Field Measurements of Spatial Air Emissions from Dairy Pastures Using an Unmanned Aircraft System
Abstract
1. Introduction
2. Materials and Methods
2.1. Unmanned Aircraft System and Air Analyzer
2.2. Test Site and Data Collection
2.3. Sampling Bag Analysis
2.4. Data Analysis and Sensitivity Tests
3. Results
3.1. Propeller Downwash
3.2. Kriging Interpolation and Sensitivity Analysis
3.3. Gas Chromatography Analysis and Quality Control
3.4. Gas and Particulate Matter Concentrations
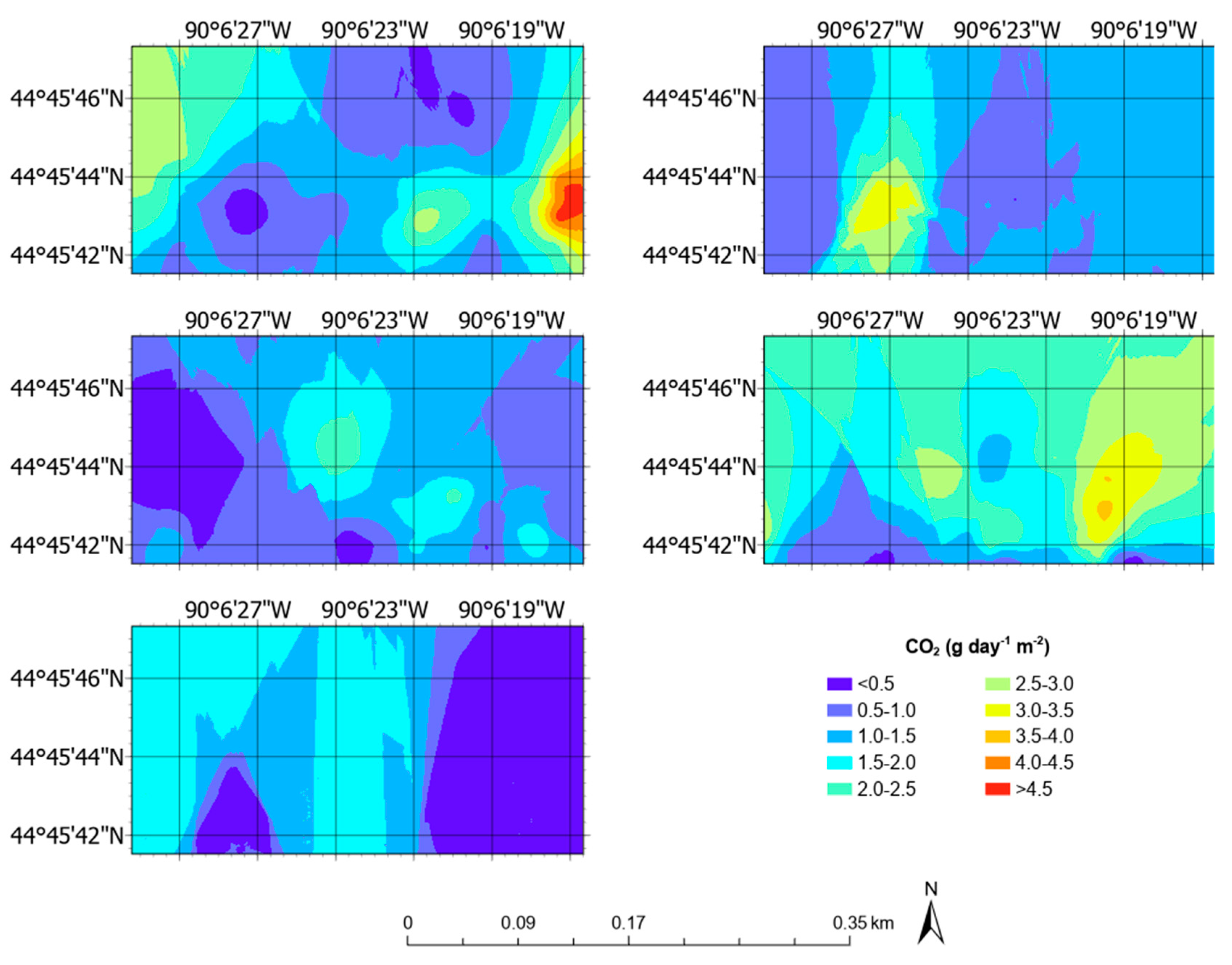
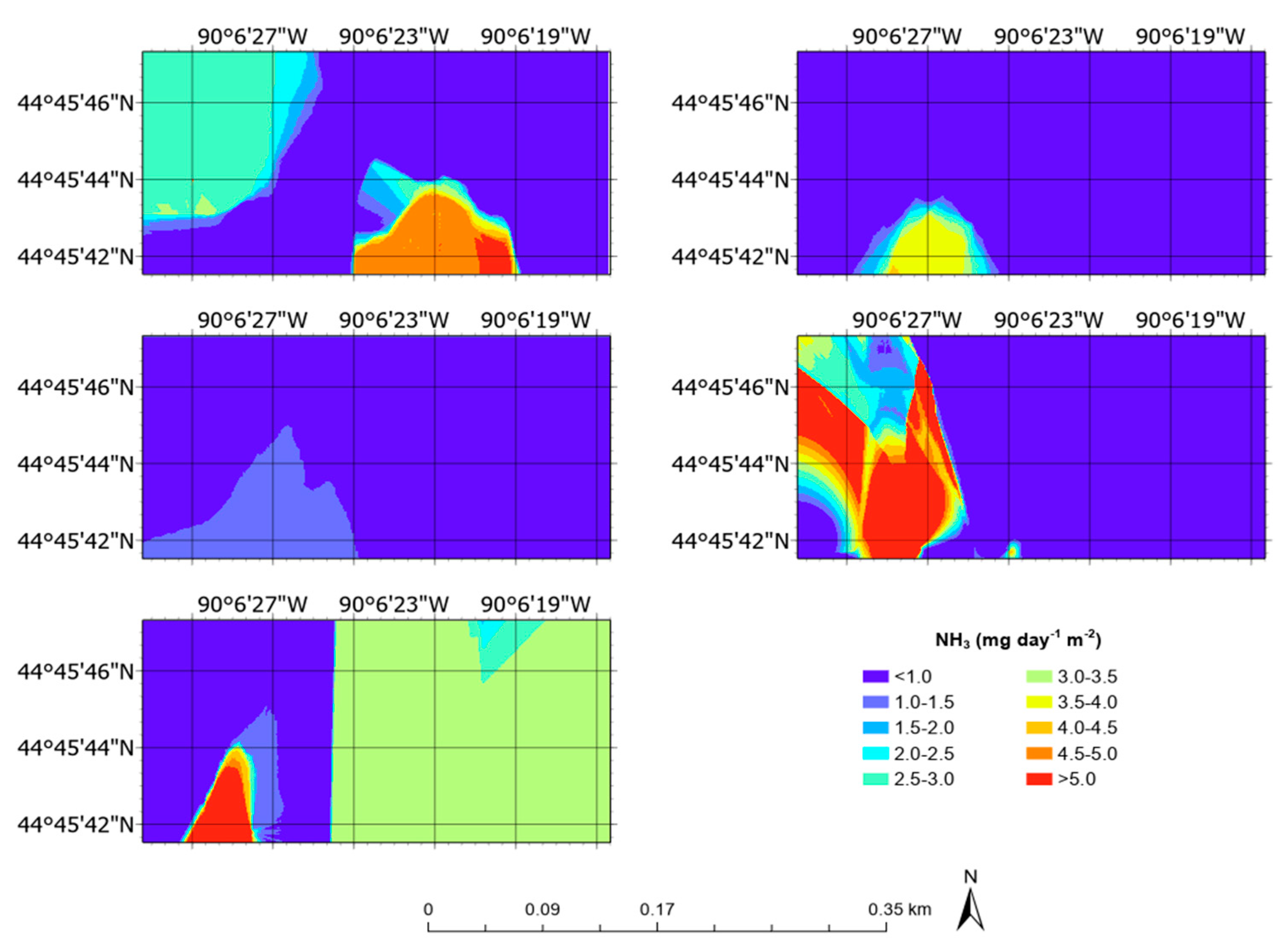
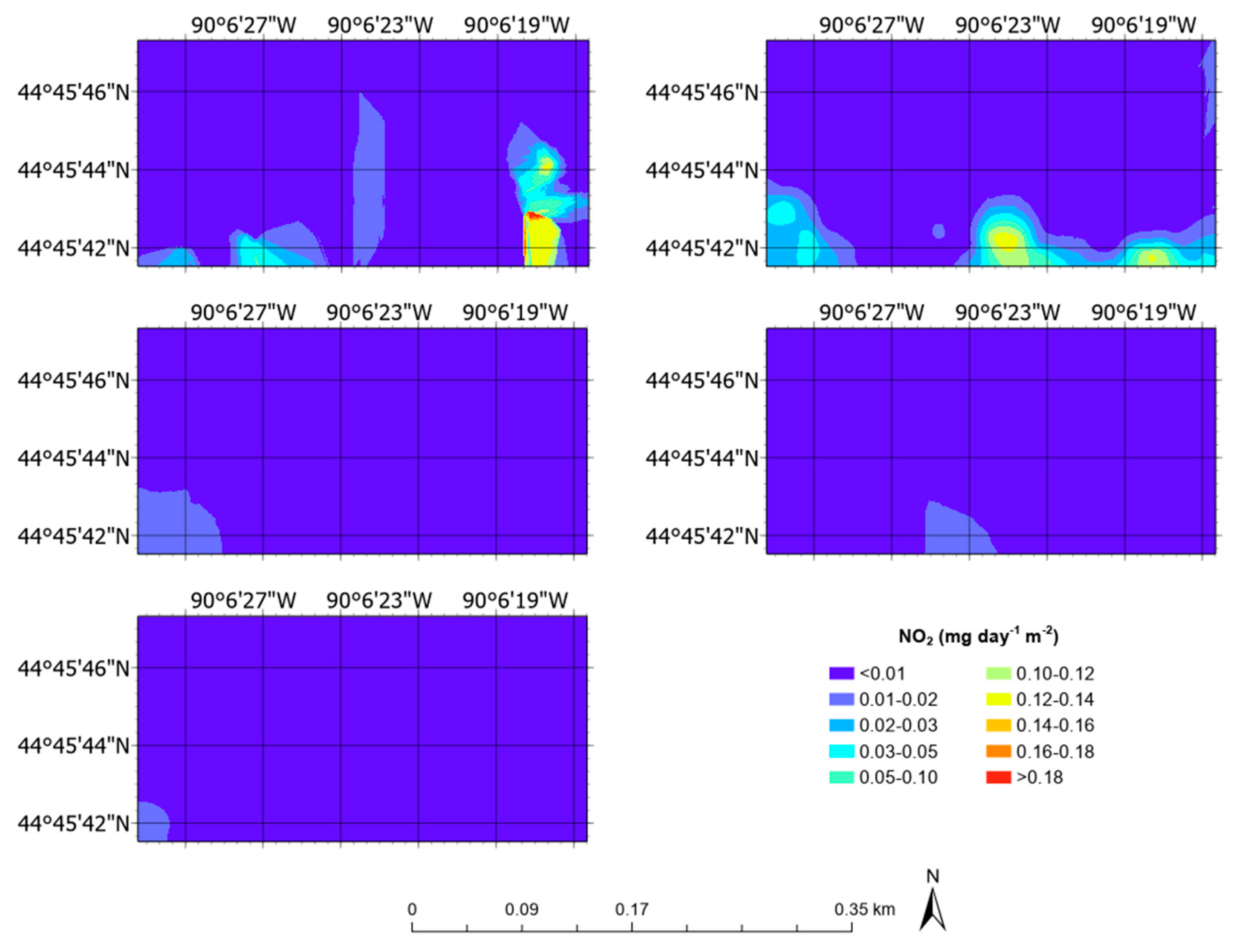
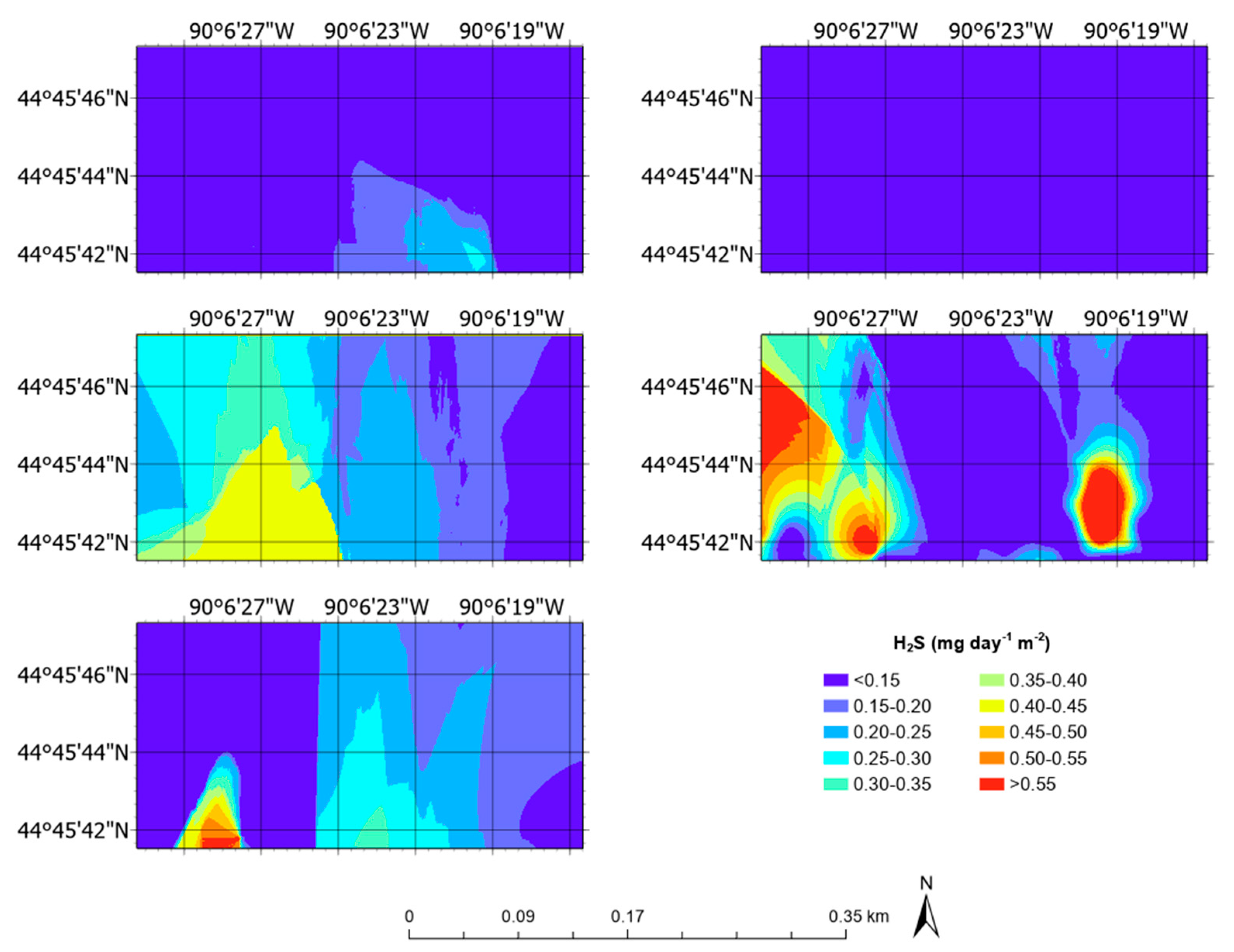
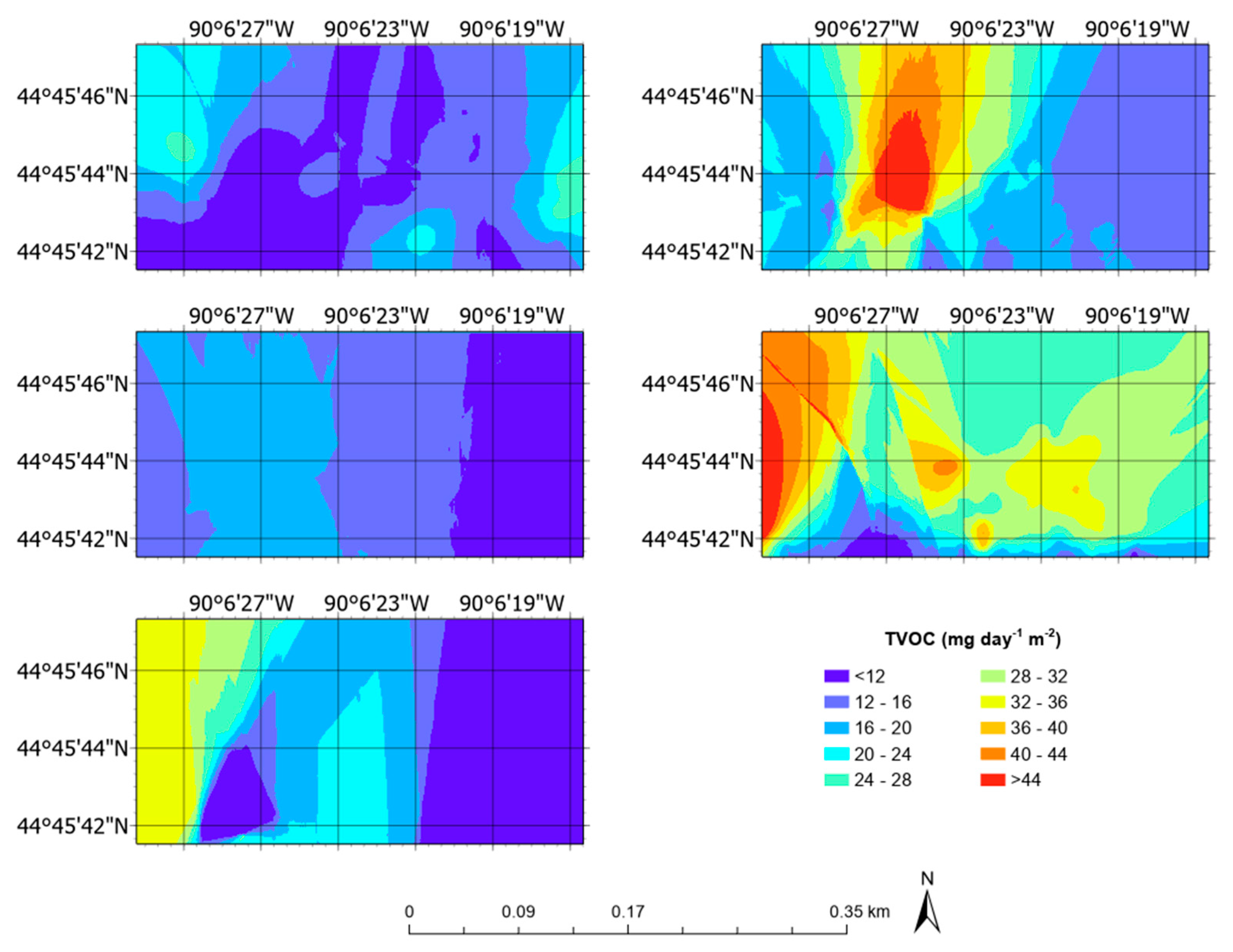
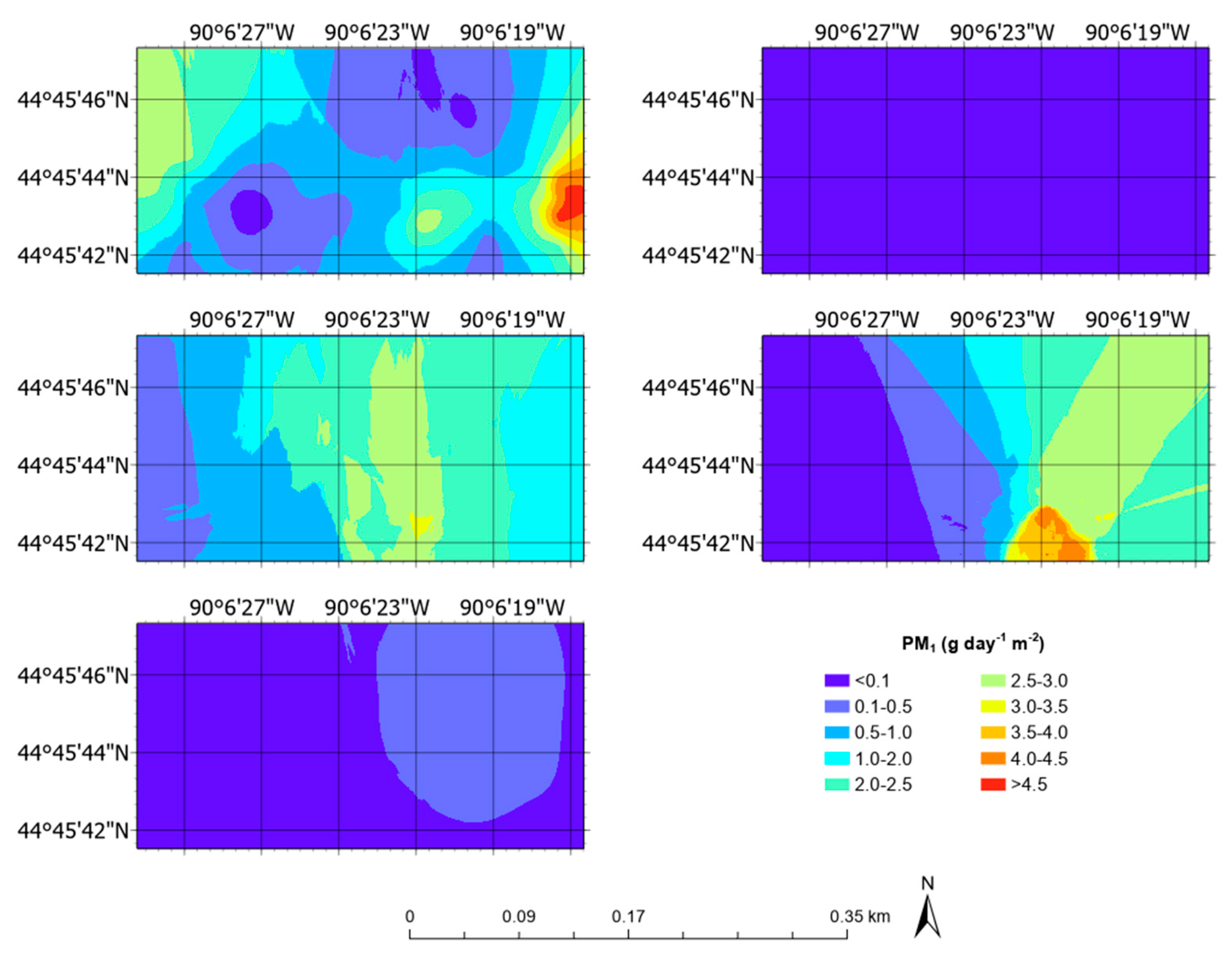
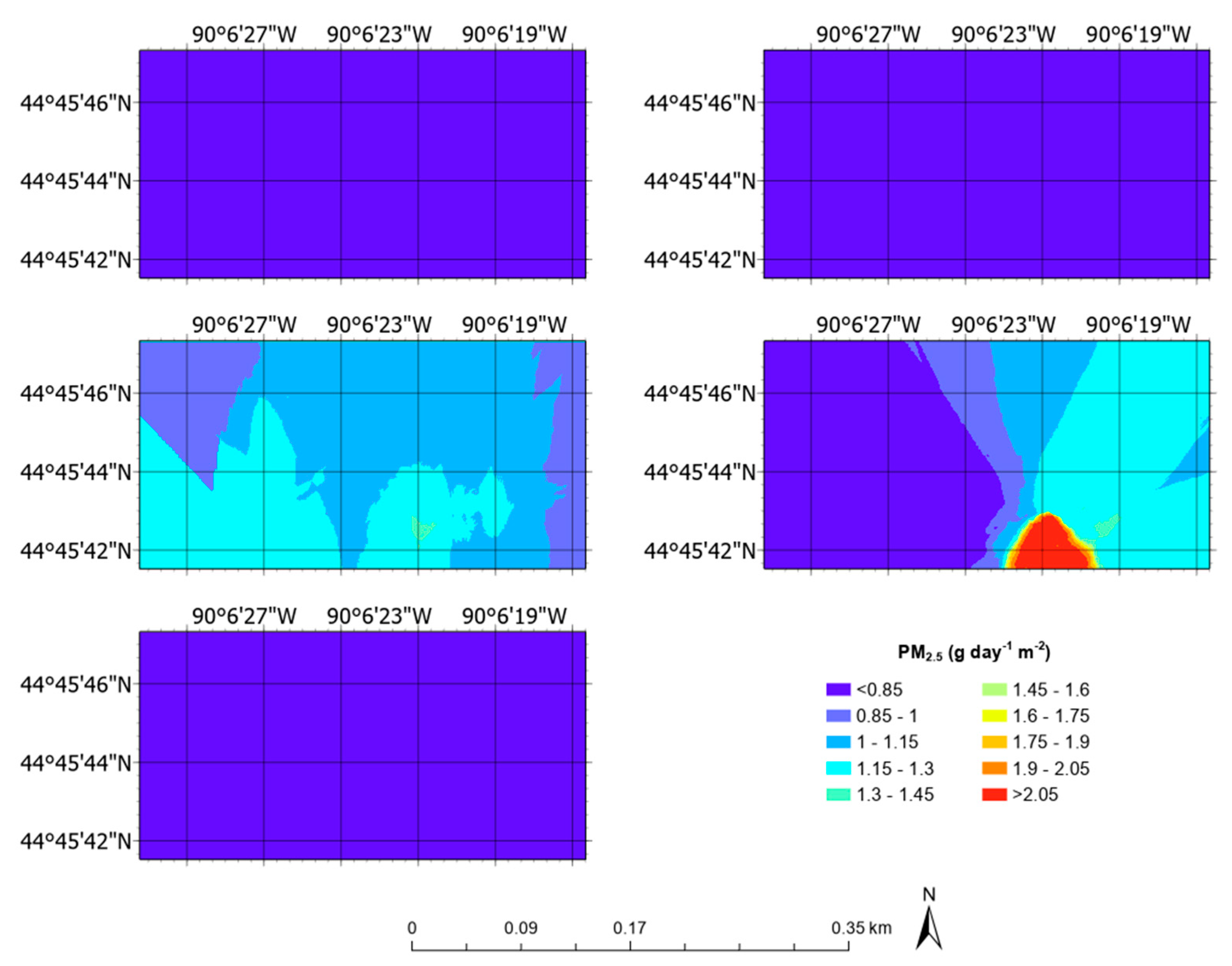
3.5. Aerial Emission Rate Maps
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAA. Small Unmanned Aircraft System (sUAS). Available online: https://www.faa.gov/air_traffic/publications/atpubs/aim_html/chap11_section_2.html (accessed on 17 April 2024).
- Vinkovic, K.; Andersen, T.; De Vries, M.; Kers, B.; Van Heuven, S.; Peters, W.; Hensen, A.; Van Den Bulk, P.; Chen, H. Evaluating the use of an unmanned aerial vehicle (UAV)-based active AirCore system to quantify methane emissions from dairy cows. Sci. Total Environ. 2022, 831, 154898. [Google Scholar] [CrossRef] [PubMed]
- Burgués, J.; Esclapez, M.D.; Doñate, S.; Pastor, L.; Marco, S. Aerial mapping of odorous gases in a wastewater treatment plant using a small drone. Remote Sens. 2021, 13, 1757. [Google Scholar] [CrossRef]
- Arroyo, P.; Gómez-Suárez, J.; Herrero, J.L.; Lozano, J. Electrochemical gas sensing module combined with unmanned aerial vehicles for air quality monitoring. Sens. Actuators B Chem. 2022, 364, 131815. [Google Scholar] [CrossRef]
- Bakirci, M. Efficient air pollution mapping in extensive regions with fully autonomous unmanned aerial vehicles: A numerical perspective. Sci. Total Environ. 2023, 909, 168606. [Google Scholar] [CrossRef] [PubMed]
- Bax, C.; Sironi, S.; Capelli, L. How can odors be measured? An overview of methods and their applications. Atmosphere 2020, 11, 92. [Google Scholar] [CrossRef]
- Burgués, J.; Doñate, S.; Esclapez, M.D.; Saúco, L.; Marco, S. Characterization of odour emissions in a wastewater treatment plant using a drone-based chemical sensor system. Sci. Total Environ. 2022, 846, 157290. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, B.; Richardson, T.; Watson, M.; Greatwood, C.; Clarke, R.; Thomas, R.; Wood, K.; Freer, J.; Thomas, H.; Liu, E.; et al. Remote sensing and identification of volcanic plumes using fixed-wing UAVs over Volcán de Fuego, Guatemala. J. Field Robot. 2019, 36, 1192–1211. [Google Scholar] [CrossRef]
- Burgues, J.; Marco, S. Environmental chemical sensing using small drones: A review. Sci. Total Environ. 2020, 748, 141172. [Google Scholar] [CrossRef]
- Rutkauskas, M.; Asenov, M.; Ramamoorthy, S.; Reid, D.T. Autonomous multi-species environmental gas sensing using drone-based Fourier-transform infrared spectroscopy. Opt. Express 2019, 27, 9578–9587. [Google Scholar] [CrossRef]
- Emran, B.; Tannant, D.; Najjaran, H. Low-Altitude Aerial Methane Concentration Mapping. Remote Sens. 2017, 9, 823. [Google Scholar] [CrossRef]
- Roldan, J.J.; Garcia-Aunon, P.; Garzon, M.; De Leon, J.; Del Cerro, J.; Barrientos, A. Heterogeneous multi-robot system for mapping environmental variables of greenhouses. Sensors 2016, 16, 1018. [Google Scholar] [CrossRef] [PubMed]
- Galfalk, M.; Nilsson Paledal, S.; Bastviken, D. Sensitive drone mapping of methane emissions without the need for supplementary ground-based measurements. ACS Earth Space Chem. 2021, 5, 2668–2676. [Google Scholar] [CrossRef]
- Ali, N.B.H.; Abichou, T.; Green, R. Comparing estimates of fugitive landfill methane emissions using inverse plume modeling obtained with surface emission monitoring (SEM), drone emission monitoring (DEM), and downwind plume emission monitoring (DWPEM). J. Air Waste Manag. Assoc. 2020, 70, 410–424. [Google Scholar] [CrossRef]
- Yuan, H.; Xiao, C.; Wang, Y.; Peng, X.; Wen, Y. Maritime vessel emission monitoring by a UAV gas sensor system. Ocean Eng. 2020, 218, 108206. [Google Scholar] [CrossRef]
- Allen, G.; Hollingsworth, P.; Kabbabe, K.; Pitt, J.R.; Mead, M.I.; Illingworth, S.; Roberts, G.; Bourn, M.; Shallcross, D.E.; Percival, C.J. The development and trial of an unmanned aerial system for the Measurement of methane flux from landfill and greenhouse gas emission hotspots. Waste Manag. 2019, 87, 883–892. [Google Scholar] [CrossRef]
- Kearney, M.; O’Riordan, E.G.; Byrne, N.; Breen, J.; Crosson, P. Mitigation of greenhouse gas emissions in pasture-based dairy-beef production systems. Agric. Syst. 2023, 211, 103748. [Google Scholar] [CrossRef]
- Munidasa, S.; Eckard, R.; Sun, X.; Cullen, B.; McGill, D.; Chen, D.; Cheng, L. Challenges and opportunities for quantifying greenhouse gas emissions through dairy cattle research in developing countries. J. Dairy Res. 2021, 88, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Pas, M.A.W.; Uddin, M.E.; Letelier, P.; Jackson, R.D.; Larson, R.A. Emission and Mitigation of greenhouse gases from dairy farms: The cow, the manure, and the field. Appl. Anim. Sci. 2019, 35, 238–254. [Google Scholar] [CrossRef]
- Reinemann, D.J.; Larson, R.; Aguirre-Villegas, H.; Passos-Fonseca, T. Comparing Greenhouse Gas Emissions of Dairy Systems. Available online: https://cias.wisc.edu/wp-content/uploads/sites/194/2019/03/ciasrb101final.pdf (accessed on 14 February 2024).
- Rotz, C.A. Modeling greenhouse gas emissions from dairy farms. J. Dairy Sci. 2018, 101, 6675–6690. [Google Scholar] [CrossRef]
- Collier, S.M.; Ruark, M.D.; Oates, L.G.; Jokelam, W.E.; Dell, C.J. Measurement of greenhouse gas flux from agricultural soils using static chambers. J. Vis. Exp. 2014, 90, 52110. [Google Scholar] [CrossRef]
- Pape, L.; Ammann, C.; Nyfeler-Brunner, A.; Spirig, C.; Hens, K.; Meisinger, F.X. An automated dynamic chamber system for surface exchange measurement of non-reactive and reactive trace gases of grassland ecosystems. Biogeosciences 2009, 6, 405–429. [Google Scholar] [CrossRef]
- ISO 21501-4; Determination of Particle Size Distribution—Single Particle Light Interaction Methods. 2023. Available online: https://www.en-standard.eu/bs-iso-21501-4-2018-a1-2023 (accessed on 12 August 2024).
- Akdeniz, N.; Janni, K.A.; Hetchler, B.P. Mitigation of multiple air emissions from swine buildings using corn cob biofilters. Trans. ASABE 2016, 59, 1413–1420. [Google Scholar] [CrossRef]
- Akdeniz, N.; Janni, K.A.; Salnikov, I.A. Biofilter performance of pine nuggets and lava rock as media. Bioresour. Technol. 2011, 102, 4974–4980. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Akdeniz, N.; Banik, C.; Koziel, J.A. Alkaline hydrolysis of poultry carcasses and health risk assessment of exposure to target volatile organic compounds. J. ASABE 2024, 67, 373–384. [Google Scholar] [CrossRef]
- Nathan, B.J.; Golston, L.M.; O’Brien, A.S.; Ross, K.; Harrison, W.A.; Tao, L.; Lary, D.J.; Johnson, D.R.; Covington, A.N.; Clark, N.N.; et al. Near-Field Characterization of methane emission variability from a compressor station using a model aircraft. Environ. Sci. Technol. 2015, 49, 7896–7903. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H. International Encyclopedia of Statistical Science-Standard Deviation; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Crazzolara, C.; Ebner, M.; Platis, A.; Miranda, T.; Bange, J.; Junginger, A. A new multicopter-based unmanned aerial system for pollen and spores collection in the atmospheric boundary layer. Atmos. Meas. Tech. 2019, 12, 1581–1598. [Google Scholar] [CrossRef]
- Burgues, J.; Hernandez, V.; Lilienthal, A.J.; Marco, S. Smelling nano aerial vehicle for gas source localization and mapping. Sensors 2019, 19, 478. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Meng, Q.H.; Wang, J.Y.; Ma, S.G. Simulate the aerodynamic olfactory effects of gas-sensitive UAVs: A numerical model and its parallel implementation. Adv. Eng. Softw. 2016, 102, 123–133. [Google Scholar] [CrossRef]
- Cosentino, N.J.; Opaza, N.E.; Lambert, F.; Osses, A.; Van Wout, E. Global-Krigger: A global Kriging interpolation toolbox with paleoclimatology examples. Adv. Earth Space Sci. 2023, 24, e2022GC010821. [Google Scholar] [CrossRef]
- Oliver, M.A.; Webster, R. The Variogram and Modelling. In Basic Steps in Geostatistics: The Variogram and Kriging; Oliver, M.A., Webster, R., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 15–42. [Google Scholar]
- Zhang, H.; Tian, Y.; Zhao, P. Dispersion curve interpolation based on Kriging method. Appl. Sci. 2023, 13, 2557. [Google Scholar] [CrossRef]
- Darvishpoor, S.; Roshanian, J.; Raissi, A.; Hassanalian, M. Configurations, flight mechanisms, and applications of unmanned aerial systems: A review. Prog. Aerosp. Sci. 2020, 121, 100694. [Google Scholar] [CrossRef]
- Hassanalian, M.; Abdelkefi, A. Classifications, applications, and design challenges of drones: A review. Prog. Aerosp. Sci. 2017, 91, 99–131. [Google Scholar] [CrossRef]
- WSCO. Summer Climate Data-Wisconsin State Climatology Office. Available online: https://climtology.nelson.wisc.edu/wisconsin-seasons/summer/ (accessed on 14 March 2024).
- NOAA. Climate Change: Atmospheric Carbon Dioxide. Available online: https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide (accessed on 14 May 2024).
- Li, M.; Weschler, C.J.; Bekö, G.; Wargocki, P.; Lucic, G.; Williams, J. Human ammonia emission rates under various indoor environmental conditions. Environ. Sci. Technol. 2020, 54, 5419–5428. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Public Health Statement Hydrogen Sulfide. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp114-c1-b.pdf (accessed on 14 March 2024).
- EPA. Air Quality and Climate Change Research. Available online: https://www.epa.gov/air-research/air-quality-and-climate-change-research (accessed on 14 March 2024).
- CAAQS. California Ambient Air Quality Standards. Available online: https://ww2.arb.ca.gov/resources/inhalable-particulate-matter-and-health (accessed on 16 September 2023).
- EPA. National Ambient Air Quality Standards (NAAQS) for PM. Available online: https://www.epa.gov/pm-pollution/national-ambient-air-quality-standards-naaqs-pm (accessed on 7 May 2024).
- EPA. Technical Overview of Volatile Organic Compounds. Available online: https://www.epa.gov/indoor-air-quality-iaq/technical-overview-volatile-organic-compounds#measurement (accessed on 16 September 2023).
- EPA. Timeline of Nitrogendioxide (NO2). Available online: https://www.epa.gov/no2-pollution/timeline-nitrogen-dioxide-NO2-national-ambient-air-quality-standards-naaqs (accessed on 16 September 2023).
- Saggar, S.; Adhikari, K.; Giltrap, D.; Luo, J.; Palmada, T.; Berben, P.; Lindsey, S.; Sprosen, M. Improving the accuracy of nitrous oxide emission factors estimated for hotspots within dairy-grazed farms. Sci. Total Environ. 2022, 806, 150608. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; De Klein, C.A.M.; Ledgard, S.F.; Saggar, S. Management options to reduce nitrous oxide emissions from intensively grazed pastures: A review. Agric. Ecosyst. Environ. 2010, 136, 282–291. [Google Scholar] [CrossRef]
- Adhikari, K.P.; Chibuike, G.; Saggar, S.; Simon, P.L.; Luo, J.; De Klein, C.A.M. Management and implications of using nitrification inhibitors to reduce nitrous oxide emissions from urine patches on grazed pasture soils—A review. Sci. Total Environ. 2021, 791, 148099. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.P.; Johnson, I.R.; Schwenke, G.; Lam, S.K.; Suter, H.C.; Eckard, R.J. Predicting ammonia volatilization from fertilized pastures used for grazing. Agric. For. Meteorol. 2020, 287, 107952. [Google Scholar] [CrossRef]
- Raparthi, N.; Barudgar, A.; Chu, M.; Ning, Z.; Phuleria, H.C. Estimating individual vehicle emission factors from near-road measurements in India. Atmos. Environ. 2023, 308, 119869. [Google Scholar] [CrossRef]
- Bezerra, B.G.; Silva, S.; Mendes, K.R.; Mutti, P.R.; Fernandes, L.S.; Marques, T.V.; Silva, C.L.C.; Campos, S.; De Lima Vieira, M.M.; Urbano, S.A.; et al. CO2 exchanges and evapotranspiration of a grazed pasture under tropical climate conditions. Agric. For. Meteorol. 2022, 323, 1090888. [Google Scholar] [CrossRef]
- Rotz, C.A.; Beegle, D.; Bernard, J.K.; Leytem, A.; Feyereisen, G.; Hagevoort, R.; Harrison, J.; Aksland, G.; Thoma, G. Fifty years of environmental progress for United States dairy farms. J. Dairy Sci. 2024, 107, 3651–3668. [Google Scholar] [CrossRef]
- Bento, C.B.; Brandani, C.B.; Filoso, S.; Martinelli, L.A.; Do Carmo, J.B. Effects of extensive-to-intensive pasture conversion on soil nitrogen availability and CO2 and N2O fluxes in a Brazilian oxisol. Agric. Ecosyst. Environ. 2021, 321, 107633. [Google Scholar] [CrossRef]
- Yan, R.R.; Tang, H.J.; Lv, S.H.; Jin, D.Y.; Xin, X.P.; Chen, B.R.; Zhang, B.H.; Yan, Y.C.; Wang, X.; Murray, P.J.; et al. Response of ecosystem CO2 fluxes to grazing intensities-a five-year experiment in the Hulunber meadow steppe of China. Sci. Rep. 2017, 7, 9491. [Google Scholar] [CrossRef]
- Murphy, B.; Crosson, P.; Kelly, A.K.; Prendiville, R. An economic and greenhouse gas emissions evaluation of pasture-based dairy calf-to-beef production systems. Agric. Sys. 2017, 154, 124–132. [Google Scholar] [CrossRef]
- Luo, J.; Kulasegarampillai, M.; Bolan, N.; Donnison, A. Control of gaseous emissions of ammonia and hydrogen sulphide from cow manure by use of natural materials. N. Z. J. Agric. Res. 2004, 47, 545–556. [Google Scholar] [CrossRef]
- Hristov, A.N.; Haigan, M.; Cole, A.; Todd, R.; McAllister, T.A.; Ndegwa, P.M.; Rotz, A. Review: Ammonia emissions from dairy farms and beef feedlots. Can. J. Anim. Sci. 2011, 91, 1–35. [Google Scholar] [CrossRef]
- McGinn, S.M.; Flesch, T.K.; Chen, D.; Crenna, B.; Denmead, O.T.; Naylor, T.; Rowell, D. Coarse particulate matter emissions from cattle feedlots in Australia. J. Environ. Qual. 2010, 39, 791–798. [Google Scholar] [CrossRef]
- Ancelet, T.; Davy, P.K.; Trompetter, W.J.; Markwitz, A. Sources of particulate matter pollution in a small New Zealand city. Atmos. Pollut. Res. 2014, 5, 572–580. [Google Scholar] [CrossRef]
- Brusca, S.; Famoso, F.; Lanzafame, R.; Mauro, S.; Garrano, A.M.C.; Monforte, P. Theoretical and experimental study pf Gaussian plume model in small scale system. Energy Procedia 2016, 101, 58–65. [Google Scholar] [CrossRef]

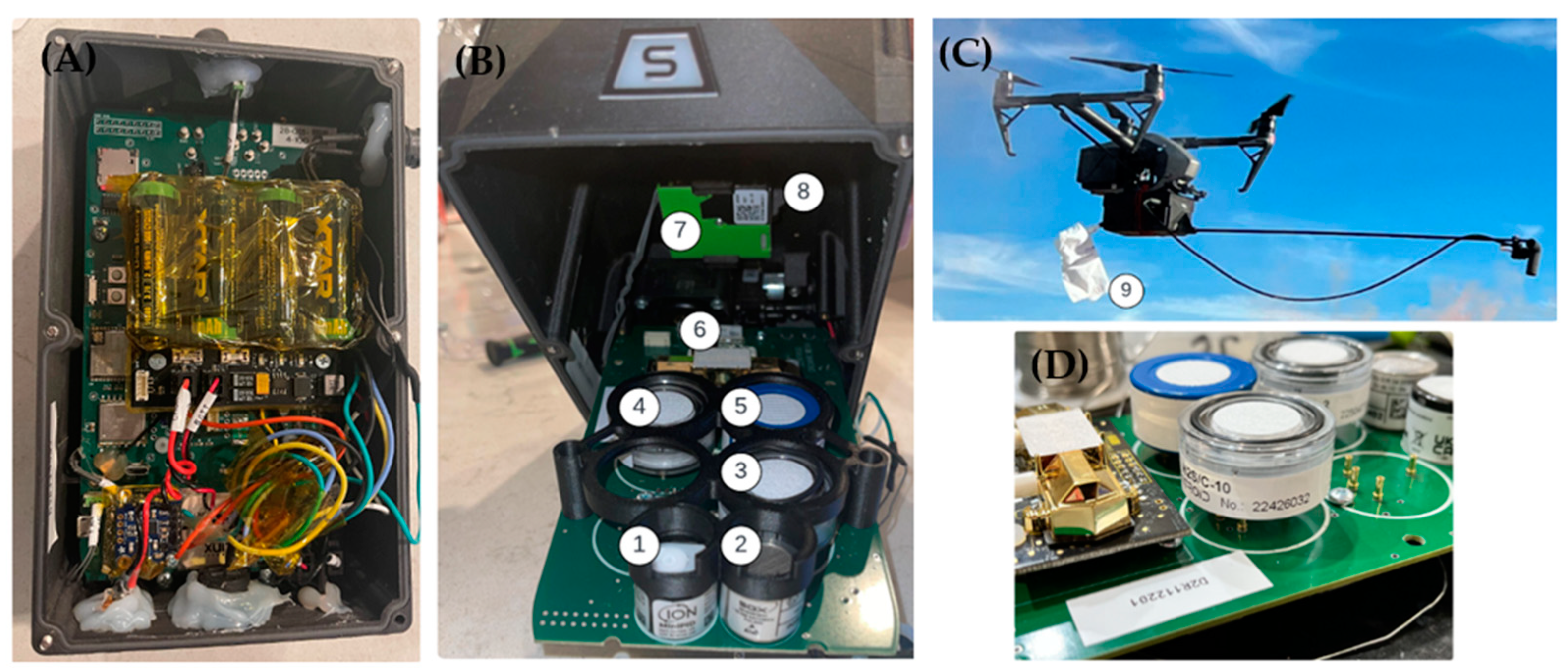

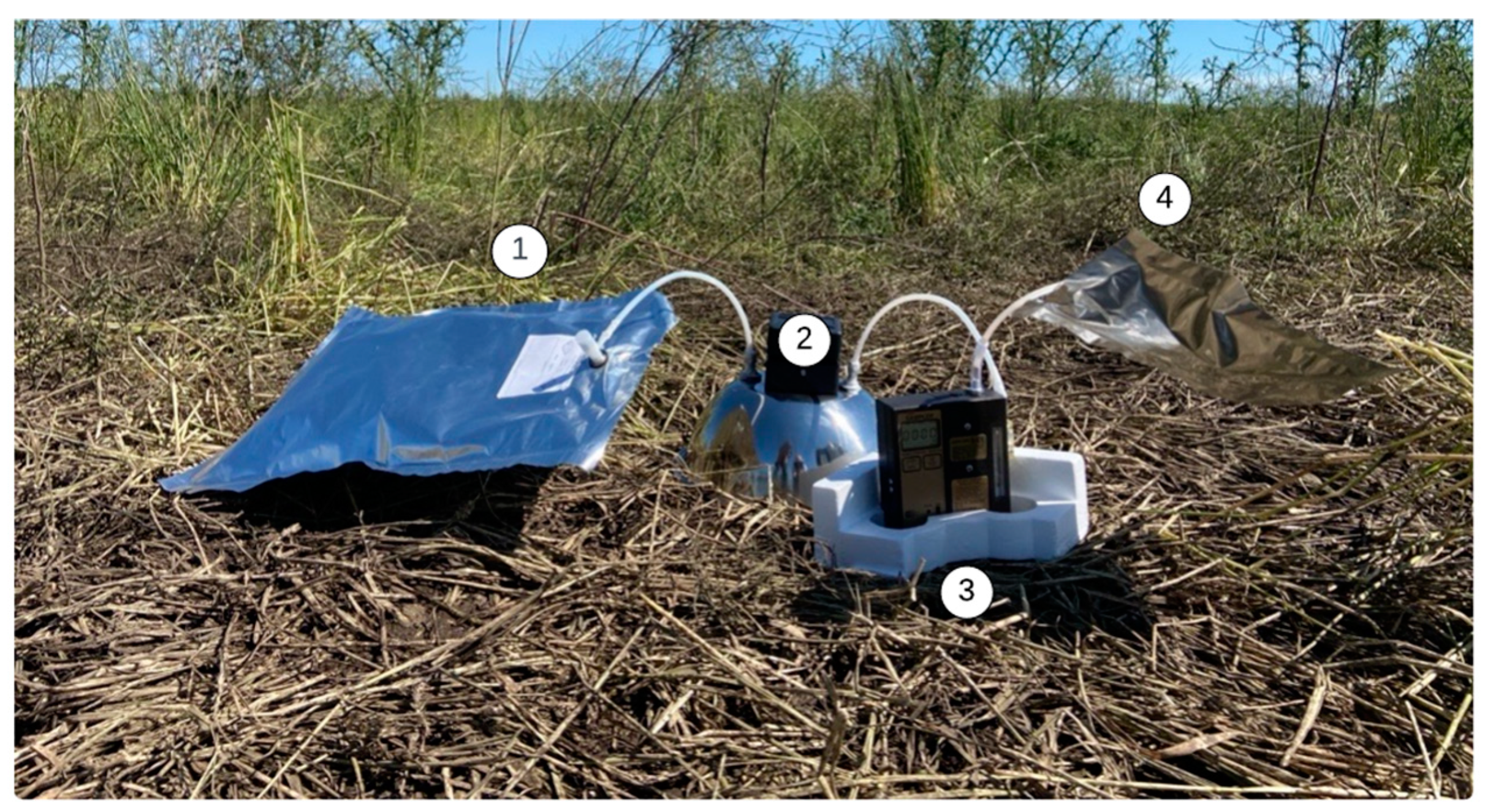
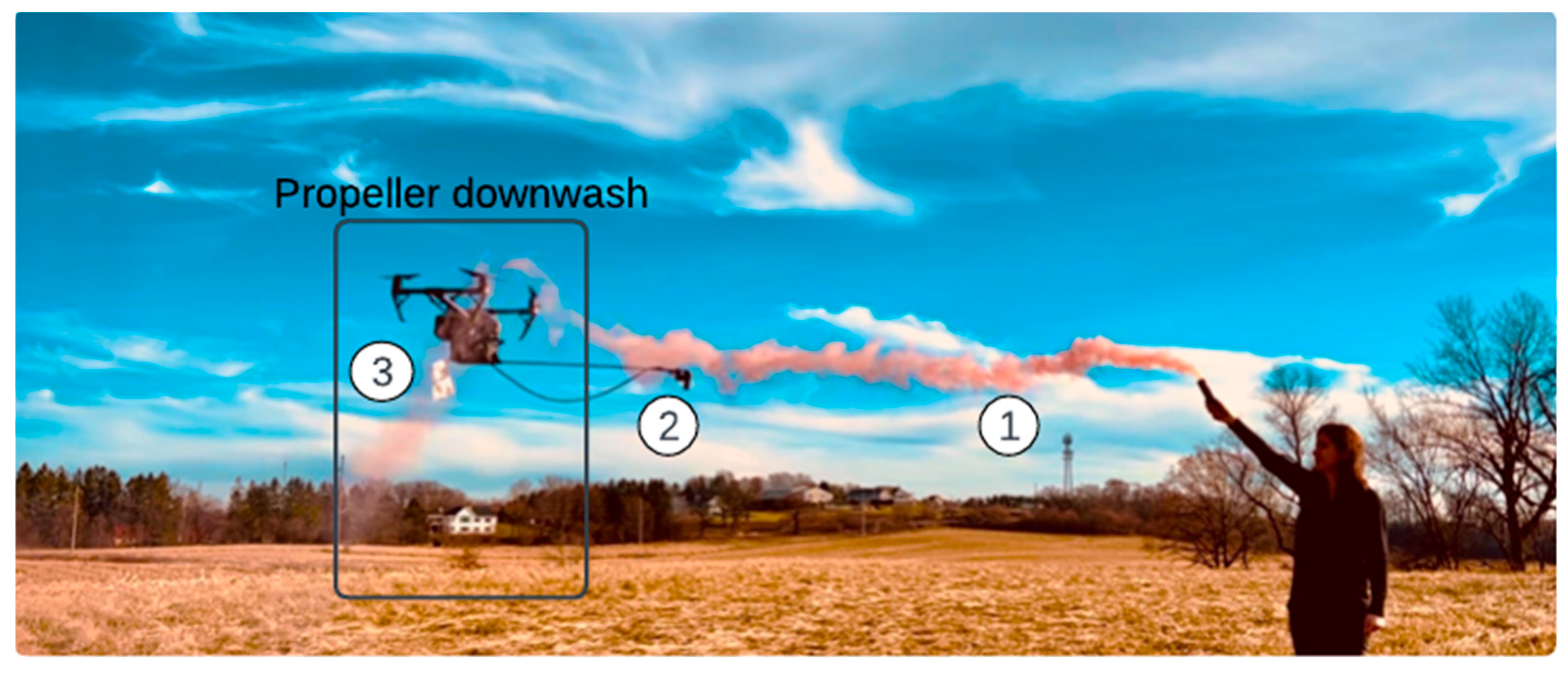

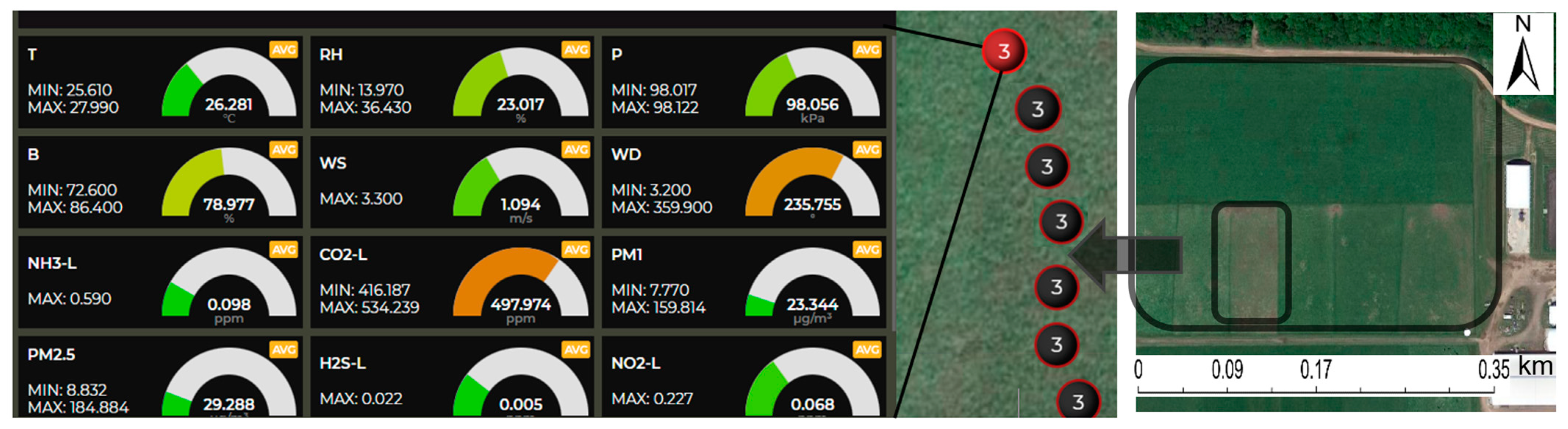

| Parameter | Sensor Type | Range |
|---|---|---|
| CO2 | NDIR | 1 to 2000 ppm |
| NH3 | EC | 0.005 ppm to 10 ppm |
| NOx | EC | 0.01 to 1 ppm |
| H2S | EC | 7 ppb to 3 ppm |
| CH4 | NDIR | 0.4 to 100 ppm |
| Total VOCs | PID | 1 ppb to 50 ppm |
| PM1, PM2.5, PM10 | Laser-scattered | 1 to 2000 µg m−3 |
| Temperature | - | 5 to 40 °C |
| Humidity | - | 10 to 90% |
| Barometric pressure | - | - |
| Inertial movement unit | Wind direction, compass | - |
| Wind speed | - | 0.3 to 67 m s−1 |
| Compound | Unit | Mean ± Stdev | Lower Bound | Upper Bound |
|---|---|---|---|---|
| CO2 | g day−1 m−2 | 1.52 ± 0.80 | 0.25 | 4.64 |
| NH3 | mg day−1 m−2 | 1.22 ± 1.02 | 0.00 | 6.60 |
| NO2 | mg day−1 m−2 | 0.01 ± 0.01 | 0.00 | 0.09 |
| H2S | mg day−1 m−2 | 0.08 ± 0.05 | 0.01 | 0.46 |
| TVOC | mg day−1 m−2 | 14.23 ± 4.71 | 3.70 | 25.73 |
| PM1 | g day−1 m−2 | 0.14 ± 0.04 | 0.00 | 5.02 |
| PM2.5 | g day−1 m−2 | 0.11 ± 0.03 | 0.04 | 0.20 |
| PM10 | g day−1 m−2 | 0.04 ± 0.02 | 0.01 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Wang, Y.; Akdeniz, N. Field Measurements of Spatial Air Emissions from Dairy Pastures Using an Unmanned Aircraft System. Remote Sens. 2024, 16, 3007. https://doi.org/10.3390/rs16163007
Yang D, Wang Y, Akdeniz N. Field Measurements of Spatial Air Emissions from Dairy Pastures Using an Unmanned Aircraft System. Remote Sensing. 2024; 16(16):3007. https://doi.org/10.3390/rs16163007
Chicago/Turabian StyleYang, Doee, Yuchuan Wang, and Neslihan Akdeniz. 2024. "Field Measurements of Spatial Air Emissions from Dairy Pastures Using an Unmanned Aircraft System" Remote Sensing 16, no. 16: 3007. https://doi.org/10.3390/rs16163007
APA StyleYang, D., Wang, Y., & Akdeniz, N. (2024). Field Measurements of Spatial Air Emissions from Dairy Pastures Using an Unmanned Aircraft System. Remote Sensing, 16(16), 3007. https://doi.org/10.3390/rs16163007






