4.2. Analysis of Variance of Mean Squares Using the F-Test
Analysis of variance revealed significant differences for most morphoagronomic and some physiological variables. All the morphoagronomic variables, except the number of plants (NP), showed significant differences among maize hybrids.
Significant differences in morphoagronomic variables among maize hybrids underscored their genetic variability, even under uniform growing conditions. This demonstrates the importance of selecting hybrids that suit specific growing conditions. These differences allow for the selection of hybrids that better meet local needs and conditions [
9,
41].
The number of plants (NP) showed no significant differences among hybrids, despite variations in other variables. This suggests that, under the growing conditions of the present study, all hybrids maintained a similar population density pattern. This is important for the consistency and reliability of the study results, since uniform population density among hybrids reduces potential sources of variation that could mask the effects of genetic traits on other variables under analysis [
42].
Several variables related to photosynthetic capacity and pigment concentration in individual leaves showed significant differences among maize hybrids at different development stages, indicating variations in photochemical efficiency, photosynthetic pigment concentration or plant vigor. The variables φEo and PIabs at 98 DAP suggest variations in photochemical efficiency, indicating hybrid adaptations to more efficient use of photosynthetically active radiation (PAR). At 112 DAP, the variables ABS/Cso, PIabs, and PSRI, and at 119 DAP, Anth, reflect differences in leaf pigment concentration and potentially the ability to protect against environmental stresses at the time of assessments. These findings are consistent with previous studies, highlighting the importance of photosynthetic capacity and pigment concentration associated with photosynthesis and leaf protection in selecting maize hybrids suitable for specific growing conditions [
43].
The UAV RGB (red, green, blue) indices played a significant role in differentiating between maize hybrids. These indices are sensitive to plant traits other than mineral nutrition and water availability, including leaf pigment concentration, organ structure, and overall plant vigor. The ANOVA results revealed significant differences in the RGB components across different color spaces among maize hybrids. These variations can be interpreted in terms of pigment concentration and plant structure. RGB components, representing the intensity of red, green, and blue, respectively, can provide information about plant pigment concentration and distribution [
44]. For example, differences in the red spectrum may indicate variations in chlorophyll concentration, a crucial pigment for photosynthesis. The green spectrum is related to the amount of green mass in plants, while the blue spectrum reflects canopy density and plant structure [
45,
46]. Thus, the differences in RGB components may be associated with variations in leaf pigment concentration, photosynthetic efficiency, canopy growth, and plant development.
Vegetation indices calculated from drone images also showed significant differences among maize hybrids at different development stages. In summary, differences in RGB components across different color spaces and VIs among maize hybrids can be explained in terms of pigment concentration, photosynthetic efficiency, canopy growth, and plant vigor. These results underscore the importance of considering multiple variables and indices when assessing crop performance, given that each can provide specific information about relevant aspects of plant production.
Across different DAP, analysis of variance revealed significant variations in several components and UAV RGB indices. At the early stage (91 DAP), statistically significant differences were observed in components such as SCI, GLI, NGRDI, VARI, and I. These variations can be attributed to the initial plant response to environmental and growth factors. As the crop progressed to 98 DAP, H and S also showed significant differences, indicating possible changes in leaf structure and pigment concentration during this period. At 112 DAP, in addition to previous components, L, a, and b also exhibited significant differences, suggesting a complex evolution in plant color and structure traits. As the crop reached 119 DAP, components u and v displayed significant differences, suggesting possible variations in plant color distribution. Vegetation indices GA, GGA, and CSI also exhibited statistically significant variations across different DAPs, reflecting changes in photosynthetic efficiency and overall plant vigor as the crop cycle progressed. These results highlight the dynamics of plant traits throughout the growth cycle and the importance of considering these changes when assessing the performance of maize hybrids at different development stages [
47].
4.3. Pearson Correlation Analysis
After ANOVA, variables with statistically significant differences were submitted to phenotypic correlation. At different stages of crop development, the results highlighted important correlations between morphoagronomic variables and UAV RGB indices. At 91 DAP, a strong positive correlation was observed between grain yield (GY) and component H (r = 0.97), suggesting a possible relationship between production and pixel hue [
48].
In the early stage at 91 DAP, significant correlations were observed between morphoagronomic variables such as grain yield (GY) and average plant height (AP), with UAV RGB components such as GLI and VARI providing crucial information. The strong positive correlation between GY and the H index suggests that crop development at this stage is related to the amount of green mass [
48]. These findings can be explained by the fact that, at this moment, the plants are in active growth, and photosynthetic efficiency, as well as pigment distribution, plays a crucial role in biomass accumulation [
5].
As the crop advanced to 98 DAP, important correlations were observed, such as the strong association between GY and the CSI. This suggests that, at this stage, crop yield is closely related to leaf pigment concentration. Additionally, significant correlations between CSI and AP indicate that leaf pigment concentration and photosynthetic capacity can influence plant morphology. These associations can be explained by the fact that, at this stage, plants are reaching maximum growth and photosynthetic capacity, and differences in morphoagronomic traits and leaf pigment concentration can affect yields.
At 112 DAP, correlations between morphoagronomic variables and components and UAV RGB indices also provide important information. The strong correlation between GY and UAV RGB variables (NGRDI, VARI, u, GGA, and CSI), with a value of |0.99|, suggests that crop yield may also be related to plant photosynthetic efficiency during the critical phase of maize development, specifically during the grain-filling phase. Furthermore, the correlation between AP and the GA index indicates the importance of leaf chlorophyll concentration for plant morphology and average plant height. At this stage, chlorophyll molecule concentration plays an essential role in yield.
Vegetation indices (VIs) derived from RGB images, such as the Normalized Green–Red Difference Index (NGRDI), serve as indirect indicators of leaf density and distribution, correlating with chlorophyll content in the leaves. Chlorophyll, being a key pigment for photosynthesis, indicates the plant’s photosynthetic capacity. Therefore, higher VIs suggest greater chlorophyll levels and photosynthetic potential. However, while VIs provide an indirect estimate of chlorophyll, they do not measure it directly. The prediction of crop yield using VIs is based on their correlation with agronomic parameters like leaf area, chlorophyll content, and plant biomass, all of which influence yield. Monitoring these parameters through the growth cycle helps identify patterns indicating yield potential.
As plants reach 119 DAP, correlations continue to provide valuable data. The strong correlation (r = |0.99|) between GY and UAV variables (SCI, NGRDI, VARI, H, u, and GA) suggests that yield is related to the color distribution of plant leaves. The negative correlation between AP and the GA index indicates that average plant height may be influenced by the percentage of pixels with the H value in the green range. These associations underscore the complexity of interactions between morphoagronomic traits, leaf pigment concentration, and plant structure in the late growth cycle.
It is important to note that the relationship between photosynthetic capacity and the bands used in each index is complex. In general, the use of different colored bands (red, green, blue) to calculate UAV RGB indices is directly associated with the absorption and reflectance of chlorophyll/carotenoids, canopy density, plant morphology, and the amount of non-photosynthetic pigments present in the leaves. For example, the VARI, which uses red, green, and blue bands, is sensitive to leaf chloroplast pigment concentration and can reflect differences in chlorophyll and carotenoid concentration, essential in the photochemical phase of photosynthesis. A strong correlation was observed between grain yield (GY) and the VARI at 98 DAP (r = 0.91), 112 DAP and 119 DAP (r = 0.99). This correlation indicates a strong and significant association between this vegetation index and crop yield [
47].
Among other morphoagronomic variables analyzed at different maize development stages, several exhibited significant correlations with UAV RGB indices. At 91 DAP, female flowering (FF) revealed a strong correlation (r = −0.91) with the VARI, suggesting the influence of leaf photosynthetic pigments in the early stages of the crop. In addition, at 91 DAP, average plant height (AP) showed a strong correlation (r = 0.76) with the L index, related to plant morphology, indicating that morphoagronomic traits are highly relevant in the early stages of maize growth. Additionally, other variables such as average ear diameter (AED), number of ears (NE), and 100-grain weight (100 GW) also showed significant correlations with several UAV RGB indices, including SCI, GLI, NGRDI, GA, and CSI. The ir values for these correlations varied, underscoring the complex relationships between plant morphology and the traits obtained from drone images, ranging from 0.73 to 0.88.
In regard to variables associated with photosynthetic capacity and leaf pigments, the only significant correlation observed was between Anth at 119 DAP and UAV RGB indices. This result demonstrates a notable association between Anth and remote sensing indices. The best correlation (r = −0.90) was observed with the GGA index. This implies that variations in the Anth concentration of maize leaves are strongly related to variations in the GGA index, which represents green traits in drone images. This strong correlation suggests that the amount of Anth pigment influences the quantity and distribution of green mass in maize plants, which, in turn, affects the reflectance of green captured by drone sensors. This relationship is important because it may indicate that the concentration of this pigment in maize leaves is strongly linked to vigor, photosynthetic efficiency, and response to variability in this species. Thus, drone images and UAV RGB indices are promising tools for assessing not only leaf pigment concentration but also the photosynthetic capacity and vigor of maize plants in the field. This information can be crucial for improving agronomic management and selecting genotypes with desirable leaf pigment concentration and plant vigor traits [
49].
4.4. Analysis of Tukey’s Test for Pairwise Mean Comparison
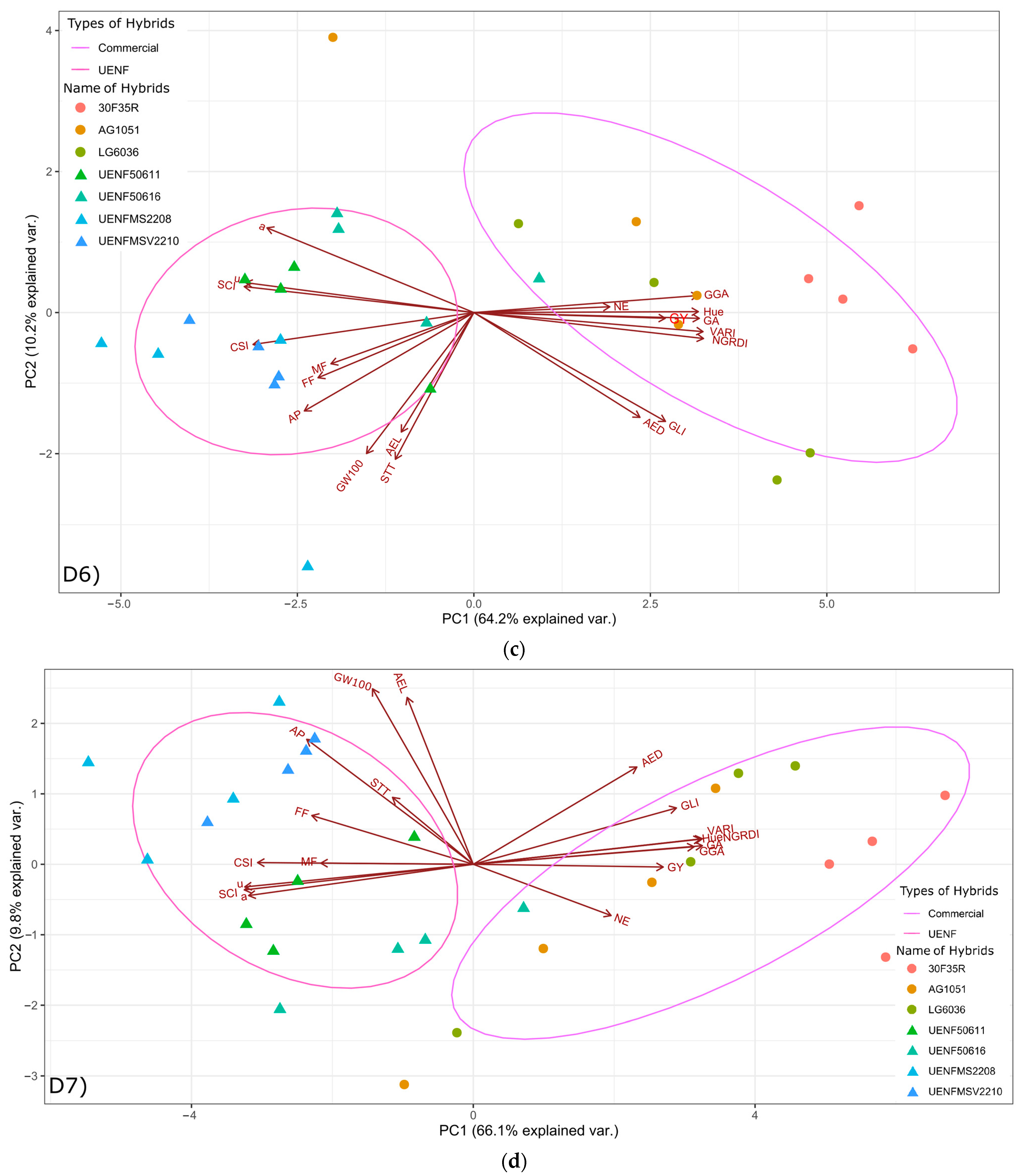
Our analysis of the Tukey’s test results revealed important information about the performance of local and commercial hybrids at different stages of crop development, especially indices that showed a strong correlation with grain yield. For example, at 112 and 119 DAP, the VARI, when compared to commercial hybrids (H6, H7, and H8), showed similar and inferior responses for local hybrids (H1, H2, H3, and H4). Hybrid H7 obtained the highest VARI value, indicating a strong association with yield. With respect to indices such as GLI, NGRDI at 112 DAP and 119 DAP, commercial hybrids (H7 and H8) demonstrated superior performance, suggesting a potential advantage in later development stages, given the positive correlation between these indices and grain yield. The SCI showed a strong negative correlation with grain yield and generally exhibited inverse clustering compared to other indices. When assessing the performance of different hybrids associated with indices related differently to agricultural yield, according to the DAP, it is essential to consider the importance of different crop development stages in the assessment.
Hybrid 30F35R (H7) showed the highest grain yield, exceeding all local hybrids. This difference is associated with the fact that the commercial hybrid is a simple variety, resulting from the crossing of two inbred lines, while local hybrids originate from interpopulation crosses. In this case, while simple hybrids capitalize on 100% of heterosis and are more uniform, interpopulation hybrids partially capture heterosis and obtain lower yields [
50].
At 119 DAP and with respect to the SCI (Soil Color Index), commercial hybrids H6, H7, and H8 showed significantly lower values when compared to their local counterparts, indicating a possible difference in the average RGB values of hybrid plots. Hybrid H7 had the highest GLI (Green Leaf Index) value, suggesting a larger number of green leaves on the plants. This difference may be related to a larger photosynthetic area, since more chlorophyll can result in higher photosynthetic carbon assimilation. H7 also showed the highest NGRDI value, indicating a greater difference between green and red bands. This may be related to leaf pigment concentration, and chlorophyll concentration (since chlorophyll molecules absorb light in the electromagnetic spectrum of red and blue, and different concentrations change the NGRDI value), which can directly influence photosynthetic capacity. For VARI (Visible Atmospherically Resistant Index), H7 once again obtained the highest value, indicating greater visible atmospheric resistance. This variable is associated with plant canopy morphology (canopy leaf area). In relation to the H index (Hue), hybrid H7 had the highest value, suggesting differences between hybrids in leaf color traits. With respect to the S index (saturation), hybrid H6 exhibited the highest value, indicating the degree of color purity in plants. High saturation indicates a pure color, and low saturation indicates a more faded color, i.e., close to a gray tone. The highest S index value may be related to the leaf vigor and greater photosynthetic capacity. In regard to the a index, hybrid H7 obtained the lowest value, indicating different a* color channel intensities, which are associated with the range of reds and magentas in plants. Hybrid H7 obtained the lowest value for the u index, indicating different u* color channel intensities, and the highest GA value (Green Area Index), suggesting a larger leaf area with greater photosynthetic capacity. Hybrid H7 also had the highest GGA index (Greener Area Index), indicating that this hybrid has the largest green leaf color area. The CSI (Crop Senescence Index), associated with early plant senescence [
51,
52], was highest in hybrid H4.
The results obtained from the indices in this study indicate that differences in leaf pigment concentration, photosynthetic capacity, canopy leaf area, and plant vigor are crucial in differentiating maize hybrids. Furthermore, there were differences in clustering between local and commercial hybrids, with H7 often obtaining the highest or lowest values in a number of indices (at 119 DAP: SCI, GLI, NGRDI, VARI, H, a, u, GA, GGA, and CSI). These results can be attributed to hybrid genetics. An analysis of variations in leaf color traits, associated with leaf area and pigment concentration, can provide important information for hybrid selection as well as correct maize crop management.
In summary, in the differentiation of maize hybrids, the Tukey’s test results underscored the importance of variables related to leaf pigment concentration, photosynthetic capacity, canopy density, and overall plant vigor. Additionally, the persistence of these differences over time indicates the lasting influence of these traits on maize crop performance. These results can contribute to hybrid selection and the development of more effective agronomic management strategies to optimize maize plant production.