Beyond Range: Innovating Fluorescence Microscopy
Abstract
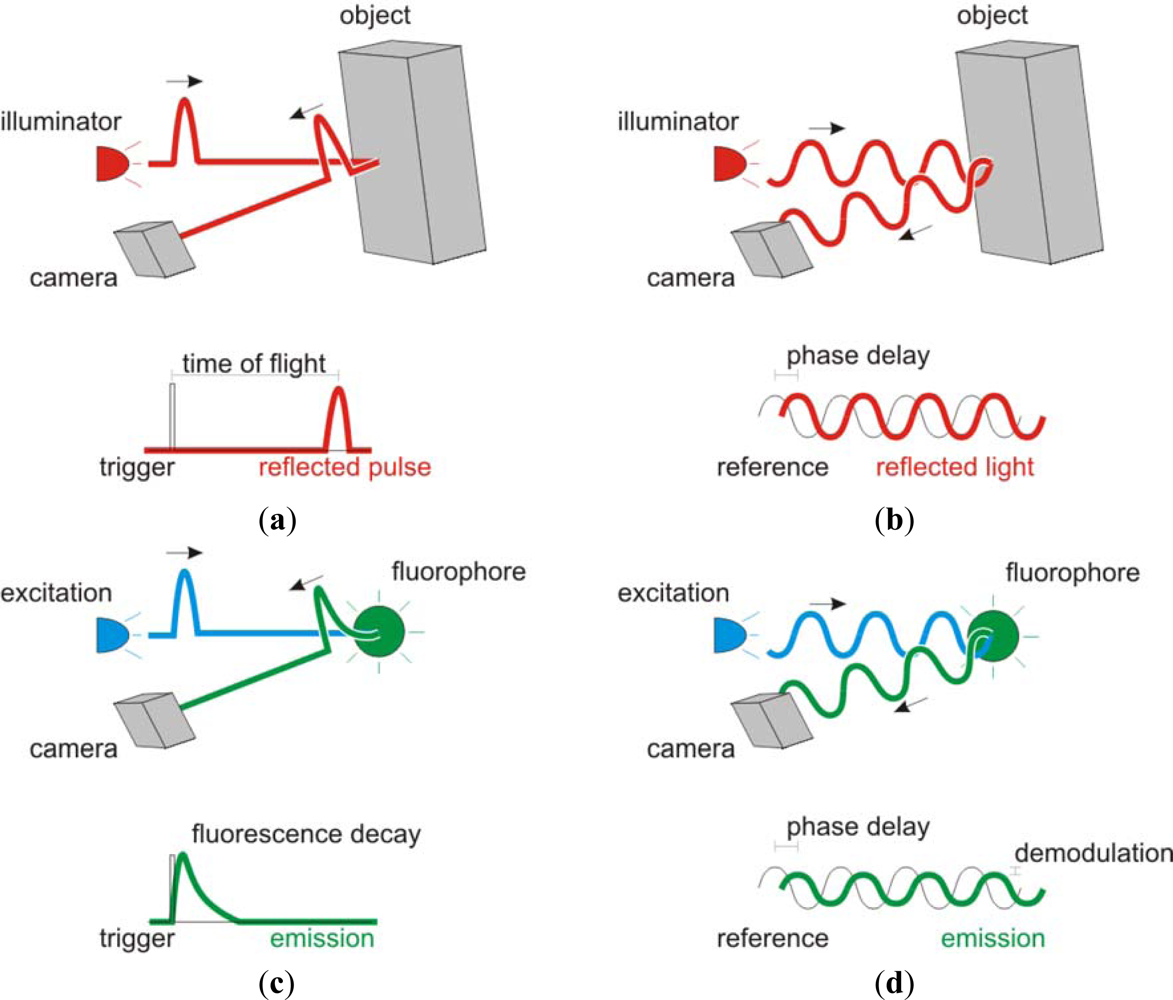
:1. Converging Communities
2. Beyond Range
3. A Vision for the Future
Acknowledgments
References
- Esposito, A.; Dohm, C.P.; Bahr, M.; Wouters, F.S. Unsupervised fluorescence lifetime imaging microscopy for high-content and high-throughput screening. Mol. Cell. Proteomics 2007, 6, 1446–1454. [Google Scholar]
- Nishikata, K.; Kimura, Y.; Takai, Y.; Ikuta, T.; Shimizu, R. Real-time lock-in imaging by a newly developed high-speed image-processing charged coupled device video camera. Rev. Sci. Instrum 2003, 74, 1393–1396. [Google Scholar]
- Mitchell, A.C.; Wall, J.E.; Murray, J.G.; Morgan, C.G. Measurement of nanosecond time-resolved fluorescence with a directly gated interline CCD camera. J. Microsc 2002, 206, 233–238. [Google Scholar]
- Mitchell, A.C.; Wall, J.E.; Murray, J.G.; Morgan, C.G. Direct modulation of the effective sensitivity of a CCD detector: A new approach to time-resolved fluorescence imaging. J. Microsc 2002, 206, 225–232. [Google Scholar]
- Kollner, M.; Wolfrum, J. How many photons are necessary for fluorescence-lifetime measurements. Chem. Phys. Lett 1992, 200, 199–204. [Google Scholar]
- Esposito, A.; Gerritsen, H.C.; Wouters, F.S. Optimizing frequency-domain fluorescence lifetime sensing for high-throughput applications: Photon economy and acquisition speed. J. Opt. Soc. Am. A 2007, 24, 3261–3273. [Google Scholar]
- Esposito, A.; Oggier, T.; Gerritsen, H.C.; Lustenberger, F.; Wouters, F.S. All-solid-state lock-in imaging for wide-field fluorescence lifetime sensing. Opt. Express 2005, 13, 9812–9821. [Google Scholar]
- Esposito, A.; Gerritsen, H.C.; Lustenberger, F.; Oggier, T.; Wouters, F.S. Innovating lifetime microscopy: A compact and simple tool for the life sciences, screening and diagnostics. J. Biomed. Opt 2006, 11, 034016. [Google Scholar]
- Mosconi, D.; Stoppa, D.; Pancheri, L.; Gonzo, L.; Simoni, A. CMOS Single-Photon Avalanche Diode Array for Time-Resolved Fluorescence Detection. Proceedings of the 32nd European Solid-State Circuits Conference, Montreux, Switzerland, 19–21 September 2006; pp. 564–567.
- Stoppa, D.; Pancheri, L.; Scandiuzzo, M.; Gonzo, L.; Dalla Betta, G.F.; Simoni, A. A CMOS 3-D imager based on single photon avalanche diode. IEEE Trans. Circuit. Syst. I 2007, 54, 4–12. [Google Scholar]
- Pancheri, L.; Stoppa, D. A SPAD-Based Pixel Linear Array for High-Speed Time-Gated Fluorescence Lifetime Imaging. Proceedings of ESSCIRC 2009, Athens, Greece, 14–18 September 2009; pp. 428–431.
- Jian, G.; Sonkusale, S. A CMOS Imager with Digital Phase Readout for Fluorescence Lifetime Imaging. Proceedings of the ESSCIRC 2011, Helsinki, Finland, 12–16 September 2011; pp. 115–118.
- Yoon, H.J.; Itoh, S.; Kawahito, S. A CMOS image sensor with in-pixel two-stage charge transfer for fluorescence lifetime imaging. IEEE Trans. Electron Dev 2009, 56, 214–221. [Google Scholar]
- Veerappan, C.; Richardson, J.; Walker, R.; Day-Uey, L.; Fishburn, M.W.; Maruyama, Y.; Stoppa, D.; Borghetti, F.; Gersbach, M.; Henderson, R.K.; et al. A 160 × 128 Single-Photon Image Sensor with On-Pixel 55 ps 10 b Time-To-Digital Converter. Proceedings of 2011 IEEE International Solid-State Circuits Conference Digest of Technical Papers (ISSCC), San Francisco, CA, USA, 20–24 February 2011; pp. 312–314.
- Li, D.-U.; Arlt, J.; Richardson, J.; Walker, R.; Buts, A.; Stoppa, D.; Charbon, E.; Henderson, R. Real-time fluorescence lifetime imaging system with a 32 × 32 0.13 m CMOS low dark-count single-photon avalanche diode array. Opt. Express 2010, 18, 10257–10269. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999. [Google Scholar]
- Buurman, E.P.; Sanders, R.; Draaijer, A.; Gerritsen, H.C.; Vanveen, J.J.F.; Houpt, P.M.; Levine, Y.K. Fluorescence lifetime imaging using a confocal laser scanning microscope. Scanning 1992, 14, 155–159. [Google Scholar]
- Lakowicz, J.R.; Balter, A. Theory of phase-modulation fluorescence spectroscopy for excited-state processes. Biophys. Chem 1982, 16, 99–115. [Google Scholar]
- Clegg, R.M.; Schneider, P.C.; Slavik, J. Fluorescence lifetime-resolved imaging microscopy: A general description of lifetime-resolved imaging measurements. In Fluorescence Microscopy and Fluorescence Probes; Plenum Press: New York, NY, USA, 1996; pp. 15–33. [Google Scholar]
- Li, D.-U.; Bonnist, E.; Renshaw, D.; Henderson, R. On-chip, time-correlated, fluorescence lifetime extraction algorithms and error analysis. J. Opt. Soc. Am. A 2008, 25, 1190–1198. [Google Scholar]
- Lange, R.; Seitz, P.; Biber, A.; Schwarte, R. Time-of-flight range imaging with a custom solid state image sensor. Proc. SPIE 1999, 3823, 180–191. [Google Scholar]
- Viarani, L.; Stoppa, D.; Gonzo, L.; Gottardi, M.; Simoni, A. A CMOS smart pixel for active 3-D vision applications. IEEE Sens. J 2004, 4, 145–152. [Google Scholar]
- Oggier, T.; Lehmann, M.; Kaufmann, R.; Schweizer, M.; Richter, M.; Metzler, P.; Lang, G.; Lustenberger, F.; Blanc, N. An all-solid-state optical range camera for 3D real-time imaging with sub-centimeter depth resolution (SwissRanger). Proc. SPIE 2004, 5249, 534–545. [Google Scholar]
- Schwartz, D.E.; Charbon, E.; Shepard, K.L. A Single-Photon Avalanche Diode Imager for Fluorescence Lifetime Applications. Proceedings of 2007 Symposium on VLSI Circuits, 14–16 June 2007; pp. 144–145.
- Rae, B.R.; Griffin, C.; McKendry, J.; Girkin, J.M.; Zhang, H.X.; Gu, E.; Renshaw, D.; Charbon, E.; Dawson, M.D.; Henderson, R.K. CMOS driven micro-pixel LEDs integrated with single photon avalanche diodes for time resolved fluorescence measurements. J. Phys. D 2008, 41, 094011. [Google Scholar]
- Gerritsen, H.C.; Agronskaia, A.V.; Bader, A.N.; Esposito, A. Time domain FLIM: Theory, instrumentation and data analysis. In FRET & FLIM Imaging Techniques; Gadella, T.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Förster, T.; Sinanoglu, O. Delocalized excitation and excitation transfer; in modern quantum chemistry. In Modern Quantum Chemistry; Academic Press: New York, NY, USA, 1965; pp. 93–137. [Google Scholar]
- Jares-Erijman, E.A.; Jovin, T.M. FRET imaging. Nature Biotech 2003, 21, 1387–1395. [Google Scholar]
- Esposito, A.; Gerritsen, H.C.; Wouters, F.S. Fluorescence lifetime heterogeneity resolution in the frequency-domain by Lifetime Moments Analysis (LiMA). Biophys. J 2005, 89, 4286–4299. [Google Scholar]
- Hanley, Q.S.; Clayton, A.H. AB-plot assisted determination of fluorophore mixtures in a fluorescence lifetime microscope using spectra or quenchers. J. Microsc 2005, 218, 62–67. [Google Scholar]
- Digman, M.; Caiolfa, V.R.; Zamai, M.; Gratton, E. The Phasor approach to fluorescence lifetime imaging analysis. Biophys. J 2007, 94, L14–16. [Google Scholar]
- Wouters, F.S.; Esposito, A. Quantitative analysis of fluorescence lifetime imaging made easy. HFSP J 2008, 2, 7. [Google Scholar]
- Elson, D.S.; Munro, I.; Requejo-Isidro, J.; McGinty, J.; Dunsby, C.; Galletly, N.; Stamp, G.W.; Neil, M.A.A.; Lever, M.J.; Kellett, P.A.; et al. Real-time time-domain fluorescence lifetime imaging including single-shot acquisition with a segmented optical image intensifier. New J. Phys 2004, 6, 180–193. [Google Scholar]
- Agronskaia, A.V.; Tertoolen, L.; Gerritsen, H.C. High frame rate fluorescence lifetime imaging. J. Phys. D 2003, 36, 1655–1662. [Google Scholar]
- Gerritsen, H.C.; Asselbergs, M.A.; Agronskaia, A.V.; Van Sark, W.G. Fluorescence lifetime imaging in scanning microscopes: acquisition speed, photon economy and lifetime resolution. J. Microsc 2002, 206, 218–224. [Google Scholar]
- Buttgen, B.; Seitz, P. Robust optical time-of-flight range imaging based on smart pixel structures. IEEE Trans. Circuit. Syst. I 2008, 55, 1512–1525. [Google Scholar]
- Garnett, M.J.; Mansfeld, J.; Godwin, C.; Matsusaka, T.; Wu, J.; Russell, P.; Pines, J.; Venkitaraman, A.R. UBE2S elongates ubiquitin chains on APC/C substrates to promote mitotic exit. Nat. Cell Biol 2009, 11, 1363–1369. [Google Scholar]
- Kola, I.; Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov 2004, 3, 711–716. [Google Scholar]
- Tadrous, P.J.; Siegel, J.; French, P.M.; Shousha, S.; Lalani el, N.; Stamp, G.W. Fluorescence lifetime imaging of unstained tissues: early results in human breast cancer. J. Pathol 2003, 199, 309–317. [Google Scholar]
- Lee, K.C.B.; Siegel, J.; Webb, S.E.D.; Leveque-Fort, S.; Cole, M.J.; Jones, R.; Dowling, K.; Lever, M.J.; French, P.M.W. Application of the stretched exponential function to fluorescence lifetime imaging. Biophys. J 2001, 81, 1265–1274. [Google Scholar]
- Esposito, A.; Bader, A.N.; Schlachter, S.C.; van den Heuvel, D.J.; Schierle, G.S.; Venkitaraman, A.R.; Kaminski, C.F.; Gerritsen, H.C. Design and application of a confocal microscope for spectrally resolved anisotropy imaging. Opt. Express 2011, 19, 2546–2555. [Google Scholar]
Share and Cite
Esposito, A. Beyond Range: Innovating Fluorescence Microscopy. Remote Sens. 2012, 4, 111-119. https://doi.org/10.3390/rs4010111
Esposito A. Beyond Range: Innovating Fluorescence Microscopy. Remote Sensing. 2012; 4(1):111-119. https://doi.org/10.3390/rs4010111
Chicago/Turabian StyleEsposito, Alessandro. 2012. "Beyond Range: Innovating Fluorescence Microscopy" Remote Sensing 4, no. 1: 111-119. https://doi.org/10.3390/rs4010111
APA StyleEsposito, A. (2012). Beyond Range: Innovating Fluorescence Microscopy. Remote Sensing, 4(1), 111-119. https://doi.org/10.3390/rs4010111



