Detection of Banana Diseases Based on Landsat-8 Data and Machine Learning
Abstract
1. Introduction
2. Materials and Methods
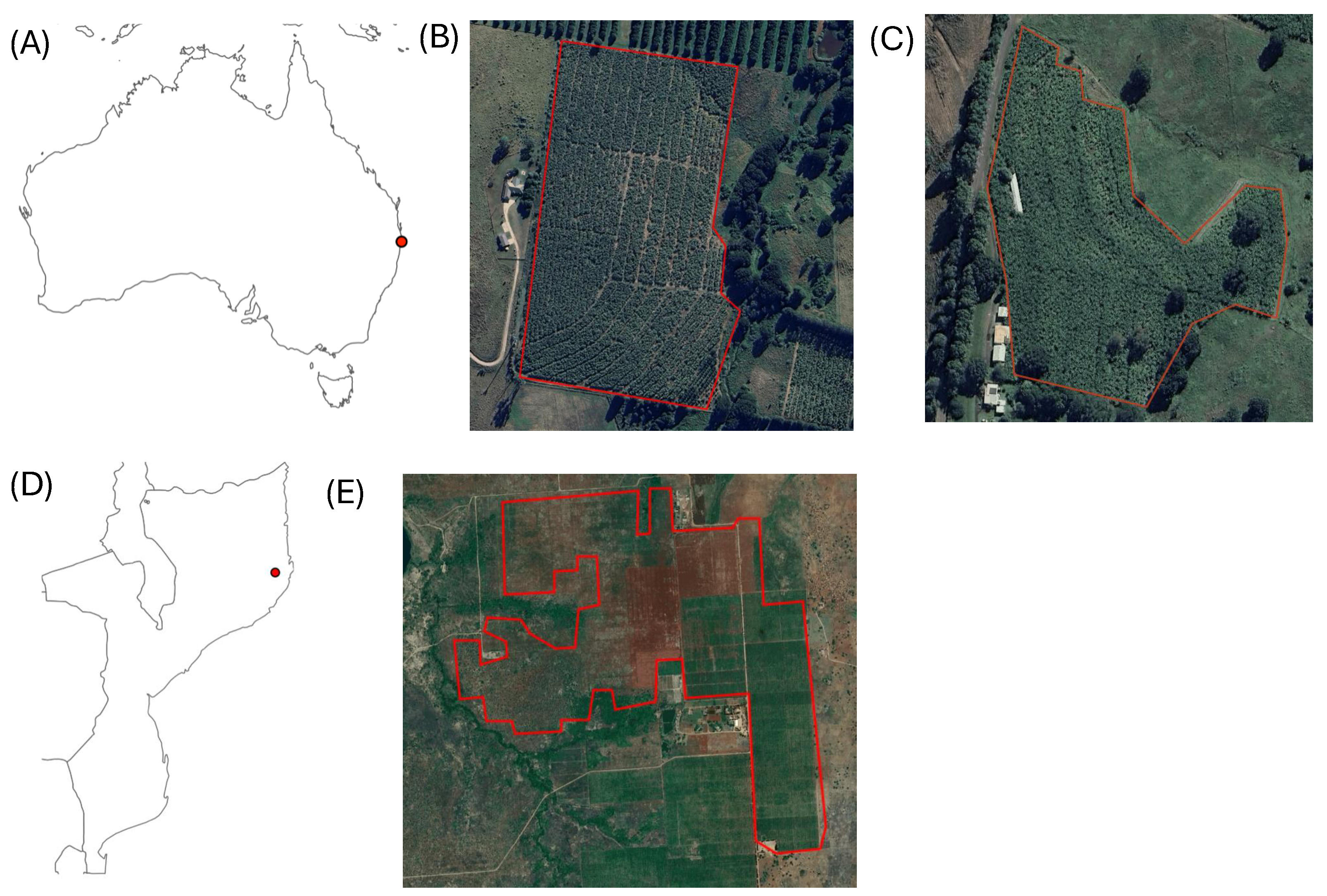
2.1. Case Studies
2.1.1. Banana Bunchy Top Disease
2.1.2. Fusarium TR4
2.2. Methodological Approach
2.3. Datasets
2.3.1. Remote Sensing Imagery
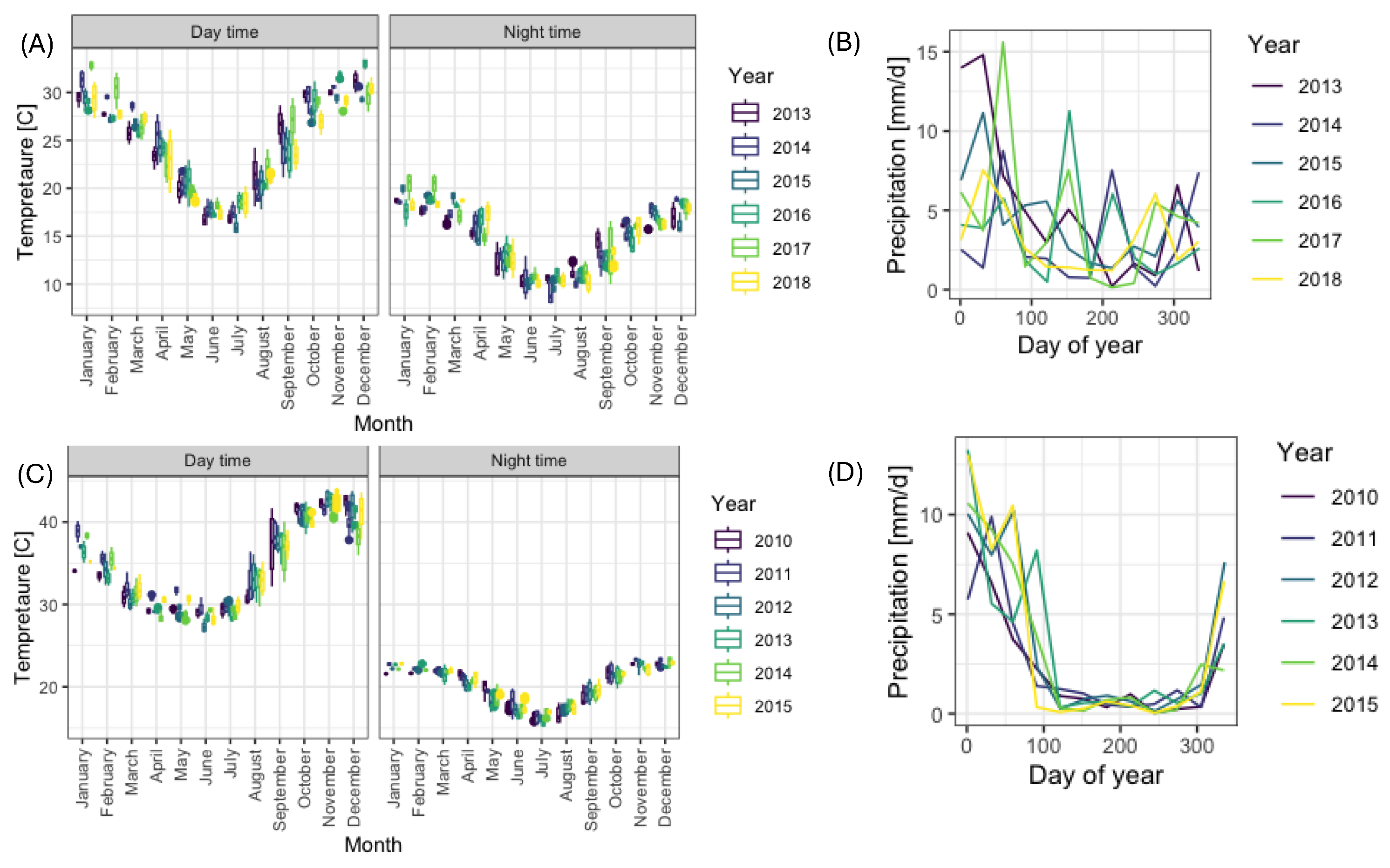
2.3.2. Temperature Data
2.3.3. Precipitation Data
2.4. Pre-Processing and Analysis
2.4.1. Plantation Boundary Delineation
2.4.2. Smoothing of Time Series Data
2.5. Vegetation Indices
Alignment of Datasets to a Common Baseline
- Spatial alignment with Landsat-8 resolution. A central component of the methodology was the development of pixel-specific ML models, wherein an independent model was trained for each individual 30 m × 30 m Landsat-8 pixel. While the meteorological predictors—MODIS-derived temperature (1 km resolution) and NASA GES DISC precipitation (10 km resolution)—originate from coarser spatial scales, they were systematically aligned to the Landsat grid to enable pixel-level modeling.
- Temporal alignment. Landsat-8 imagery, with its 16-day revisit cycle, was used to derive VIs, which were linearly interpolated to regular 10-day intervals to enhance temporal resolution. Daily temperature and monthly precipitation data were incorporated through a lag-window approach, where meteorological conditions from day to informed each VI prediction.
2.6. Machine Learning Algorithms
2.6.1. Decision Tree (DT) Model
2.6.2. Support Vector Machines (SVMs)
2.6.3. Random Forest (RF) Model
2.6.4. Training and Testing of ML Models
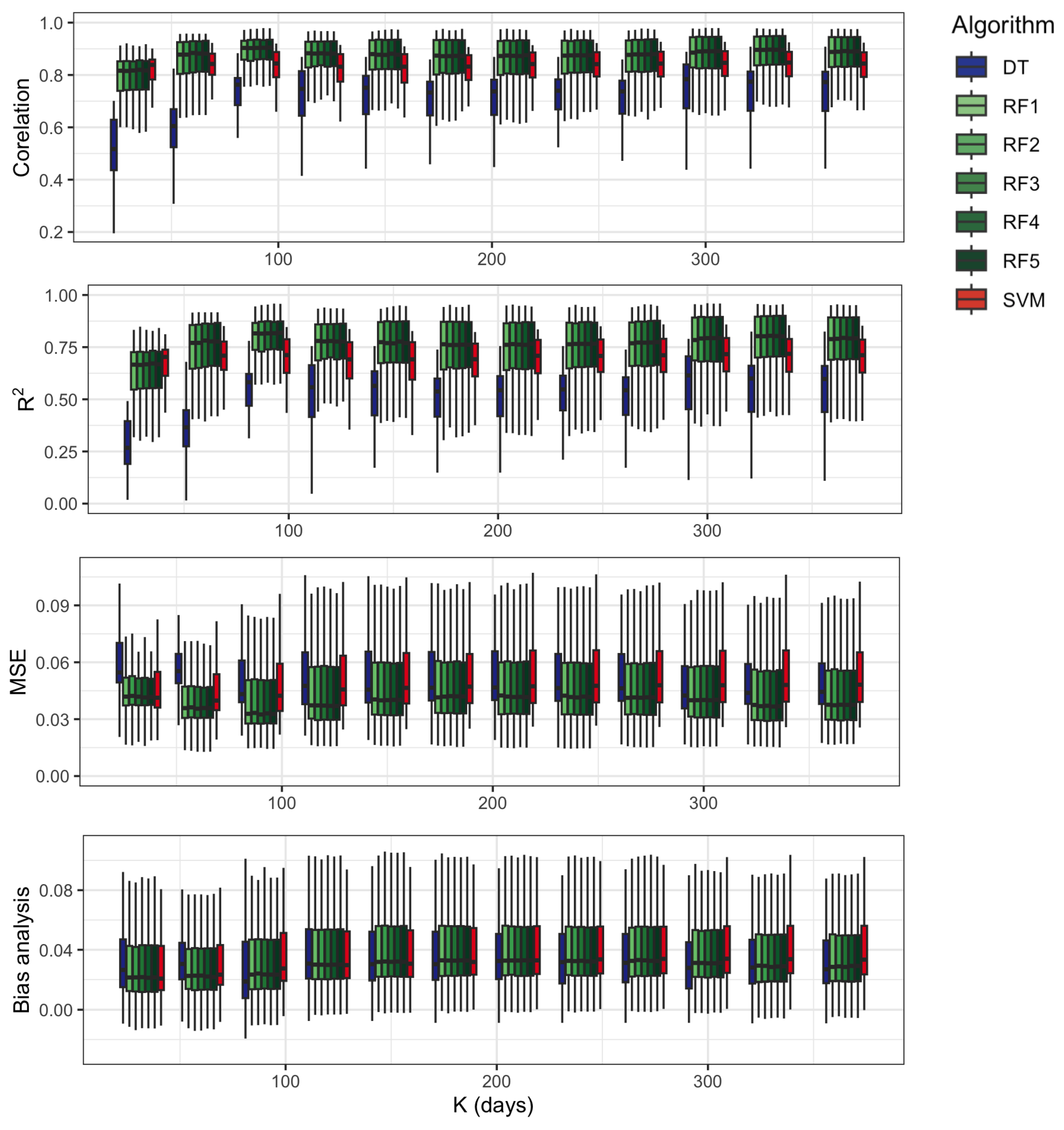
2.7. Model Accuracy Assessment
3. Results
3.1. Year-to-Year Dynamics of Temperature and Precipitation
3.2. Selection of ML Model and Hyperparameters
3.3. Effect of Seasonal Variation on VIs
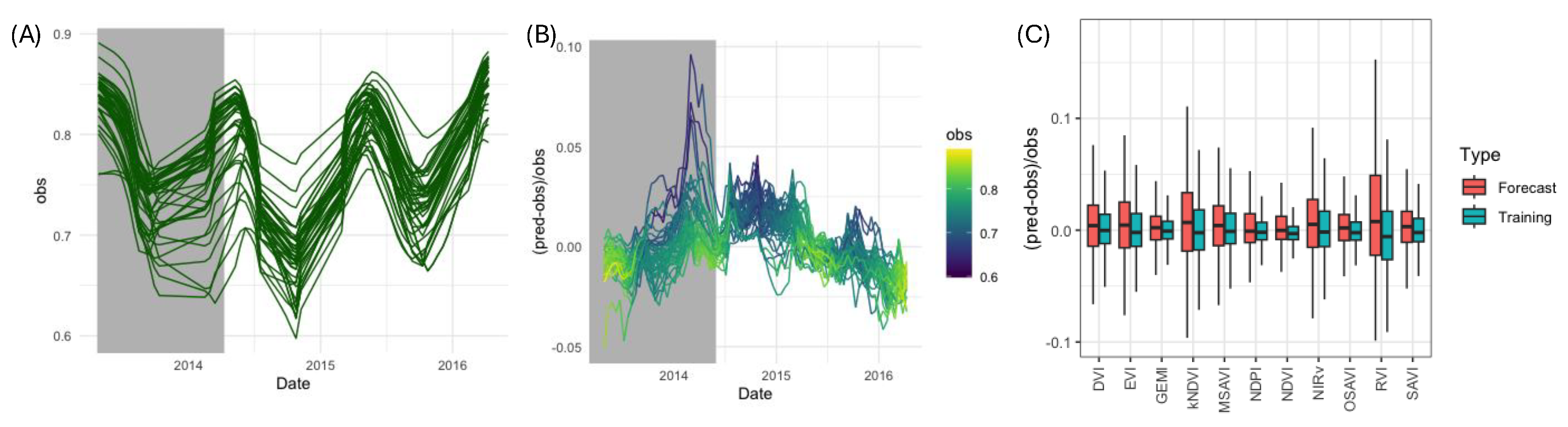
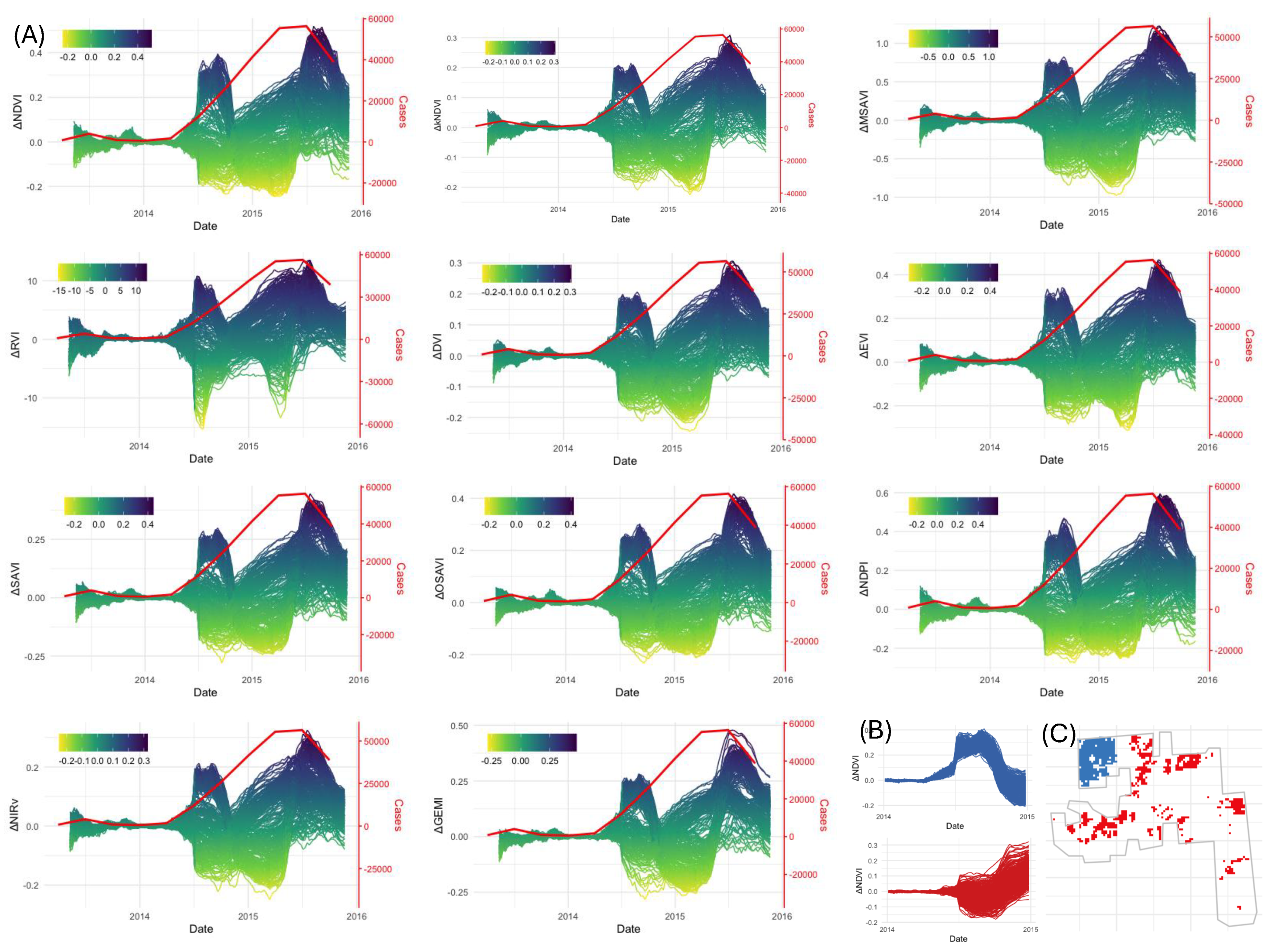
3.4. Detecting BBTD Presence at the NSW1 Banana Plantation
3.5. Detecting TR4 Presence
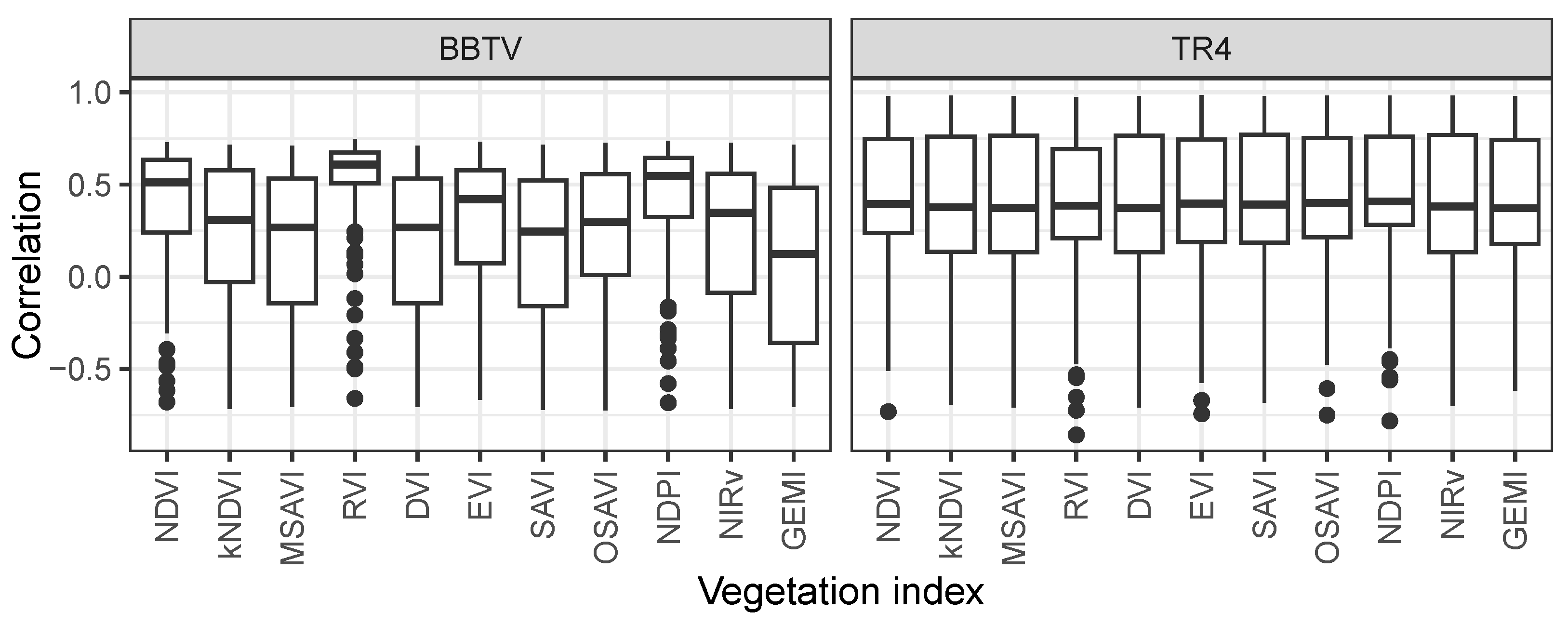
3.6. Performance of VIs
4. Discussion
4.1. VIs, Phenology, and the Challenge of Disease Detection
4.2. Pixel-Level Modeling to Account for Phenological and Structural Variability
4.3. Linking VI Anomalies to Observed Disease Dynamics
4.4. Limitations and Future Opportunities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Voora, V.; Larrea, C.; Bermudez, S. Global Market Report: Bananas; International Institute for Sustainable Development: Winnipeg, MB, Canada, 2020. [Google Scholar]
- Ploetz, R.C. Management of Fusarium wilt of banana: A review with special reference to tropical race 4. Crop Prot. 2015, 73, 7–15. [Google Scholar] [CrossRef]
- De Bellaire, L.d.L.; Fouré, E.; Abadie, C.; Carlier, J. Black Leaf Streak Disease is challenging the banana industry. Fruits 2010, 65, 327–342. [Google Scholar] [CrossRef][Green Version]
- Marin, D.H.; Romero, R.A.; Guzmán, M.; Sutton, T.B. Black Sigatoka: An increasing threat to banana cultivation. Plant Dis. 2003, 87, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.; Liu, S.; Edwards, J.; Villalta, O.; Aurambout, J.P.; Kriticos, D.; Drenth, A.; De Barro, P. An assessment of the benefits of yellow Sigatoka (Mycosphaerella musicola) control in the Queensland Northern Banana Pest Quarantine Area. NeoBiota 2013, 18, 67–81. [Google Scholar] [CrossRef]
- Dahal, G.; Hughes, J.; Lockhart, B. Status of banana streak disease in Africa: Problems and future research needs. Integr. Pest Manag. Rev. 1998, 3, 85–97. [Google Scholar] [CrossRef]
- Tripathi, L.; Mwangi, M.; Abele, S.; Aritua, V.; Tushemereirwe, W.K.; Bandyopadhyay, R. Xanthomonas wilt: A threat to banana production in East and Central Africa. Plant Dis. 2009, 93, 440–451. [Google Scholar] [CrossRef]
- Kema, G.H.; Drenth, A.; Dita, M.; Jansen, K.; Vellema, S.; Stoorvogel, J.J. Fusarium wilt of banana, a recurring threat to global banana production. Front. Plant Sci. 2021, 11, 628888. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.L. Banana bunchy top: An economically important tropical plant virus disease. Adv. Virus Res. 1987, 33, 301–325. [Google Scholar] [PubMed]
- Jeger, M.; Eden-Green, S.; Thresh, J.; Johanson, A.; Waller, J.; Brown, A. Banana diseases. In Bananas and Plantains; Springer: Berlin/Heidelberg, Germany, 1995; pp. 317–381. [Google Scholar]
- Drenth, A.; Kema, G. The vulnerability of bananas to globally emerging disease threats. Phytopathology 2021, 111, 2146–2161. [Google Scholar] [CrossRef]
- Christaki, E. New technologies in predicting, preventing and controlling emerging infectious diseases. Virulence 2015, 6, 558–565. [Google Scholar] [CrossRef]
- Gupta, S.; Tripathi, A.K. Fruit and vegetable disease detection and classification: Recent trends, challenges, and future opportunities. Eng. Appl. Artif. Intell. 2024, 133, 108260. [Google Scholar] [CrossRef]
- Kashyap, B.; Kumar, R. Sensing methodologies in agriculture for monitoring biotic stress in plants due to pathogens and pests. Inventions 2021, 6, 29. [Google Scholar] [CrossRef]
- Roy, D.P.; Wulder, M.A.; Loveland, T.R.; Woodcock, C.E.; Allen, R.G.; Anderson, M.C.; Helder, D.; Irons, J.R.; Johnson, D.M.; Kennedy, R.; et al. Landsat-8: Science and product vision for terrestrial global change research. Remote Sens. Environ. 2014, 145, 154–172. [Google Scholar] [CrossRef]
- Ma, H.; Jing, Y.; Huang, W.; Shi, Y.; Dong, Y.; Zhang, J.; Liu, L. Integrating Early Growth Information to Monitor Winter Wheat Powdery Mildew Using Multi-Temporal Landsat-8 Imagery. Sensors 2018, 18, 3290. [Google Scholar] [CrossRef]
- Wang, H.; Pu, R.; Zhu, Q.; Ren, L.; Zhang, Z. Mapping health levels of Robinia pseudoacacia forests in the Yellow River delta, China, using IKONOS and Landsat 8 OLI imagery. Int. J. Remote Sens. 2015, 36, 1114–1135. [Google Scholar] [CrossRef]
- Long, L.; Chen, Y.; Song, S.; Zhang, X.; Jia, X.; Lu, Y.; Liu, G. Remote Sensing Monitoring of Pine Wilt Disease Based on Time-Series Remote Sensing Index. Remote Sens. 2023, 15, 360. [Google Scholar] [CrossRef]
- Wikantika, K.; Ghazali, M.F.; Dwivany, F.M.; Susantoro, T.M.; Yayusman, L.F.; Sunarwati, D.; Sutanto, A. A Study on the Distribution Pattern of Banana Blood Disease (BBD) and Fusarium Wilt Using Multispectral Aerial Photos and a Handheld Spectrometer in Subang, Indonesia. Diversity 2023, 15, 1046. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Ba, Y.; Lyu, X.; Zhang, M.; Li, M. Banana Fusarium Wilt Disease Detection by Supervised and Unsupervised Methods from UAV-Based Multispectral Imagery. Remote Sens. 2022, 14, 1231. [Google Scholar] [CrossRef]
- Mora, J.J.; Selvaraj, M.G.; Alvarez, C.I.; Safari, N.; Blomme, G. From pixels to plant health: Accurate detection of banana Xanthomonas wilt in complex African landscapes using high-resolution UAV images and deep learning. Discov. Appl. Sci. 2024, 6, 377. [Google Scholar] [CrossRef]
- Gomez Selvaraj, M.; Vergara, A.; Montenegro, F.; Alonso Ruiz, H.; Safari, N.; Raymaekers, D.; Ocimati, W.; Ntamwira, J.; Tits, L.; Omondi, A.B.; et al. Detection of banana plants and their major diseases through aerial images and machine learning methods: A case study in DR Congo and Republic of Benin. ISPRS J. Photogramm. Remote Sens. 2020, 169, 110–124. [Google Scholar] [CrossRef]
- Alabi, T.R.; Adewopo, J.; Duke, O.P.; Kumar, P.L. Banana Mapping in Heterogenous Smallholder Farming Systems Using High-Resolution Remote Sensing Imagery and Machine Learning Models with Implications for Banana Bunchy Top Disease Surveillance. Remote Sens. 2022, 14, 5206. [Google Scholar] [CrossRef]
- Moradi, S.; Bokani, A.; Hassan, J. UAV-based Smart Agriculture: A Review of UAV Sensing and Applications. In Proceedings of the 2022 32nd International Telecommunication Networks and Applications Conference (ITNAC), Wellington, New Zealand, 30 November–2 December 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 181–184. [Google Scholar] [CrossRef]
- Reddy Maddikunta, P.K.; Hakak, S.; Alazab, M.; Bhattacharya, S.; Gadekallu, T.R.; Khan, W.Z.; Pham, Q.V. Unmanned Aerial Vehicles in Smart Agriculture: Applications, Requirements, and Challenges. IEEE Sensors J. 2021, 21, 17608–17619. [Google Scholar] [CrossRef]
- Tagami, G.N.; van Westerhoven, A.C.; Seidl, M.F.; van der Sluis, J.; Cozzarelli, M.; Balarezo Camminati, D.; Pflücker, R.; Márquez Rosillo, C.; Clercx, L.; Kema, G.H.J. Aerial Mapping of the Peruvian Chira Valley Banana Production System to Monitor the Expansion of Fusarium Wilt Caused by Tropical Race 4. PhytoFrontiers 2024, 4, 196–204. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Strengthening the Preparedness of West and Central Africa to Control Banana Bunch Top Virus and Banana Fusarium Wilt (TR4); FAO: Rome, Italy, 2024; Available online: https://www.fao.org/transboundary-plant-pests-diseases/news/detail/strengthening-the-preparedness-of-west-and-central-africa-to-control-banana-bunch-top-virus-and-banana-fusarium-wilt-(tr4)/en (accessed on 3 July 2025).
- Qazi, J. Banana bunchy top virus and the bunchy top disease. J. Gen. Plant Pathol. 2016, 82, 2–11. [Google Scholar] [CrossRef]
- Hooks, C.; Wright, M.; Kabasawa, D.; Manandhar, R.; Almeida, R. Effect of banana bunchy top virus infection on morphology and growth characteristics of banana. Ann. Appl. Biol. 2008, 153, 1–9. [Google Scholar] [CrossRef]
- Nelson, S.C.; Messing, R.; Hamasaki, R.; Gaskill, D.; Nishijima, W. Banana bunchy top: Detailed signs and symptoms. In Knowledge Creation Diffusion Utilization; Cooperative Extension Service, College of Tropical Agriculture and Human Resources, University of Hawai’i at Mānoa: Honolulu, HI, USA, 2004; pp. 1–22. [Google Scholar]
- Chabi, M.; Dassou, A.G.; Adoukonou-Sagbadja, H.; Thomas, J.; Omondi, A.B. Variation in Symptom Development and Infectivity of Banana Bunchy Top Disease among Four Cultivars of Musa sp. Crops 2023, 3, 158–169. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Health (PLH); Bragard, C.; Baptista, P.; Chatzivassiliou, E.; Di Serio, F.; Gonthier, P.; Jaques Miret, J.A.; Justesen, A.F.; MacLeod, A.; Magnusson, C.S.; et al. Pest categorisation of Fusarium oxysporum f. sp. cubense Tropical Race 4. EFSA J. 2022, 20, e07092. [Google Scholar]
- Garcia-Bastidas, F. Fusarium oxysporum f. sp. cubense Tropical Race 4 (Foc TR4); CABI Compendium: Oxfordshire, UK, 2022. [Google Scholar]
- Varghese, A.; Drovandi, C.; Mira, A.; Mengersen, K. Estimating a novel stochastic model for within-field disease dynamics of banana bunchy top virus via approximate Bayesian computation. PLoS Comput. Biol. 2020, 16, e1007878. [Google Scholar] [CrossRef]
- Australian Bureau of Meteorology. New South Wales Climate Averages and Extremes. 2023. Available online: http://www.bom.gov.au/climate/current/annual/nsw/summary.shtml (accessed on 3 July 2025).
- Viljoen, A.; Mostert, D.; Chiconela, T.; Beukes, I.; Fraser, C.; Dwyer, J.; Murray, H.; Amisse, J.; Matabuana, E.L.; Tazan, G.; et al. Occurrence and spread of the banana fungus Fusarium oxysporum f. sp. cubense TR4 in Mozambique. S. Afr. J. Sci. 2020, 116, 1–11. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Mozambique: Climate and Agro-Ecological Zones. 2022. Available online: https://www.fao.org/gaez/en/pages/national-zones (accessed on 3 July 2025).
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote. Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Wan, Z.; Hook, S.; Hulley, G. MOD11A1 MODIS/Terra Land Surface Temperature/Emissivity Daily L3 Global 1km SIN Grid V006; NASA: Washington, DC, USA, 2015. [CrossRef]
- Huffman, G.J.; Bolvin, D.T.; Braithwaite, D.; Hsu, K.; Joyce, R.; Xie, P.; Yoo, S.H. NASA global precipitation measurement (GPM) integrated multi-satellite retrievals for GPM (IMERG). Algorithm Theor. Basis Doc. (ATBD) Version 2015, 4, 2020–05. [Google Scholar]
- Frasso, G.; Eilers, P.H. L- and V-curves for optimal smoothing. Stat. Model. 2014, 15, 91–111. [Google Scholar] [CrossRef]
- Eilers, P.H.; Pesendorfer, V.; Bonifacio, R. Automatic smoothing of remote sensing data. In Proceedings of the 2017 9th International Workshop on the Analysis of Multitemporal Remote Sensing Images (MultiTemp), Brugge, Belgium, 27–29 June 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–3. [Google Scholar] [CrossRef]
- Retkute, R.; Thurston, W.; Cressman, K.; Gilligan, C.A. A framework for modelling desert locust population dynamics and large-scale dispersal. PLoS Comput. Biol. 2024, 20, e1012562. [Google Scholar] [CrossRef] [PubMed]
- Camps-Valls, G.; Campos-Taberner, M.; Moreno-Martínez, Á.; Walther, S.; Duveiller, G.; Cescatti, A.; Mahecha, M.D.; Muñoz-Marí, J.; García-Haro, F.J.; Guanter, L.; et al. A unified vegetation index for quantifying the terrestrial biosphere. Sci. Adv. 2021, 7, eabc7447. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Kang, Y.; Özdoğan, M.; Zipper, S.; Román, M.; Walker, J.; Hong, S.; Marshall, M.; Magliulo, V.; Moreno, J.; Alonso, L.; et al. How Universal Is the Relationship between Remotely Sensed Vegetation Indices and Crop Leaf Area Index? A Global Assessment. Remote Sens. 2016, 8, 597. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Wang, C.; Chen, J.; Wu, J.; Tang, Y.; Shi, P.; Black, T.A.; Zhu, K. A snow-free vegetation index for improved monitoring of vegetation spring green-up date in deciduous ecosystems. Remote Sens. Environ. 2017, 196, 1–12. [Google Scholar] [CrossRef]
- Badgley, G.; Anderegg, L.D.; Berry, J.A.; Field, C.B. Terrestrial gross primary production: Using NIRV to scale from site to globe. Glob. Change Biol. 2019, 25, 3731–3740. [Google Scholar] [CrossRef]
- Pinty, B.; Verstraete, M. GEMI: A non-linear index to monitor global vegetation from satellites. Vegetatio 1992, 101, 15–20. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. NASA Spec. Publ. 1974, 351, 309. [Google Scholar]
- Pearson, R.L.; Miller, L.D. Remote mapping of standing crop biomass for estimation of the productivity of the shortgrass prairie, Pawnee National Grasslands, Colorado. In Proceedings of the Eighth International Symposium on Remote Sensing of Environment, Ann Arbor, MI, USA, 2–6 October 1972. [Google Scholar]
- Liu, H.Q.; Huete, A. A feedback based modification of the NDVI to minimize canopy background and atmospheric noise. IEEE Trans. Geosci. Remote Sens. 1995, 33, 457–465. [Google Scholar] [CrossRef]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Quinlan, J.R. Induction of decision trees. Mach. Learn. 1986, 1, 81–106. [Google Scholar] [CrossRef]
- Kuhn, M. Building Predictive Models inRUsing thecaretPackage. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Meyer, D.; Dimitriadou, E.; Hornik, K.; Weingessel, A.; Leisch, F.; Chang, C.C.; Lin, C.C. e1071: Misc Functions of the Department of Statistics, Probability Theory Group (Formerly: E1071). R Package Version 1.7-16. 2024. Available online: https://CRAN.R-project.org/package=e1071 (accessed on 3 July 2025).
- Belgiu, M.; Drăguţ, L. Random forest in remote sensing: A review of applications and future directions. ISPRS J. Photogramm. Remote Sens. 2016, 114, 24–31. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Zou, X.; Mõttus, M. Sensitivity of Common Vegetation Indices to the Canopy Structure of Field Crops. Remote Sens. 2017, 9, 994. [Google Scholar] [CrossRef]
- Tan, Y.; Sun, J.Y.; Zhang, B.; Chen, M.; Liu, Y.; Liu, X.D. Sensitivity of a Ratio Vegetation Index Derived from Hyperspectral Remote Sensing to the Brown Planthopper Stress on Rice Plants. Sensors 2019, 19, 375. [Google Scholar] [CrossRef] [PubMed]
- Aeberli, A.; Phinn, S.; Johansen, K.; Robson, A.; Lamb, D.W. Characterisation of Banana Plant Growth Using High-Spatiotemporal-Resolution Multispectral UAV Imagery. Remote Sens. 2023, 15, 679. [Google Scholar] [CrossRef]
- Allen, R.; Dettmann, E.; Johns, G.; Turner, D. Estimation of leaf emergence rates of bananas. Aust. J. Agric. Res. 1988, 39, 53. [Google Scholar] [CrossRef]
- Gonçalves, L.R.; Oliveira, C.W.; Meireles, A.C.M. Spatial distribution of evapotranspiration by fractional vegetation cover index on irrigated cropland banana (Musa Spp.) in the semiarid. Remote Sens. Appl. Soc. Environ. 2023, 29, 100878. [Google Scholar] [CrossRef]
- Lamour, J.; Le Moguédec, G.; Naud, O.; Lechaudel, M.; Taylor, J.; Tisseyre, B. Evaluating the drivers of banana flowering cycle duration using a stochastic model and on farm production data. Precis. Agric. 2020, 22, 873–896. [Google Scholar] [CrossRef]
- Lamour, J.; Naud, O.; Lechaudel, M.; Le Moguédec, G.; Taylor, J.; Tisseyre, B. Spatial analysis and mapping of banana crop properties: Issues of the asynchronicity of the banana production and proposition of a statistical method to take it into account. Precis. Agric. 2019, 21, 897–921. [Google Scholar] [CrossRef]
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P.; et al. Sentinel-2: ESA’s Optical High-Resolution Mission for GMES Operational Services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- Retkute, R.; Gilligan, C.A. Developing a spatio-temporal model for banana bunchy top disease: Leveraging remote sensing and survey data. Front. Plant Sci. 2025, 16, 1521620. [Google Scholar] [CrossRef]


| Index | Formula | Ref. |
|---|---|---|
| Normalized difference vegetation index | [51] | |
| Kernel NDVI | [44] | |
| Ratio vegetation index | [52] | |
| Difference vegetation index | [45] | |
| Enhanced vegetation index | [53] | |
| Soil-adjusted vegetation index | [54] | |
| Modified soil-adjusted vegetation index | [47] | |
| Optimized soil-adjusted vegetation index | [55] | |
| Normalized difference phenology index | [48] | |
| Near-infrared reflectance of vegetation | [49] | |
| Global environment monitoring index | [50] | |
| Case | Split | Date Range | Size |
|---|---|---|---|
| No disease | Training | Apr 2013 to Marc 2014 | 25 |
| No disease | Forecasting | Apr 2014 to Marc 2016 | 50 |
| BBTD | Training | Apr 2013 to Mar 2015 | 100 |
| BBTD | Testing | Apr 2015 to Dec 2015 | 31 |
| BBTD | Forecasting | Jan 2016 to Dec 2019 | 200 |
| Fusarium TR4 | Training | Jun 2013 to Apr 2014 | 48 |
| Fusarium TR4 | Forecasting | May 2014 to Oct 2015 | 79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Retkute, R.; Crew, K.S.; Thomas, J.E.; Gilligan, C.A. Detection of Banana Diseases Based on Landsat-8 Data and Machine Learning. Remote Sens. 2025, 17, 2308. https://doi.org/10.3390/rs17132308
Retkute R, Crew KS, Thomas JE, Gilligan CA. Detection of Banana Diseases Based on Landsat-8 Data and Machine Learning. Remote Sensing. 2025; 17(13):2308. https://doi.org/10.3390/rs17132308
Chicago/Turabian StyleRetkute, Renata, Kathleen S. Crew, John E. Thomas, and Christopher A. Gilligan. 2025. "Detection of Banana Diseases Based on Landsat-8 Data and Machine Learning" Remote Sensing 17, no. 13: 2308. https://doi.org/10.3390/rs17132308
APA StyleRetkute, R., Crew, K. S., Thomas, J. E., & Gilligan, C. A. (2025). Detection of Banana Diseases Based on Landsat-8 Data and Machine Learning. Remote Sensing, 17(13), 2308. https://doi.org/10.3390/rs17132308







