Towards an Improved High-Throughput Phenotyping Approach: Utilizing MLRA and Dimensionality Reduction Techniques for Transferring Hyperspectral Proximal-Based Model to Airborne Images
Abstract
1. Introduction
2. Materials and Methods
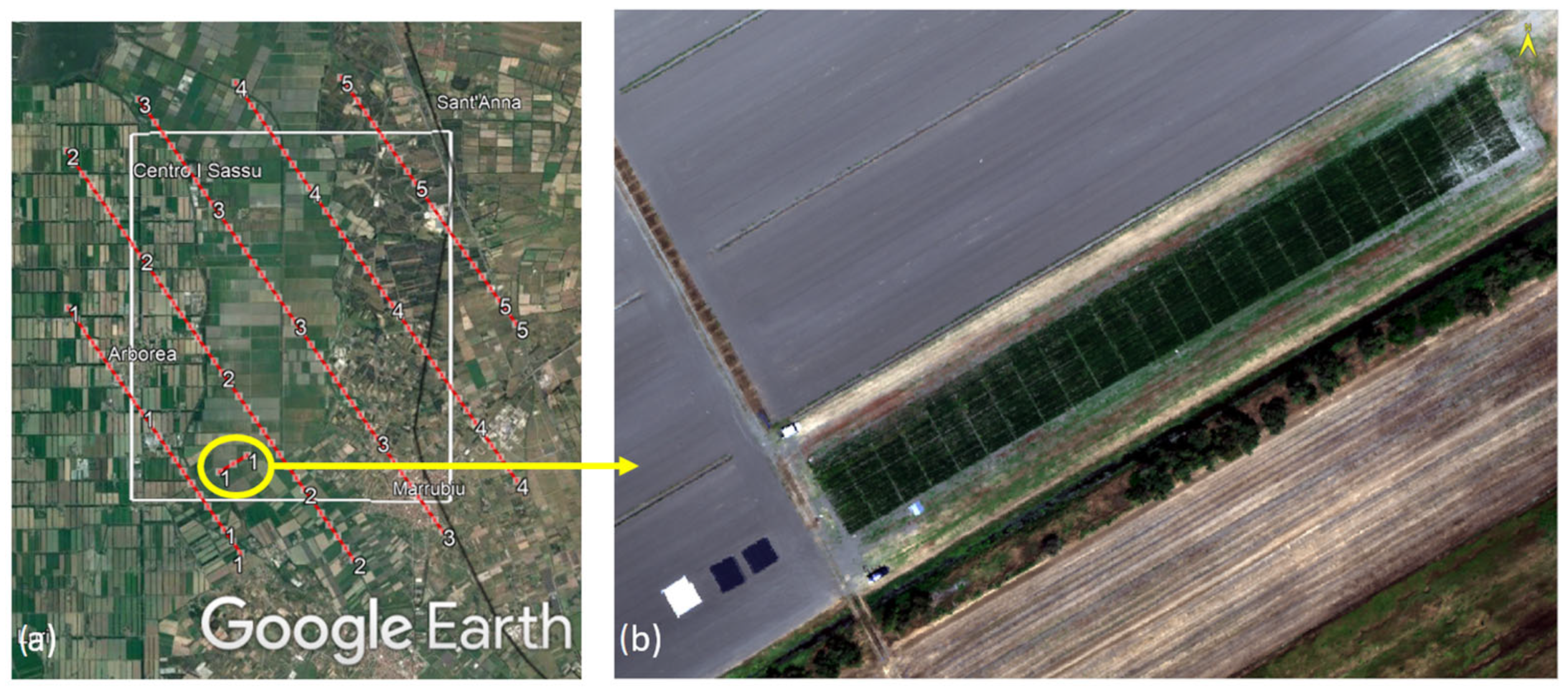
2.1. Experimental Design
2.2. Crop Trait Measurements
- (i)
- LAI: LAI was measured using an LI-COR LAI2200 plant analyser along transects according to an A-10 × B-A scheme, in which A and B represent above and below canopy measurements for each experimental plot. The average of 10 LAI2200 measurements made within each plot was used to describe the LAI in each experimental plot.
- (ii)
- LCC and CCC: LCC was measured using an MC-100 chlorophyll content meter based on the ratio of light transmittance through the plant leaves at 653 and 931 nm spectral bands. LCC measurements were conducted on the last fully developed leaf of 10 randomly selected plants within each experimental plot. CCC was then computed by multiplying LCC and the corresponding LAI values [9].
- (iii)
- LWC and CWC: For LWC determination, five plants for each experimental plot of V3 and V4 varieties were considered, totalling 24 samples. A set of three leaf discs with an 8 mm diameter was sampled on the last fully developed leaf of the selected plants using a handheld punch. The samples were then oven-dried at 50 °C for about three days until reaching a constant weight to retrieve LWC. This destructive sampling of LWC and LMA took place on 29 April, 13 May, and 30 May. CWC was retrieved based on multiplying LWC by LAI values [9].
- (iv)
- LNC and CNC: The aforementioned leaf discs were used to determine leaf nitrogen concentration (Nmass) via dry combustion using the CN element analyser. LNC was then calculated by multiplying Nmass and leaf mass area. Subsequently, CNC was retrieved by multiplying LNC and the corresponding LAI values [9].
2.3. Spectral Data Acquisition
2.3.1. Proximal Sensing—Spectrometer
2.3.2. Manned Airborne Remote Sensing—Aircraft
2.3.3. Spectra Pre-Processing
2.4. DR Analysis
2.5. MLRA Model Generation
2.6. Model Transferability to Airborne Image
2.7. Phenotyping Analysis
3. Results
3.1. MLRA Model Generation for Crop Trait Estimation
3.2. MLRAs Model Transferability
3.3. Wheat Phenotyping
4. Discussion
4.1. Canopy vs. Leaf Traits Accuracy Retrieval
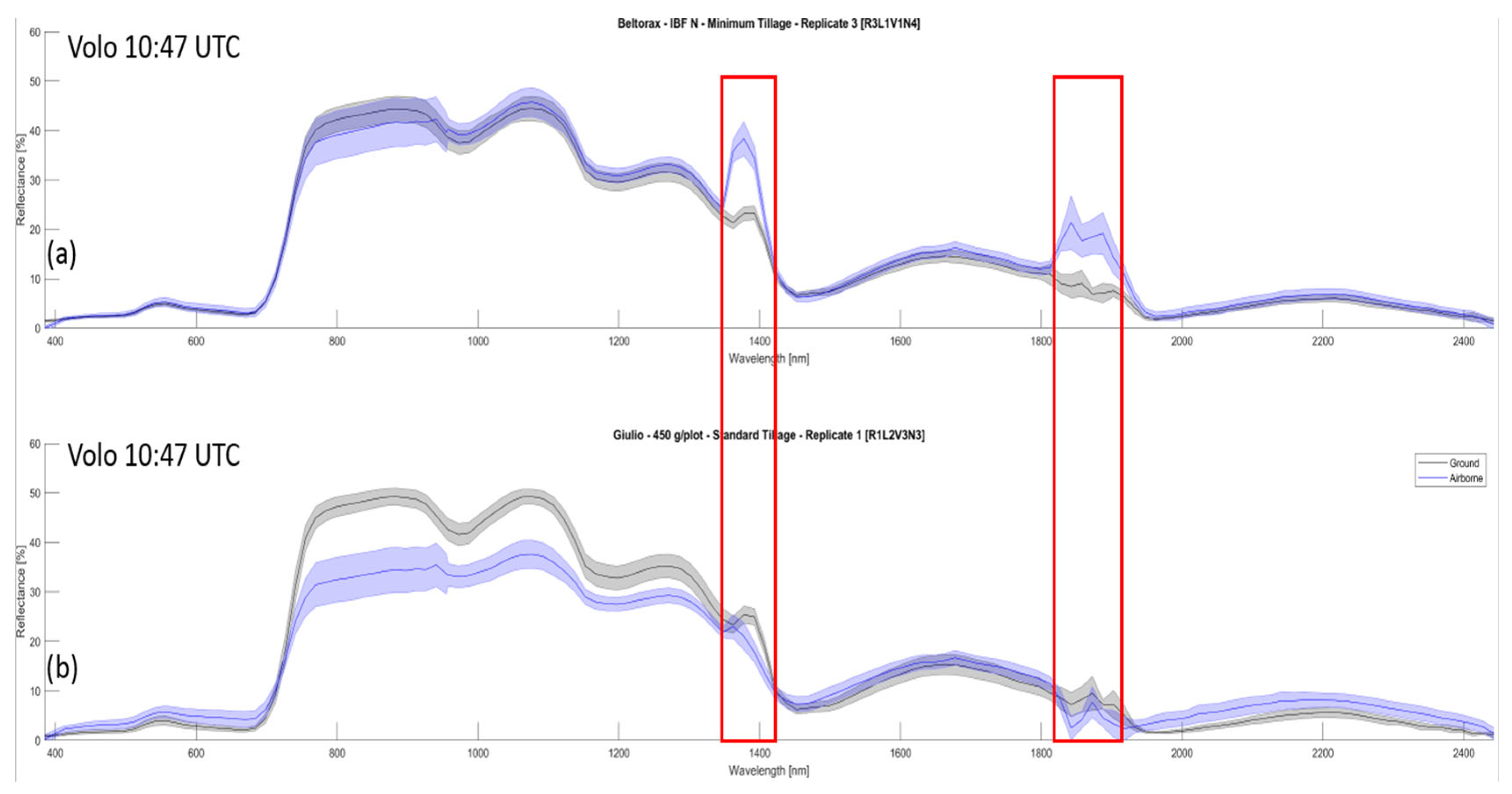
4.2. Tranferability from Ground to Aerial Data
4.3. Phenotyping Considerations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. CASI-SASI Pre-Processing


Appendix B. VIP and PCA Dimensionality Reduction Techniques
Appendix C. Hyperspectral Vegetation Indices and Spectral Features
| VI | Formula | Reference |
|---|---|---|
| CARI | R700 × abs(a × 670 + R670 + b)/R670 × (a2 + 1)0.5 a = (R700 − R550)/150 b = R550 − (a × 550) | [37] |
| CARTER1 | R695/R420 | [38] |
| CARTER2 | R695/R760 | [38] |
| CARTER3 | R605/R760 | [38] |
| CARTER4 | R710/R760 | [38] |
| CARTER5 | R695/R670 | [38] |
| CIgreen | (R780/R550) − 1 | [39] |
| CIrededge | (R780/R710) − 1 | [39] |
| DATT1 | (R850 − R710)/(R850 − R680) | [40] |
| DATT2 | R850/R710 | [40] |
| DVI | R800 − R680 | [41] |
| EVI | 2.5 × ((R800 − R670)/(R800 − (6 × R670) − (7.5 × R475) + 1)) | [42] |
| GI | R554/R677 | [43] |
| GNDVI | (R800 − R550)/(R800 + R550) | [44] |
| MCARI | ((R700 − R670) − 0:2 × (R700 − R550)) × (R700/R670) | [45] |
| MCARI_d_OSAVI | MCARI/OSAVI | [45] |
| mNDVI705 | (R750 − R705)/(R750 + R705 − 2 × R445) | [46] |
| MSAVI | 0.5 × (2 × R800 + 1 − ((2 × R800 + 1)2 − 8 × (R800 − R670))0.5) | [47] |
| MTCI | (R754 − R709)/(R709 − R681) | [48] |
| NDCI | (R762 − R527)/(R762 + R527) | [49] |
| NDNI | (log(1/R1510) − log(1/R1680))/(log(1/R1510) + log(1/R1680)) | [50] |
| NRI1510 | (R1510 − R660)/(R1510 + R660) | [51] |
| NDRE | (R800 − R739)/(R800 + R739) | [52] |
| NDVI | (R800 − R680)/(R800 + R680) | [53] |
| NDVI705 | (R750 − R705)/(R750 + R705) | [54] |
| NVI | (R777 − R747)/R673 | [55] |
| OSAVI | (1 + 0.16) × (R800 − R670)/(R800 + R670 + 0.16) | [56] |
| PRI | (R531 − R570)/(R531 + R570) | [57] |
| PSSR | R800/R635 | [58] |
| REP | 700 + (((40 × (R670 + R780)/2) − R700)/(R740 − R700)) | [55] |
| SPVI | 0.4 × 3.7 × (R800 − R670) − 1.2 × ((R530 − R670)2)0.5 | [59] |
| SR | R800/R680 | [60] |
| TCARI | 3 × ((R700 − R670) − 0.2 × (R700 − R550) × (R700/R670)) | [61] |
| TCARI2 | 3 × ((R750 − R705) − 0.2 × (R750 − R550) × (R750/R705)) | [62] |
| TCARI_d_OSAVI | TCARI/OSAVI | [61] |
| TVI | 0.5 × (120 × (R750 − R550) − 200 × (R670 − R550)) | [63] |
| VOGI1 | R740/R720 | [64] |
| VOGI2 | (R734 − R747)/(R715 + R726) | [64] |
| VOGI3 | D715/D705 | [64] |
| DWI | (R816 − R2218)/(R816 + R2218) | [40] |
| MSGR | (R753 − R708)/(R708 − R681) | [48] |
| NDII | (R819 − R1600)/(R819 + R1600) | [65] |
| NMDGI | (R860 − R1640 − R2130)/(R860 + R1640 − R2130) | [66] |
| NDWGI1 | (R820 − R1650)/(R820 + R1650) | [65] |
| NDWGI2 | (R860 − R1240)/(R860 + R1240) | [67] |
| RDGI | 100 × (R1116 − (min(R1120, R1150)))/(R1116) | [68] |
| RGI1 | R1600/R820 | [69] |
| RGI2 | R900/R970 | [70] |
| RGI3 | R860/R1240 | [71] |
| TDGI | 0.02 × (R670 − R550) + 0.01 × (R670 − R480) | [72] |
| WI | R900/R970 | [73] |
| WLHGI | R676 − (0.5 × (R746 + R665)) | [74] |

Appendix D. Performance Metrics for All the Tested Solutions (MLRA and DR)
| LAI (#232) | CWC (#61) | CCC (#64) | CNC (#44) | LWC (#61) | LCC (#64) | LNC (#44) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | nRMSE | RPD | R2 | nRMSE | RPD | R2 | nRMSE | RPD | R2 | nRMSE | RPD | R2 | nRMSE | RPD | R2 | nRMSE | RPD | R2 | nRMSE | RPD | ||
| Full spectra | PLSR | 0.72 | 11.97 | 1.89 | 0.77 | 10.89 | 2.12 | 0.7 | 14.61 | 1.83 | 0.74 | 14.38 | 1.97 | 0.58 | 17.55 | 1.91 | 0.34 | 16.69 | 1.41 | 0.2 | 17.61 | 1.59 |
| RF | 0.59 | 14.59 | 1.55 | 0.42 | 17.42 | 1.32 | 0.5 | 18.86 | 1.42 | 0.42 | 21.41 | 1.32 | 0.38 | 22.23 | 1.27 | 0.14 | 19.31 | 1.06 | 0.06 | 17.61 | 1.06 | |
| SVR | 0.69 | 12.75 | 1.77 | 0.46 | 16.69 | 1.38 | 0.52 | 18.59 | 1.43 | 0.37 | 22.46 | 1.25 | 0.36 | 22.23 | 1.25 | 0.23 | 18.11 | 1.14 | 0.01 | 17.61 | 1.06 | |
| GPR | 0.68 | 13.46 | 1.68 | 0.45 | 17.42 | 1.32 | 0.53 | 18.78 | 1.42 | 0.49 | 20.38 | 1.38 | 0.37 | 23.23 | 1.24 | 0.21 | 18.2 | 1.13 | 0.01 | 17.61 | 1.06 | |
| NN | 0.82 | 9.66 | 2.34 | 0.63 | 14.52 | 1.58 | 0.66 | 16 | 1.67 | 0.69 | 15.86 | 1.78 | 0.39 | 22.23 | 1.25 | 0.34 | 17.37 | 1.18 | 0.09 | 17.61 | 1.07 | |
| Full Spectra—VIPs | PLSR | 0.72 | 11.9 | 1.9 | 0.76 | 10.89 | 2.27 | 0.72 | 13.9 | 2.01 | 0.74 | 14.42 | 1.96 | 0.62 | 23.4 | 2.45 | 0.35 | 16.48 | 1.43 | 0.21 | 17.6 | 1.6 |
| RF | 0.61 | 14.24 | 1.58 | 0.47 | 16.69 | 1.38 | 0.58 | 17.3 | 1.54 | 0.44 | 20.96 | 1.35 | 0.4 | 23.4 | 1.22 | 0.22 | 18.09 | 1.14 | 0.09 | 17.61 | 1.06 | |
| SVR | 0.69 | 12.7 | 1.78 | 0.45 | 16.69 | 1.38 | 0.62 | 16.61 | 1.6 | 0.47 | 20.55 | 1.37 | 0.37 | 22.7 | 1.26 | 0.34 | 17.03 | 1.21 | 0.07 | 17.61 | 1.06 | |
| GPR | 0.68 | 13.16 | 1.71 | 0.51 | 15.97 | 1.44 | 0.61 | 17.12 | 1.56 | 0.51 | 19.78 | 1.43 | 0.43 | 22 | 1.3 | 0.29 | 17.32 | 1.19 | 0.02 | 17.61 | 1.06 | |
| NN | 0.79 | 10.33 | 2.18 | 0.72 | 14.51 | 1.59 | 0.68 | 15.12 | 1.76 | 0.8 | 12.57 | 2.25 | 0.62 | 17.55 | 1.63 | 0.38 | 16.28 | 1.26 | 0.15 | 17.61 | 1.06 | |
| Dimensionally-reduced spectra—PCA20 | PLSR | 0.81 | 9.9 | 2.28 | 0.8 | 7.26 | 3.18 | 0.76 | 12.92 | 2.29 | 0.74 | 14.38 | 1.96 | 0.58 | 18.25 | 1.91 | 0.33 | 16.68 | 1.41 | 0.2 | 17.61 | 1.59 |
| RF | 0.76 | 11.39 | 1.98 | 0.7 | 14.51 | 1.59 | 0.73 | 14.23 | 1.87 | 0.62 | 17.36 | 1.62 | 0.4 | 21.06 | 1.29 | 0.28 | 17.4 | 1.18 | 0.06 | 17.62 | 1.06 | |
| SVR | 0.76 | 11.18 | 2.02 | 0.63 | 14.51 | 0.59 | 0.65 | 16.5 | 1.61 | 0.47 | 21.91 | 1.29 | 0.39 | 22.23 | 1.28 | 0.27 | 17.46 | 1.18 | 0.22 | 17.62 | 1.06 | |
| GPR | 0.75 | 12.07 | 1.87 | 0.7 | 14.51 | 1.59 | 0.67 | 17.22 | 1.55 | 0.59 | 22.35 | 1.26 | 0.46 | 21.06 | 1.31 | 0.27 | 17.67 | 1.16 | 0.06 | 17.62 | 1.06 | |
| NN | 0.82 | 9.48 | 2.38 | 0.79 | 7.26 | 3 | 0.72 | 14.12 | 1.89 | 0.67 | 16.28 | 1.73 | 0.54 | 18.72 | 1.48 | 0.27 | 17.74 | 1.16 | 0.09 | 17.62 | 1.06 | |
| Dimensionally-reduced spectra—VIs | PLSR | 0.74 | 11.5 | 1.96 | 0.77 | 7.26 | 3.18 | 0.76 | 11.77 | 2.26 | 0.69 | 15.63 | 1.81 | 0.51 | 18.72 | 1.51 | 0.39 | 14.14 | 1.46 | 0.08 | 17.62 | 1.06 |
| RF | 0.75 | 11.39 | 1.98 | 0.76 | 14.51 | 1.59 | 0.68 | 14.99 | 1.78 | 0.61 | 17.56 | 1.61 | 0.52 | 23.4 | 1.22 | 0.22 | 18.45 | 1.12 | 0.2 | 17.61 | 1.06 | |
| SVR | 0.74 | 11.51 | 1.96 | 0.53 | 14.51 | 1.59 | 0.58 | 17.56 | 1.52 | 0.51 | 19.66 | 1.43 | 0.53 | 18.72 | 1.46 | 0.25 | 17.89 | 1.15 | 0.13 | 17.61 | 1.06 | |
| GPR | 0.72 | 12.23 | 1.85 | 0.62 | 14.51 | 1.59 | 0.57 | 17.56 | 1.52 | 0.48 | 20.71 | 1.36 | 0.45 | 21.06 | 1.34 | 0.22 | 18.08 | 1.14 | 0.16 | 17.61 | 1.06 | |
| NN | 0.8 | 10.03 | 2.25 | 0.77 | 14.51 | 1.59 | 0.72 | 13.99 | 1.9 | 0.67 | 16.17 | 1.74 | 0.64 | 16.38 | 1.66 | 0.31 | 17.19 | 1.2 | 0.41 | 15.47 | 1.2 | |
| Dimensionally-reduced spectra—VIs—VIPs | PLSR | 0.74 | 11.45 | 1.97 | 0.76 | 7.26 | 3.18 | 0.75 | 13.37 | 2.15 | 0.73 | 14.68 | 1.92 | 0.62 | 16.36 | 1.75 | 0.29 | 17.25 | 1.34 | 0.35 | 17.61 | 1.59 |
| RF | 0.75 | 11.21 | 2.01 | 0.8 | 10.16 | 2.27 | 0.73 | 13.91 | 1.92 | 0.65 | 16.5 | 1.71 | 0.55 | 23.4 | 1.22 | 0.25 | 18 | 1.14 | 0.23 | 17.61 | 1.06 | |
| SVR | 0.77 | 10.79 | 2.09 | 0.67 | 13.06 | 1.76 | 0.68 | 15.03 | 1.77 | 0.57 | 18.51 | 1.52 | 0.57 | 23.4 | 1.22 | 0.26 | 17.19 | 1.61 | 0.24 | 17.61 | 1.06 | |
| GPR | 0.77 | 11.18 | 2.02 | 0.66 | 13.79 | 1.67 | 0.7 | 14.91 | 1.79 | 0.59 | 18.27 | 1.54 | 0.56 | 23.4 | 1.22 | 0.22 | 18.19 | 1.13 | 0.24 | 17.61 | 1.06 | |
| NN | 0.8 | 10.18 | 2.22 | 0.78 | 20.89 | 2.12 | 0.73 | 13.79 | 1.93 | 0.71 | 15.02 | 1.88 | 0.64 | 11.7 | 2.45 | 0.33 | 16.83 | 1.22 | 0.28 | 17.61 | 1.06 | |
| Dimensionally-reduced spectra—Features | PLSR | 0.77 | 10.75 | 2.1 | 0.62 | 7.26 | 3.18 | 0.53 | 13.48 | 1.98 | 0.63 | 17.04 | 1.66 | 0.49 | 15.21 | 1.82 | 0.22 | 14.2 | 1.45 | 0.09 | 17.61 | 0.97 |
| RF | 0.77 | 10.76 | 2.1 | 0.72 | 14.51 | 1.59 | 0.69 | 14.69 | 1.81 | 0.59 | 17.84 | 1.58 | 0.58 | 23.4 | 1.22 | 0.29 | 17.25 | 1.19 | 0.21 | 17.61 | 1.06 | |
| SVR | 0.74 | 11.53 | 1.96 | 0.59 | 14.51 | 1.56 | 0.58 | 17.27 | 1.54 | 0.55 | 18.81 | 1.5 | 0.52 | 18.72 | 1.44 | 0.25 | 17.8 | 1.6 | 0.07 | 17.81 | 1.04 | |
| GPR | 0.73 | 12 | 1.88 | 0.66 | 7.26 | 1.67 | 0.61 | 17.23 | 1.55 | 0.58 | 18.67 | 1.51 | 0.59 | 18.72 | 1.5 | 0.21 | 18.23 | 1.13 | 0.08 | 27.37 | 0.68 | |
| NN | 0.78 | 10.76 | 2.1 | 0.65 | 7.26 | 1.7 | 0.66 | 15.57 | 1.71 | 0.65 | 16.79 | 1.68 | 0.58 | 18.72 | 1.53 | 0.19 | 18.97 | 1.08 | 0.12 | 17.61 | 1.06 | |
| Dimensionally-reduced spectra—Features—VIPs | PLSR | 0.77 | 10.75 | 2.1 | 0.71 | 14.51 | 1.59 | 0.72 | 13.98 | 2.14 | 0.68 | 15.91 | 1.77 | 0.67 | 16.38 | 1.88 | 0.3 | 17.16 | 1.34 | 0.17 | 17.61 | 1.06 |
| RF | 0.79 | 10.39 | 2.17 | 0.77 | 10.89 | 2.12 | 0.73 | 13.79 | 1.93 | 0.63 | 17.1 | 1.65 | 0.66 | 11.7 | 2.45 | 0.37 | 16.27 | 1.26 | 0.32 | 17.61 | 1.06 | |
| SVR | 0.77 | 11.01 | 2.05 | 0.72 | 12.34 | 1.87 | 0.68 | 14.96 | 1.78 | 0.61 | 17.46 | 1.62 | 0.6 | 23.4 | 1.22 | 0.42 | 15.58 | 1.32 | 0.18 | 17.61 | 1.06 | |
| GPR | 0.76 | 11.2 | 2.02 | 0.72 | 12.34 | 1.87 | 0.66 | 16.95 | 1.57 | 0.6 | 17.93 | 1.57 | 0.66 | 11.7 | 2.45 | 0.39 | 16.13 | 1.28 | 0.18 | 17.61 | 1.06 | |
| NN | 0.82 | 9.64 | 2.34 | 0.78 | 10.89 | 2.12 | 0.73 | 13.71 | 1.94 | 0.64 | 16.78 | 1.68 | 0.69 | 11.7 | 2.45 | 0.36 | 16.59 | 1.24 | 0.11 | 17.61 | 1.06 | |
Appendix E. Performance of MLRA Transferability to Aerial Data
| LAI (#89) | CWC (#22) | CCC (#24) | CNC (#22) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | nRMSE | RPD | PBIAS | R2 | nRMSE | RPD | PBIAS | R2 | nRMSE | RPD | PBIAS | R2 | nRMSE | RPD | PBIAS | ||
| Full spectra | PLSR | - | - | - | - | 0.19 | 45.75 | 0.58 | −30.50 | 0.43 | 43.88 | 0.71 | −26.00 | 0.51 | 21.27 | 1.23 | 10.00 |
| RF | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| SVR | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| GPR | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| NN | 0.62 | 19.46 | 1.15 | −12.50 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Full Spectra—VIPs | PLSR | - | - | - | - | 0.63 | 30.50 | 0.87 | −16.10 | 0.00 | 85.02 | 0.36 | 19.20 | 0.64 | 18.34 | 1.43 | 9.00 |
| RF | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| SVR | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| GPR | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| NN | 0.69 | 14.95 | 1.50 | 7.30 | - | - | - | - | - | - | - | - | 0.49 | 52.85 | 0.50 | −42.10 | |
| Dimensionally-reduced spectra—PCA20 | PLSR | 0.52 | 20.18 | 1.11 | −10.00 | 0.21 | 45.70 | 0.60 | −30.10 | 0.45 | 37.26 | 0.83 | −19.70 | 0.53 | 21.23 | 1.25 | −9.30 |
| RF | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| SVR | 0.52 | 22.34 | 1.00 | −13.50 | - | - | - | - | - | - | - | - | - | - | - | - | |
| GPR | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| NN | 0.55 | 18.56 | 1.21 | 9.80 | 0.26 | 30.50 | 0.87 | 9.80 | - | - | - | - | - | - | - | - | |
| Dimensionally-reduced spectra—VIs | PLSR | - | - | - | - | 0.39 | 15.25 | 1.74 | 1.20 | 0.00 | 85.02 | 0.36 | 19.20 | 0.29 | 49.07 | 0.54 | 40.00 |
| RF | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| SVR | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| GPR | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| NN | 0.00 | 25.59 | 0.88 | 0.40 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Dimensionally-reduced spectra—VIs—VIPs | PLSR | - | - | - | - | 0.55 | 30.50 | 0.87 | 16.20 | 0.02 | 44.61 | 0.69 | 21.30 | 0.04 | 35.45 | 0.74 | −20.00 |
| RF | 0.32 | 39.64 | 0.57 | −31.90 | 0.22 | 61.00 | 0.43 | −40.60 | - | - | - | - | - | - | - | - | |
| SVR | 0.03 | 37.12 | 0.60 | −27.40 | - | - | - | - | - | - | - | - | - | - | - | - | |
| GPR | 0.12 | 27.57 | 0.81 | −15.90 | - | - | - | - | - | - | - | - | - | - | - | - | |
| NN | 0.00 | 26.13 | 0.86 | −7.80 | 0.00 | 76.25 | 0.35 | −47.60 | - | - | - | - | - | - | - | - | |
| Dimensionally-reduced spectra—Features | PLSR | 0.55 | 34.41 | 0.65 | −27.80 | 0.23 | 61.00 | 0.43 | −35.10 | 0.00 | 85.02 | 0.36 | 19.20 | 0.25 | 53.13 | 0.9 | −44.00 |
| RF | 0.61 | 33.33 | 0.67 | −27.60 | - | - | - | - | - | - | - | - | - | - | - | - | |
| SVR | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| GPR | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| NN | 0.31 | 47.21 | 0.48 | −35.80 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Dimensionally-reduced spectra—Features—VIPs | PLSR | 0.18 | 72.61 | 0.31 | −63.00 | 0.67 | 30.50 | 0.87 | 5.10 | 0.13 | 85.22 | 0.36 | −57.80 | 0.55 | 23.82 | 1.10 | −10.00 |
| RF | 0.66 | 27.21 | 0.83 | −21.40 | 0.46 | 61.00 | 0.43 | −36.60 | - | - | - | - | - | - | - | - | |
| SVR | 0.31 | 35.5 | 0.63 | −26.10 | - | - | - | - | - | - | - | - | - | - | - | - | |
| GPR | 0.31 | 27.93 | 0.80 | −16.70 | - | - | - | - | - | - | - | - | - | - | - | - | |
| NN | 0.07 | 63.06 | 0.36 | −47.10 | 0.34 | 45.75 | 0.58 | −26.30 | - | - | - | - | - | - | - | - | |
References
- FAO. World Food Situation—FAO Cereal Supply and Demand Brief (Release Date: 03/09/2020); Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2020. [Google Scholar]
- Heidarian Dehkordi, R.; El Jarroudi, M.; Kouadio, L.; Meersmans, J.; Beyer, M. Monitoring Wheat Leaf Rust and Stripe Rust in Winter Wheat Using High-Resolution Uav-Based Red-Green-Blue Imagery. Remote Sens. 2020, 12, 3696. [Google Scholar] [CrossRef]
- Lee, H.; Wang, J.; Leblon, B. Using Linear Regression, Random Forests, and Support Vector Machine with Unmanned Aerial Vehicle Multispectral Images to Predict Canopy Nitrogen Weight in Corn. Remote Sens. 2020, 12, 2071. [Google Scholar] [CrossRef]
- Herrmann, I.; Berger, K. Remote and Proximal Assessment of Plant Traits. Remote Sens. 2021, 13, 1893. [Google Scholar] [CrossRef]
- Tagliabue, G.; Boschetti, M.; Bramati, G.; Candiani, G.; Colombo, R.; Nutini, F.; Pompilio, L.; Rivera-Caicedo, J.P.; Rossi, M.; Rossini, M.; et al. Hybrid Retrieval of Crop Traits from Multi-Temporal PRISMA Hyperspectral Imagery. ISPRS J. Photogramm. Remote Sens. 2022, 187, 362–377. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Venteo, A.B.; Portalés, E.; Berger, K.; Tagliabue, G.; Garcia, J.L.; Pérez-Suay, A.; Rivera-Caicedo, J.P.; Verrelst, J. Prototyping Crop Traits Retrieval Models for CHIME: Dimensionality Reduction Strategies Applied to PRISMA Data. Remote Sens. 2022, 14, 2448. [Google Scholar] [CrossRef] [PubMed]
- Heidarian Dehkordi, R.; Nutini, F.; Mereu, S.; Candiani, G.; De Peppo, M.; Boschetti, M. Retrieving Biophysical and Biochemical Crop Traits Using Continuum-Removed Absorption Features from Hyperspectral Proximal Sensing. In Proceedings of the 12th IEEE GRSS Workshop on Hyperspectral Image and Signal Processing: Evolution in Remote Sensing WHISPERS, Rome, Italy, 13–16 September 2022. [Google Scholar] [CrossRef]
- Wang, S.; Guan, K.; Wang, Z.; Ainsworth, E.A.; Zheng, T.; Townsend, P.A.; Liu, N.; Nafziger, E.; Masters, M.D.; Li, K.; et al. Airborne Hyperspectral Imaging of Nitrogen Deficiency on Crop Traits and Yield of Maize by Machine Learning and Radiative Transfer Modeling. Int. J. Appl. Earth Obs. Geoinf. 2021, 105, 102617. [Google Scholar] [CrossRef]
- Candiani, G.; Tagliabue, G.; Panigada, C.; Verrelst, J.; Picchi, V.; Caicedo, J.P.R.; Boschetti, M. Evaluation of Hybrid Models to Estimate Chlorophyll and Nitrogen Content of Maize Crops in the Framework of the Future CHIME Mission. Remote Sens. 2022, 14, 1792. [Google Scholar] [CrossRef]
- Pullanagari, R.R.; Dehghan-Shoar, M.; Yule, I.J.; Bhatia, N. Field Spectroscopy of Canopy Nitrogen Concentration in Temperate Grasslands Using a Convolutional Neural Network. Remote Sens. Environ. 2021, 257, 112353. [Google Scholar] [CrossRef]
- Meacham-Hensold, K.; Montes, C.M.; Wu, J.; Guan, K.; Fu, P.; Ainsworth, E.A.; Pederson, T.; Moore, C.E.; Brown, K.L.; Raines, C.; et al. High-Throughput Field Phenotyping Using Hyperspectral Reflectance and Partial Least Squares Regression (PLSR) Reveals Genetic Modifications to Photosynthetic Capacity. Remote Sens. Environ. 2019, 231, 111176. [Google Scholar] [CrossRef]
- Ruiz Hidalgo, D.; Bacca Cortés, B.; Caicedo Bravo, E. Dimensionality Reduction of Hyperspectral Images of Vegetation and Crops Based on Self-Organized Maps. Inf. Process. Agric. 2021, 8, 310–327. [Google Scholar] [CrossRef]
- Verrelst, J.; Malenovský, Z.; Van der Tol, C.; Camps-Valls, G.; Gastellu-Etchegorry, J.P.; Lewis, P.; North, P.; Moreno, J. Quantifying Vegetation Biophysical Variables from Imaging Spectroscopy Data: A Review on Retrieval Methods. Surv. Geophys. 2019, 40, 589–629. [Google Scholar] [CrossRef]
- Cogliati, S.; Sarti, F.; Chiarantini, L.; Cosi, M.; Lorusso, R.; Lopinto, E.; Miglietta, F.; Genesio, L.; Guanter, L.; Damm, A.; et al. The PRISMA Imaging Spectroscopy Mission: Overview and First Performance Analysis. Remote Sens. Environ. 2021, 262, 112499. [Google Scholar] [CrossRef]
- Stevens, A.; Ramirez-Lopez, L.; Hans, G. An introduction to the prospectr package. J. Stat. Softw. 2019, 89, 1–23. [Google Scholar]
- Verrelst, J.; Rivera-Caicedo, J.P.; Reyes-Muñoz, P.; Morata, M.; Amin, E.; Tagliabue, G.; Panigada, C.; Hank, T.; Berger, K. Mapping Landscape Canopy Nitrogen Content from Space Using PRISMA Data. ISPRS J. Photogramm. Remote Sens. 2021, 178, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-Regression: A Basic Tool of Chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Akarachantachote, N.; Seree, C.; Kidakan, S. Cutoff threshold of variable importance in projection for variable selection. Int. J. Pure Appl. Math. 2014, 94, 307–322. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 2065. [Google Scholar] [CrossRef] [PubMed]
- Ranghetti, M.; Boschetti, M.; Ranghetti, L.; Tagliabue, G.; Panigada, C.; Gianinetto, M.; Verrelst, J.; Candiani, G. Assessment of Maize Nitrogen Uptake from PRISMA Hyperspectral Data through Hybrid Modelling. Eur. J. Remote Sens. 2022, 56, 2117650. [Google Scholar] [CrossRef]
- Yang, H.; Du, J. Classification of Desert Steppe Species Based on Unmanned Aerial Vehicle Hyperspectral Remote Sensing and Continuum Removal Vegetation Indices. Optik 2021, 247, 167877. [Google Scholar] [CrossRef]
- Cho, M.A.; Skidmore, A.K. A New Technique for Extracting the Red Edge Position from Hyperspectral Data: The Linear Extrapolation Method. Remote Sens. Environ. 2006, 101, 181–193. [Google Scholar] [CrossRef]
- Roy, D.P.; Li, Z.; Zhang, H.K. Adjustment of Sentinel-2 Multi-Spectral Instrument (MSI) Red-Edge Band Reflectance to Nadir BRDF Adjusted Reflectance (NBAR) and Quantification of Red-Edge Band BRDF Effects. Remote Sens. 2017, 9, 1325. [Google Scholar] [CrossRef]
- Lehnert, L.W.; Meyer, H.; Obermeier, W.A.; Silva, B.; Regeling, B.; Bendix, J. Hyperspectral Data Analysis in R: The hsdar Package. J. Stat. Softw. 2014, 89, 1–23. [Google Scholar] [CrossRef]
- Geladi, P.; Kowalski, B.R. Partial least-squares regression: A tutorial. Anal. Chim. Acta. 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Iqbal, F.; Lucieer, A.; Barry, K. Poppy Crop Capsule Volume Estimation Using UAS Remote Sensing and Random Forest Regression. Int. J. Appl. Earth Obs. Geoinf. 2018, 73, 362–373. [Google Scholar] [CrossRef]
- Zhang, F.; O’Donnell, L.J. Support vector regression. Mach. Learn. Methods Appl. Brain Disord. 2020, 123–140. [Google Scholar] [CrossRef]
- Rasmussen, C.E.; Williams, C.K.I. Gaussian Processes for Machine Learning; MIT Press: Cambridge, MA, USA, 2006; Volume 2–3. [Google Scholar]
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef]
- Heidarian Dehkordi, R.; Pelgrum, H.; Meersmans, J. High Spatio-Temporal Monitoring of Century-Old Biochar Effects on Evapotranspiration through the ETLook Model: A Case Study with UAV and Satellite Image Fusion Based on Additive Wavelet Transform (AWT). GISci. Remote Sens. 2022, 59, 111–141. [Google Scholar] [CrossRef]
- Bellon-Maurel, V.; Fernandez-Ahumada, E.; Palagos, B.; Roger, J.M.; McBratney, A. Critical Review of Chemometric Indicators Commonly Used for Assessing the Quality of the Prediction of Soil Attributes by NIR Spectroscopy. TrAC-Trends Anal. Chem. 2010, 29, 1073–1081. [Google Scholar] [CrossRef]
- Bartholomeus, H.M.; Schaepman, M.E.; Kooistra, L.; Stevens, A.; Hoogmoed, W.B.; Spaargaren, O.S.P. Spectral Reflectance Based Indices for Soil Organic Carbon Quantification. Geoderma 2008, 145, 28–36. [Google Scholar] [CrossRef]
- Chen, H.; Ma, Y.; Zhu, A.; Wang, Z.; Zhao, G.; Wei, Y. Soil Salinity Inversion Based on Differentiated Fusion of Satellite Image and Ground Spectra. Int. J. Appl. Earth Obs. Geoinf. 2021, 101, 102360. [Google Scholar] [CrossRef]
- Kim, T.K. T test as a parametric statistic. Korean J. Anesthesiol. 2015, 68, 540. [Google Scholar] [CrossRef]
- Munappy, A.R.; Bosch, J.; Olsson, H.H.; Arpteg, A.; Brinne, B. Data management for production quality deep learning models: Challenges and solutions. J. Syst. Softw. 2022, 191, 111359. [Google Scholar] [CrossRef]
- Kim, M.; Daughtry, C.; Chappelle, E.; McMurtrey, J.; Walthall, C. The use of high spectral resolution bands for estimating absorbed photosynthetically active radiation (Apar). In Proceedings of the Sixth Symposium on Physical Measurements and Signatures in Remote Sensing, Val D’Isere, France, 17–21 January 1994; p. 299306. [Google Scholar]
- Carter, G.A. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Int. J. Remote Sens. 1994, 15, 697703. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Datt, B. Visible/near infrared reflectance and chlorophyll content in Eucalyptus leaves. Int. J. Remote Sens. 1999, 20, 27412759. [Google Scholar] [CrossRef]
- Tian, Y.C.; Yao, X.; Yang, J.; Cao, W.X.; Hannaway, D.B.; Zhu, Y. Assessing newly developed and published vegetation indices for estimating rice leaf nitrogen concentration with ground- and space-based hyperspectral reflectance. Field Crops. Res. 2011, 120, 299–310. [Google Scholar] [CrossRef]
- Huete, A.; Liu, H.; Batchily, K.; van Leeuwen, W. A comparison of vegetation indices over a global set of TM images for EOS-MODIS. Remote Sens. Environ. 1997, 59, 440451. [Google Scholar] [CrossRef]
- Smith, R.; Adams, J.; Stephens, D.; Hick, P. Forecasting wheat yield in a mediterranean-type environment from the NOAA satellite. Aust. J. Agric. Res. 1995, 46, 113–125. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289298. [Google Scholar] [CrossRef]
- Daughtry, C.; Walthall, C.; Kim, M.; de Colstoun, E.; McMurtrey, J.E., III. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229239. [Google Scholar] [CrossRef]
- Sims, D.; Gamon, J. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.; Kerr, Y.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119126. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P.J. The MERIS terrestrial chlorophyll index. Int. J. Remote Sens. 2004, 25, 54035413. [Google Scholar] [CrossRef]
- Liang, L.; Di, L.; Zhang, L.; Deng, M.; Qin, Z.; Zhao, S.; Lin, H. Estimation of crop LAI using hyperspectral vegetation indices and a hybrid inversion method. Remote Sens. Environ. 2015, 165, 123–134. [Google Scholar] [CrossRef]
- Serrano, L.; Peñuelas, J.; Ustin, S.L. Remote sensing of nitrogen and lignin in mediterranean vegetation from AVIRIS data: Decomposing biochemical from structural signals. Remote Sens. Environ. 2002, 81, 355364. [Google Scholar] [CrossRef]
- Herrmann, I.; Karnieli, A.; Bonfil, D.J.; Cohen, Y.; Alchanatis, V. SWIR-based spectral indices for assessing nitrogen content in potato fields. Int. J. Remote Sens. 2010, 31, 5127–5143. [Google Scholar] [CrossRef]
- Siegmann, B.; Jarmer, T.; Lilienthal, H.; Richter, N.; Selige, T.; Höfled, B. Comparison of narrow band vegetation indices and empirical models from hyperspectral remote sensing data for the assessment of wheat nitrogen concentration. In Proceedings of the the 8th EARSeL Workshop on Imaging Spectroscopy, Nantes, France, 8–10 April 2013; pp. 1–2. [Google Scholar]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127150. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Quantitative estimation of chlorophylla using reflectance spectra: Experiments with autumn chestnut and maple leaves. J. Photochem. Photobiol. B Biol. 1994, 22, 247252. [Google Scholar] [CrossRef]
- Gupta, R.K.; Vijayan, D.; Prasad, T.S. New hyperspectral vegetation characterization parameters. Adv. Space Res. 2001, 28, 201–206. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95107. [Google Scholar] [CrossRef]
- Gamon, J.; Nuelas, J.P.; Field, C. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 3544. [Google Scholar] [CrossRef]
- Blackburn, G.A. Quantifying chlorophylls and caroteniods at leaf and canopy scales: An evaluation of some hyperspectral approaches. Remote Sens. Environ. 1998, 66, 273285. [Google Scholar] [CrossRef]
- Vincini, M.; Frazzi, E.; D’Alessio, P. Angular dependence of maize and sugar beet VIs from directional CHRIS/PROBA data. In Proceedings of the Fourth ESA CHRIS PROBA Workshop, ESRIN, Frascati, Italy, 19–21 September 2006; p. 1921. [Google Scholar]
- Jordan, C.F. Derivation of leaf-area index from quality of light on forest floor. Ecology 1969, 50, 663. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Estimating chlorophyll content from hyperspectral vegetation indices: Modeling and validation. Agric. For. Meteorol. 2008, 148, 12301241. [Google Scholar] [CrossRef]
- Broge, N.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2001, 76, 156172. [Google Scholar] [CrossRef]
- Vogelmann, J.E.; Rock, B.N.; Moss, D.M. Red edge spectral measurements from sugar maple leaves. Int. J. Remote Sens. 1993, 14, 15631575. [Google Scholar] [CrossRef]
- Hardinsky, M.A.; Lemas, V.; Smart, R.M. The influence of soil salinity, growth form, and leaf moisture on the spectral reflectance of Spartina alternifolia canopies. Photogramm. Eng. Remote Sens. 1983, 49, 77–83. [Google Scholar]
- Wang, L.; Qu, J. NMDI: A normalized multi-band drought index for monitoring soil and vegetation moisture with satellite remote sensing. Geophys. Res. Lett. 2007, 34, L20405. [Google Scholar] [CrossRef]
- Gao, B.C. NDWI—A normalized difference water index for remote sensing of vegetationliquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Rollin, E.M.; Milton, E.J. Processing of high spectral resolution reflectance data for the retrieval of canopy water content information. Remote Sens. Environ. 1998, 65, 86–92. [Google Scholar] [CrossRef]
- Hunt, E.R.; Rock, B.N. Detection of changes in leaf water content using near- and middle-rnfrared reflectances. Remote Sens. Environ. 1989, 30, 43–54. [Google Scholar] [CrossRef]
- Peñuelas, J.; Pinol, J.; Ogaya, R.; Filella, I. Estimation of plant water concentration by the reflectance water index WI (R900/R970). Int. J. Remote Sens. 1997, 18, 2869–2875. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Ustin, S.L. Modeling canopy water content for carbon estimates from MODIS data at land EOS validation sites. In IEEE 2001 International Geoscience and Remote Sensing Symposium (Cat. No.01CH37217), Proceedings of the IGARSS 2001—Scanning the Present and Resolving the Future, Sydney, Australia, 9–13 July 2001; IEEE: Cham, Switzerland, 2001; Volume 1, pp. 342–344. [Google Scholar] [CrossRef]
- Hunt, E.R.; Doraiswamy, P.C.; McMurtrey, J.E.; Daughtry, C.S.T.; Perry, E.M.; Akhmedov, B. A visible band index for remote sensing leaf chlorophyll content at the Canopy scale. Int. J. Appl. Earth Obs. Geoinf. 2013, 21, 103–112. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Savé, R. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Gower, J.F.R. Observations of in situ fluorescence of chlorophyll-a in Saanich Inlet. Bound.-Layer Meteorol. 1980, 18, 235–245. [Google Scholar] [CrossRef]








| Feature | Property | Description |
|---|---|---|
| Absorption Features (AFs) and Reflectance Peak Features (RpFs) | Area | sum of band depth values within the identified feature |
| Width | wavelength difference between upper and lower FWHM | |
| RMSE_GC_L (Distance to left) | distances to the Gaussian curve from the maximum towards its left | |
| RMSE_GC_R (Distance to right) | distances to the Gaussian curve from the maximum towards its right | |
| Max value | observed maximum value within the feature | |
| Max value wavelength | wavelength of the Max value | |
| Red-edge Region (RE) | RE_min_R | observed minimum reflectance value within the red spectral channel |
| RE_min_wl | wavelength of the RE_min_R | |
| RE_infl_R | reflectance value at the red-edge inflection point | |
| RE_infl_wl | wavelength of the RE_infl_R | |
| RE_shld_R | reflectance value at the near-infrared shoulder | |
| RE_shld_wl | wavelength of the RE_shld_R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidarian Dehkordi, R.; Candiani, G.; Nutini, F.; Carotenuto, F.; Gioli, B.; Cesaraccio, C.; Boschetti, M. Towards an Improved High-Throughput Phenotyping Approach: Utilizing MLRA and Dimensionality Reduction Techniques for Transferring Hyperspectral Proximal-Based Model to Airborne Images. Remote Sens. 2024, 16, 492. https://doi.org/10.3390/rs16030492
Heidarian Dehkordi R, Candiani G, Nutini F, Carotenuto F, Gioli B, Cesaraccio C, Boschetti M. Towards an Improved High-Throughput Phenotyping Approach: Utilizing MLRA and Dimensionality Reduction Techniques for Transferring Hyperspectral Proximal-Based Model to Airborne Images. Remote Sensing. 2024; 16(3):492. https://doi.org/10.3390/rs16030492
Chicago/Turabian StyleHeidarian Dehkordi, Ramin, Gabriele Candiani, Francesco Nutini, Federico Carotenuto, Beniamino Gioli, Carla Cesaraccio, and Mirco Boschetti. 2024. "Towards an Improved High-Throughput Phenotyping Approach: Utilizing MLRA and Dimensionality Reduction Techniques for Transferring Hyperspectral Proximal-Based Model to Airborne Images" Remote Sensing 16, no. 3: 492. https://doi.org/10.3390/rs16030492
APA StyleHeidarian Dehkordi, R., Candiani, G., Nutini, F., Carotenuto, F., Gioli, B., Cesaraccio, C., & Boschetti, M. (2024). Towards an Improved High-Throughput Phenotyping Approach: Utilizing MLRA and Dimensionality Reduction Techniques for Transferring Hyperspectral Proximal-Based Model to Airborne Images. Remote Sensing, 16(3), 492. https://doi.org/10.3390/rs16030492










