Mapping Wheat Stem Sawfly (Cephus cinctus Norton) Infestations in Spring and Winter Wheat Fields via Multiway Modelling of Multitemporal Sentinel 2 Images
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Selection and Ground Reference Data Collection
2.2. Sentinel 2 Imagery Acquisition and Time Standardization
2.3. Sampled WSS Infestation and Available Sentinel 2 Imagery
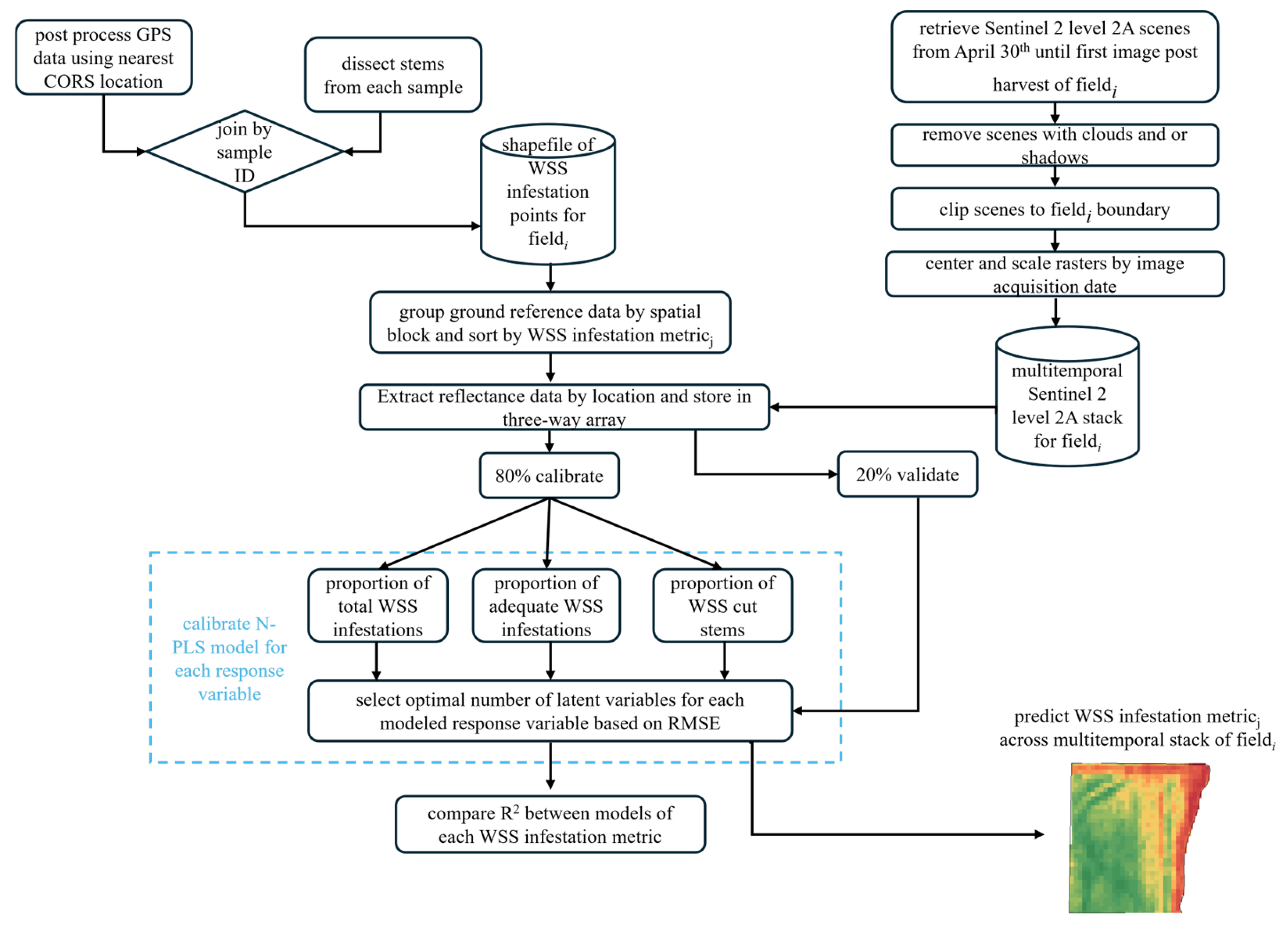
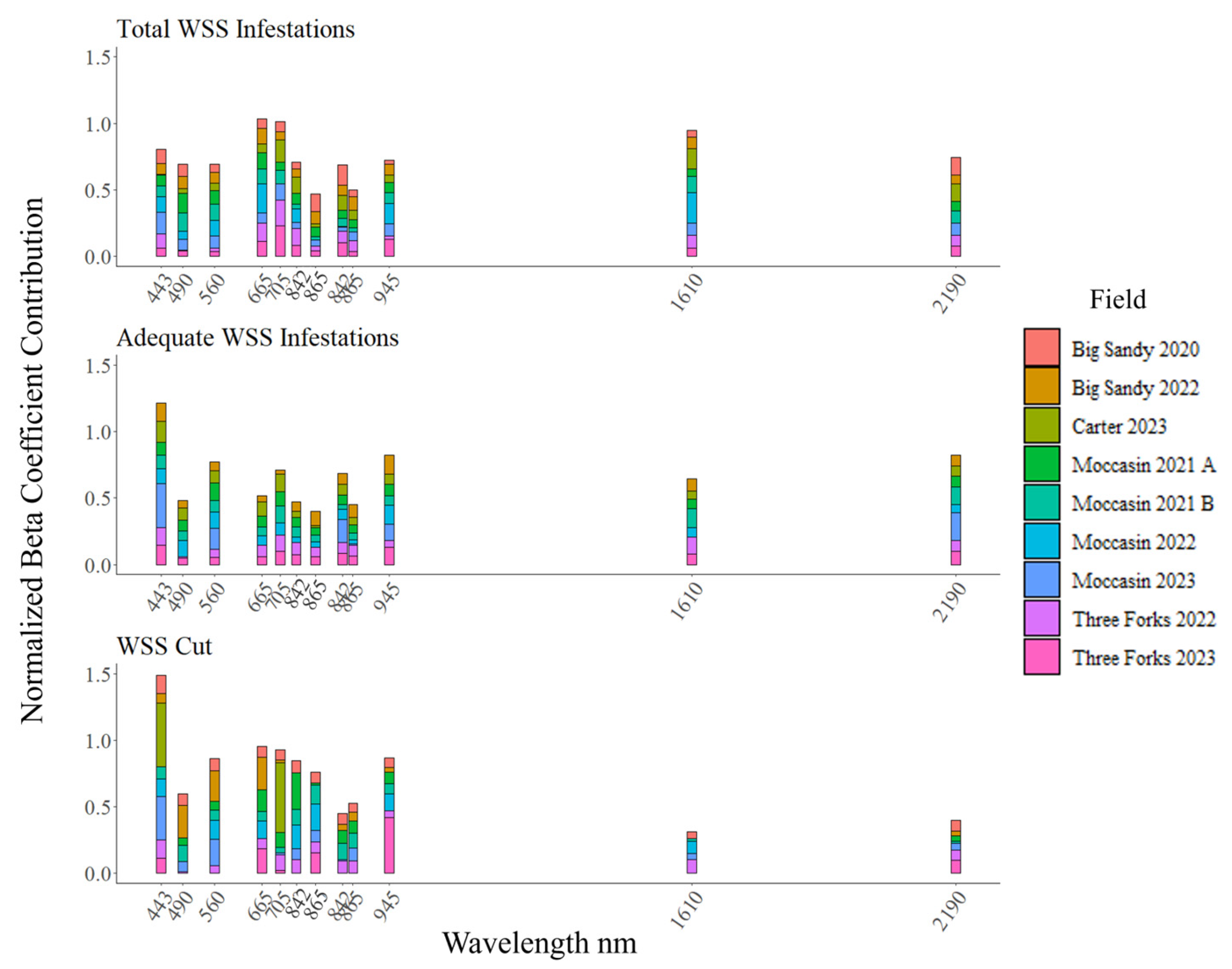
2.4. Sparse Multiway Partial Least Squares Regression Models
3. Results
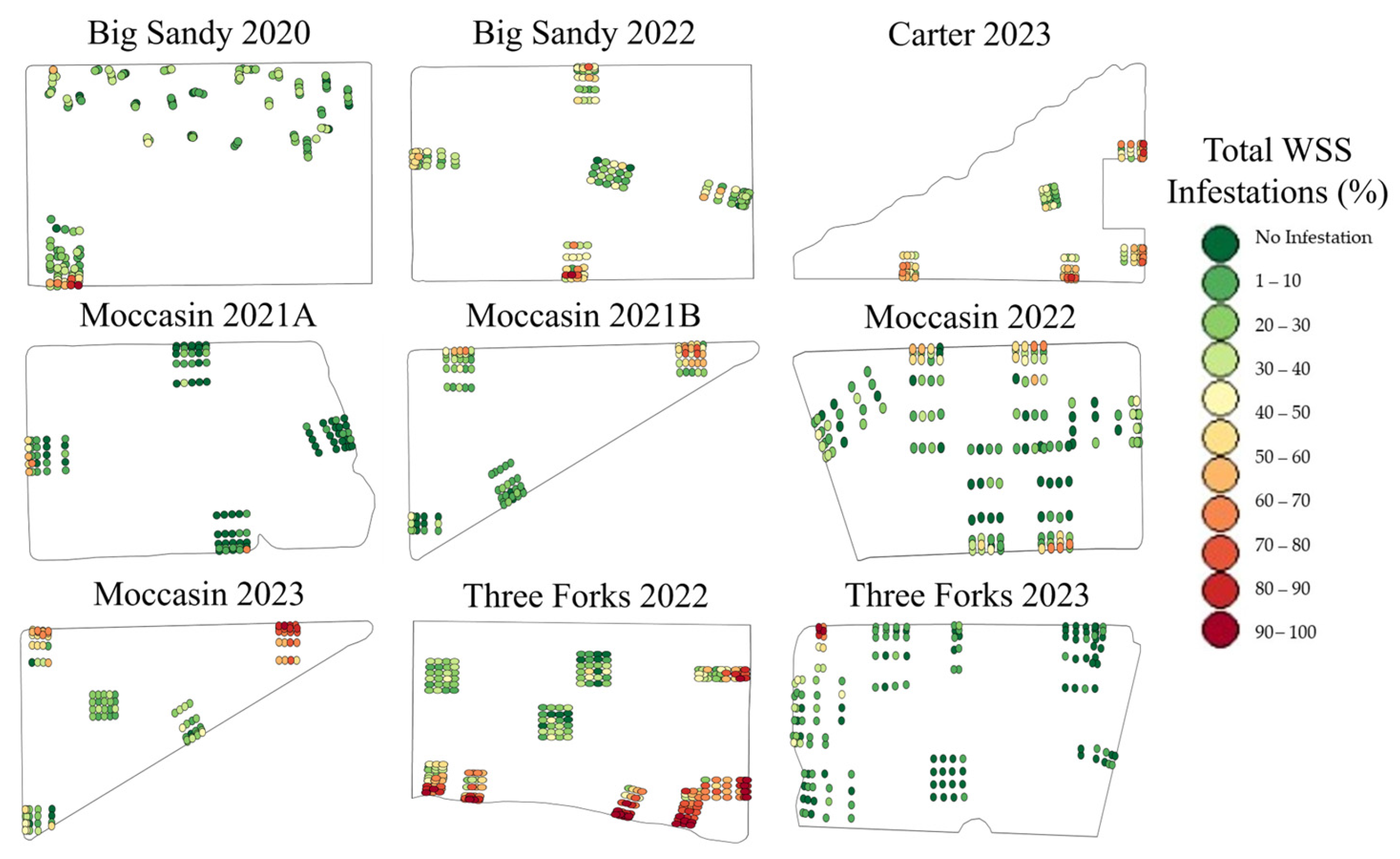
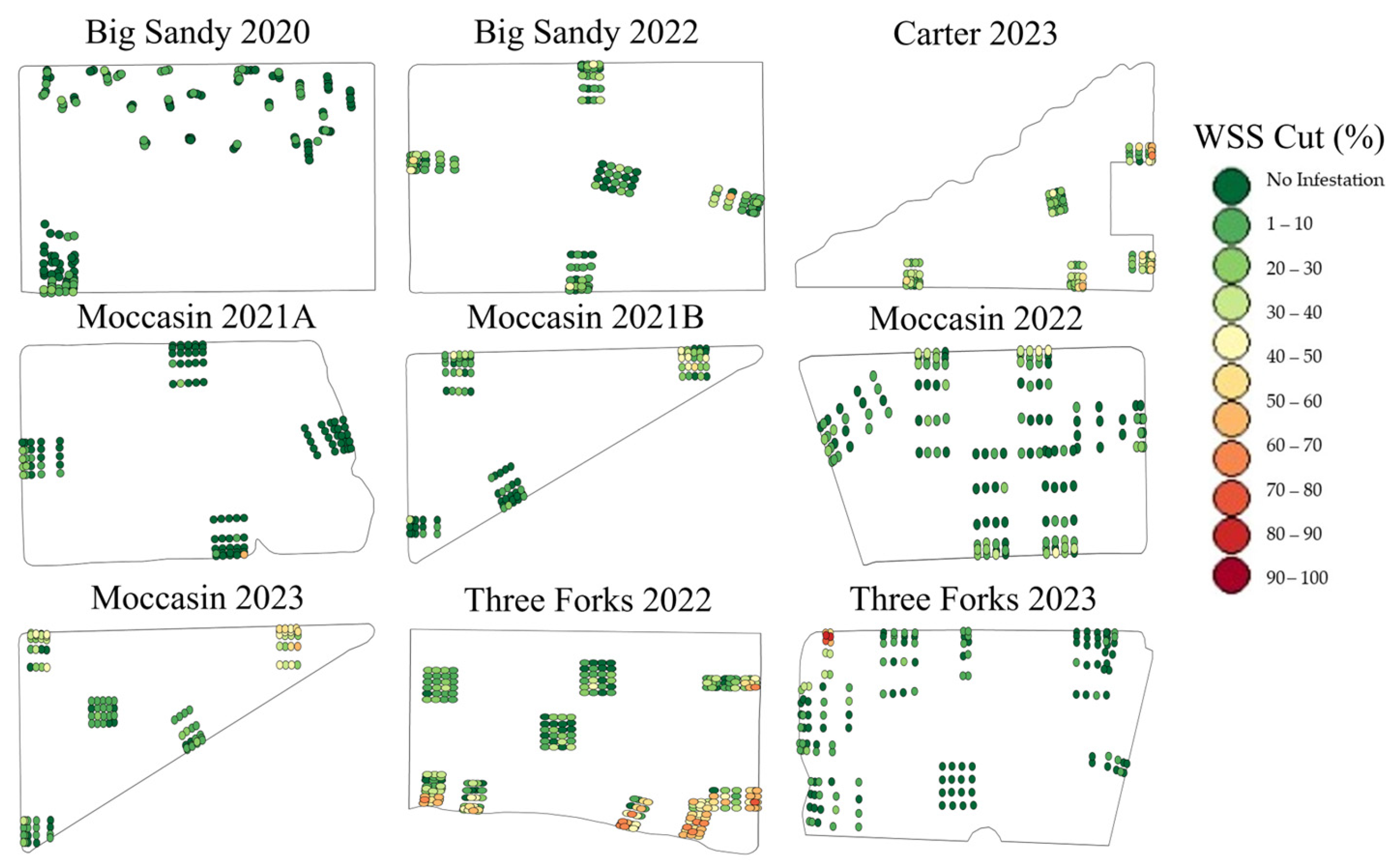
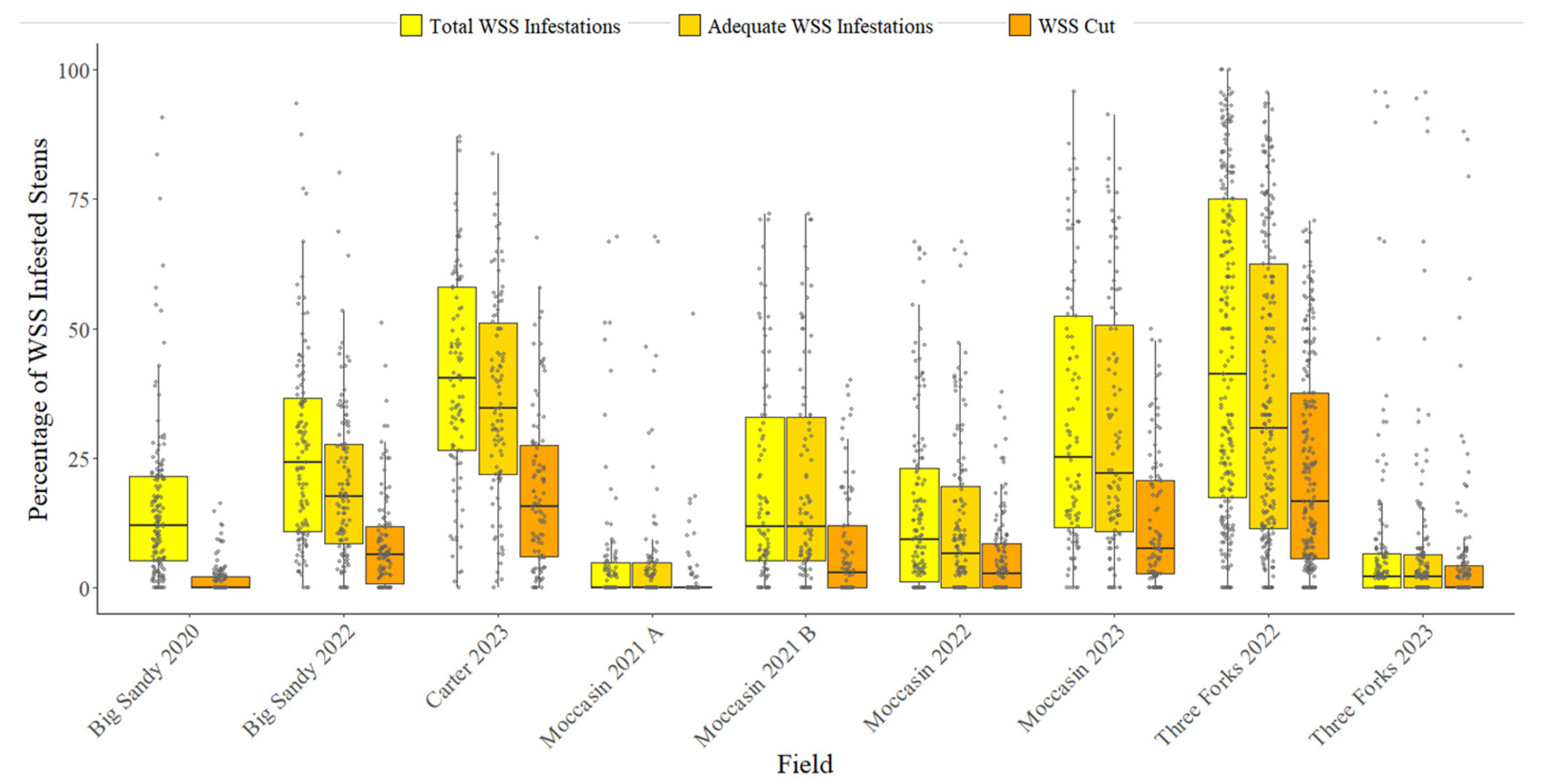
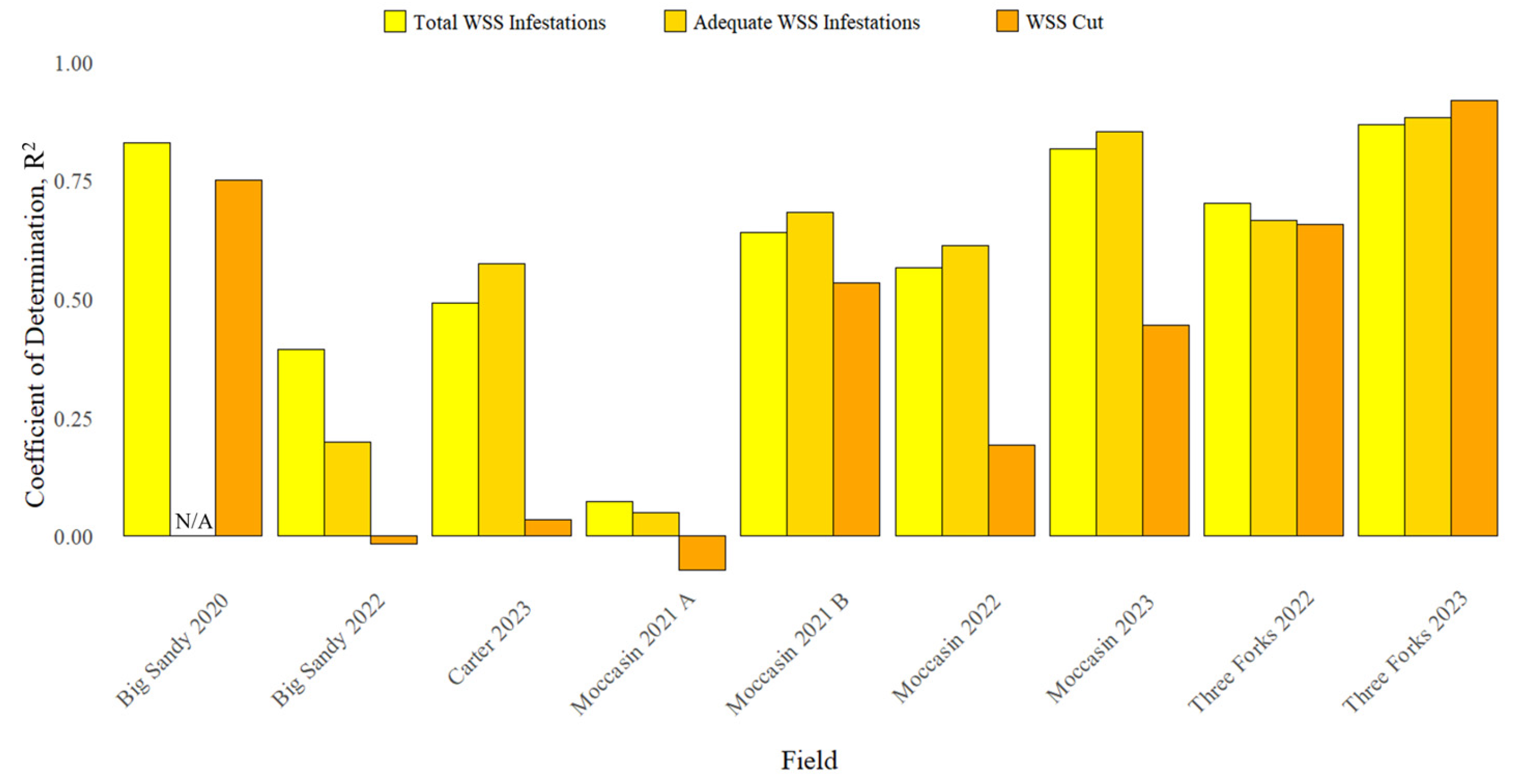
3.1. Surveyed WSS Infestation
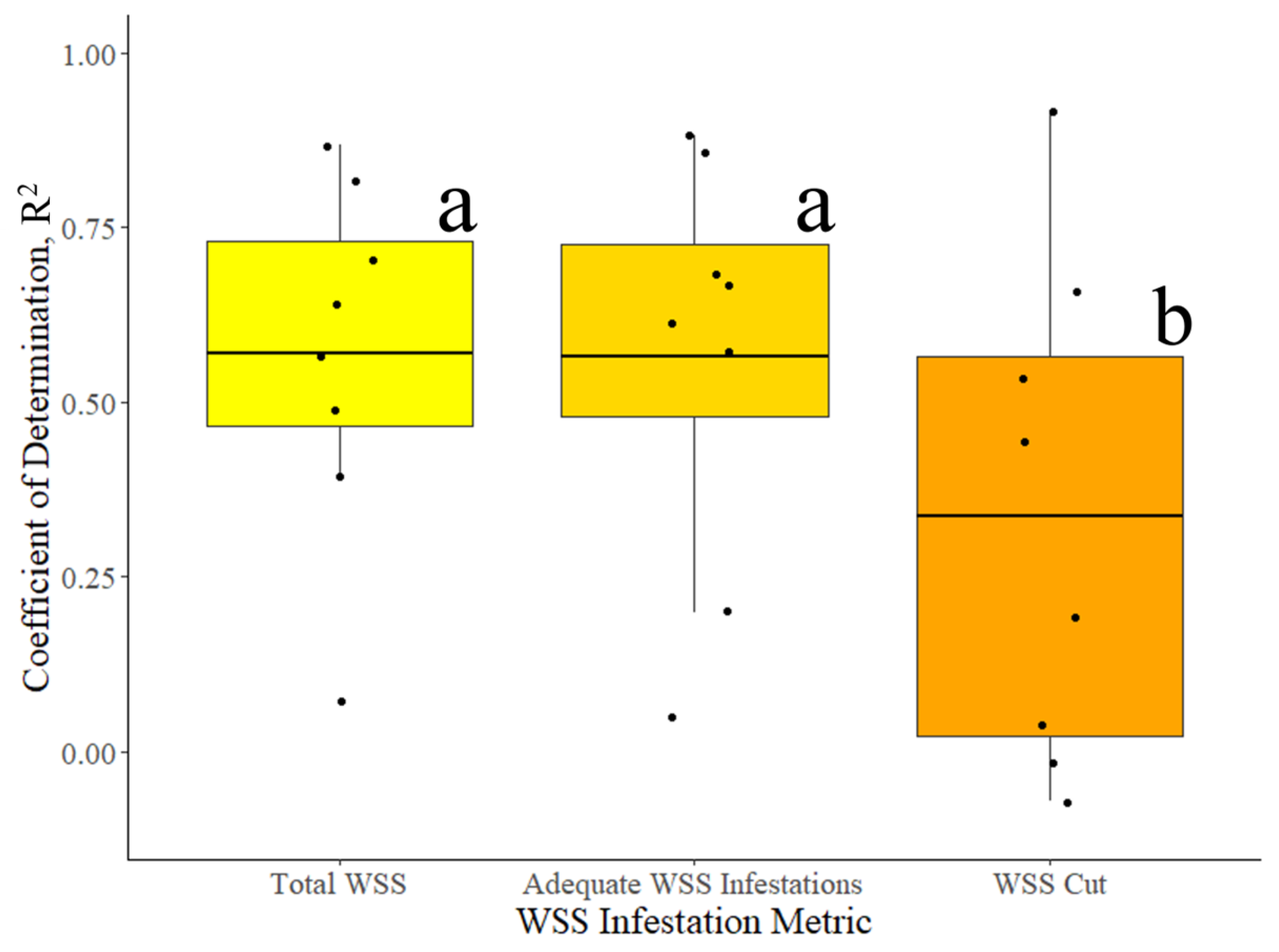
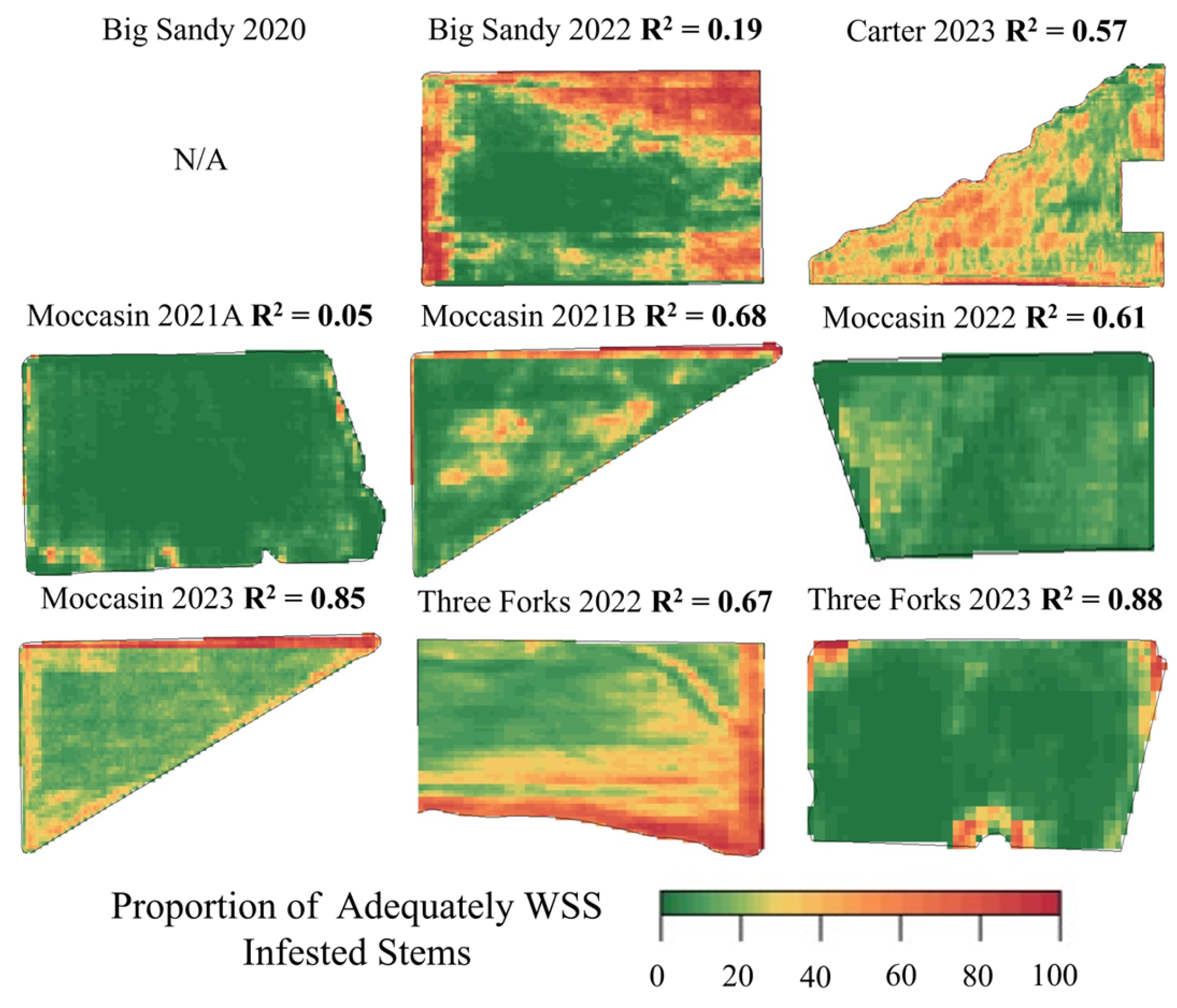
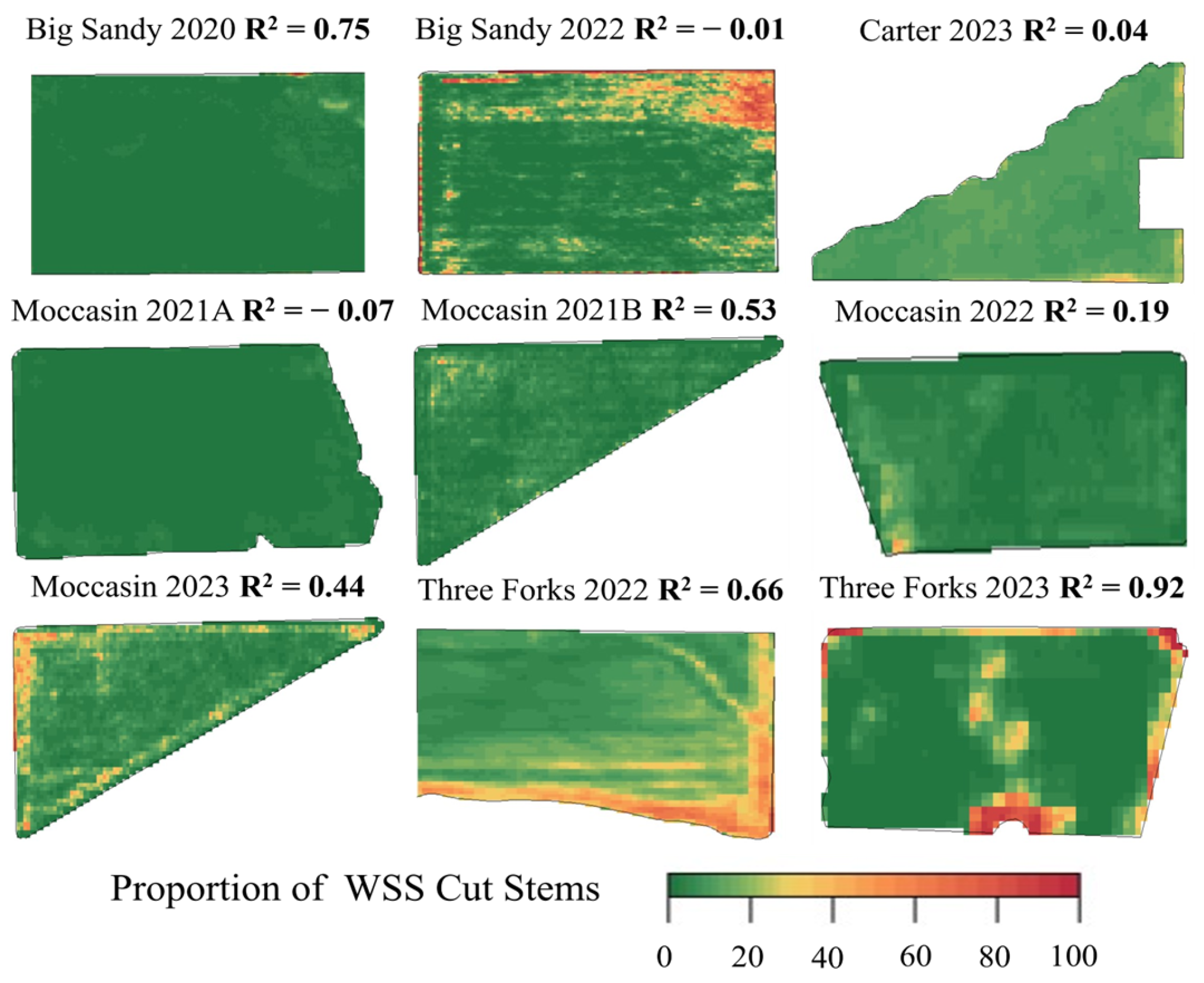
3.2. Modeled WSS Infestation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


| Field | No. Latent Variables | No. Time-Points | No. Bands | Calibration RRMSE | Validation RRMSE | Calibration r2 | Validation r2 | Calibration R2 | Validation R2 |
|---|---|---|---|---|---|---|---|---|---|
| Big Sandy 2020 | 10 | 9 | 5 | 0.528 | 0.438 | 0.835 | 0.921 | 0.698 | 0.830 |
| Big Sandy 2022 | 8 | 9 | 11 | 0.557 | 0.626 | 0.606 | 0.663 | 0.353 | 0.393 |
| Carter 2023 | 7 | 7 | 3 | 0.318 | 0.358 | 0.788 | 0.759 | 0.612 | 0.491 |
| Moccasin 2021A | 9 | 1 | 11 | 0.647 | 5.039 | 0.948 | 0.513 | 0.894 | 0.073 |
| Moccasin 2021B | 7 | 1 | 10 | 0.528 | 0.754 | 0.854 | 0.859 | 0.720 | 0.641 |
| Moccasin 2022 | 5 | 1 | 2 | 0.884 | 0.924 | 0.648 | 0.791 | 0.375 | 0.567 |
| Moccasin 2023 | 9 | 1 | 9 | 0.396 | 0.323 | 0.874 | 0.911 | 0.759 | 0.816 |
| Three Forks 2022 | 2 | 1 | 7 | 0.338 | 0.309 | 0.893 | 0.874 | 0.753 | 0.702 |
| Three Forks 2023 | 10 | 1 | 3 | 0.757 | 0.935 | 0.942 | 0.942 | 0.885 | 0.869 |
| Field | No. Latent Variables | No. Time-Points | No. Bands | Calibration RRMSE | Validation RRMSE | Calibration r2 | Validation r2 | Calibration R2 | Validation R2 |
|---|---|---|---|---|---|---|---|---|---|
| Big Sandy 2020 | * N/A | ||||||||
| Big Sandy 2022 | 10 | 4 | 12 | 0.598 | 0.818 | 0.587 | 0.488 | 0.297 | 0.199 |
| Carter 2023 | 10 | 1 | 5 | 0.379 | 0.352 | 0.725 | 0.817 | 0.513 | 0.574 |
| Moccasin 2021A | 10 | 11 | 11 | 0.626 | 4.51 | 0.951 | 0.404 | 0.891 | 0.049 |
| Moccasin 2021B | 2 | 1 | 11 | 0.577 | 0.689 | 0.819 | 0.873 | 0.668 | 0.682 |
| Moccasin 2022 | 6 | 16 | 11 | 0.969 | 1.015 | 0.666 | 0.799 | 0.392 | 0.612 |
| Moccasin 2023 | 6 | 1 | 1 | 0.422 | 0.301 | 0.866 | 0.938 | 0.739 | 0.854 |
| Three Forks 2022 | 2 | 4 | 8 | 0.395 | 0.413 | 0.877 | 0.823 | 0.744 | 0.665 |
| Three Forks 2023 | 10 | 4 | 11 | 0.856 | 0.810 | 0.922 | 0.941 | 0.848 | 0.882 |
| Field | No. Latent Variables | No. Time-points | No. Bands | Calibration RRMSE | Validation RRMSE | Calibration r2 | Validation r2 | Calibration R2 | Validation R2 |
|---|---|---|---|---|---|---|---|---|---|
| Big Sandy 2020 | 7 | 10 | 11 | 0.905 | 0.682 | 0.709 | 0.868 | 0.428 | 0.752 |
| Big Sandy 2022 | 10 | 9 | 1 | 0.816 | 2.275 | 0.515 | 0.426 | 0.219 | −0.016 |
| Carter 2023 | 1 | 3 | 2 | 0.783 | 1.001 | 0.358 | 0.421 | 0.087 | 0.035 |
| Moccasin 2021A | 9 | 4 | 2 | 0.851 | 9.25 | 0.872 | 0.216 | 0.705 | −0.072 |
| Moccasin 2021B | 10 | 11 | 1 | 0.936 | 0.984 | 0.728 | 0.757 | 0.489 | 0.534 |
| Moccasin 2022 | 7 | 4 | 1 | 0.913 | 1.568 | 0.715 | 0.49 | 0.427 | 0.191 |
| Moccasin 2023 | 9 | 12 | 7 | 0.507 | 0.994 | 0.874 | 0.735 | 0.756 | 0.444 |
| Three Forks 2022 | 2 | 4 | 8 | 0.574 | 0.528 | 0.762 | 0.814 | 0.566 | 0.657 |
| Three Forks 2023 | 10 | 7 | 1 | 1.144 | 0.788 | 0.877 | 0.965 | 0.749 | 0.918 |
References
- Beres, B.L.; Dosdall, L.M.; Weaver, D.K.; Cárcamo, H.A.; Spaner, D.M. Biology and Integrated Management of Wheat Stem Sawfly and the Need for Continuing Research. Can. Entomol. 2011, 143, 105–125. [Google Scholar] [CrossRef]
- Criddle, N. The Western-Stem Sawfly and its Control. Dom. Can. Dept. Ag. 1922, 6, 1–8. [Google Scholar]
- Weaver, D. Wheat Stem Sawfly (Cephus cinctus Norton). In Advances in Understanding Insect Pests Affecting Wheat and Other Cereals; Eigenbrode, S.D., Rashed, A., Eds.; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2023; pp. 93–134. [Google Scholar]
- Weaver, D.; Tallman, S.; Lane, T. Wheat Stem Sawfly Best Management Practices; MSU Extension Bulletin EB0244; USDA-NRCS Agronomy Technical Note MT-95; Montana State University: Bozeman, MT, USA, 2024. [Google Scholar] [CrossRef]
- Morrill, W.L.; Gabor, J.W.; Kushnak, G.D. Wheat Stem Sawfly (Hymenoptera: Cephidae): Damage and Detection. J. Econ. Entomol. 1992, 85, 2413–2417. [Google Scholar] [CrossRef]
- Norton, E. Notes on North American Tenthredinidæ, with Descriptions of New Species. Trans. Amer. Entomol. Soc. (1867–1877) 1872, 4, 77–86. [Google Scholar] [CrossRef]
- Criddle, N. The Life Habits of Cephus cinctus Norton in Manitoba. Can. Entomol. 1923, 55, 1–4. [Google Scholar] [CrossRef]
- Fletcher, J. The Western Wheat Stem Sawfly (Cephus pygmaeus L.). Dom. Can. Dept. Ag. Rep. Dom. Entomol. 1896, 1, 147–149. [Google Scholar]
- Ainslie, C.N. The Western Grass-Stem Sawfly a Pest of Small Grains. In U.S. Department of Agriculture Technology Bulletin; US Department of Agriculture: Washington, DC, USA, 1929; Volume 157, pp. 1–23. [Google Scholar]
- Bekkerman, A.; Weaver, D.K. Modeling Joint Dependence of Managed Ecosystems Pests: The Case of the Wheat Stem Sawfly. J. Ag. Res. Econ. 2018, 43, 172–194. [Google Scholar]
- Beres, B.L.; Cárcamo, H.A.; Byers, J.R. Effect of Wheat Stem Sawfly Damage on Yield and Quality of Selected Canadian Spring Wheat. J. Econ. Entomol. 2007, 100, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Cockrell, D.M.; Randolph, T.; Peirce, E.; Peairs, F.B. Survey of Wheat Stem Sawfly (Hymenoptera: Cephidae) Infesting Wheat in Eastern Colorado. J. Econ. Entomol. 2021, 114, 998–1004. [Google Scholar] [CrossRef]
- Lesieur, V.; Martin, J.-F.; Weaver, D.K.; Hoelmer, K.A.; Smith, D.R.; Morrill, W.L.; Kadiri, N.; Peairs, F.B.; Cockrell, D.M.; Randolph, T.L.; et al. Phylogeography of the Wheat Stem Sawfly, Cephus cinctus Norton (Hymenoptera: Cephidae): Implications for Pest Management. PLoS ONE 2016, 11, e0168370. [Google Scholar] [CrossRef]
- McCullough, C.T.; Hein, G.L.; Bradshaw, J.D. Phenology and Dispersal of the Wheat Stem Sawfly (Hymenoptera: Cephidae) into Winter Wheat Fields in Nebraska. J. Econ. Entomol. 2020, 113, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Olfert, O.; Weiss, R.M.; Catton, H.; Cárcamo, H.; Meers, S. Bioclimatic Assessment of Abiotic Factors Affecting Relative Abundance and Distribution of Wheat Stem Sawfly (Hymenoptera: Cephidae) in Western Canada. Can. Entomol. 2019, 151, 16–33. [Google Scholar] [CrossRef]
- Shanower, T.; Waters, D. A Survey of Five Stem-Feeding Insect Pests of Wheat in the Northern Great Plains. J. Entomol. Sci. 2006, 41, 40–48. [Google Scholar] [CrossRef]
- Achhami, B.; Reddy, G.; Sherman, J.; Peterson, R.; Weaver, D. Effect of Precipitation and Temperature on Larval Survival of Cephus cinctus (Hymenoptera: Cephidae) in Barley Cultivars. J. Econ. Entomol. 2020, 113, 1982–1989. [Google Scholar] [CrossRef]
- Varella, A.C.; Talbert, L.E.; Achhami, B.B.; Blake, N.K.; Hofland, M.L.; Sherman, J.D.; Lamb, P.F.; Reddy, G.V.P.; Weaver, D.K. Characterization of Resistance to Cephus cinctus (Hymenoptera: Cephidae) in Barley Germplasm. J. Econ. Entomol. 2018, 111, 923–930. [Google Scholar] [CrossRef]
- Ainslie, C.N. The Western Grass-Stem Sawfly. In U.S. Department of Agriculture Bulletin; US Department of Agriculture: Washington, DC, USA, 1920; Volume 841, pp. 1–28. [Google Scholar]
- Cockrell, D.M.; Griffin-Nolan, R.J.; Rand, T.A.; Altilmisani, N.; Ode, P.J.; Peairs, F. Host Plants of the Wheat Stem Sawfly (Hymenoptera: Cephidae). Env. Entomol. 2017, 46, 847–854. [Google Scholar] [CrossRef]
- Peirce, E.S.; Rand, T.A.; Cockrell, D.M.; Ode, P.J.; Peairs, F.B. Effects of Landscape Composition on Wheat Stem Sawfly (Hymenoptera: Cephidae) and Its Associated Braconid Parasitoids. J. Econ. Entomol. 2021, 114, 72–81. [Google Scholar] [CrossRef]
- Rand, T.A.; Kula, R.R.; Gaskin, J.F. Evaluating the Use of Common Grasses by the Wheat Stem Sawfly (Hymenoptera: Cephidae) and Its Native Parasitoids in Rangeland and Conservation Reserve Program Grasslands. J. Econ. Entomol. 2024, 117, 858–864. [Google Scholar] [CrossRef]
- Strand, J.; Peterson, R.; Sterling, T.; Weaver, D. Agroecological Importance of Smooth Brome in Managing Wheat Stem Sawfly (Hymenoptera: Cephidae) via Associated Braconid Parasitoids. J. Econ. Entomol. 2024, 117, 2344–2354. [Google Scholar] [CrossRef]
- Wallace, L.E.; McNeal, F.H. Stem Sawflies of Economic Importance in Grain Crops in the United States; Agricultural Research Service, U.S. Department of Agriculture: Washington, DC, USA, 1966.
- Morrill, W.L.; Kushnak, G.D. Planting Date Influence on the Wheat Stem Sawfly (Hymenoptera: Cephidae) in Spring Wheat. J. Ag. Urban Entomol. 1999, 16, 123–128. [Google Scholar]
- Roemhild, G.R. Morphological Resistance of Some of the Gramineae to the Wheat Stem Sawfly (Cephus cinctus Norton). Master’s Thesis, College of Agriculture, Montana State University, Bozeman, MT, USA, 1954. [Google Scholar]
- Morrill, W.L.; Kushnak, G.D.; Bruckner, P.L.; Gabor, J.W. Wheat Stem Sawfly (Hymenoptera: Cephidae) Damage, Rates of Parasitism, and Overwinter Survival in Resistant Wheat Lines. J. Econ. Entomol. 1994, 87, 1373–1376. [Google Scholar] [CrossRef]
- Holmes, N.D. Population Dynamics of the Wheat Stem Sawfly, Cephus cinctus (Hymenoptera: Cephidae), in Wheat. Can. Entomol. 1982, 114, 775–788. [Google Scholar] [CrossRef]
- Villacorta, A.; Bell, R.A.; Callenbach, J.A. Influence of High Temperature and Light on Postdiapause Development of the Wheat Stem Sawfly. J. Econ. Entomol. 1971, 64, 749–751. [Google Scholar] [CrossRef]
- Holmes, N.D. Effects of Moisture, Gravity, and Light on the Behavior of Larvae of the Wheat Stem Sawfly, Cephus cinctus (Hymenoptera: Cephidae). Can. Entomol. 1975, 107, 391–401. [Google Scholar] [CrossRef]
- Beres, B.L.; Cárcamo, H.A.; Bremer, E. Evaluation of Alternative Planting Strategies to Reduce Wheat Stem Sawfly (Hymenoptera: Cephidae) Damage to Spring Wheat in the Northern Great Plains. J. Econ. Entomol. 2009, 102, 2137–2145. [Google Scholar] [CrossRef]
- Seamans, H.L. The Value of Trap Crops in the Control of the Wheat Stem Sawfly in Alberta. Annual Report Entomological Society of Ontario; CABI Pub.: Toronto, ON, Canada, 1928; pp. 59–64. [Google Scholar]
- Weaver, D.; Sing, S.; Runyon, J.; Morrill, W. Potential Impact of Cultural Practices on Wheat Stem Sawfly (Hymenoptera: Cephidae) and Associated Parasitoids. J. Ag. Urban Entomol. 2004, 21, 271–287. [Google Scholar]
- Beres, B.L.; Meers, S.M.; Delaney, K.J.; Cárcamo, H.A.; Miller, P.R.; Spaner, D.M.; Weaver, D.K. Stem Height and Harvest Management Influence Conservation Biological Control of Wheat Stem Sawfly by Endemic Parasitoids. Can. J. Plant Sci. 2025, 105, 1–12. [Google Scholar] [CrossRef]
- Cavallini, L.; Peterson, R.; Weaver, D. Cowpea Extrafloral Nectar Has Potential to Provide Ecosystem Services Lost in Agricultural Intensification and Support Native Parasitoids That Suppress the Wheat Stem Sawfly. J. Econ. Entomol. 2023, 116, 752–760. [Google Scholar] [CrossRef]
- Reis, D.A.; Hofland, M.L.; Peterson, R.K.D.; Weaver, D.K. Effects of Sucrose Supplementation and Generation on Life-History Traits of Bracon cephi and Bracon lissogaster, Parasitoids of the Wheat Stem Sawfly. Phys. Entomol. 2019, 44, 266–274. [Google Scholar] [CrossRef]
- Morrill, W.L.; Weaver, D.K.; Johnson, G.D. Trap Strip and Field Border Modification for Management of the Wheat Stem Sawfly (Hymenoptera: Cephidae). J. Entomol. Sci. 2001, 36, 34–45. [Google Scholar] [CrossRef]
- Delaney, K.J.; Weaver, D.K.; Peterson, R.K.D. Photosynthesis and Yield Reductions from Wheat Stem Sawfly (Hymenoptera: Cephidae): Interactions with Wheat Solidness, Water Stress, and Phosphorus Deficiency. J. Econ. Entomol. 2010, 103, 516–524. [Google Scholar] [CrossRef]
- Sjolie, D.M.; Willenborg, C.J.; Vankosky, M.A. The Causes of Wheat Stem Sawfly (Hymenoptera: Cephidae) Larval Mortality in the Canadian Prairies. Can. Entomol. 2024, 156, e3. [Google Scholar] [CrossRef]
- Dahiya, S.; Sihag, S.; Chaudhary, C. Lodging: Significance and Preventive Measures for Increasing Crop Production. Int. J. Chem. Stud. 2018, 6, 700–705. [Google Scholar]
- Cárcamo, H.; Entz, T.; Beres, B. Estimating Cephus Cinctus Wheat Stem Cutting Damage—Can We Cut Stem Counts? J. Ag. Urban Entomol. 2007, 24, 117–124. [Google Scholar] [CrossRef]
- Lestina, J.; Cook, M.; Kumar, S.; Morisette, J.; Ode, P.J.; Peairs, F. MODIS Imagery Improves Pest Risk Assessment: A Case Study of Wheat Stem Sawfly (Cephus cinctus, Hymenoptera: Cephidae) in Colorado, USA. Environ. Entomol. 2016, 45, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Nansen, C.; Macedo, T.; Swanson, R.; Weaver, D.K. Use of Spatial Structure Analysis of Hyperspectral Data Cubes for Detection of Insect-Induced Stress in Wheat Plants. Int. J. Remote Sens. 2009, 30, 2447–2464. [Google Scholar] [CrossRef]
- Ermatinger, L.S.; Powell, S.L.; Peterson, R.K.D.; Weaver, D.K. Multitemporal Hyperspectral Characterization of Wheat Infested by Wheat Stem Sawfly, Cephus cinctus Norton. Remote Sens. 2024, 16, 3505. [Google Scholar] [CrossRef]
- Haack, B.N. Landsat: A Tool for Development. World Dev. 1982, 10, 899–909. [Google Scholar] [CrossRef]
- Segarra, J.; Buchaillot, M.L.; Araus, J.L.; Kefauver, S.C. Remote Sensing for Precision Agriculture: Sentinel-2 Improved Features and Applications. Agronomy 2020, 10, 641. [Google Scholar] [CrossRef]
- Bhattarai, G.P.; Schmid, R.B.; McCornack, B.P. Remote Sensing Data to Detect Hessian Fly Infestation in Commercial Wheat Fields. Sci. Rep. 2019, 9, 6109. [Google Scholar] [CrossRef]
- Yang, Z.; Rao, M.N.; Elliott, N.C.; Kindler, S.D.; Popham, T.W. Differentiating Stress Induced by Greenbugs and Russian Wheat Aphids in Wheat Using Remote Sensing. Comp. Electr. Ag. 2009, 67, 64–70. [Google Scholar] [CrossRef]
- Macedo, T.B.; Weaver, D.K.; Peterson, R.K.D. Photosynthesis in wheat at the grain filling stage is altered by larval wheat stem sawfly (Hymenoptera: Cephidae) injury and reduced water availability. J. Entomol. Sci. 2007, 42, 228–238. [Google Scholar] [CrossRef]
- Macedo, T.B.; Peterson, R.K.; Weaver, D.K.; Morrill, W.L. Wheat Stem Sawfly, Cephus cinctus Norton, Impact on Wheat Primary Metabolism: An Ecophysiological Approach. Environ. Entomol. 2005, 34, 719–726. [Google Scholar] [CrossRef]
- Achhami, B.; Reddy, G.; Sherman, J.; Weaver, D.; Peterson, R. Antixenosis, Antibiosis, and Potential Yield Compensatory Response in Barley Cultivars Exposed to Wheat Stem Sawfly (Hymenoptera: Cephidae) Under Field Conditions. J. Insect Sci. 2020, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, S.; Ghiraldini, A.; Dramis, A.; Dal Ferro, N.; Morari, F. Estimation of Hail Damage Using Crop Models and Remote Sensing. Remote Sens. 2021, 13, 2655. [Google Scholar] [CrossRef]
- Nansen, C.; Weaver, D.K.; Sing, S.E.; Runyon, J.B.; Morrill, W.L.; Grieshop, M.J.; Shannon, C.L.; Johnson, M.L. Within-Field Spatial Distribution of Cephus cinctus (Hymenoptera: Cephidae) Larvae in Montana Wheat Fields. Can. Entomol. 2005, 137, 202–214. [Google Scholar] [CrossRef]
- Nansen, C.; Macedo, T.B.; Weaver, D.K.; Peterson, R.K.D. Spatiotemporal Distributions of Wheat Stem Sawfly Eggs and Larvae in Dryland Wheat Fields. Can. Entomol. 2005, 137, 428–440. [Google Scholar] [CrossRef]
- Sing, S.E. Spatial and Biotic Interactions of the Wheat Stem Sawfly with Wild Oat and Montana Dryland Spring Wheat. Ph.D. Thesis, College of Agriculture, Montana State University, Bozeman, MT, USA, 2002. [Google Scholar]
- Buteler, M.; Weaver, D.K. Host Selection by the Wheat Stem Sawfly in Winter Wheat and the Role of Semiochemicals Mediating Oviposition Preference. Entomol. Exp. Appl. 2012, 143, 138–147. [Google Scholar] [CrossRef]
- Boryan, C.; Yang, Z.; Mueller, R.; Craig, M. Monitoring US Agriculture: The US Department of Agriculture, National Agricultural Statistics Service, Cropland Data Layer Program. Geocarto Int. 2011, 26, 341–358. [Google Scholar] [CrossRef]
- Brooks, M.; Bolker, B.; Kristensen, K.; Maechler, M.; Magnusson, A.; McGillycuddy, M.; Skaug, H.; Nielsen, A.; Berg, C.; van Bentham, K.; et al. glmmTMB: Generalized Linear Mixed Models Using Template Model Builder. 2024. Available online: https://cran.r-project.org/web/packages/glmmTMB/index.html (accessed on 23 September 2024).
- Lenth, R.V.; Banfai, B.; Bolker, B.; Buerkner, P.; Giné-Vázquez, I.; Herve, M.; Jung, M.; Love, J.; Miguez, F.; Piaskowski, J.; et al. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. 2024. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 23 September 2024).
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-Scale Geospatial Analysis for Everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Main-Knorn, M.; Pflug, B.; Louis, J.; Debaecker, V.; Müller-Wilm, U.; Gascon, F. Sen2Cor for Sentinel-2. In Proceedings of the Image and Signal Processing for Remote Sensing XXIII, Warsaw, Poland, 4 October 2017; SPIE: Washington, DC, USA, 2017; Volume 10427, pp. 37–48. [Google Scholar] [CrossRef]
- Miller, P.; Lanier, W.; Brandt, S. Using Growing Degree Days to Predict Plant Stages 2001. Available online: https://landresources.montana.edu/soilfertility/documents/PDF/pub/GDDPlantStagesMT200103AG.pdf (accessed on 13 September 2024).
- PRISM Climate Group. Available online: https://prism.oregonstate.edu/ (accessed on 30 August 2024).
- Wold, S.; Geladi, P.; Esbensen, K.; Öhman, J. Multi-Way Principal Components-and PLS-Analysis. J. Chemom. 1987, 1, 41–56. [Google Scholar] [CrossRef]
- Bro, R. Multiway Calibration. Multilinear PLS. J. Chemom. 1996, 10, 47–61. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer Series in Statistics; Springer: New York, NY, USA, 2009; ISBN 978-0-387-84857-0. [Google Scholar]
- Roberts, D.R.; Bahn, V.; Ciuti, S.; Boyce, M.S.; Elith, J.; Guillera-Arroita, G.; Hauenstein, S.; Lahoz-Monfort, J.J.; Schröder, B.; Thuiller, W.; et al. Cross-Validation Strategies for Data with Temporal, Spatial, Hierarchical, or Phylogenetic Structure. Ecography 2017, 40, 913–929. [Google Scholar] [CrossRef]
- R Core Team. R Language Definition; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Hervas, D. sNPLS. 2022. Available online: https://cran.r-project.org/web/packages/sNPLS/index.html (accessed on 23 September 2024).
- Hervás, D.; Prats-Montalbán, J.M.; García-Cañaveras, J.C.; Lahoz, A.; Ferrer, A. Sparse N-Way Partial Least Squares by L1-Penalization. Chemom. Intell. Lab. Syst. 2019, 185, 85–91. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection via the Lasso. J. R. Stat. Soc. Ser. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Goodarzi, M.; Freitas, M.P. On the use of PLS and N-PLS in MIA-QSAR: Azole Antifungals. Intell. Lab. Syst. 2009, 96, 59–62. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. 2025. Available online: https://cran.r-project.org/web/packages/lme4/index.html. (accessed on 23 September 2024).
- Lopez-Fornieles, E.; Brunel, G.; Rancon, F.; Gaci, B.; Metz, M.; Devaux, N.; Taylor, J.; Tisseyre, B.; Roger, J.M. Potential of Multiway PLS (N-PLS) Regression Method to Analyse Time-Series of Multispectral Images: A Case Study in Agriculture. Remote Sens. 2022, 14, 216. [Google Scholar] [CrossRef]
- Payne, E.H.; Hardin, J.W.; Egede, L.E.; Ramakrishnan, V.; Selassie, A.; Gebregziabher, M. Approaches for Dealing with Various Sources of Overdispersion in Modeling Count Data: Scale Adjustment versus Modeling. Stat. Methods Med. Res. 2017, 26, 1802–1823. [Google Scholar] [CrossRef]
- Buteler, M.; Peterson, R.K.D.; Hofland, M.L.; Weaver, D.K. Multiple Decrement Life Table Reveals That Host Plant Resistance and Parasitism Are Major Causes of Mortality for the Wheat Stem Sawfly. Environ. Entomol. 2015, 44, 1571–1580. [Google Scholar] [CrossRef]
- Buteler, M.; Weaver, D.K.; Bruckner, P.L.; Carlson, G.R.; Berg, J.E.; Lamb, P.F. Using Agronomic Traits and Semiochemical Production in Winter Wheat Cultivars to Identify Suitable Trap Crops for the Wheat Stem Sawfly. Can. Entomol. 2010, 142, 222–233. [Google Scholar] [CrossRef]
- Buteler, M.; Peterson, R.K.D.; Weaver, D.K. Oviposition Behavior of the Wheat Stem Sawfly When Encountering Plants Infested With Cryptic Conspecifics. Environ. Entomol. 2009, 38, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Ajadi, O.A.; Liao, H.; Jaacks, J.; Delos Santos, A.; Kumpatla, S.P.; Patel, R.; Swatantran, A. Landscape-Scale Crop Lodging Assessment across Iowa and Illinois Using Synthetic Aperture Radar (SAR) Images. Remote Sens. 2020, 12, 3885. [Google Scholar] [CrossRef]
- Hu, X.; Sun, L.; Gu, X.; Sun, Q.; Wei, Z.; Pan, Y.; Chen, L. Assessing the Self-Recovery Ability of Maize after Lodging Using UAV-LiDAR Data. Remote Sens. 2021, 13, 2270. [Google Scholar] [CrossRef]




| Field Name | Longitude | Latitude | Crop | Summer Precipitation (mm) a | No. of Wheat Samples | No. of Cloud-Free Images b | Planting Date | Harvest Date |
|---|---|---|---|---|---|---|---|---|
| Big Sandy 2020 | 110°21′41.76″W | 48°16′9.84″N | Spring Wheat | 165.0 | 145 | 10 | 28 April 2020 | 23 August 2020 |
| Big Sandy 2022 | 110°23′39.84″W | 48°16′3.72″N | Spring Wheat | 117.3 | 115 | 10 | 11 April 2022 | 13 August 2022 |
| Carter 2023 | 110°57′51.12″W | 47°45′35.28″N | Winter Wheat | 221.6 | 98 | 8 | N/A | 26 July 2023 |
| Moccasin 2021 A | 109°52′39.72″W | 47°5′47.40″N | Winter Wheat | 135.2 | 100 | 15 | N/A | 30 July 2021 |
| Moccasin 2021 B | 109°51′33.84″W | 47°4′5.88″N | Winter Wheat | 135.2 | 90 | 17 | 27 September 2021 | 30 July 2021 |
| Moccasin 2022 | 109°52′3.36″W | 47°4′2.64″N | Winter Wheat | 179.9 | 139 | 16 | N/A | 4 August 2022 |
| Moccasin 2023 | 109°51′33.84″W | 47°4′5.88″N | Winter Wheat | 297.5 | 100 | 16 | N/A | 8 August 2023 |
| Three Forks 2022 | 111°36′7.20″W | 46°0′57.96″N | Spring Wheat | 177.5 | 225 | 12 | 5 April 2022 | 20 August2022 |
| Three Forks 2023 | 111°35′38.04″W | 45°59′22.20″N | Winter Wheat | 178.3 | 146 | 13 | 15 September 2023 | 21 August 2023 |
| Field | Total WSS Infestations | Adequate WSS Infestations | WSS Cut |
|---|---|---|---|
| Big Sandy 2020 | 15.64 A ± 15.68 | N/A | 1.51 B ± 2.97 |
| Big Sandy 2022 | 26.78 A ± 18.98 | 20.13 B ± 15.20 | 8.59 C ± 9.59 |
| Carter 2023 | 40.75 A ± 20.52 | 36.43 B ± 19.58 | 18.8 C ± 15.46 |
| Moccasin 2021 A | 5.92 A ± 13.56 | 5.48 A ± 12.66 | 1.75 B ± 6.26 |
| Moccasin 2021 B | 20.35 A ± 20.40 | 20.24 A ± 20.39 | 7.76 B ± 10.88 |
| Moccasin 2022 | 15.18 A ± 17.29 | 12.31 B ± 15.60 | 5.57 C ± 7.89 |
| Moccasin 2023 | 32.42 A ± 25.36 | 31.26 A ± 25.0 | 12.79 B ± 13.13 |
| Three Forks 2022 | 45.67 A ± 31.01 | 36.90 B ± 28.80 | 22.92 C ± 20.30 |
| Three Forks 2023 | 8.18 A ± 18.12 | 7.89 A ± 17.65 | 5.72 B ± 14.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermatinger, L.S.; Powell, S.L.; Peterson, R.K.D.; Weaver, D.K. Mapping Wheat Stem Sawfly (Cephus cinctus Norton) Infestations in Spring and Winter Wheat Fields via Multiway Modelling of Multitemporal Sentinel 2 Images. Remote Sens. 2025, 17, 1950. https://doi.org/10.3390/rs17111950
Ermatinger LS, Powell SL, Peterson RKD, Weaver DK. Mapping Wheat Stem Sawfly (Cephus cinctus Norton) Infestations in Spring and Winter Wheat Fields via Multiway Modelling of Multitemporal Sentinel 2 Images. Remote Sensing. 2025; 17(11):1950. https://doi.org/10.3390/rs17111950
Chicago/Turabian StyleErmatinger, Lochlin S., Scott L. Powell, Robert K. D. Peterson, and David K. Weaver. 2025. "Mapping Wheat Stem Sawfly (Cephus cinctus Norton) Infestations in Spring and Winter Wheat Fields via Multiway Modelling of Multitemporal Sentinel 2 Images" Remote Sensing 17, no. 11: 1950. https://doi.org/10.3390/rs17111950
APA StyleErmatinger, L. S., Powell, S. L., Peterson, R. K. D., & Weaver, D. K. (2025). Mapping Wheat Stem Sawfly (Cephus cinctus Norton) Infestations in Spring and Winter Wheat Fields via Multiway Modelling of Multitemporal Sentinel 2 Images. Remote Sensing, 17(11), 1950. https://doi.org/10.3390/rs17111950






