Monitoring of Cotton Boll Opening Rate Based on UAV Multispectral Data
Abstract
:1. Introduction
2. Study Area and Data
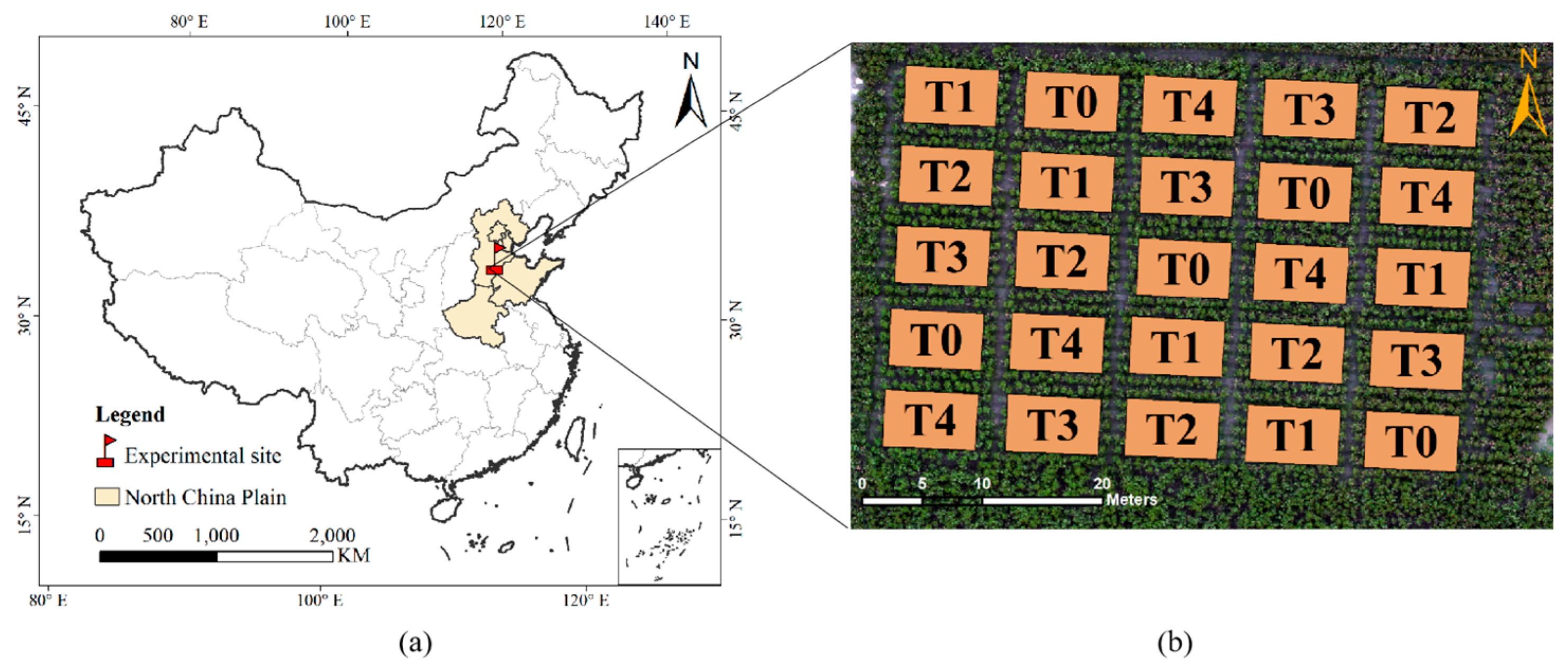
2.1. Study Area and Design of the Experiment
2.2. Data Collection and Processing
2.2.1. Field Data Collection
2.2.2. Acquisition and Pre-Processing of the UAV Remote Sensing Data
3. Methods
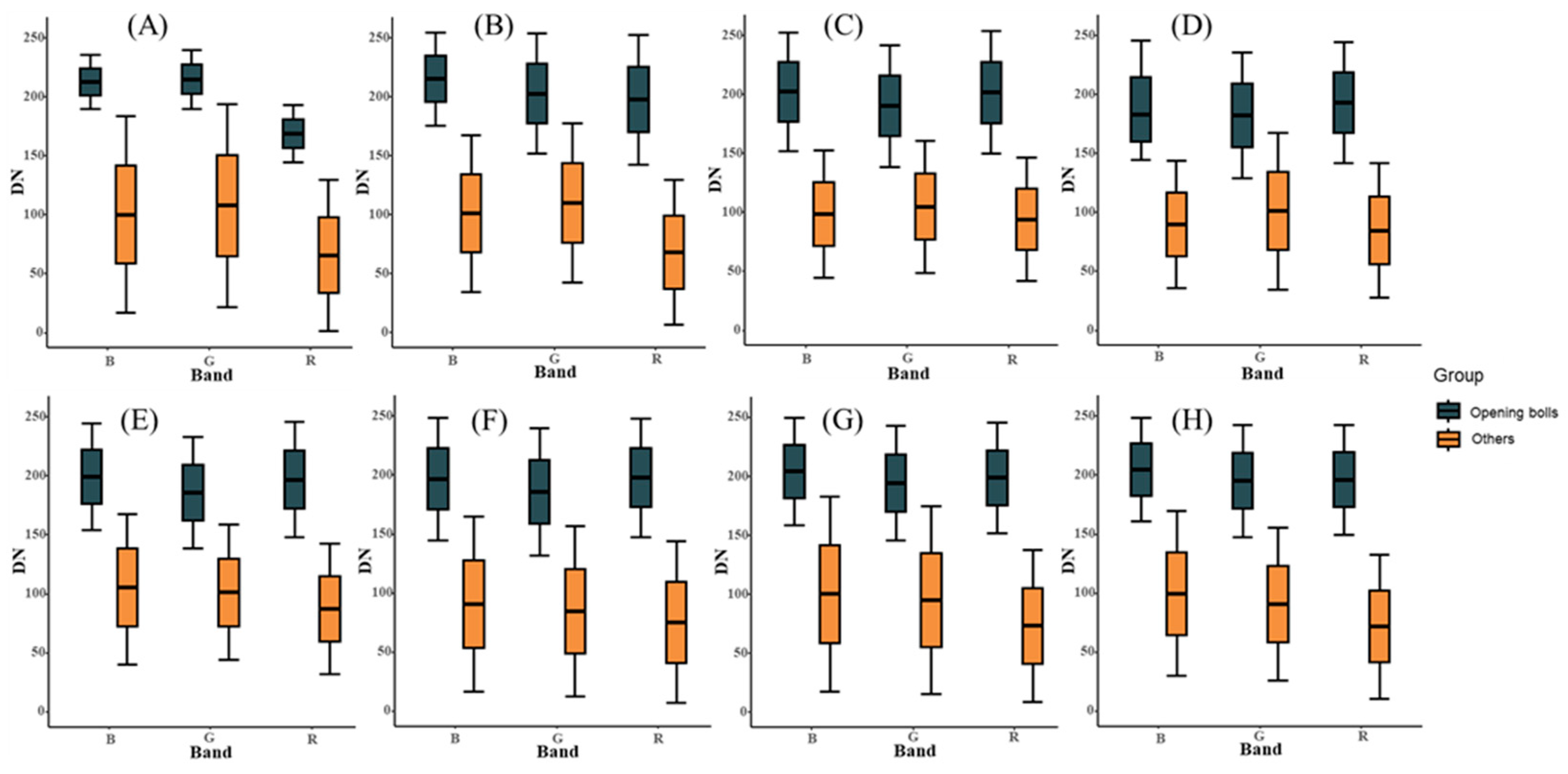
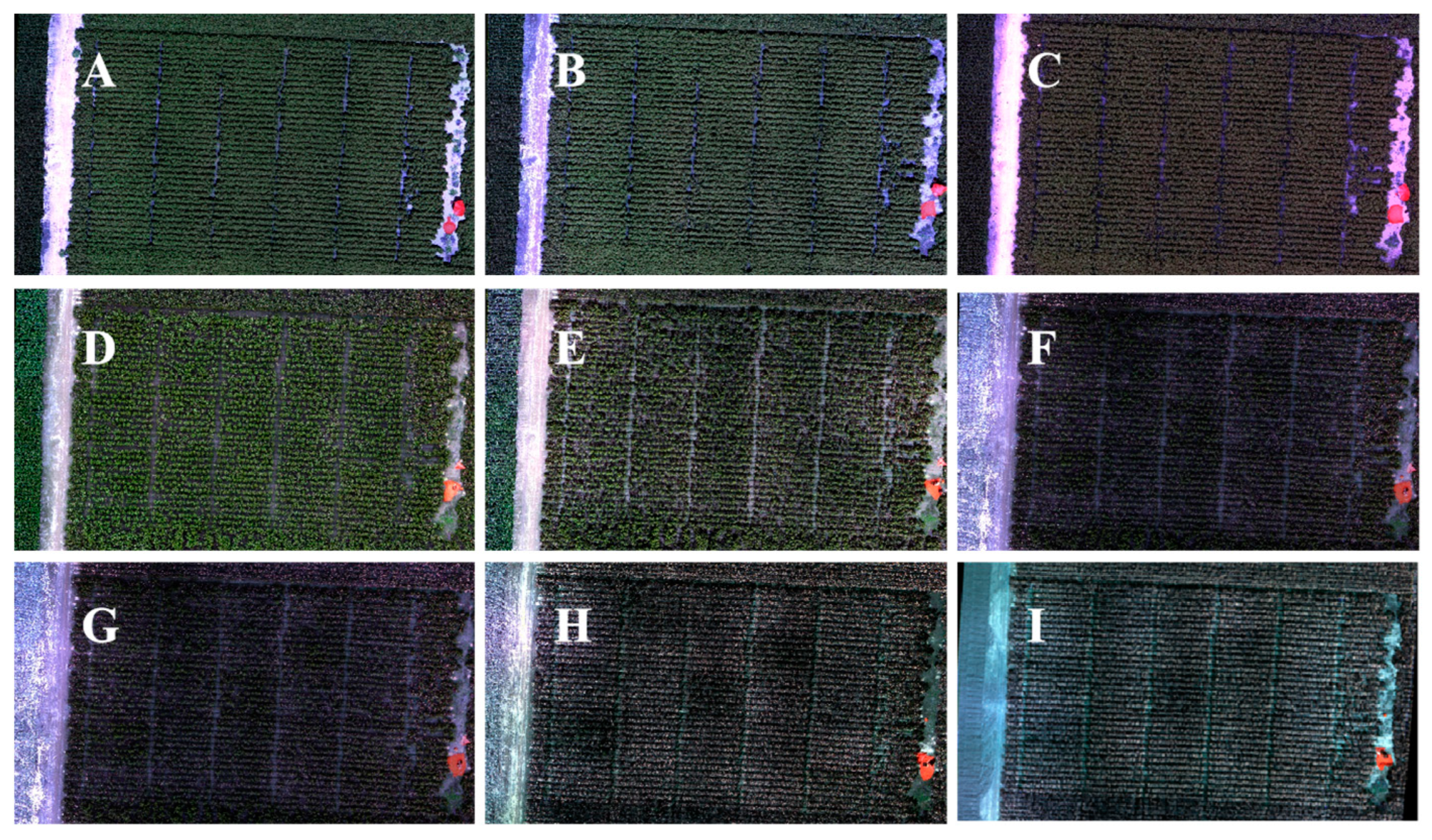
3.1. Opening Boll Area Extraction
3.2. Selection of VIs
3.3. Modeling and Algorithm Accuracy Evaluation
3.3.1. Calculating the Rate of the Vegetation Index Change
3.3.2. Linear Regression
3.3.3. Partial Least Squares Regression
3.3.4. Random Forest
3.3.5. Evaluation of Model Accuracy
4. Results
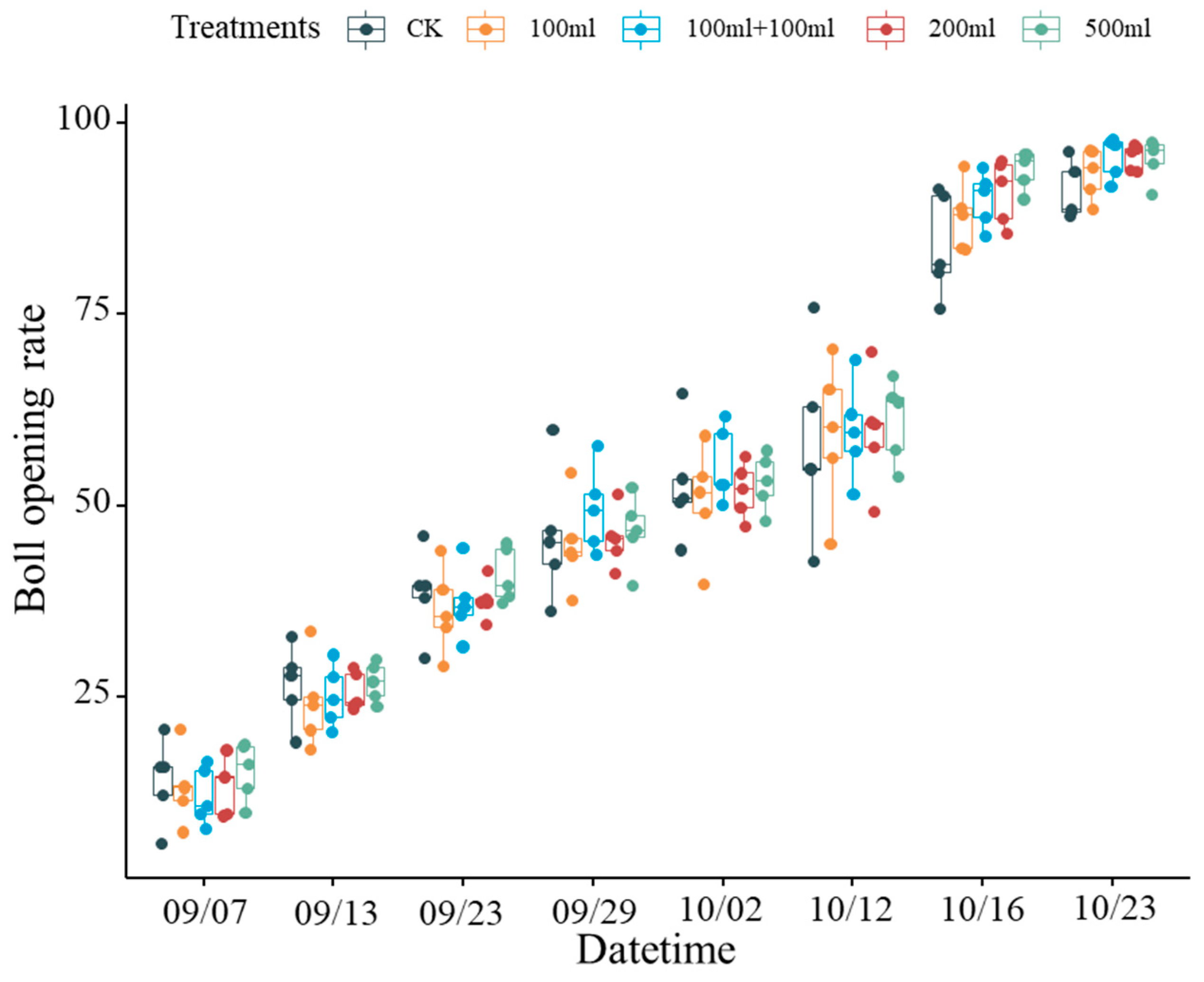
4.1. Variations of the Boll Opening Rate
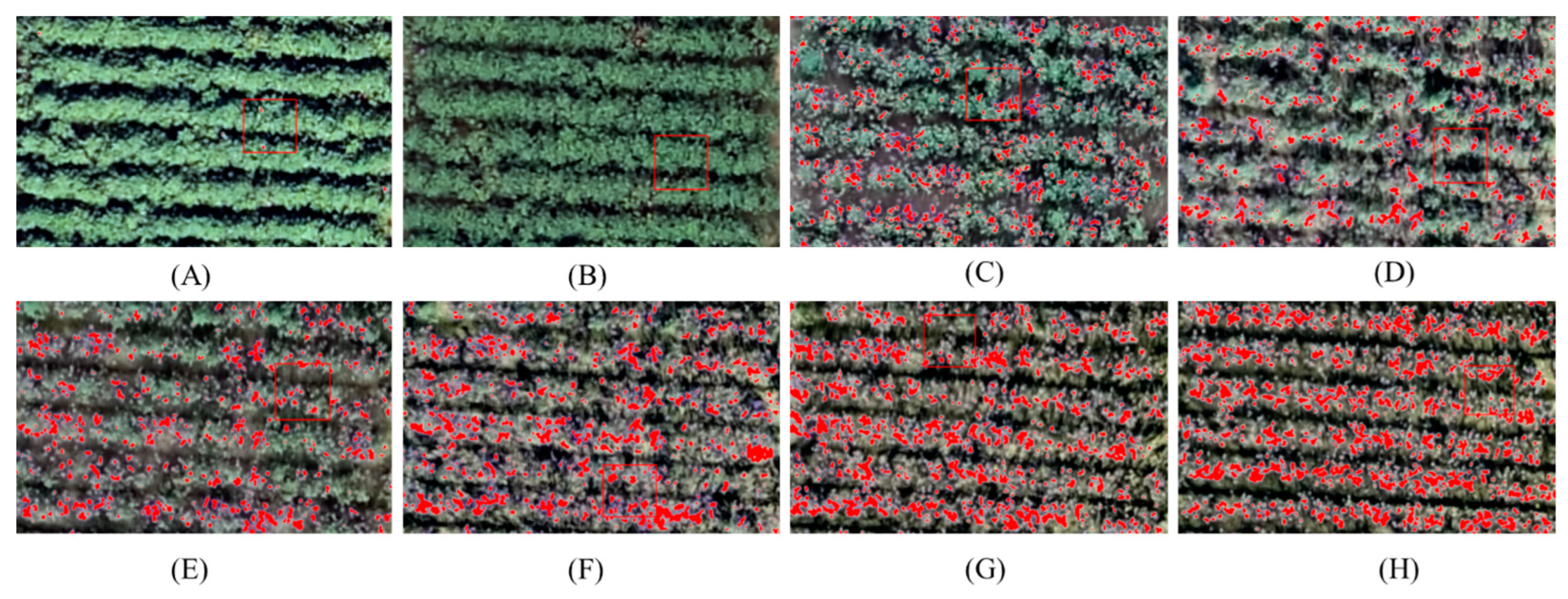
4.2. Extract the Opening Bolls Area Based on the Threshold
4.3. Correlation between Vegetation Indices and the Opening Bolls’ Area
4.4. Regression Equations of the Opening Bolls’ Area Estimation Model Based on Each of the Vegetation Indices
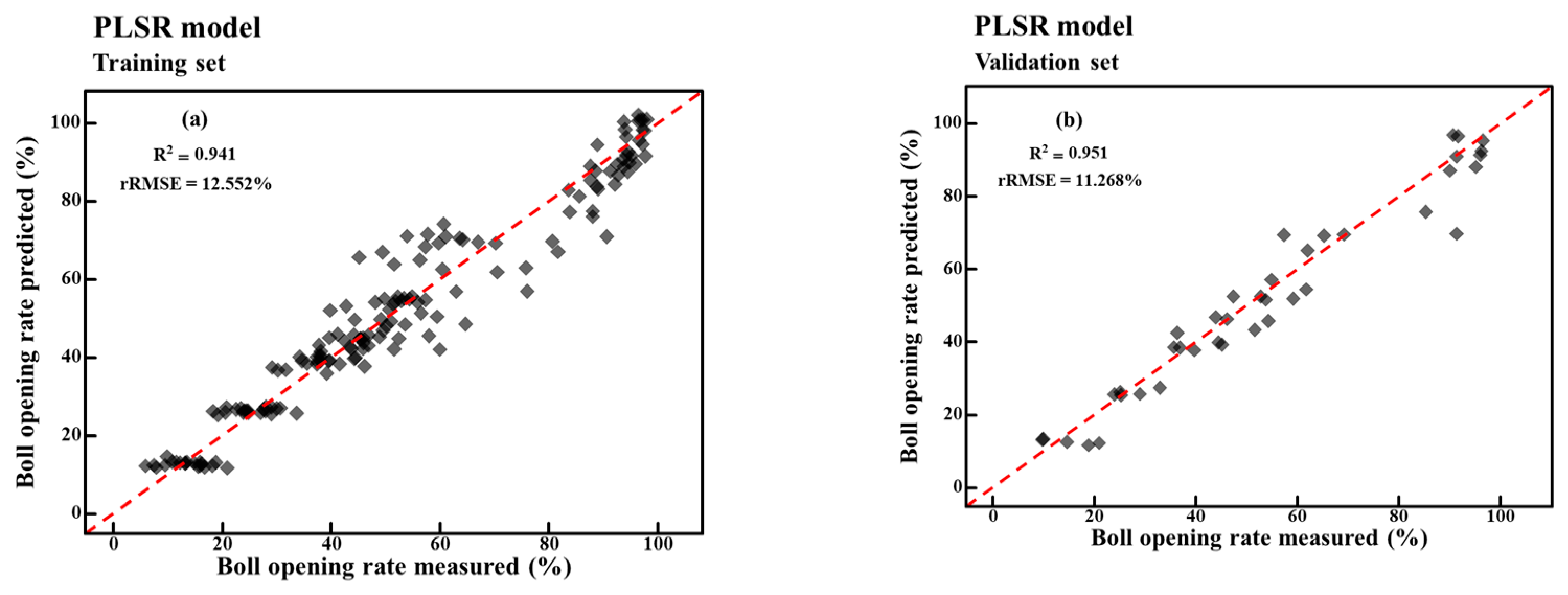
4.5. Boll Opening Rate Model Based on PLSR
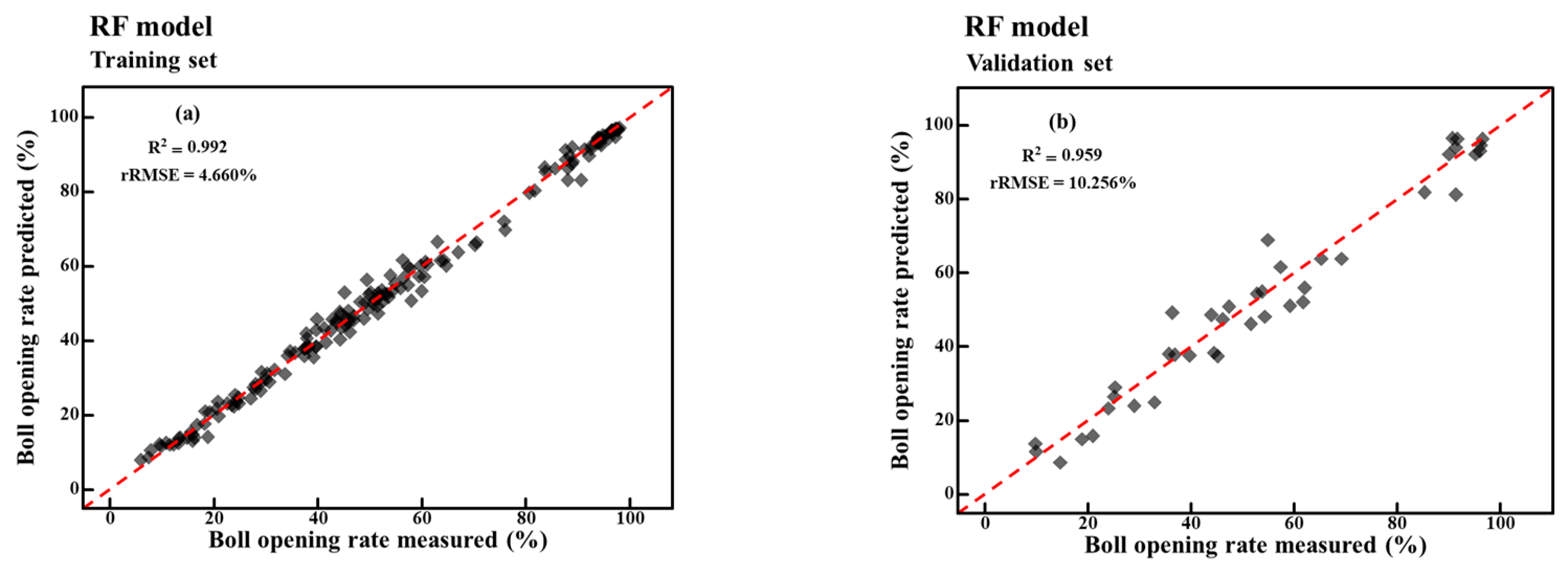
4.6. Boll Opening Rate Model Based on RF
5. Discussion
5.1. The Rate of Change of the Vegetation Index Is a More Appropriate Method for the Cotton Boll Opening Rate
5.2. The Vegetation Index with a Good Prediction Capability for the Boll Opening Rate
5.3. The Method with Unique Advantages for Predicting the Boll Opening Rate
5.4. Limitations of This Study and Suggestions for the Future Boll Opening Rate Estimation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, L.; Liusheng, D.; Zhaohu, L.; Baomin, W.; Zhongpei, H. Physiological bases of chemical accelerated boll maturation and defoliation in cotton. Plant Physiol. J. 2004, 40, 758–762. [Google Scholar]
- Gwathmey, C.O.; Bange, M.P.; Brodrick, R. Cotton crop maturity: A compendium of measures and predictors. Field Crops Res. 2016, 191, 41–53. [Google Scholar] [CrossRef]
- Snipes, C.E.; Baskin, C.C. Influence of early defoliation on cotton yield, seed quality, and fiber properties. Field Crops Res. 1994, 37, 137–143. [Google Scholar] [CrossRef]
- Joel, C.F.; Keith, L.E.; Randy, W.; Alexander, M.S. Timing defoliation applications for maximum yields and optimum quality in cotton containing a fruiting gap. Crop Sci. 2004, 44, 158–164. [Google Scholar]
- Zheng, N.; Zhai, W.; Zhang, S.; Zhang, W.; Jiang, G.; Han, R.; Qi, F. Factors affecting cotton maturation and senescence processes and the corre-sponding regulative strategies. Plant Physiol. J. 2014, 50, 1310–1314. [Google Scholar]
- Dorigo, W.A.; Zurita-Milla, R.; de Wit, A.J.W.; Brazile, J.; Singh, R.; Schaepman, M.E. A review on reflective remote sensing and data assimilation techniques for enhanced agroecosystem modeling. Int. J. Appl. Earth Obs. 2007, 9, 165–193. [Google Scholar] [CrossRef]
- Huang, Y.; Thomson, S.J.; Hoffmann, W.C.; Lan, Y.; Fritz, B.K. Development and prospect of unmanned aerial vehicle technologies for agricultural production management. Int. J. Agric. Biol. Eng. 2013, 6, 1. [Google Scholar]
- Ren, Y.; Meng, Y.; Huang, W.; Ye, H.; Han, Y.; Kong, W.; Zhou, X.; Cui, B.; Xing, N.; Guo, A. Novel vegetation indices for cotton boll opening status estimation using sentinel-2 data. Remote Sens. 2020, 12, 1712. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, X.; Li, J. MODIS data based NDVI Seasonal dynamics in agro-ecosystems of south bank Hangzhouwan bay. Afr. J. Agric. Res. 2012, 6, 4025–4033. [Google Scholar]
- Yeom, J.; Jung, J.; Chang, A.; Maeda, M.; Landivar, J. Automated open cotton boll detection for yield estimation using unmanned aircraft vehicle (UAV) data. Remote Sens. 2019, 10, 1895. [Google Scholar] [CrossRef]
- Gwathmey, C.O.; Tyler, D.D.; Yin, X. Prospects for monitoring cotton crop maturity with normalized difference vegetation index. Agron. J. 2010, 102, 1352–1360. [Google Scholar] [CrossRef]
- Harris, F.A.; English, P.J.; Sudbrink, D.; Nichols, S.P.; Snipes, C.E.; Wills, G.; Hanks, J. Remote-Sensing Measures of Cotton Maturity—Cutout and Boll Opening. In Proceedings of the Beltwide Cotton Conferences, San Antonio, TX, USA, 5–8 January 2004; pp. 1869–1875. [Google Scholar]
- Xu, W.; Yang, W.; Chen, S.; Wu, C.; Chen, P.; Lan, Y. Establishing a model to predict the single boll weight of cotton in northern Xinjiang by using high resolution UAV remote sensing data. Comput. Electron. Agric. 2020, 179, 105762. [Google Scholar] [CrossRef]
- Zhu, C.; Ding, J.; Zhang, Z.; Wang, Z. Exploring the potential of UAV hyperspectral image for estimating soil salinity: Effects of optimal band combination algorithm and random forest. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 279, 121416. [Google Scholar] [CrossRef] [PubMed]
- Maimaitijiang, M.; Sagan, V.; Sidike, P.; Daloye, A.M.; Erkbol, H.; Fritschi, F.B. Crop monitoring using Satellite/UAV data fusion and machine learning. Remote Sens. 2020, 12, 1357. [Google Scholar] [CrossRef]
- Xiang, H.; Tian, L. Development of a low-cost agricultural remote sensing system based on an autonomous unmanned aerial vehicle (UAV). Biosyst. Eng. 2011, 108, 174–190. [Google Scholar] [CrossRef]
- Homolová, L.; Malenovský, Z.; Clevers, J.G.P.W.; García-Santos, G.; Schaepman, M.E. Review of optical-based remote sensing for plant trait mapping. Ecol. Complex. 2013, 15, 1–16. [Google Scholar] [CrossRef]
- Jin, X.; Liu, S.; Baret, F.; Hemerlé, M.; Comar, A. Estimates of plant density of wheat crops at emergence from very low altitude UAV imagery. Remote Sens. Environ. 2017, 198, 105–114. [Google Scholar] [CrossRef]
- Xun, L.; Zhang, J.; Yao, F.; Cao, D. Improved identification of cotton cultivated areas by applying instance-based transfer learning on the time series of MODIS NDVI. Catena 2022, 213, 106130. [Google Scholar] [CrossRef]
- Feng, A.; Zhou, J.; Vories, E.; Sudduth, K.A. Evaluation of cotton emergence using UAV-based imagery and deep learning. Comput. Electron. Agric. 2020, 177, 105711. [Google Scholar] [CrossRef]
- Cuaran, J.; Leon, J. Crop monitoring using unmanned aerial vehicles: A review. Agric. Rev. 2021, 42, 121–132. [Google Scholar] [CrossRef]
- Feng, L.; Chen, S.; Zhang, C.; Zhang, Y.; He, Y. A comprehensive review on recent applications of unmanned aerial vehicle remote sensing with various sensors for high-throughput plant phenotyping. Comput. Electron. Agric. 2021, 182, 106033. [Google Scholar] [CrossRef]
- Durate, A.; Borralho, N.; Cabral, P.; Caetano, M. Recent advances in forest insect pests and diseases monitoring using uav-based data: A systematic review. Forests 2022, 13, 911. [Google Scholar] [CrossRef]
- Fu, Z.; Jiang, J.; Gao, Y.; Krienke, B.; Wang, M.; Zhong, K.; Cao, Q.; Tian, Y.; Zhu, Y.; Cao, W.; et al. Wheat growth monitoring and yield estimation based on multi-rotor unmanned aerial vehicle. Remote Sens. 2020, 12, 508. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Y.; Zhao, Z.; Xie, M.; Hou, D. Estimation of relative chlorophyll content in spring wheat based on multi-temporal UAV remote sensing. Agronomy 2023, 13, 211. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Xiao, Z.; Zeng, X.; Tang, S.; Lin, F.; Zhang, L.; Meng, X.; Liu, S. Cotton blight identification with ground framed canopy photo-assisted multispectral UAV images. Agronomy 2023, 13, 1222. [Google Scholar] [CrossRef]
- Chen, P.; Xu, W.; Zhan, Y.; Yang, W.; Wang, J.; Lan, Y. Evaluation of cotton defoliation rate and establishment of spray prescription map using remote sensing imagery. Remote Sens. 2022, 14, 4206. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Wang, S.; Wu, Y. Extraction of vegetation information from visible unmanned aerial vehicle images. Tran. Chin. Society Agric. Eng. 2015, 31, 152–159. [Google Scholar] [CrossRef]
- Hunt, E.R.J.; Cavigelli, M.; Daughtry, C.S.T.; McMurtrey, J.I.; Walthall, C.L. Evaluation of digital photography from model aircraft for remote sensing of crop biomass and nitrogen status. Precis. Agric. 2005, 6, 359–378. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P.J. Evaluation of the MERIS terrestrial chlorophyll index. Adv. Space Res. 2004, 39, 100–104. [Google Scholar] [CrossRef]
- Takebe, M.; Yoneyama, T.; Inada, K.; Murakami, T. Spectral reflectance ratio of rice canopy for estimating crop nitrogen status. Plant Soil. 1990, 122, 295–297. [Google Scholar] [CrossRef]
- Kanemasu, E.T. Seasonal canopy reflectance patterns of wheat, sorghum, and soybean. Remote Sens. Environ. 1974, 3, 43–47. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Woebbecke, D.; Meyer, G.; Mortensen, D. Color indicesfor weed identification under various soil, residue, and lighting conditions. Trans. ASAE 1995, 38, 259–269. [Google Scholar] [CrossRef]
- Meyer, G.E.; Hindman, T.; Laksmi, K. Machine vision detection parameters for plant species identification. SPIE Proc. 1999, 14, 3243. [Google Scholar] [CrossRef]
- Meyer, G.E.; Neto, J.C. Verification of color vegetation indices for automated crop imaging applications. Comput. Electron. Agric. 2008, 63, 282–293. [Google Scholar] [CrossRef]
- Jordan, C.F. Derivation of leaf-area index from quality of light on the forest floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- David, R.M.; Rosser, N.J.; Donoghue, D.N.M. Improving above ground biomass estimates of Southern Africa dryland forests by combining Sentinel-1 SAR and Sentinel-2 multispectral imagery. Remote Sens. Environ. 2022, 282, 113232. [Google Scholar] [CrossRef]
- Xu, W.; Chen, P.; Zhan, Y.; Chen, S.; Zhang, L.; Lan, Y. Cotton yield estimation model based on machine learning using time series UAV remote sensing data. Int. J. Appl. Earth Obs. 2021, 104, 102511. [Google Scholar] [CrossRef]
- Barnes, E.M.; Clarke, T.R.; Richards, S.E.; Colaizzi, P.D.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.; Riley, E. Coincident Detection of Crop Water Stress, Nitrogen Status and Canopy Density Using Ground-Based Multispectral Data. In Proceedings of the Fifth International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2000. [Google Scholar]
- Ramos, M.A.P.; Osco, P.L.; Furuya, D.E.G.; Gonçalves, W.N.; Santana, D.C.; Teodoro, L.P.R.; Junior, C.A.; Capristo-Silva, G.F.; Li, J.; Baio, F.H.R.; et al. A random forest ranking approach to predict yield in maize with uav-based vegetation spectral indices. Comput. Electron. Agric. 2020, 178, 105791. [Google Scholar] [CrossRef]
- Srinet, R.; Nandy, S.; Patel, N.R. Estimating leaf area index and light extinction coefficient using Random Forest regression algorithm in a tropical moist deciduous forest, India. Ecol. Inform. 2019, 52, 94–102. [Google Scholar] [CrossRef]
- Teluguntla, P.; Thenkabail, P.S.; Oliphant, A.; Xiong, J.; Gumma, M.K.; Congalton, R.G.; Yadav, K.; Huete, A. A 30-m landsat-derived cropland extent product of Australia and China using random forest machine learning algorithm on Google Earth Engine cloud computing platform. ISPRS J. Photogramm. 2018, 144, 325–340. [Google Scholar] [CrossRef]
- Zeng, Y.; Hao, D.; Huete, A.; Dechant, B.; Berry, J.; Chen, J.M.; Joiner, J.; Frankenberg, C.; Bond-Lamberty, B.; Ryu, Y. Optical vegetation indices for monitoring terrestrial ecosystems globally. Nat. Rev. Earth Environ. 2022, 3, 477–493. [Google Scholar] [CrossRef]
- Li, F.; Li, D.; Elsayed, S.; Hu, Y.; Schmidhalter, U. Using optimized three-band spectral indices to assess canopy N uptake in corn and wheat. Eur. J. Agron. 2021, 127, 126286. [Google Scholar] [CrossRef]
- Wang, F.; Huang, J.; Tang, Y.; Wang, X. New vegetation index and its application in estimating leaf area index of rice. Rice Sci. 2007, 14, 195–203. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, P.; Khan, M.S.; Semwal, M. Multi-Temporal High Resolution Unmanned Aerial Vehicle (UAV) Multispectral Imaging for Menthol Mint Crop Monitoring. In Proceedings of the 6th International Conference for Convergence in Technology (I2CT), Maharashtra, India, 2–4 April 2021; Volume 161, p. 20593513. [Google Scholar] [CrossRef]
- Jiang, Z.; Huete, A.R.; Chen, J.; Chen, Y.; Li, J.; Yan, G.; Zhang, X. Analysis of NDVI and scaled difference vegetation index retrievals of vegetation fraction. Remote Sens. Environ. 2006, 101, 366–378. [Google Scholar] [CrossRef]
- Maestrini, B.; Basso, B. Predicting spatial patterns of within-field crop yield variability. Field Crop. Res. 2018, 219, 106–112. [Google Scholar] [CrossRef]
- Tenreiro, T.R.; García-Vila, M.; Gómez, J.A.; Jiménez-Berni, J.A.; Fereres, E. Using NDVI for the assessment of canopy cover in agricultural crops within modelling research. Comput. Electron. Agric. 2021, 182, 106038. [Google Scholar] [CrossRef]
- Yang, H.; Yin, H.; Li, F.; Hu, Y.; Yu, K. Machine learning models fed with optimized spectral indices to advance crop nitrogen monitoring. Field Crop. Res. 2023, 293, 108844. [Google Scholar] [CrossRef]
- Yiru, M.; Xiangyu, C.; Changping, H.; Tongyu, H.; Xin, L.; Ze, Z. Monitoring defoliation rate and boll-opening rate of machine-harvested cotton based on UAV RGB images. Eur. J. Agron. 2023, 151, 126976. [Google Scholar]



| Aircraft Parameters | Camera Parameters | ||
|---|---|---|---|
| Takeoff weight | 1487 g | FOV | 62.7° |
| Diagonal distance | 350 mm | Focal length | 5.74 mm |
| Maximum flying altitude | 6000 m | Aperture | f/2.2 |
| Max ascent speed | 6 m/s | RGB sensor ISO | 200–800 |
| Max descent speed | 3 m/s | Monochrome sensor gain | 1–8× |
| Max speed | 50 km/h | Max image size | 1600 × 1300 |
| Max flight time | 27 min | Photo format | JPEG/TIFF |
| Operating temperature | 0 °C to 40 °C | Supported file systems | FAT32(32 GB); exFAT(>32 GB) |
| Operating frequency | 5.72 to 5.85 GHz | Operating temperature | 0 °C to 40 °C |
| Vegetation Index | Formula | References |
|---|---|---|
| Normalized Difference Vegetation Index (NDVI) | (NIR − R)/(NIR + R) | [28] |
| Normalized Difference Red Edge (NDRE) | (NIR-RE)/(NIR + RE) | [29] |
| Visible-band Difference Vegetation Index (VDVI) | (2 × G − R − B)/(2 × G + R + B) | [30] |
| Green Normalized Difference Vegetation Index (GNDVI) | (NIR − G)/(NIR + G) | [31] |
| Simple Ratio Index (SR) | NIR/R | [32] |
| Green Ratio Vegetation Index (GRVI) | NIR/G | [33] |
| Red Green Ratio Index (RGRI) | R/G | [34] |
| Difference Vegetation Index (DVI) | NIR − R | [35] |
| Excess Green Vegetation Index (EXG) | 2 × G − R − B | [36] |
| Excess Red Vegetation Index (EXR) | 1.4 × R − G | [37] |
| Red Edge Soil-Adjusted Vegetation Index (RESAVI) | 1.5 × (NIR − RE)/(NIR + RE + 0.5) | [38] |
| Enhanced Vegetation Index (EVI) | 2.5 × (NIR − R)/(NIR + 6 × R − 7.5 × B + 1) | [39] |
| Date | Overall Accuracy | Kappa Coefficient |
|---|---|---|
| 7 September | 99.82% | 0.945 |
| 13 September | 99.86% | 0.958 |
| 23 September | 99.16% | 0.966 |
| 29 September | 99.83% | 0.991 |
| 2 October | 99.93% | 0.997 |
| 12 October | 99.98% | 0.999 |
| 16 October | 99.82% | 0.994 |
| 23 October | 99.64% | 0.988 |
| Vegetation Index | r |
|---|---|
| DVI | −0.654 *** |
| EVI | −0.835 *** |
| EXG | −0.815 *** |
| EXR | 0.814 *** |
| GNDVI | −0.835 *** |
| GRVI | −0.838 *** |
| NDRE | −0.750 *** |
| NDVI | −0.835 *** |
| RESAVI | −0.810 *** |
| RGRI | 0.777 *** |
| SR | −0.838 *** |
| VDVI | −0.794 *** |
| Vegetation Index | Model | Training Set | Validation Set | ||
|---|---|---|---|---|---|
| R2 | rRMSE (%) | R2 | rRMSE (%) | ||
| EVI | y = 88.580x + 2.305 | 0.811 | 22.496 | 0.839 | 20.165 |
| GNDVI | y = 53.333x + 24.562 | 0.901 | 16.318 | 0.909 | 15.225 |
| GRVI | y = 97.183x + 3.581 | 0.825 | 21.666 | 0.841 | 19.968 |
| NDVI | y = 101.316x + 15.372 | 0.912 | 15.387 | 0.929 | 13.414 |
| SR | y = 81.510x − 1.543 | 0.724 | 27.222 | 0.74 | 25.421 |
| Data | 1 Comps | 2 Comps | 3 Comps | 4 Comps | 5 Comps |
|---|---|---|---|---|---|
| X dimension | 98.56 | 99.24 | 99.84 | 100.98 | 100.00 |
| Y dimension | 89.78 | 93.50 | 94.12 | 94.17 | 94.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xiao, C.; Wang, Y.; Li, K.; Yu, K.; Geng, J.; Li, Q.; Yang, J.; Zhang, J.; Zhang, M.; et al. Monitoring of Cotton Boll Opening Rate Based on UAV Multispectral Data. Remote Sens. 2024, 16, 132. https://doi.org/10.3390/rs16010132
Wang Y, Xiao C, Wang Y, Li K, Yu K, Geng J, Li Q, Yang J, Zhang J, Zhang M, et al. Monitoring of Cotton Boll Opening Rate Based on UAV Multispectral Data. Remote Sensing. 2024; 16(1):132. https://doi.org/10.3390/rs16010132
Chicago/Turabian StyleWang, Yukun, Chenyu Xiao, Yao Wang, Kexin Li, Keke Yu, Jijia Geng, Qiangzi Li, Jiutao Yang, Jie Zhang, Mingcai Zhang, and et al. 2024. "Monitoring of Cotton Boll Opening Rate Based on UAV Multispectral Data" Remote Sensing 16, no. 1: 132. https://doi.org/10.3390/rs16010132
APA StyleWang, Y., Xiao, C., Wang, Y., Li, K., Yu, K., Geng, J., Li, Q., Yang, J., Zhang, J., Zhang, M., Lu, H., Du, X., Du, M., Tian, X., & Li, Z. (2024). Monitoring of Cotton Boll Opening Rate Based on UAV Multispectral Data. Remote Sensing, 16(1), 132. https://doi.org/10.3390/rs16010132







