Assessment of Habitat Suitability for Oedaleus decorus asiaticus Using MaxEnt and Frequency Ratio Model in Xilingol League, China
Abstract
1. Introduction
2. Materials and Methods
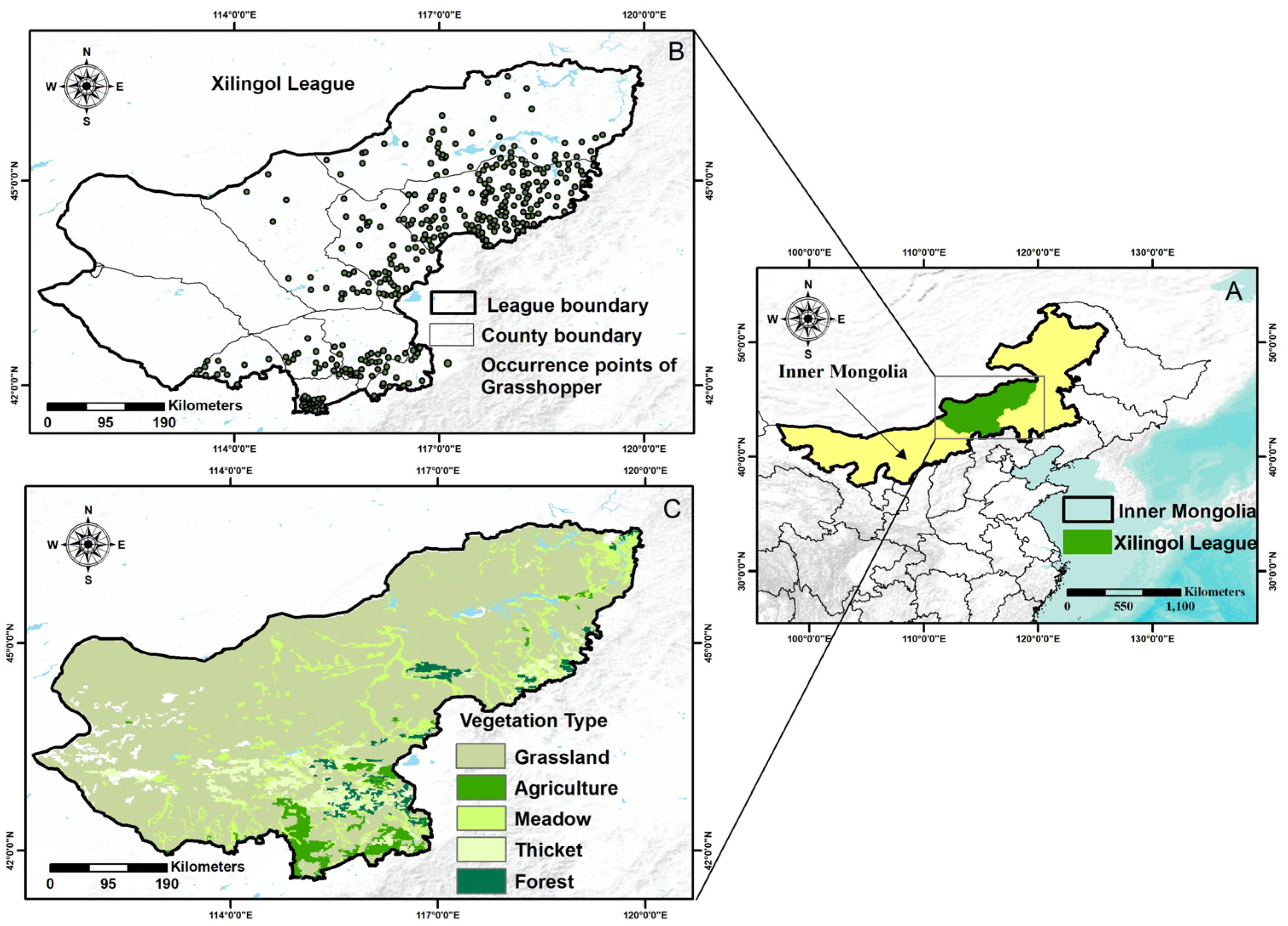
2.1. Study Area
2.2. Data Acquisition and Processing
2.2.1. Satellite Data
2.2.2. Meteorological Data
2.2.3. Soil and Other Geospatial Data
2.2.4. Field Survey Data
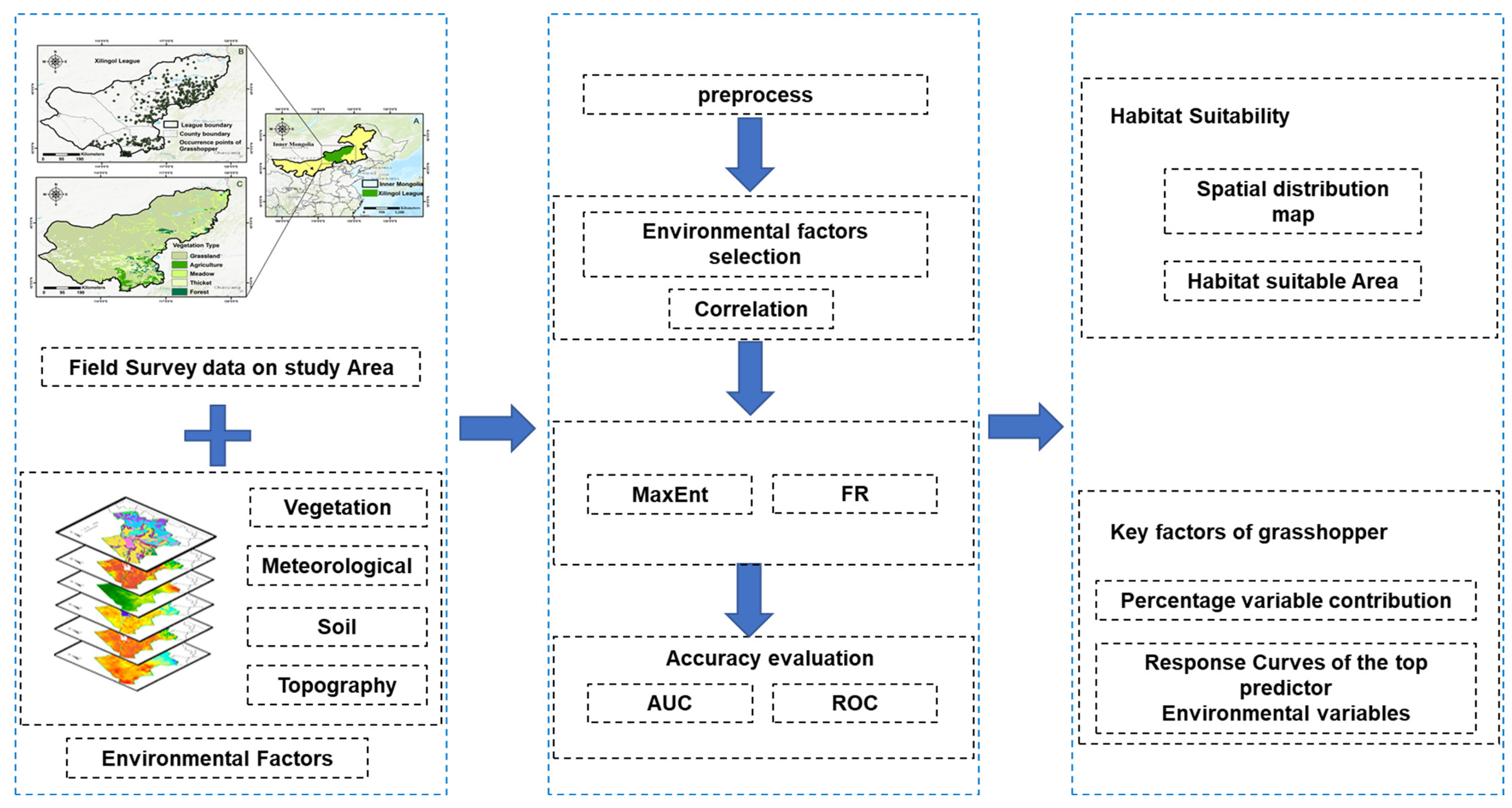
2.3. Data Analysis
2.3.1. Determination of Grasshopper Development Stage
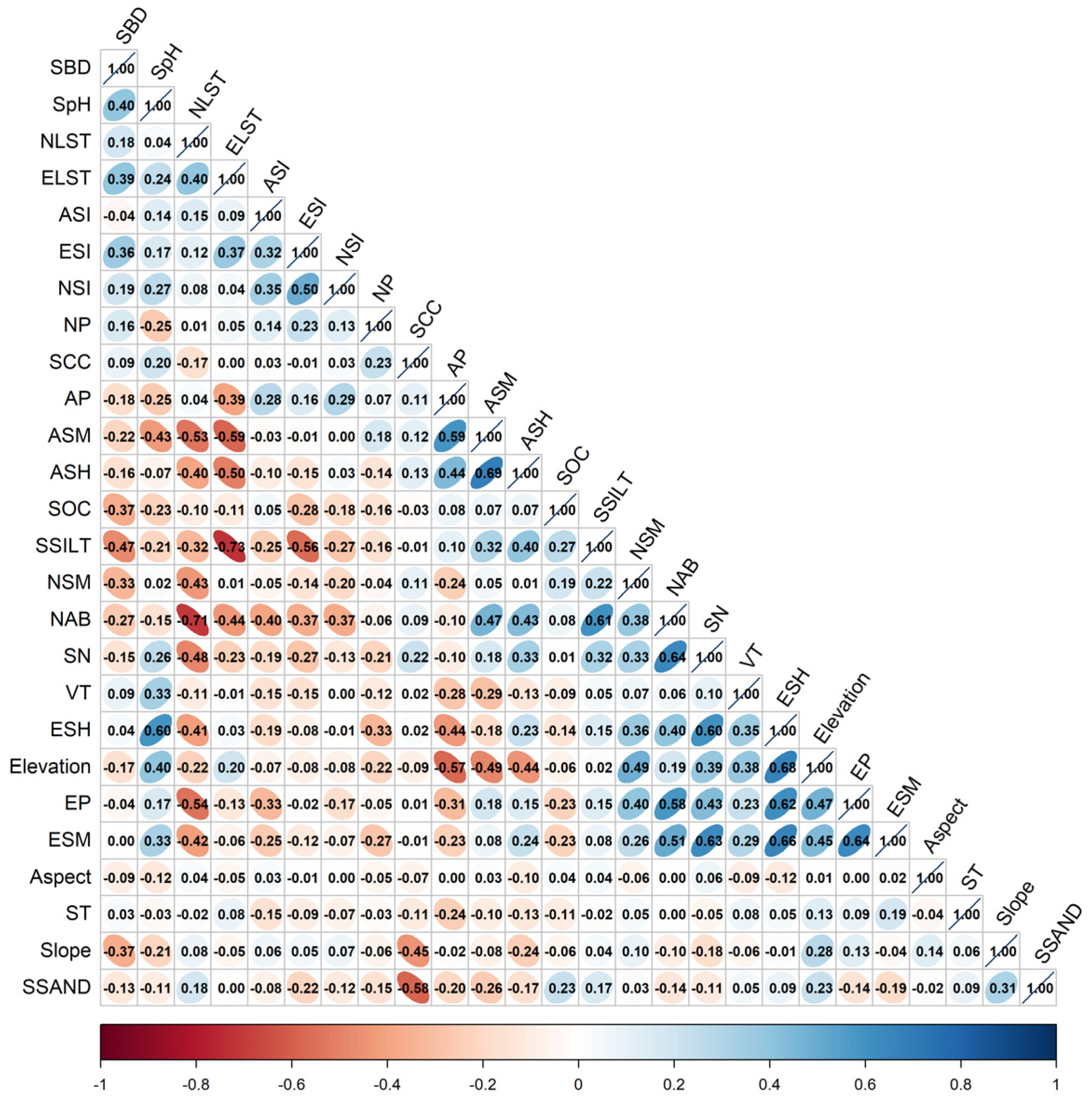
2.3.2. Selection of Environmental Factors
2.3.3. Extraction Method of Habitat Suitability
- MaxEnt approach
- 2.
- Frequency ratio (FR) approach
3. Results
3.1. Habitat Suitability Results Using the MaxEnt Approach
3.2. Habitat Suitability Results Using the FR Approach
3.3. Accuracy Evaluation
3.4. Contribution of Habitat Factors Affecting O. d. asiaticus Distribution
3.5. The Influence of Principal Contributing Factors on Grasshopper Presence in Xilingol League
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASI | Soil salinity index in the adult stage |
| SBD | Soil bulk density |
| ESI | Soil salinity index in the egg stage |
| ELST | Mean land surface temperature in the egg stage |
| NSI | Soil salinity index in the nymph stage |
| SSILT | Soil silt content |
| SCC | Soil clay content |
| SpH | Soil pH |
| NLST | Mean land surface temperature in the nymph stage |
| NAB | Above-ground biomass in the nymph stage |
| SOC | Soil organic carbon |
| SN | Soil nitrogen content |
| ST | Soil type |
| VT | Vegetation type |
| SSAND | Soil sand content |
| EP | Mean precipitation in the egg stage |
| ASM | Soil moisture in the adult stage |
| NSM | Soil moisture in the nymph stage |
| AP | Mean precipitation in the adult stage |
| ESM | Soil moisture in the egg stage |
| NP | Mean precipitation in the nymph stage |
| ASH | Mean specific humidity in the adult stage |
| ESH | Mean specific humidity in the egg stage |
| GIS | Geographic information system |
References
- Sun, Z.; Ye, H.; Huang, W.; Qimuge, E.; Bai, H.; Nie, C.; Lu, L.; Qian, B.; Wu, B. Assessment on Potential Suitable Habitats of the Grasshopper Oedaleus decorus asiaticus in North China Based on MaxEnt Modeling and Remote Sensing Data. Insects 2023, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.M.; Jonas, J.; Laws, A.N.; Branson, D.H.; Pennings, S.C.; Prather, C.M.; Strickland, M.S. Functional and Taxonomic Diversity of Grasshoppers Differentially Shape Above- and Below-ground Communities and Their Function. Funct. Ecol. 2021, 35, 167–180. [Google Scholar] [CrossRef]
- Olfert, O.; Weiss, R.M.; Giffen, D.; Vankosky, M.A. Modeling Ecological Dynamics of a Major Agricultural Pest Insect (Melanoplus sanguinipes; Orthoptera: Acrididae): A Cohort-Based Approach Incorporating the Effects of Weather on Grasshopper Development and Abundance. J. Econ. Entomol. 2021, 114, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, N.; Gexigeduren; He, B.; Liu, C.-Y.; Li, Y.; Zhang, H.-Y.; Chen, X.-Y.; Lin, H. Construction of a GeogDetector-Based Model System to Indicate the Potential Occurrence of Grasshoppers in Inner Mongolia Steppe Habitats. Bull. Entomol. Res. 2015, 105, 335–346. [Google Scholar] [CrossRef]
- Cease, A.J.; Elser, J.J.; Ford, C.F.; Hao, S.; Kang, L.; Harrison, J.F. Heavy Livestock Grazing Promotes Locust Outbreaks by Lowering Plant Nitrogen Content. Science 2012, 335, 467–469. [Google Scholar] [CrossRef]
- Du, B.; Ding, X.; Ji, C.; Lin, K.; Guo, J.; Lu, L.; Dong, Y.; Huang, W.; Wang, N. Estimating Leymus chinensis Loss Caused by Oedaleus decorus asiaticus Using an Unmanned Aerial Vehicle (UAV). Remote Sens. 2023, 15, 4352. [Google Scholar] [CrossRef]
- Kang, L.; Chen, Y. Dynamics of Grasshopper Communities Under Different Grazing Intensities in Inner Mongolian Steppes. Insect Sci. 1995, 2, 265–281. [Google Scholar] [CrossRef]
- Wen, F.; Lu, L.; Nie, C.; Sun, Z.; Liu, R.; Huang, W.; Ye, H. Analysis of Spatiotemporal Variation in Habitat Suitability for Oedaleus Decorus Asiaticus Bei-Bienko on the Mongolian Plateau Using Maxent and Multi-Source Remote Sensing Data. Insects 2023, 14, 492. [Google Scholar] [CrossRef]
- Zhang, L.; Hunter, D. Management of Locusts and Grasshoppers in China. JOR 2017, 26, 155–159. [Google Scholar] [CrossRef]
- Brunelle, T.; Chakir, R.; Carpentier, A.; Dorin, B.; Goll, D.; Guilpart, N.; Maggi, F.; Makowski, D.; Nesme, T.; Roosen, J.; et al. Reducing Chemical Inputs in Agriculture Requires a System Change. Commun. Earth Environ. 2024, 5, 369. [Google Scholar] [CrossRef]
- Guo, J.; Lu, L.; Dong, Y.; Huang, W.; Zhang, B.; Du, B.; Ding, C.; Ye, H.; Wang, K.; Huang, Y.; et al. Spatiotemporal Distribution and Main Influencing Factors of Grasshopper Potential Habitats in Two Steppe Types of Inner Mongolia, China. Remote Sens. 2023, 15, 866. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, Y.; Huang, W.; Guo, J.; Wang, N.; Ding, X. Extraction and Analysis of Grasshopper Potential Habitat in Hulunbuir Based on the Maximum Entropy Model. Remote Sens. 2024, 16, 746. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, F.; Liu, L.; Du, X.; Ren, B.; Guo, A.; Geng, Y.; Ruan, C.; Ye, H.; Huang, W.; et al. Automatic System for Crop Pest and Disease Dynamic Monitoring and Early Forecasting. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 4410–4418. [Google Scholar] [CrossRef]
- Sivanpillai, R.; Latchininsky, A.V. Special Section Guest Editorial: Advances in Remote Sensing Applications for Locust Habitat Monitoring and Management. J. Appl. Remote Sens. 2015, 8, 084801. [Google Scholar] [CrossRef]
- Zhang, F.; Geng, M.; Wu, Q.; Liang, Y. Study on the Spatial-Temporal Variation in Evapotranspiration in China from 1948 to 2018. Sci. Rep. 2020, 10, 17139. [Google Scholar] [CrossRef]
- Huang, K.H.J.; Huang, K.H.J.; Huang, K.H.J. Remote Sensing of Locust and Grasshopper Plague in China: A Review. In Proceedings of the 2016 Fifth International Conference on Agro-Geoinformatics (Agro-Geoinformatics), Tianjin, China, 18–20 July 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1–6. [Google Scholar]
- Guo, J.; Huang, W.; Dong, Y.; Lin, K.; Zhou, Y.; Wang, N.; Hua, R.; Hao, Z.; Ding, X.; Zhao, F. Spatiotemporal Monitoring of Grasshopper Habitats Using Multi-Source Data: Combined with Landscape and Spatial Heterogeneity. Int. J. Appl. Earth Obs. Geoinf. 2024, 130, 103838. [Google Scholar] [CrossRef]
- Waldner, F.; Ebbe, M.; Cressman, K.; Defourny, P. Operational Monitoring of the Desert Locust Habitat with Earth Observation: An Assessment. ISPRS Int. J. Geo-Inf. 2015, 4, 2379–2400. [Google Scholar] [CrossRef]
- Wang, B.; Deveson, E.D.; Waters, C.; Spessa, A.; Lawton, D.; Feng, P.; Liu, D.L. Future Climate Change Likely to Reduce the Australian Plague Locust (Chortoicetes terminifera) Seasonal Outbreaks. Sci. Total Environ. 2019, 668, 947–957. [Google Scholar] [CrossRef]
- Clissold, F.J.; Simpson, S.J. Temperature, Food Quality and Life History Traits of Herbivorous Insects. Curr. Opin. Insect Sci. 2015, 11, 63–70. [Google Scholar] [CrossRef]
- Poniatowski, D.; Beckmann, C.; Löffler, F.; Münsch, T.; Helbing, F.; Samways, M.J.; Fartmann, T. Relative Impacts of Land-use and Climate Change on Grasshopper Range Shifts Have Changed over Time. Glob. Ecol. Biogeogr. 2020, 29, 2190–2202. [Google Scholar] [CrossRef]
- Prinster, A.J.; Resasco, J.; Nufio, C.R. Weather Variation Affects the Dispersal of Grasshoppers beyond Their Elevational Ranges. Ecol. Evol. 2020, 10, 14411–14422. [Google Scholar] [CrossRef] [PubMed]
- Renier, C.; Waldner, F.; Jacques, D.; Babah Ebbe, M.; Cressman, K.; Defourny, P. A Dynamic Vegetation Senescence Indicator for Near-Real-Time Desert Locust Habitat Monitoring with MODIS. Remote Sens. 2015, 7, 7545–7570. [Google Scholar] [CrossRef]
- Stige, L.C.; Chan, K.-S.; Zhang, Z.; Frank, D.; Stenseth, N.C. Thousand-Year-Long Chinese Time Series Reveals Climatic Forcing of Decadal Locust Dynamics. Proc. Natl. Acad. Sci. USA 2007, 104, 16188–16193. [Google Scholar] [CrossRef] [PubMed]
- Wysiecki, M.L.D.; Arturi, M.; Torrusio, S.; Cigliano, M.M. Influence of Weather Variables and Plant Communities on Grasshopper Density in the Southern Pampas, Argentina. J. Insect Sci. 2011, 11, 109. [Google Scholar] [CrossRef]
- Humbert, J.-Y.; Delley, S.; Arlettaz, R. Grassland Intensification Dramatically Impacts Grasshoppers: Experimental Evidence for Direct and Indirect Effects of Fertilisation and Irrigation. Agric. Ecosyst. Environ. 2021, 314, 107412. [Google Scholar] [CrossRef]
- Propastin, P. Satellite-Based Monitoring System for Assessment of Vegetation Vulnerability to Locust Hazard in the River Ili Delta (Lake Balkhash, Kazakhstan). J. Appl. Remote Sens. 2013, 7, 075094. [Google Scholar] [CrossRef]
- Tian, H.; Stige, L.C.; Cazelles, B.; Kausrud, K.L.; Svarverud, R.; Stenseth, N.C.; Zhang, Z. Reconstruction of a 1,910-y-Long Locust Series Reveals Consistent Associations with Climate Fluctuations in China. Proc. Natl. Acad. Sci. USA 2011, 108, 14521–14526. [Google Scholar] [CrossRef]
- Veran, S.; Simpson, S.J.; Sword, G.A.; Deveson, E.; Piry, S.; Hines, J.E.; Berthier, K. Modeling Spatiotemporal Dynamics of Outbreaking Species: Influence of Environment and Migration in a Locust. Ecology 2015, 96, 737–748. [Google Scholar] [CrossRef]
- Leonard, A.; Egonyu, J.P.; Tanga, C.M.; Kyamanywa, S.; Tonnang, H.Z.E.; Azrag, A.G.A.; Khamis, F.M.; Ekesi, S.; Subramanian, S. Predicting the Current and Future Distribution of the Edible Long-Horned Grasshopper Ruspolia differens (Serville) Using Temperature-Dependent Phenology Models. J. Therm. Biol. 2021, 95, 102786. [Google Scholar] [CrossRef]
- Leins, J.A.; Banitz, T.; Grimm, V.; Drechsler, M. High-Resolution PVA along Large Environmental Gradients to Model the Combined Effects of Climate Change and Land Use Timing: Lessons from the Large Marsh Grasshopper. Ecol. Model. 2021, 440, 109355. [Google Scholar] [CrossRef]
- Fartmann, T.; Poniatowski, D.; Holtmann, L. Habitat Availability and Climate Warming Drive Changes in the Distribution of Grassland Grasshoppers. Agric. Ecosyst. Environ. 2021, 320, 107565. [Google Scholar] [CrossRef]
- Branson, D.H. Effects of Altered Seasonality of Precipitation on Grass Production and Grasshopper Performance in a Northern Mixed Prairie. Environ. Entomol. 2017, 46, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.B.; Graham, S.I.; Nufio, C.R. Grasshopper Species’ Seasonal Timing Underlies Shifts in Phenological Overlap in Response to Climate Gradients, Variability and Change. J. Anim. Ecol. 2021, 90, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Kistner-Thomas, E.; Kumar, S.; Jech, L.; Woller, D.A. Modeling Rangeland Grasshopper (Orthoptera: Acrididae) Population Density Using a Landscape-Level Predictive Mapping Approach. J. Econ. Entomol. 2021, 114, 1557–1567. [Google Scholar] [CrossRef]
- Li, L.; Zhao, C.; Zhao, X.; Wang, D.; Li, Y. Pattern of Plant Communities’ Influence to Grasshopper Abundance Distribution in Heterogeneous Landscapes at the Upper Reaches of Heihe River, Qilian Mountains, China. Environ. Sci. Pollut. Res. 2022, 29, 13177–13187. [Google Scholar] [CrossRef]
- Yadav, S.; Stow, A.; Dudaniec, R.Y. Elevational Partitioning in Species Distribution, Abundance and Body Size of Australian Alpine Grasshoppers (Kosciuscola). Austral Ecol. 2020, 45, 609–620. [Google Scholar] [CrossRef]
- Burt, P.J.A.; Colvin, J.; Smith, S.M. Remote Sensing of Rainfall by Satellite as an Aid to Oedaleus senegalensis (Orthoptera: Acrididae) Control in the Sahel. Bull. Entomol. Res. 1995, 85, 455–462. [Google Scholar] [CrossRef]
- Ni, S.-X.; Wang, J.-C.; Jiang, J.-J.; Zha, Y. Rangeland Grasshoppers in Relation to Soils in the Qinghai Lake Region, China. Pedosphere 2007, 17, 84–89. [Google Scholar] [CrossRef]
- Ozment, K.A.; Welti, E.A.R.; Shaffer, M.; Kaspari, M. Tracking Nutrients in Space and Time: Interactions between Grazing Lawns and Drought Drive Abundances of Tallgrass Prairie Grasshoppers. Ecol. Evol. 2021, 11, 5413–5423. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, K.; Zhao, C.; Zhang, Q. Analysis of Spatial Pattern among Grasshopper and Vegetation in Heihe Based on GIS. Phys. Procedia 2012, 33, 1261–1268. [Google Scholar] [CrossRef][Green Version]
- Meynard, C.N.; Lecoq, M.; Chapuis, M.; Piou, C. On the Relative Role of Climate Change and Management in the Current Desert Locust Outbreak in East Africa. Glob. Change Biol. 2020, 26, 3753–3755. [Google Scholar] [CrossRef] [PubMed]
- Ortego, J.; Aguirre, M.P.; Noguerales, V.; Cordero, P.J. Consequences of Extensive Habitat Fragmentation in Landscape-level Patterns of Genetic Diversity and Structure in the M Editerranean Esparto Grasshopper. Evol. Appl. 2015, 8, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Lozano, F.J.; Suárez-Seoane, S.; Kelly, M.; Luis, E. A Multi-Scale Approach for Modeling Fire Occurrence Probability Using Satellite Data and Classification Trees: A Case Study in a Mountainous Mediterranean Region. Remote Sens. Environ. 2008, 112, 708–719. [Google Scholar] [CrossRef]
- Norberg, A.; Abrego, N.; Blanchet, F.G.; Adler, F.R.; Anderson, B.J.; Anttila, J.; Araújo, M.B.; Dallas, T.; Dunson, D.; Elith, J.; et al. A Comprehensive Evaluation of Predictive Performance of 33 Species Distribution Models at Species and Community Levels. Ecol. Monogr. 2019, 89, e01370. [Google Scholar] [CrossRef]
- Padalia, H.; Srivastava, V.; Kushwaha, S.P.S. Modeling Potential Invasion Range of Alien Invasive Species, Hyptis suaveolens (L.) Poit. in India: Comparison of MaxEnt and GARP. Ecol. Inform. 2014, 22, 36–43. [Google Scholar] [CrossRef]
- Farashi, A.; Kaboli, M.; Karami, M. Predicting Range Expansion of Invasive Raccoons in Northern Iran Using ENFA Model at Two Different Scales. Ecol. Inform. 2013, 15, 96–102. [Google Scholar] [CrossRef]
- Lee, M.-J.; Kang, J.; Jeon, S. Application of Frequency Ratio Model and Validation for Predictive Flooded Area Susceptibility Mapping Using GIS. In Proceedings of the 2012 IEEE International Geoscience and Remote Sensing Symposium, Munich, Germany, 22–27 July 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 895–898. [Google Scholar]
- Jebur, M.N.; Pradhan, B.; Tehrany, M.S. Optimization of Landslide Conditioning Factors Using Very High-Resolution Airborne Laser Scanning (LiDAR) Data at Catchment Scale. Remote Sens. Environ. 2014, 152, 150–165. [Google Scholar] [CrossRef]
- Shah, A.A.; Ullah, A.; Khan, N.A.; Shah, M.H.; Ahmed, R.; Hassan, S.T.; Tariq, M.A.U.R.; Xu, C. Identifying Obstacles Encountered at Different Stages of the Disaster Management Cycle (DMC) and Its Implications for Rural Flooding in Pakistan. Front. Environ. Sci. 2023, 11, 1088126. [Google Scholar] [CrossRef]
- Arabameri, A.; Pradhan, B.; Rezaei, K.; Lee, C.-W. Assessment of Landslide Susceptibility Using Statistical- and Artificial Intelligence-Based FR–RF Integrated Model and Multiresolution DEMs. Remote Sens. 2019, 11, 999. [Google Scholar] [CrossRef]
- Rehman, A.; Song, J.; Haq, F.; Mahmood, S.; Ahamad, M.I.; Basharat, M.; Sajid, M.; Mehmood, M.S. Multi-Hazard Susceptibility Assessment Using the Analytical Hierarchy Process and Frequency Ratio Techniques in the Northwest Himalayas, Pakistan. Remote Sens. 2022, 14, 554. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, J.; Tong, Z.; Han, A.; Zhi, F. Ecological Security Assessment of Xilingol Grassland in China Using DPSIRM Model. Ecol. Indic. 2022, 143, 109336. [Google Scholar] [CrossRef]
- Yang, W.; Zhen, L. Household Perceptions of Factors That Affect Food Consumption in Grassland Areas: A Case Study in the Xilin Gol Grassland, China. Environ. Res. Lett. 2020, 15, 115007. [Google Scholar] [CrossRef]
- Jia, M.; Zhen, L. Food Consumption Characteristics and Influencing Factors in a Grassland Transect of Inner Mongolia Based on the Emergy Method. Foods 2022, 11, 3637. [Google Scholar] [CrossRef]
- Haiyan, D.A.I.; Haimei, W.A.N.G. Influence of Rainfall Events on Soil Moisture in a Typical Steppe of Xilingol. Phys. Chem. Earth Parts A B C 2021, 121, 102964. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, H.-Y.; He, B.; Gexigeduren; Xin, Z.-Y.; Lin, H. Spatiotemporal Heterogeneity of the Potential Occurrence of Oedaleus Decorus Asiaticus in Inner Mongolia Steppe Habitats. J. Arid. Environ. 2015, 116, 33–43. [Google Scholar] [CrossRef]
- Lu, L.; Kong, W.; Eerdengqimuge; Ye, H.; Sun, Z.; Wang, N.; Du, B.; Zhou, Y.; Weijun; Huang, W. Detecting Key Factors of Grasshopper Occurrence in Typical Steppe and Meadow Steppe by Integrating Machine Learning Model and Remote Sensing Data. Insects 2022, 13, 894. [Google Scholar] [CrossRef]
- Wang, F.; Chen, X.; Luo, G.; Ding, J.; Chen, X. Detecting Soil Salinity with Arid Fraction Integrated Index and Salinity Index in Feature Space Using Landsat TM Imagery. J. Arid Land 2013, 5, 340–353. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Bujang, M.A.; Baharum, N. A Simplified Guide to Determination of Sample Size Requirements for Estimating the Value of Intraclass Correlation Coefficient: A Review. Arch. Orofac. Sci. 2017, 12, 1–11. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the Black Box: An Open-source Release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, Y.; Huang, W.; Ren, B.; Deng, Q.; Shi, Y.; Bai, J.; Ren, Y.; Geng, Y.; Ma, H. Overwintering Distribution of Fall Armyworm (Spodoptera frugiperda) in Yunnan, China, and Influencing Environmental Factors. Insects 2020, 11, 805. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.U.; Ullah, W.; Bai, S.; Ullah, S.; Jan, M.A.; Khan, M.; Tayyab, M. GIS-Based Flood Susceptibility Mapping Using Bivariate Statistical Model in Swat River Basin, Eastern Hindukush Region, Pakistan. Front. Environ. Sci. 2023, 11, 1178540. [Google Scholar] [CrossRef]
- Wan, G.-Z.; Wang, L.; Jin, L.; Chen, J. Evaluation of Environmental Factors Affecting the Quality of Codonopsis Pilosula Based on Chromatographic Fingerprint and MaxEnt Model. Ind. Crops Prod. 2021, 170, 113783. [Google Scholar] [CrossRef]
- Ni, S.; Wu, T. Monitoring the Intensity of Locust Damage to Vegetation Using Hyper-Spectra Data Obtained at Ground Surface. In Proceedings of the Remote Sensing and Modeling of Ecosystems for Sustainability IV, San Diego, CA, USA, 26–30 August 2007; Volume 66790. [Google Scholar]
- Sillero, N. What Does Ecological Modelling Model? A Proposed Classification of Ecological Niche Models Based on Their Underlying Methods. Ecol. Model. 2011, 222, 1343–1346. [Google Scholar] [CrossRef]




| Category | Factors | Development Stage | Data Source | Spatial Resolution | Temporal Resolution |
|---|---|---|---|---|---|
| Meteorology | Mean LST | Egg Nymph | MOD11A1 | 1 km | 1 day |
| Mean Specific Humidity | Egg Adult | FLDAS | 11,132 m | 1 day | |
| Mean Precipitation | Egg Nymph Adult | GPM | 11,132 m | Monthly | |
| Vegetation | Above-ground Biomass | Nymph | MOD13A2 | 1 km | 16 days |
| Vegetation Type | Static Factor | Chinese Academy of Sciences | 1 km | ||
| Soil | Soil Moisture | Egg Nymph Adult | FLDAS | 11,132 m | Monthly |
| Soil Salinity index | Egg Nymph Adult | MOD09A1 | 1 km | 8 days | |
| Soil Sand | Static Factor | Soil Grids | 250 m | ||
| Soil Organic Carbon | Static Factor | Soil Grids | 250 m | ||
| Soil Ph | Static Factor | Soil Grids | 250 m | ||
| Soil Silt | Static Factor | Soil Grids | 250 m | ||
| Soil Bulk Density | Static Factor | ||||
| Soil Nitrogen | Static Factor | Soil Grids | 250 m | ||
| Soil Clay Content | Static Factor | Soil Grids | 250 m | ||
| Soil Type | Static Factor | Chinese Academy of Sciences | 1 km | ||
| Topographic | Elevation | Static Factor | Chinese Academy of Sciences | 90 m | |
| Slope | Static Factor | Chinese Academy of Sciences | 90 m | ||
| Aspect | Static Factor | Chinese Academy of Sciences | 90 m |
| Factor | Class | Points | % Points | Class Area | % Class Area | FR | RF |
|---|---|---|---|---|---|---|---|
| ESI | 1 | 62,000,000 | 80.52 | 64,116 | 31.60 | 2.55 | 0.75 |
| 2 | 12,000,000 | 15.58 | 49,731 | 24.51 | 0.64 | 0.19 | |
| 3 | 3,000,000 | 3.90 | 39,270 | 19.36 | 0.20 | 0.06 | |
| 4 | 0 | 0.00 | 35,977 | 17.73 | 0.00 | 0.00 | |
| 5 | 0 | 0.00 | 13,773 | 6.79 | 0.00 | 0.00 | |
| EP | 1 | 0 | 0.00 | 37,060 | 18.27 | 0.00 | 0.00 |
| 2 | 1,000,000 | 1.30 | 36,785 | 18.13 | 0.07 | 0.01 | |
| 3 | 3,000,000 | 3.90 | 43,345 | 21.37 | 0.18 | 0.03 | |
| 4 | 28,000,000 | 36.36 | 52,691 | 25.97 | 1.40 | 0.27 | |
| 5 | 45,000,000 | 58.44 | 32,986 | 16.26 | 3.59 | 0.68 | |
| Slope | 1 | 20,000,000 | 25.97 | 87,820 | 43.85 | 0.59 | 0.04 |
| 2 | 24,000,000 | 31.17 | 68,383 | 34.14 | 0.91 | 0.07 | |
| 3 | 28,000,000 | 36.36 | 34,314 | 17.13 | 2.12 | 0.16 | |
| 4 | 3,000,000 | 3.90 | 9201 | 4.59 | 0.85 | 0.06 | |
| 5 | 2,000,000 | 2.60 | 574 | 0.29 | 9.06 | 0.67 | |
| ASM | 1 | 0 | 0.00 | 47,304 | 23.38 | 0.00 | 0.00 |
| 2 | 12,000,000 | 15.58 | 70,317 | 34.75 | 0.45 | 0.08 | |
| 3 | 42,000,000 | 54.55 | 60,071 | 29.69 | 1.84 | 0.31 | |
| 4 | 23,000,000 | 29.87 | 16,735 | 8.27 | 3.61 | 0.61 | |
| 5 | 0 | 0.00 | 7915 | 3.91 | 0.00 | 0.00 | |
| NSI | 1 | 35,000,000 | 45.45 | 34,491 | 17.00 | 2.67 | 0.57 |
| 2 | 37,000,000 | 48.05 | 57,516 | 28.35 | 1.69 | 0.36 | |
| 3 | 4,000,000 | 5.19 | 45,617 | 22.49 | 0.23 | 0.05 | |
| 4 | 1,000,000 | 1.30 | 43,141 | 21.27 | 0.06 | 0.01 | |
| 5 | 0 | 0.00 | 22,102 | 10.89 | 0.00 | 0.00 | |
| ESM | 1 | 0 | 0.00 | 30,855 | 15.25 | 0.00 | 0.00 |
| 2 | 5,000,000 | 6.49 | 57,184 | 28.26 | 0.23 | 0.05 | |
| 3 | 30,000,000 | 38.96 | 58,428 | 28.88 | 1.35 | 0.29 | |
| 4 | 39,000,000 | 50.65 | 38,746 | 19.15 | 2.65 | 0.56 | |
| 5 | 3,000,000 | 3.90 | 17,129 | 8.47 | 0.46 | 0.10 | |
| ELST | 1 | 37,000,000 | 48.05 | 30,451 | 15.01 | 3.20 | 0.58 |
| 2 | 15,000,000 | 19.48 | 44,908 | 22.14 | 0.88 | 0.16 | |
| 3 | 10,000,000 | 12.99 | 35,115 | 17.31 | 0.51 | 0.09 | |
| 4 | 13,000,000 | 16.88 | 51,435 | 25.35 | 0.84 | 0.15 | |
| 5 | 2,000,000 | 2.60 | 40,958 | 20.19 | 0.13 | 0.02 | |
| AP | 1 | 0 | 0.00 | 47,891 | 23.61 | 0.00 | 0.00 |
| 2 | 2,000,000 | 2.60 | 56,521 | 27.86 | 0.09 | 0.02 | |
| 3 | 9,000,000 | 11.69 | 33,344 | 16.44 | 0.71 | 0.12 | |
| 4 | 45,000,000 | 58.44 | 38,543 | 19.00 | 3.08 | 0.52 | |
| 5 | 21,000,000 | 27.27 | 26,568 | 13.10 | 2.08 | 0.35 | |
| SBD | 1 | 0 | 0.00 | 1241 | 0.61 | 0.00 | 0.00 |
| 2 | 7,000,000 | 9.09 | 10,980 | 5.41 | 1.68 | 0.31 | |
| 3 | 44,000,000 | 57.14 | 44,682 | 22.03 | 2.59 | 0.48 | |
| 4 | 24,000,000 | 31.17 | 60,234 | 29.69 | 1.05 | 0.19 | |
| 5 | 2,000,000 | 2.60 | 85,723 | 42.26 | 0.06 | 0.01 | |
| ASI | 1 | 30,000,000 | 38.96 | 37,877 | 18.67 | 2.09 | 0.44 |
| 2 | 37,000,000 | 48.05 | 47,880 | 23.60 | 2.04 | 0.43 | |
| 3 | 9,000,000 | 11.69 | 43,911 | 21.65 | 0.54 | 0.11 | |
| 4 | 1,000,000 | 1.30 | 42,279 | 20.84 | 0.06 | 0.01 | |
| 5 | 0 | 0.00 | 30,920 | 15.24 | 0.00 | 0.00 | |
| Elevation | 1 | 34,000,000 | 44.16 | 40,635 | 20.03 | 2.20 | 0.46 |
| 2 | 28,000,000 | 36.36 | 58,161 | 28.67 | 1.27 | 0.26 | |
| 3 | 7,000,000 | 9.09 | 47,141 | 23.24 | 0.39 | 0.08 | |
| 4 | 2,000,000 | 2.60 | 36,669 | 18.08 | 0.14 | 0.03 | |
| 5 | 6,000,000 | 7.79 | 20,261 | 9.99 | 0.78 | 0.16 | |
| ASH | 1 | 3,000,000 | 3.90 | 45,378 | 22.37 | 0.17 | 0.03 |
| 2 | 3,000,000 | 3.90 | 40,005 | 19.72 | 0.20 | 0.03 | |
| 3 | 10,000,000 | 12.99 | 51,111 | 25.19 | 0.52 | 0.09 | |
| 4 | 38,000,000 | 49.35 | 42,492 | 20.95 | 2.36 | 0.41 | |
| 5 | 23,000,000 | 29.87 | 23,881 | 11.77 | 2.54 | 0.44 | |
| SSAND | 1 | 0 | 0.00 | 1240 | 0.61 | 0.00 | 0.00 |
| 2 | 3,000,000 | 3.90 | 32,407 | 15.98 | 0.24 | 0.06 | |
| 3 | 22,000,000 | 28.57 | 66,413 | 32.74 | 0.87 | 0.22 | |
| 4 | 32,000,000 | 41.56 | 69,927 | 34.47 | 1.21 | 0.31 | |
| 5 | 20,000,000 | 25.97 | 32,873 | 16.20 | 1.60 | 0.41 | |
| SCC | 1 | 0 | 0.00 | 1240 | 0.61 | 0.00 | 0.00 |
| 2 | 37,000,000 | 48.05 | 62,791 | 30.95 | 1.55 | 0.41 | |
| 3 | 24,000,000 | 31.17 | 76,572 | 37.75 | 0.83 | 0.22 | |
| 4 | 10,000,000 | 12.99 | 42,880 | 21.14 | 0.61 | 0.16 | |
| 5 | 6,000,000 | 7.79 | 19,377 | 9.55 | 0.82 | 0.21 | |
| NP | 1 | 0 | 0.00 | 69,863 | 34.44 | 0.00 | 0.00 |
| 2 | 22,000,000 | 28.57 | 69,085 | 34.05 | 0.84 | 0.13 | |
| 3 | 38,000,000 | 49.35 | 37,945 | 18.70 | 2.64 | 0.40 | |
| 4 | 15,000,000 | 19.48 | 15,587 | 7.68 | 2.54 | 0.39 | |
| 5 | 2,000,000 | 2.60 | 10,387 | 5.12 | 0.51 | 0.08 | |
| NSM | 1 | 0 | 0.00 | 41,029 | 19.68 | 0.00 | 0.00 |
| 2 | 17,000,000 | 22.08 | 63,030 | 30.23 | 0.73 | 0.13 | |
| 3 | 29,000,000 | 37.66 | 47,875 | 22.96 | 1.64 | 0.28 | |
| 4 | 15,000,000 | 19.48 | 37,859 | 18.16 | 1.07 | 0.19 | |
| 5 | 16,000,000 | 20.78 | 18,699 | 8.97 | 2.32 | 0.40 | |
| ESH | 1 | 4,000,000 | 5.19 | 21,928 | 10.81 | 0.48 | 0.13 |
| 2 | 14,000,000 | 18.18 | 40,834 | 20.13 | 0.90 | 0.24 | |
| 3 | 22,000,000 | 28.57 | 65,204 | 32.14 | 0.89 | 0.23 | |
| 4 | 37,000,000 | 48.05 | 64,211 | 31.65 | 1.52 | 0.40 | |
| 5 | 0 | 0.00 | 10,690 | 5.27 | 0.00 | 0.00 | |
| ST | 1 | 0 | 0.00 | 22 | 0.01 | 0.00 | 0.00 |
| 2 | 0 | 0.00 | 204 | 0.10 | 0.00 | 0.00 | |
| 3 | 2,000,000 | 2.70 | 1706 | 0.87 | 3.09 | 0.35 | |
| 4 | 13,000,000 | 17.57 | 11,203 | 5.74 | 3.06 | 0.34 | |
| 5 | 53,000,000 | 71.62 | 112,340 | 57.55 | 1.24 | 0.14 | |
| 6 | 0 | 0.00 | 27,235 | 13.95 | 0.00 | 0.00 | |
| 7 | 0 | 0.00 | 23,786 | 12.19 | 0.00 | 0.00 | |
| 8 | 0 | 0.00 | 309 | 0.16 | 0.00 | 0.00 | |
| 9 | 0 | 0.00 | 1118 | 0.57 | 0.00 | 0.00 | |
| 10 | 6,000,000 | 8.11 | 10,629 | 5.45 | 1.49 | 0.17 | |
| 11 | 0 | 0.00 | 2549 | 1.31 | 0.00 | 0.00 | |
| 12 | 0 | 0.00 | 2748 | 1.41 | 0.00 | 0.00 | |
| 13 | 0 | 0.00 | 1049 | 0.54 | 0.00 | 0.00 | |
| 14 | 0 | 0.00 | 292 | 0.15 | 0.00 | 0.00 | |
| SpH | 1 | 0 | 0.00 | 1240 | 0.61 | 0.00 | 0.00 |
| 2 | 3,000,000 | 3.90 | 6147 | 3.03 | 1.29 | 0.28 | |
| 3 | 15,000,000 | 19.48 | 25,802 | 12.72 | 1.53 | 0.34 | |
| 4 | 47,000,000 | 61.04 | 93,060 | 45.87 | 1.33 | 0.29 | |
| 5 | 12,000,000 | 15.58 | 76,611 | 37.77 | 0.41 | 0.09 | |
| VT | 1 | 66,000,000 | 86.84 | 154,887 | 78.96 | 1.10 | 0.28 |
| 2 | 4,000,000 | 5.26 | 20,207 | 10.30 | 0.51 | 0.13 | |
| 3 | 2,000,000 | 2.63 | 4297 | 2.19 | 1.20 | 0.31 | |
| 4 | 4,000,000 | 5.26 | 9790 | 4.99 | 1.05 | 0.27 | |
| 5 | 0 | 0.00 | 6967 | 3.55 | 0.00 | 0.00 | |
| SSILT | 1 | 0 | 0.00 | 1241 | 0.61 | 0.00 | 0.00 |
| 2 | 14,000,000 | 18.18 | 28,233 | 13.93 | 1.30 | 0.33 | |
| 3 | 28,000,000 | 36.36 | 64,612 | 31.89 | 1.14 | 0.29 | |
| 4 | 29,000,000 | 37.66 | 72,076 | 35.57 | 1.06 | 0.27 | |
| 5 | 6,000,000 | 7.79 | 36,445 | 17.99 | 0.43 | 0.11 | |
| NAB | 1 | 9,000,000 | 11.69 | 108,993 | 53.73 | 0.22 | 0.03 |
| 2 | 40,000,000 | 51.95 | 61,521 | 30.33 | 1.71 | 0.21 | |
| 3 | 22,000,000 | 28.57 | 21,327 | 10.51 | 2.72 | 0.33 | |
| 4 | 3,000,000 | 3.90 | 7791 | 3.84 | 1.01 | 0.12 | |
| 5 | 3,000,000 | 3.90 | 3218 | 1.59 | 2.46 | 0.30 | |
| NLST | 1 | 8,000,000 | 10.39 | 10,658 | 5.25 | 1.98 | 0.32 |
| 2 | 13,000,000 | 16.88 | 24,280 | 11.97 | 1.41 | 0.23 | |
| 3 | 26,000,000 | 33.77 | 46,658 | 23.00 | 1.47 | 0.24 | |
| 4 | 28,000,000 | 36.36 | 58,846 | 29.01 | 1.25 | 0.20 | |
| 5 | 2,000,000 | 2.60 | 62,425 | 30.77 | 0.08 | 0.01 | |
| SN | 1 | 0 | 0.00 | 27,935 | 13.77 | 0.00 | 0.00 |
| 2 | 29,000,000 | 37.66 | 71,763 | 35.38 | 1.06 | 0.23 | |
| 3 | 32,000,000 | 41.56 | 65,365 | 32.22 | 1.29 | 0.28 | |
| 4 | 13,000,000 | 16.88 | 30,436 | 15.00 | 1.13 | 0.25 | |
| 5 | 3,000,000 | 3.90 | 7361 | 3.63 | 1.07 | 0.24 | |
| SOC | 1 | 3,000,000 | 3.90 | 83,425 | 41.12 | 0.09 | 0.01 |
| 2 | 37,000,000 | 48.05 | 63,045 | 31.08 | 1.55 | 0.21 | |
| 3 | 21,000,000 | 27.27 | 35,701 | 17.60 | 1.55 | 0.21 | |
| 4 | 13,000,000 | 16.88 | 16,954 | 8.36 | 2.02 | 0.28 | |
| 5 | 3,000,000 | 3.90 | 3735 | 1.84 | 2.12 | 0.29 | |
| Aspect | 1 | 14,000,000 | 18.18 | 36,358 | 18.15 | 1.00 | 0.20 |
| 2 | 21,000,000 | 27.27 | 38,029 | 18.99 | 1.44 | 0.29 | |
| 3 | 12,000,000 | 15.58 | 36,619 | 18.28 | 0.85 | 0.17 | |
| 4 | 13,000,000 | 16.88 | 39,951 | 19.95 | 0.85 | 0.17 | |
| 5 | 17,000,000 | 22.08 | 49,335 | 24.63 | 0.90 | 0.18 |
| Environmental Factor | Percentage Contribution |
|---|---|
| ASH | 23.8% |
| VT | 19.7% |
| NAB | 8.8% |
| SSAND | 8.4% |
| EP | 5.7% |
| NP | 4.4% |
| SBD | 3.7% |
| Elevation | 3% |
| ST | 2.9% |
| Total | 80.4% |
| Factor | Min RF | Max RF | Max–Min RF | (Max–Min) Min RF | PR Value |
|---|---|---|---|---|---|
| ESI | 0.00 | 0.75 | 0.75 | 0.09 | 8.71 |
| EP | 0.00 | 0.68 | 0.68 | 0.09 | 7.93 |
| Slope | 0.04 | 0.67 | 0.63 | 0.09 | 7.24 |
| ASM | 0.00 | 0.61 | 0.61 | 0.09 | 7.09 |
| NSI | 0.00 | 0.57 | 0.57 | 0.09 | 6.64 |
| ESM | 0.00 | 0.56 | 0.56 | 0.09 | 6.54 |
| ELST | 0.02 | 0.58 | 0.55 | 0.09 | 6.40 |
| AP | 0.00 | 0.52 | 0.52 | 0.09 | 5.97 |
| SBD | 0.00 | 0.48 | 0.48 | 0.09 | 5.58 |
| ASI | 0.00 | 0.44 | 0.44 | 0.09 | 5.11 |
| Elevation | 0.03 | 0.46 | 0.43 | 0.09 | 4.98 |
| ASH | 0.03 | 0.44 | 0.41 | 0.09 | 4.73 |
| SSAND | 0.00 | 0.41 | 0.41 | 0.09 | 4.73 |
| SCC | 0.00 | 0.41 | 0.41 | 0.09 | 4.72 |
| NP | 0.00 | 0.40 | 0.40 | 0.09 | 4.68 |
| NSM | 0.00 | 0.40 | 0.40 | 0.09 | 4.66 |
| ESH | 0.00 | 0.40 | 0.40 | 0.09 | 4.64 |
| ST | 0.00 | 0.35 | 0.35 | 0.09 | 4.03 |
| SpH | 0.00 | 0.34 | 0.34 | 0.09 | 3.89 |
| SSILT | 0.00 | 0.33 | 0.33 | 0.09 | 3.84 |
| VT | 0.00 | 0.00 | 0.00 | 0.09 | 3.59 |
| NAB | 0.03 | 0.33 | 0.31 | 0.09 | 3.56 |
| NLST | 0.01 | 0.32 | 0.31 | 0.09 | 3.54 |
| SN | 0.00 | 0.28 | 0.28 | 0.09 | 3.28 |
| SOC | 0.01 | 0.29 | 0.28 | 0.09 | 3.19 |
| Aspect | 0.20 | 0.29 | 0.09 | 0.09 | 1.00 |
| Habitat Factor | Type Name | Suitability |
|---|---|---|
| VT | Northwest stipa grassland | 0.99 |
| VT | Leymus chinensis tufted grassland | 0.97 |
| VT | Tiger hazelnut bush | 0.96 |
| VT | Birch forest | 0.94 |
| ST | Chestnut soil | 0.68 |
| ST | Meadow soil | 0.62 |
| ST | Tidal soil | 0.61 |
| ST | Gray forest | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, R.; Huang, W.; Dong, Y.; Guo, J.; Dildar, Z.; Rahman, Z.U.; Zhang, Y.; Zhang, X.; Du, B.; Yue, F. Assessment of Habitat Suitability for Oedaleus decorus asiaticus Using MaxEnt and Frequency Ratio Model in Xilingol League, China. Remote Sens. 2025, 17, 846. https://doi.org/10.3390/rs17050846
Ahmed R, Huang W, Dong Y, Guo J, Dildar Z, Rahman ZU, Zhang Y, Zhang X, Du B, Yue F. Assessment of Habitat Suitability for Oedaleus decorus asiaticus Using MaxEnt and Frequency Ratio Model in Xilingol League, China. Remote Sensing. 2025; 17(5):846. https://doi.org/10.3390/rs17050846
Chicago/Turabian StyleAhmed, Raza, Wenjiang Huang, Yingying Dong, Jing Guo, Zeenat Dildar, Zahid Ur Rahman, Yan Zhang, Xianwei Zhang, Bobo Du, and Fangzheng Yue. 2025. "Assessment of Habitat Suitability for Oedaleus decorus asiaticus Using MaxEnt and Frequency Ratio Model in Xilingol League, China" Remote Sensing 17, no. 5: 846. https://doi.org/10.3390/rs17050846
APA StyleAhmed, R., Huang, W., Dong, Y., Guo, J., Dildar, Z., Rahman, Z. U., Zhang, Y., Zhang, X., Du, B., & Yue, F. (2025). Assessment of Habitat Suitability for Oedaleus decorus asiaticus Using MaxEnt and Frequency Ratio Model in Xilingol League, China. Remote Sensing, 17(5), 846. https://doi.org/10.3390/rs17050846









