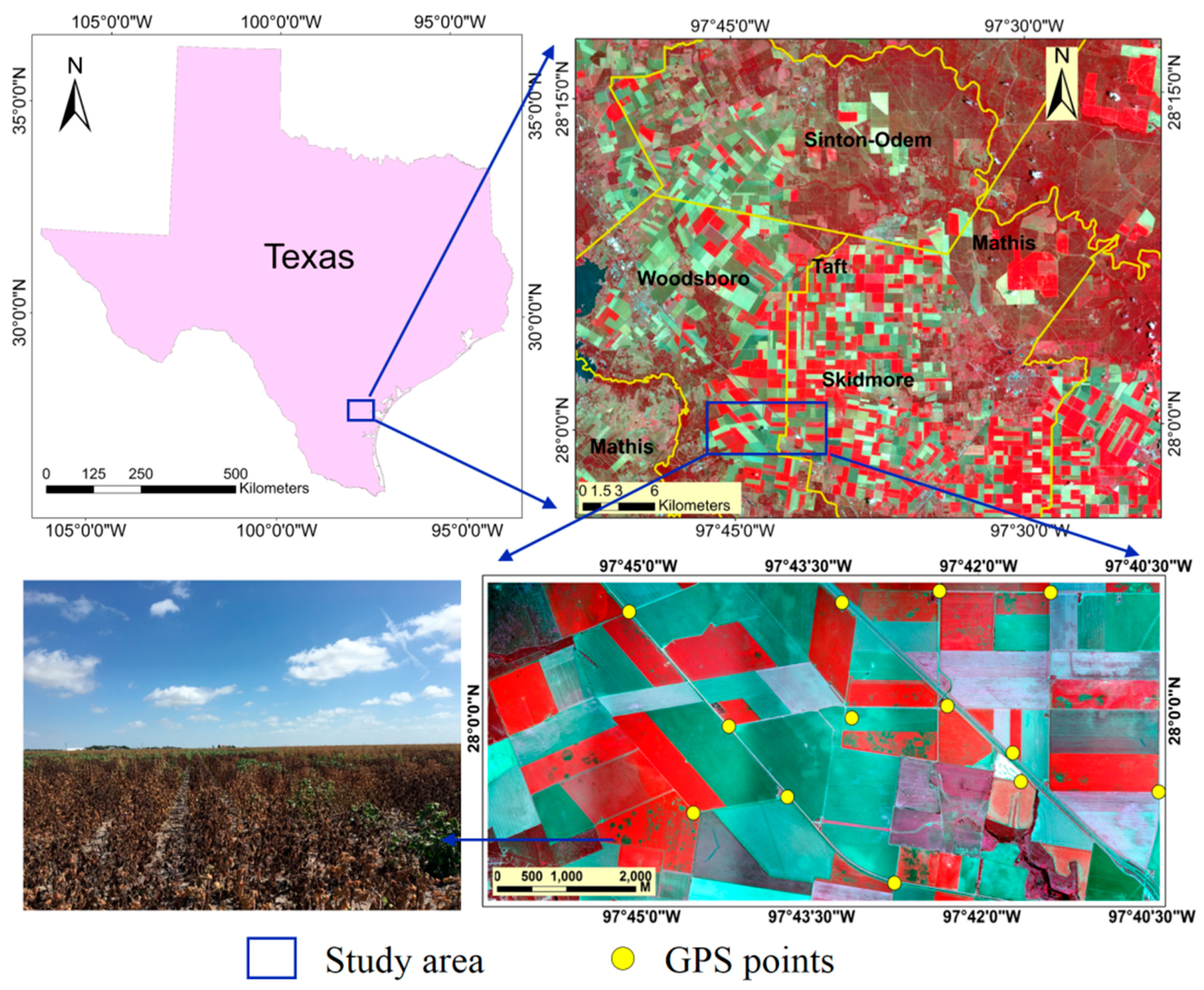
2.1. Study Area
2.2. Image Acquisition and Processing
2.2.1. Airborne Multispectral Imagery
2.2.2. Sentinel-2A Satellite Imagery
2.3. Image Classification and Analysis
2.3.1. Cotton Field Identification
2.3.2. Classification of Cotton Root Rot
2.3.3. Accuracy Assessment
3. Results
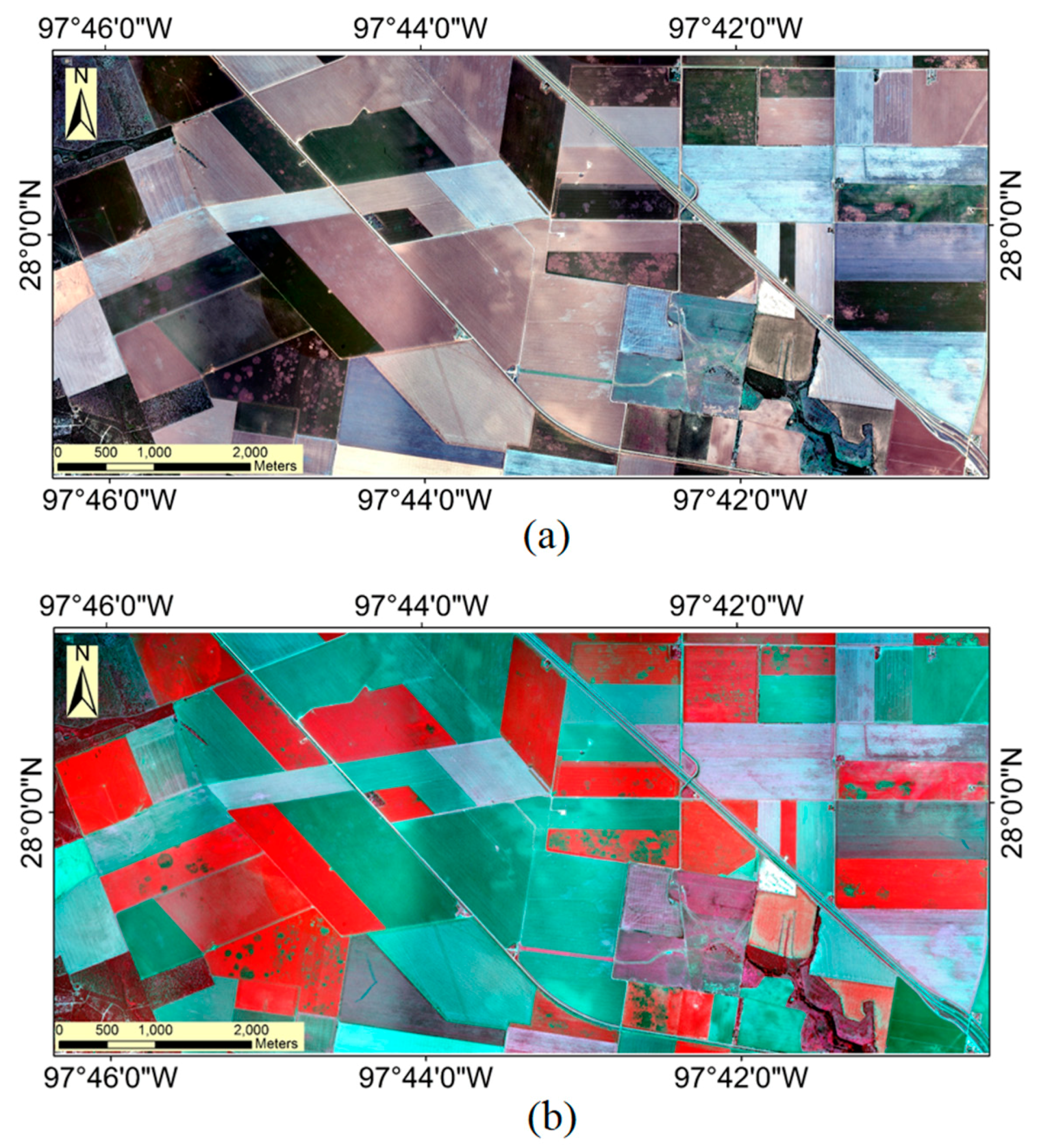
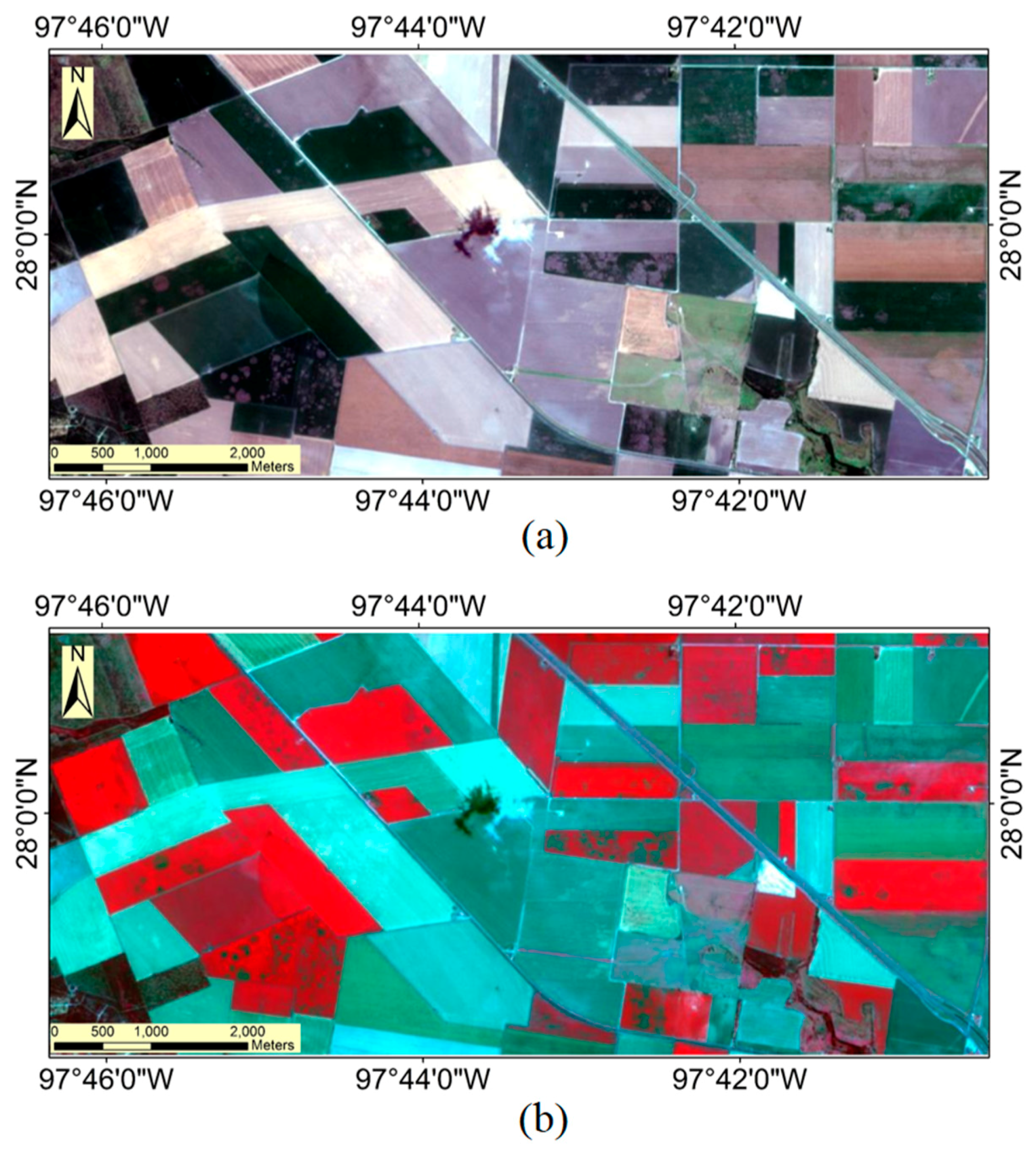
3.1. Airborne Multispectral Image Classification
3.2. Sentinel-2A Image Classification
3.3. Classification Accuracy Assessment
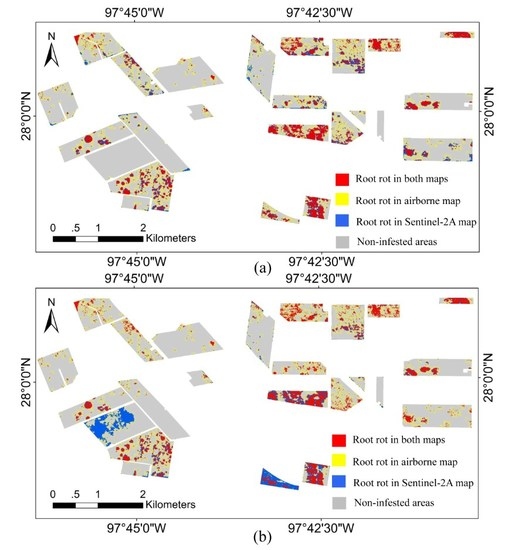
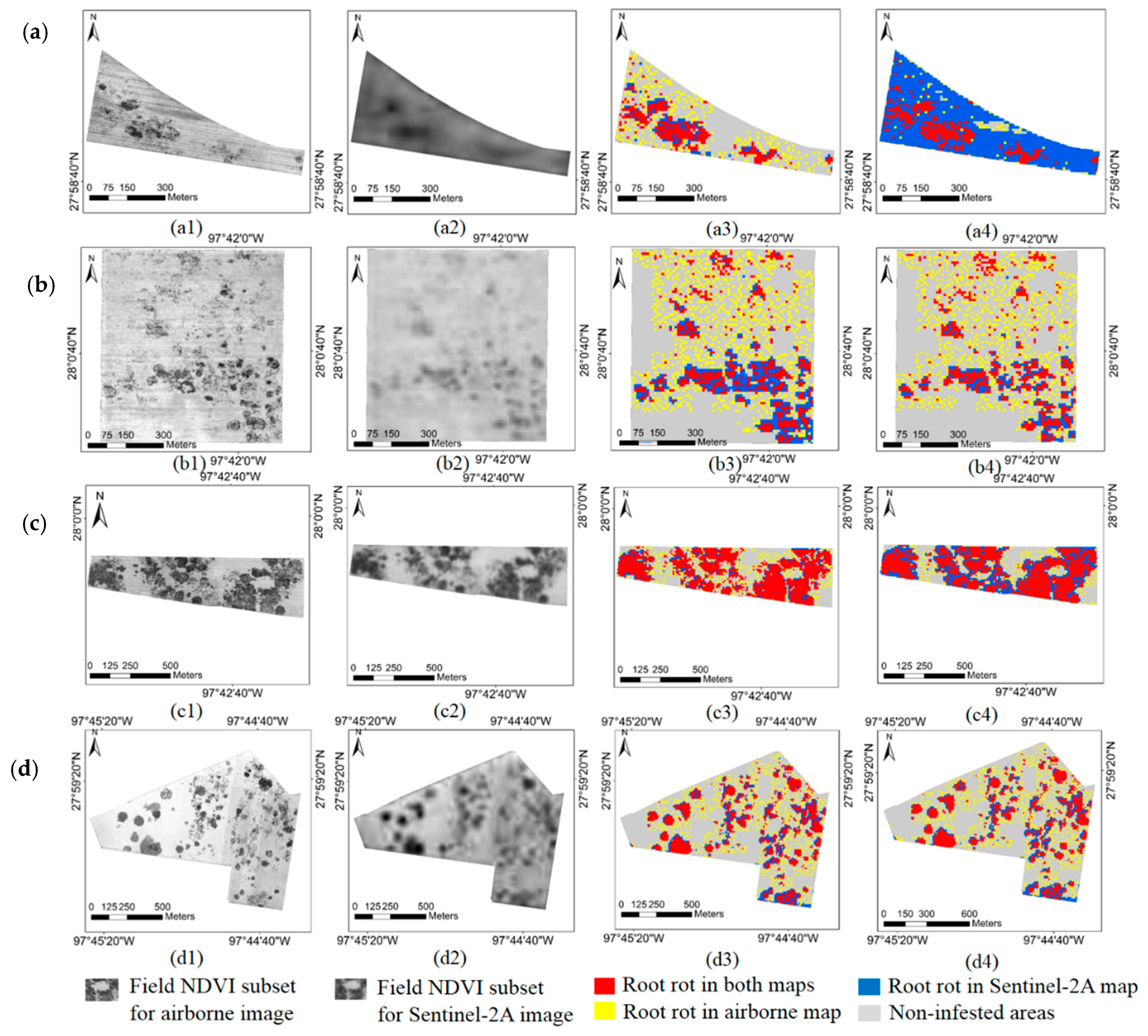
3.4. Overlapped Root Rot Area between Airborne Image and Sentinel-2A Image
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Percy, R.G. Potential range of Phymatotrichum omnivorum as determined by edaphic factors. Plant Dis. 1983, 67, 981–983. [Google Scholar] [CrossRef]
- Pammel, L.H. Root rot of cotton or “Cotton blight”. Texas Agric. Exp. Stn. Ann. Bull. 1888, 4, 50–65. [Google Scholar]
- Cribben, C.D.; Thomasson, J.A.; Ge, Y.; Morgan, C.L.S.; Yang, C.; Isakeit, T.; Nichols, R.L. Site-specific relationships between cotton root rot and soil properties. J. Cotton Sci. 2016, 20, 67–75. [Google Scholar]
- Ezekiel, W.N.; Taubenhaus, J.J. Cotton crop losses from Phymatotrichum root rot. J. Agric. Res. 1934, 49, 843–858. [Google Scholar]
- Yang, C.; Fernandez, C.J.; Everitt, J.H. Mapping Phymatotrichum root rot of cotton using airborne three-band digital imagery. Trans. ASAE 2005, 48, 1619–1626. [Google Scholar] [CrossRef]
- Yang, C.; Odvody, G.N.; Fernandez, C.J.; Landivar, J.A.; Minzenmayer, R.R.; Nichols, R.L.; Thomasson, J.A. Monitoring cotton root rot progression within a growing season using airborne multispectral imagery. J. Cotton Sci. 2014, 18, 85–93. [Google Scholar]
- Isakeit, T.; Minzenmayer, R.R.; Sansone, C.G. Flutriafol control of cotton root rot caused by Phymatotrichopsis omnivore. In Proceedings of the Beltwide Cotton Conference, San Antonio, TX, USA, 5–8 January 2009; The National Cotton Council: Memphis, TN, USA; pp. 130–133. [Google Scholar]
- Isakeit, T.; Minzenmayer, R.R.; Abrameit, A.; Moore, G.; Scasta, J.D. Control of Phymatotrichopsis root rot of cotton with flutriafol. In Proceedings of the Beltwide Cotton Conference, New Orleans, LA, USA, 4–7 January 2010; The National Cotton Council: Memphis, TN, USA; pp. 200–203. [Google Scholar]
- Isakeit, T.; Minzenmayer, R.R.; Drake, D.R.; Morgan, G.D.; Mott, D.A.; Fromme, D.D.; Multer, W.L.; Jungman, M.; Abrameit, A. Fungicide management of cotton root rot (Phymatotrichopsis omnivora): 2011 results. In Proceedings of the Beltwide Cotton Conference, Orlando, FL, USA, 3–6 January 2012; The National Cotton Council: Memphis, TN, USA; pp. 235–238. [Google Scholar]
- Taubenhaus, J.J.; Ezekiel, W.N.; Neblette, C.B. Airplane photography in the study of cotton root rot. Phytopathology 1929, 19, 1025–1029. [Google Scholar]
- Nixon, P.R.; Lyda, S.D.; Heilman, M.D.; Bowen, R.L. Incidence and Control of Cotton Root Rot Observed with Color Infrared Photography; Texas A&M Agricultural Experiment Station: College Station, TX, USA, 1975. [Google Scholar]
- Nixon, P.R.; Escobar, D.E.; Bowen, R.L. A multispectral false-color video imaging system for remote sensing applications. In Proceedings of the 11th Biennial Workshop on Color Aerial Photography and Videography in the Plant Sciences and Related Fields, Weslaco, TX, USA, 27 April–1 May 1987; pp. 295–305. [Google Scholar]
- Yang, C.; Odvody, G.N.; Fernandez, C.J.; Landivar, J.A.; Minzenmayer, R.R.; Nichols, R.L. Evaluating unsupervised and supervised image classification methods for mapping cotton root rot. Precis. Agric. 2015, 16, 201–215. [Google Scholar] [CrossRef]
- Song, H.B.; Yang, C.; Zhang, J.; He, D.J.; Thomasson, J.A. Combining fuzzy set theory and nonlinear stretching enhancement for unsupervised classification of cotton root rot. J. Appl. Remote Sens. 2015, 9, 096013. [Google Scholar] [CrossRef]
- Yang, C.; Odvody, G.N.; Thomasson, J.A.; Isakeit, T.; Nichols, R.L. Change detection of cotton root rot infection over 10-year intervals using airborne multispectral imagery. Comput. Electron. Agric. 2016, 123, 154–162. [Google Scholar] [CrossRef]
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P.; et al. Sentinel-2: ESA’s optical high-resolution mission for GMES operational services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- Immitzer, M.; Vuolo, F.; Atzberger, C. First experience with Sentinel-2 data for crop and tree species classifications in Central Europe. Remote Sens. 2016, 8, 166. [Google Scholar] [CrossRef]
- Vuolo, F.; Żółtak, M.; Pipitone, C.; Zappa, L.; Wenng, H.; Immitzer, M.; Weiss, M.; Baret, F.; Atzberger, C. Data service platform for Sentinel-2 surface reflectance and value-added products: System use and examples. Remote Sens. 2016, 8, 938. [Google Scholar] [CrossRef]
- Eitel, J.U.; Vierling, L.A.; Litvak, M.E.; Long, D.S.; Schulthess, U.; Ager, A.A.; Krofcheck, D.J.; Stoscheck, L. Broadband red-edge information from satellites improves early stress detection in a New Mexico conifer woodland. Remote Sens. Environ. 2011, 115, 3640–3646. [Google Scholar] [CrossRef]
- Schuster, C.; Förster, M.; Kleinschmit, B. Testing the red edge channel for improving land-use classifications based on high-resolution multi-spectral satellite data. Int. J. Remote Sens. 2012, 33, 5583–5599. [Google Scholar] [CrossRef]
- Verrelst, J.; Muñoz, J.; Alonso, L.; Delegido, J.; Rivera, J.P.; Camps-Valls, G.; Moreno, J. Machine learning regression algorithms for biophysical parameter retrieval: Opportunities for Sentinel-2 and-3. Remote Sens. Environ. 2012, 118, 127–139. [Google Scholar] [CrossRef]
- Delegido, J.; Verrelst, J.; Alonso, L.; Moreno, J. Evaluation of Sentinel-2 red-edge bands for empirical estimation of green LAI and chlorophyll content. Sensors 2011, 11, 7063–7081. [Google Scholar] [CrossRef] [PubMed]
- Clevers, J.G.; Gitelson, A.A. Remote estimation of crop and grass chlorophyll and nitrogen content using red-edge bands on Sentinel-2 and -3. Int. J. Appl. Earth Obs. Geoinf. 2013, 23, 344–351. [Google Scholar] [CrossRef]
- Sibanda, M.; Mutanga, O.; Rouget, M. Examining the potential of Sentinel-2 MSI spectral resolution in quantifying above ground biomass across different fertilizer treatments. ISPRS J. Photogramm. Remote Sens. 2015, 110, 55–65. [Google Scholar] [CrossRef]
- Malenovsky, Z.; Rott, H.; Cihlar, J.; Schaepman, M.E.; Garcia-Santos, G.; Fernandes, R.; Berger, M. Sentinels for science: Potential of Sentinel-1, -2, and -3 missions for scientific observations of ocean, cryosphere, and land. Remote Sens. Environ. 2012, 120, 91–101. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, Y.; Ling, F.; Wang, Q.; Li, W.; Li, X. Water bodies’ mapping from Sentinel-2 imagery with modified normalized difference water index at 10-m spatial resolution produced by sharpening the SWIR band. Remote Sens. 2016, 8, 354. [Google Scholar] [CrossRef] [Green Version]
- Congalton, R.G.; Green, K. Assessing the Accuracy of Remotely Sensed Data: Principles and Practices; Lewis Publishers: Boca Raton, FL, USA, 2008. [Google Scholar]
- Yang, C.; Fernandez, C.J.; Everitt, J.H. Comparison of airborne multispectral and hyperspectral imagery for mapping cotton root rot. Biosyst. Eng. 2010, 107, 131–139. [Google Scholar] [CrossRef]
- Lechner, A.M.; Stein, A.; Jones, S.D.; Ferwerda, J.G. Remote sensing of small and linear features: Quantifying the effects of patch size and length, grid position and detectability on land cover mapping. Remote Sens. Environ. 2009, 113, 2194–2204. [Google Scholar] [CrossRef]
- Congalton, R.G.; Birch, K.; Jones, R.; Schriever, J. Evaluating remotely sensed techniques for mapping riparian vegetation. Comput. Electron. Agric. 2002, 37, 113–126. [Google Scholar] [CrossRef]
- Lausch, A.; Herzog, F. Applicability of landscape metrics for the monitoring of landscape change: Issues of scale, resolution and interpretability. Ecol. Indic. 2002, 2, 3–15. [Google Scholar] [CrossRef]
- Jensen, J.R.; Cowen, D.C. Remote sensing of urban/suburban infrastructure and socio-economic attributes. Photogramm. Eng. Remote Sens. 1999, 65, 611–622. [Google Scholar]
- Lacaux, J.; Tourre, Y.; Vignolles, C.; Ndione, J.; Lafaye, M. Classification of ponds from high-spatial resolution remote sensing: Application to Rift Valley Fever epidemics in Senegal. Remote Sens. Environ. 2007, 106, 66–74. [Google Scholar] [CrossRef]
- Townsend, P.A.; Lookingbill, T.R.; Kingdon, C.C.; Gardner, R.H. Spatial pattern analysis for monitoring protected areas. Remote Sens. Environ. 2009, 113, 1410–1420. [Google Scholar] [CrossRef]
- Radoux, J.; Chomé, G.; Jacques, D.C.; Waldner, F.; Bellemans, N.; Matton, N.; Lamarche, C.; Raphaël, A.; Defourny, P. Sentinel-2’s potential for sub-pixel landscape feature detection. Remote Sens. 2016, 8, 488. [Google Scholar] [CrossRef]
- Joseph, G. How well do we understand Earth observation electro-optical sensor parameters? ISPRS J. Photogramm. Remote Sens. 2000, 55, 9–12. [Google Scholar] [CrossRef]
- Schowengerdt, A.R. Remote Sensing: Models and Methods for Image Processing; Elsevier Inc.: Amsterdam, The Netherlands, 2007; pp. 300–304. [Google Scholar]
- Liang, S.L.; Fang, H.L.; Chen, M.Z. Atmospheric Correction of Landsat ETM+ Land Surface Imagery—Part I: Methods. IEEE Trans. Geosci. Remote Sens. 2001, 39, 2490–2498. [Google Scholar] [CrossRef]
- Kharat, S.A.; Musande, V.B. Cotton crop discrimination using landsat-8 data. IJCSIT. Int. J. Comput. Sci. Inf. Technol. 2015, 6, 4381–4384. [Google Scholar]
- Ustunera, M.; Sanli, F.B.; Abdikan, S.; Esetlili, M.T.; Kurucu, Y. Crop type classification using vegetation indices of RAPIDEYE imagery. ISPRS Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2014, XL-7, 195–198. [Google Scholar] [CrossRef]
- Wu, M.; Yang, C.; Song, X.; Hoffmann, W.C.; Huang, W.; Niu, Z.; Wang, C.; Li, W. Evaluation of orthomosics and digital surface models derived from aerial imagery for crop type mapping. Remote Sens. 2017, 9, 239. [Google Scholar] [CrossRef]

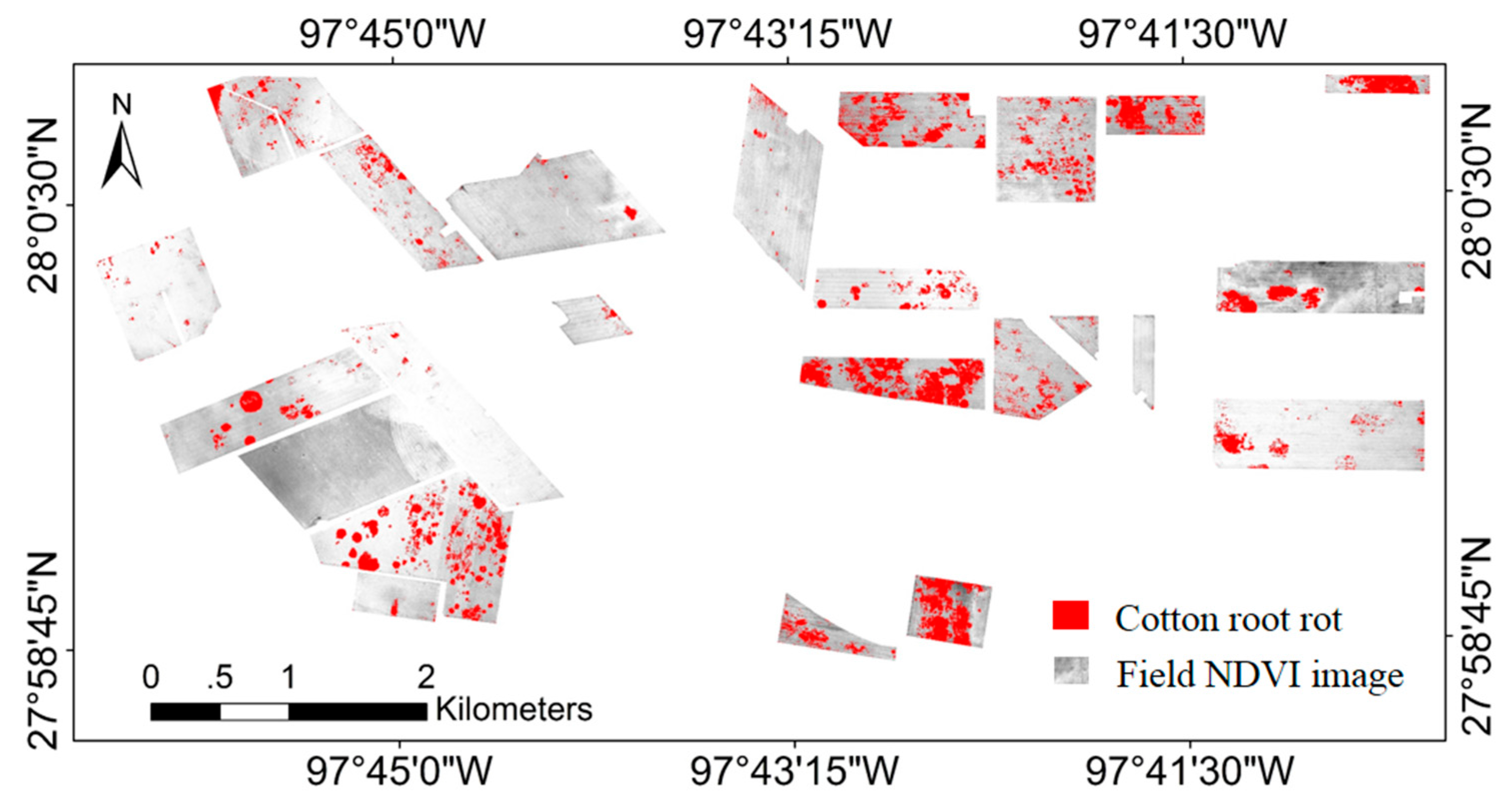
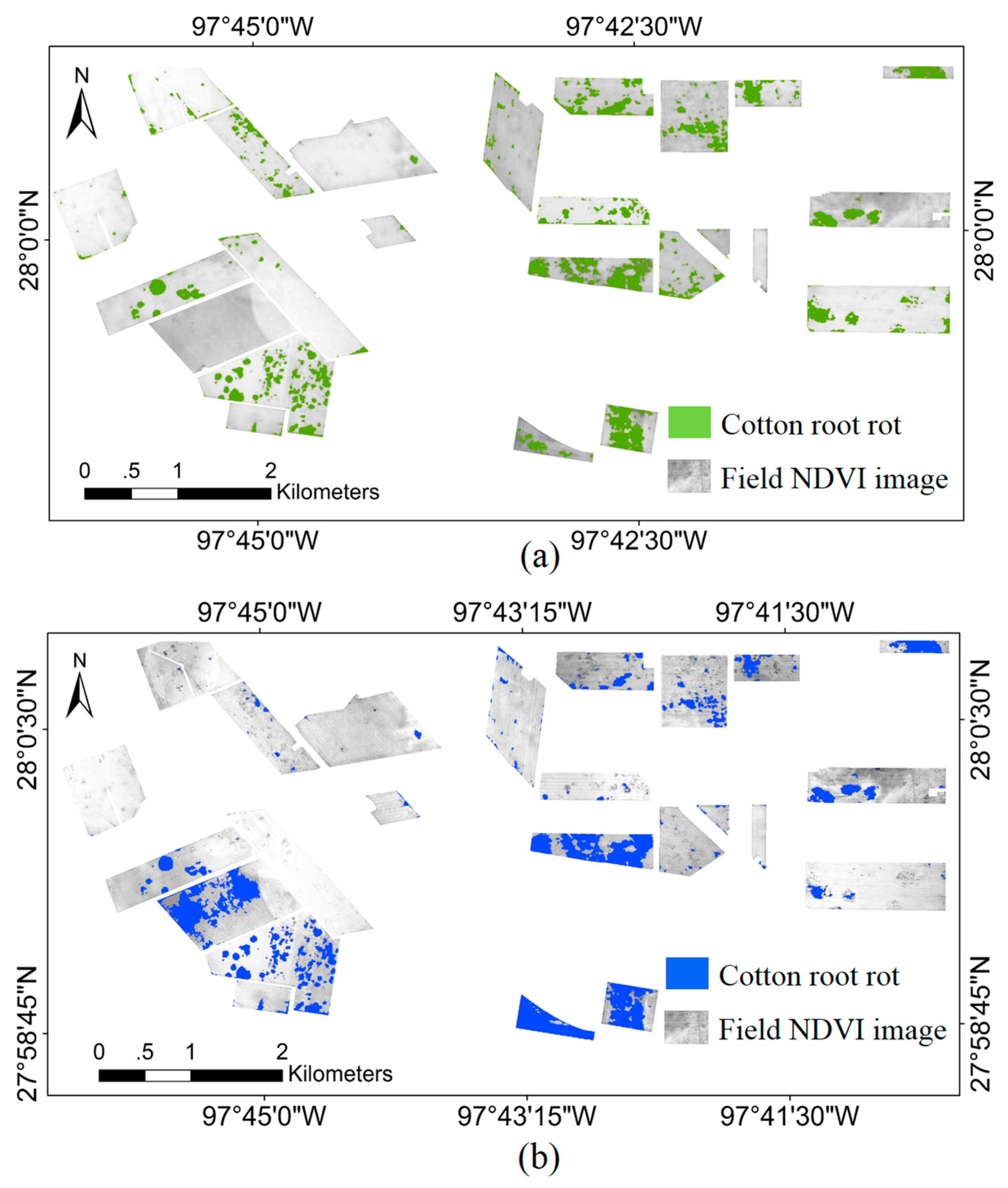
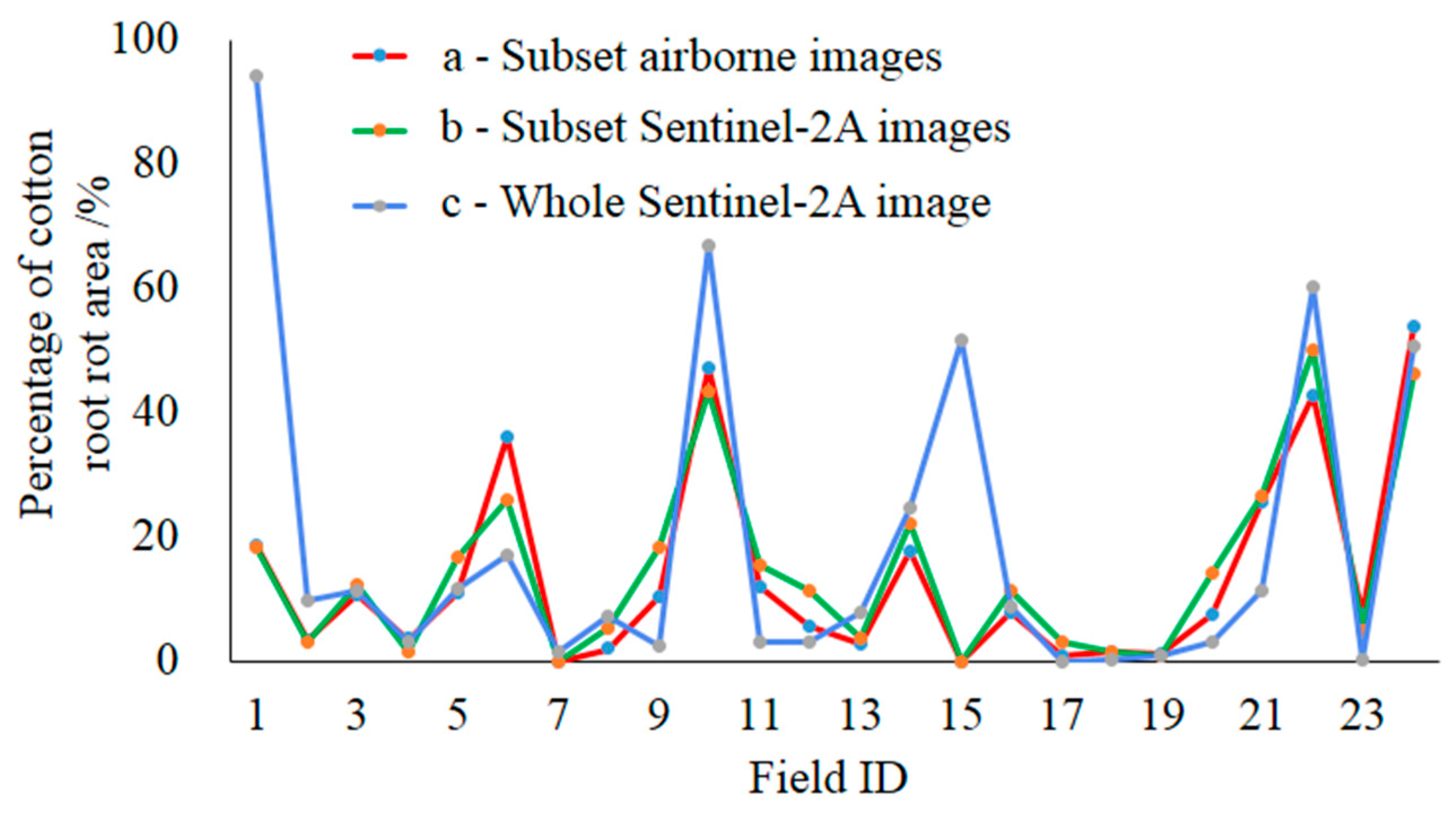
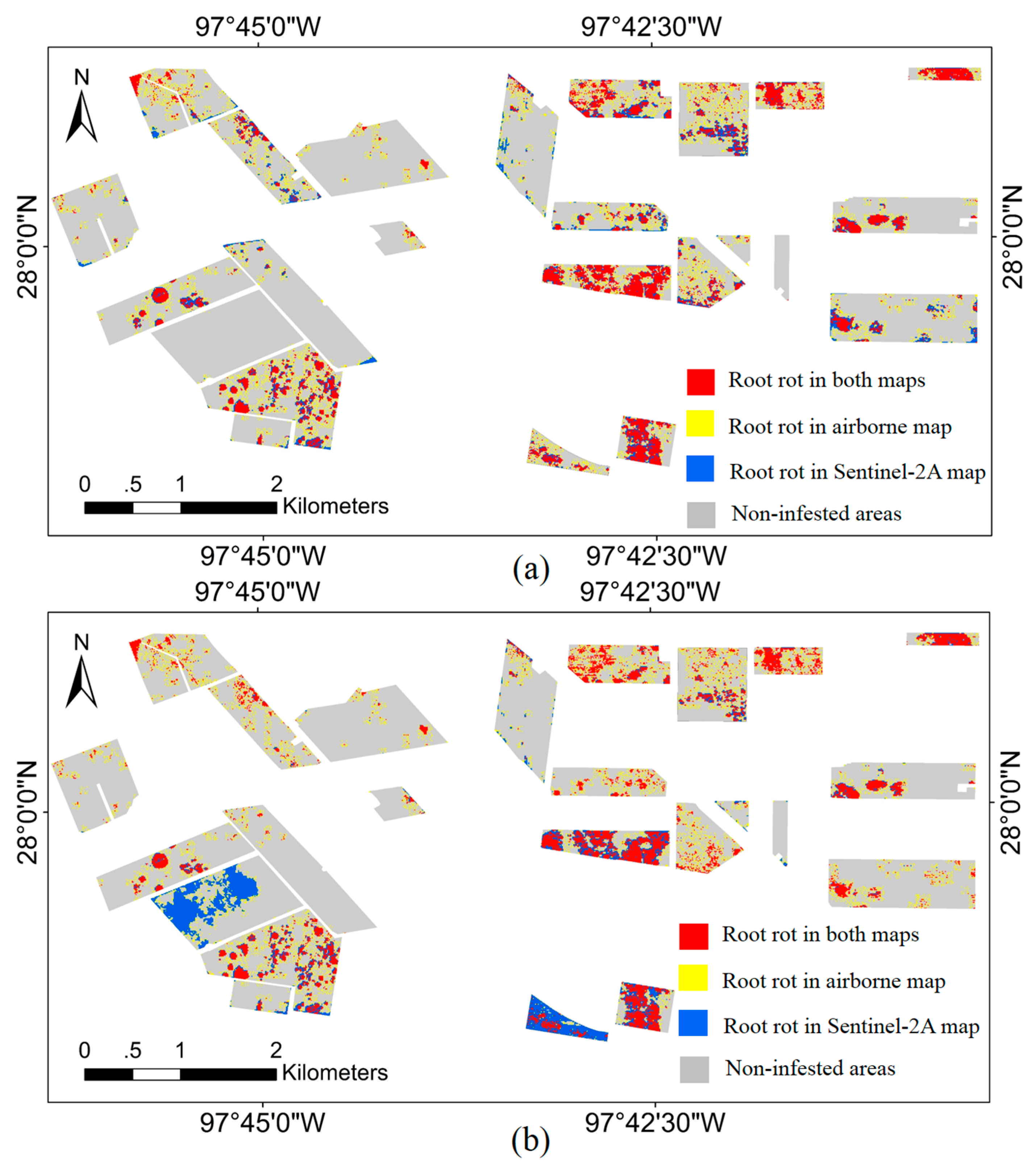
| Field ID | Field Area (ha) | Cotton Root Rot-Infested Area (ha) | Field ID | Field Area (ha) | Cotton Root Rot-Infested Area (ha) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0.81 m | 10 m | 0.81 m | 10 m | 0.81 m | 10 m | 0.81 m | 10 m | ||
| 1 | 16.67 | 16.39 | 3.17 | 3.06 | 13 | 17.07 | 17.01 | 0.46 | 0.47 |
| 2 | 6.12 | 5.98 | 0.25 | 0.2 | 14 | 88.76 | 89.28 | 15.9 | 15.82 |
| 3 | 54.63 | 54.78 | 5.75 | 5.8 | 15 | 81.78 | 82.36 | 0 | 0 |
| 4 | 12.72 | 12.53 | 0.42 | 0.44 | 16 | 59.67 | 60.14 | 4.67 | 4.69 |
| 5 | 55.51 | 55.83 | 6.27 | 6.06 | 17 | 65.05 | 64.78 | 0.51 | 0.51 |
| 6 | 20.3 | 20.38 | 7.6 | 7.37 | 18 | 53.64 | 53.68 | 0.81 | 0.78 |
| 7 | 9.44 | 9.77 | 0 | 0.02 | 19 | 76.37 | 76.36 | 0.91 | 0.96 |
| 8 | 58.99 | 58.79 | 1.09 | 1.13 | 20 | 42.74 | 43.09 | 3.32 | 3.25 |
| 9 | 34.19 | 33.73 | 3.57 | 3.54 | 21 | 38.98 | 39.04 | 9.9 | 10.01 |
| 10 | 38.49 | 38.47 | 18.1 | 18.13 | 22 | 25.2 | 25.01 | 10.6 | 10.7 |
| 11 | 33.94 | 33.82 | 3.95 | 4.08 | 23 | 51.17 | 51.16 | 3.97 | 3.82 |
| 12 | 77.11 | 76.71 | 4.41 | 4.34 | 24 | 9.88 | 10.19 | 5.58 | 5.48 |
| Field ID | Field Area (ha) | Cotton Root Rot-Infected Area (ha) | Field ID | Field Area (ha) | Cotton Root Rot-Infected Area (ha) | ||
|---|---|---|---|---|---|---|---|
| Subset Images | Whole Image | Subset Images | Whole Image | ||||
| 1 | 16.39 | 2.98 | 15.73 | 13 | 17.01 | 0.64 | 1.31 |
| 2 | 5.98 | 0.18 | 0.59 | 14 | 89.28 | 19.84 | 22 |
| 3 | 54.78 | 6.79 | 6.18 | 15 | 82.36 | 0 | 42.22 |
| 4 | 12.53 | 0.2 | 0.4 | 16 | 60.14 | 6.7 | 5.15 |
| 5 | 55.83 | 9.24 | 6.37 | 17 | 64.78 | 1.95 | 0 |
| 6 | 20.38 | 5.26 | 3.46 | 18 | 53.68 | 0.86 | 0.09 |
| 7 | 9.77 | 0 | 0.15 | 19 | 76.36 | 0.52 | 0.52 |
| 8 | 58.79 | 3.12 | 4.24 | 20 | 43.09 | 6.03 | 1.24 |
| 9 | 33.73 | 6.15 | 0.8 | 21 | 39.04 | 10.38 | 4.38 |
| 10 | 38.47 | 16.69 | 25.77 | 22 | 25.01 | 12.56 | 15.19 |
| 11 | 33.82 | 5.18 | 0.98 | 23 | 51.16 | 2.53 | 0.07 |
| 12 | 76.71 | 8.74 | 2.22 | 24 | 10.19 | 4.72 | 5 |
| Classification Category | Actual Category | User’s Accuracy | ||
|---|---|---|---|---|
| Infested (pixels) | Non-Infested (pixels) | Total (pixels) | ||
| Infested (pixels) | 9920 | 3561 | 13,481 | 73.59% |
| Non-infested (pixels) | 1999 | 78,235 | 80,234 | 97.51% |
| Total (pixels) | 11,919 | 81,796 | 93,715 | |
| Producer’s accuracy | 83.23% | 96.65% | ||
| Classified Category | Actual category | User’s Accuracy | ||
|---|---|---|---|---|
| Infested (pixels) | Non-Infested (pixels) | Total (pixels) | ||
| Infested (pixels) | 8810 | 5899 | 14,709 | 59.90% |
| Non-infested (pixels) | 3168 | 85,051 | 88,219 | 96.41% |
| Total (pixels) | 11,978 | 90,950 | 102,928 | |
| Producer’s accuracy | 73.55% | 93.51% | ||
| Zone | Number of Fragments | Minimum Area (m2) | Maximum Area (m2) | Average Area (m2) | Total Area (ha) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Subset Images | Whole Image | Subset Images | Whole Image | Subset Images | Whole Image | Subset Images | Whole Image | ||
| Red | 499 | 337 | 100 | 102,100 | 105,400 | 1698 | 2243 | 84.72 | 75.58 |
| Yellow | 1268 | 1160 | 100 | 10,300 | 15,100 | 214 | 312 | 27.10 | 36.23 |
| Blue | 1028 | 664 | 100 | 15,900 | 259,700 | 500 | 1092 | 51.35 | 72.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Yang, C.; Wu, M.; Zhao, C.; Yang, G.; Hoffmann, W.C.; Huang, W. Evaluation of Sentinel-2A Satellite Imagery for Mapping Cotton Root Rot. Remote Sens. 2017, 9, 906. https://doi.org/10.3390/rs9090906
Song X, Yang C, Wu M, Zhao C, Yang G, Hoffmann WC, Huang W. Evaluation of Sentinel-2A Satellite Imagery for Mapping Cotton Root Rot. Remote Sensing. 2017; 9(9):906. https://doi.org/10.3390/rs9090906
Chicago/Turabian StyleSong, Xiaoyu, Chenghai Yang, Mingquan Wu, Chunjiang Zhao, Guijun Yang, Wesley Clint Hoffmann, and Wenjiang Huang. 2017. "Evaluation of Sentinel-2A Satellite Imagery for Mapping Cotton Root Rot" Remote Sensing 9, no. 9: 906. https://doi.org/10.3390/rs9090906
APA StyleSong, X., Yang, C., Wu, M., Zhao, C., Yang, G., Hoffmann, W. C., & Huang, W. (2017). Evaluation of Sentinel-2A Satellite Imagery for Mapping Cotton Root Rot. Remote Sensing, 9(9), 906. https://doi.org/10.3390/rs9090906