Describing Polyps Behavior of a Deep-Sea Gorgonian, Placogorgia sp., Using a Deep-Learning Approach
Abstract
1. Introduction
2. Materials and Methods
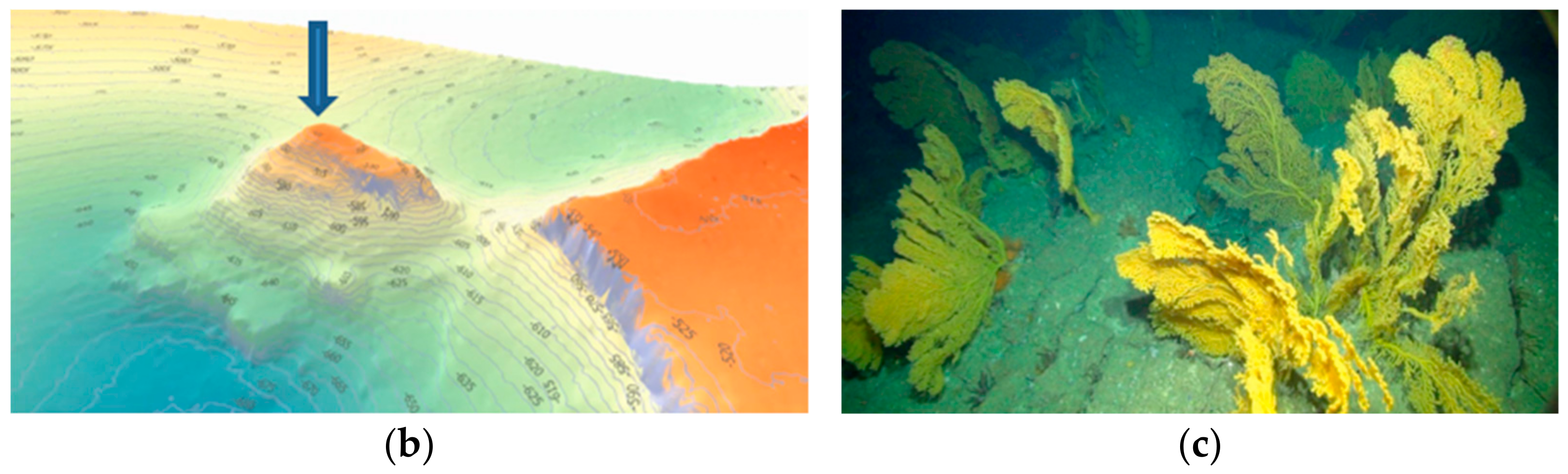
2.1. Study Area
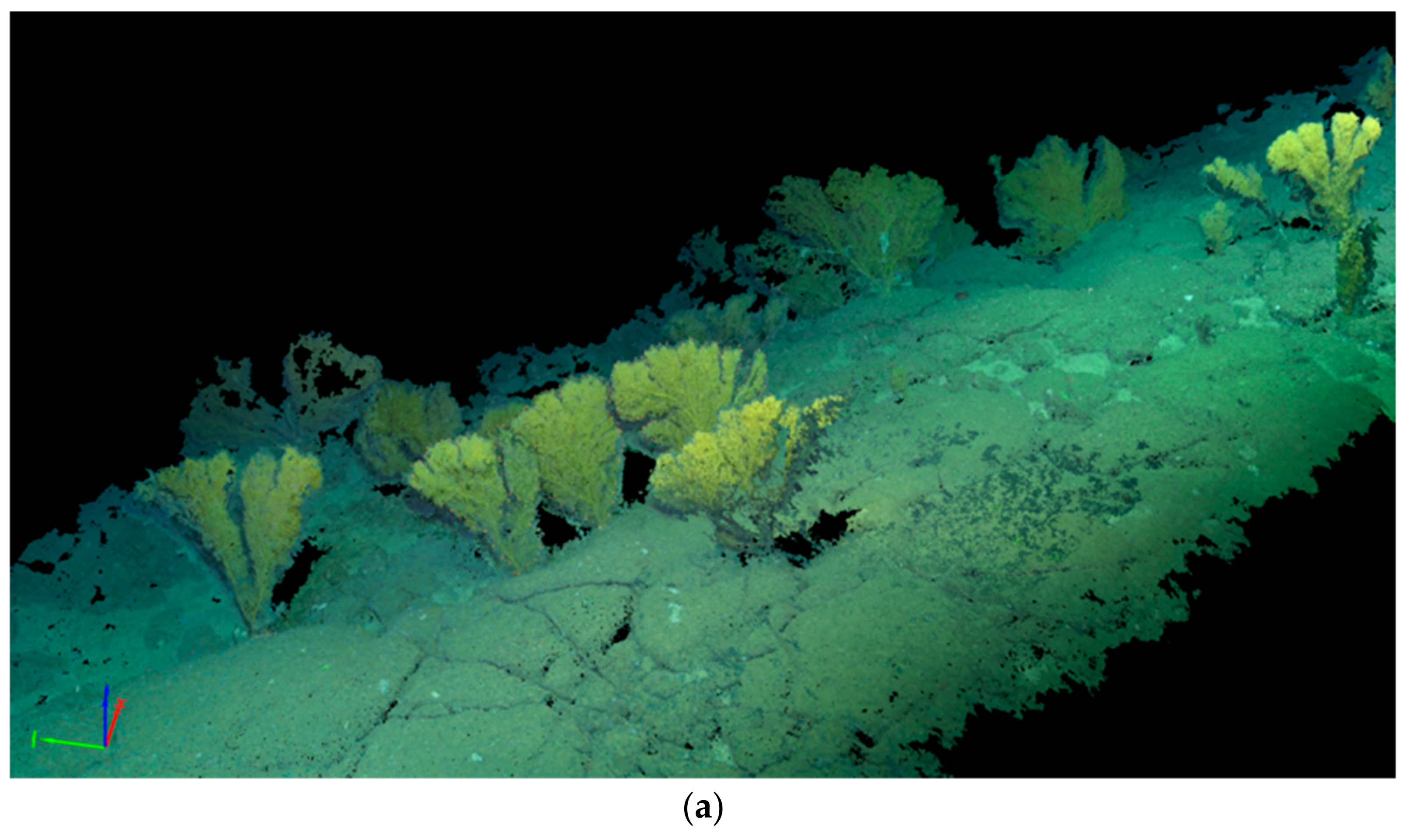
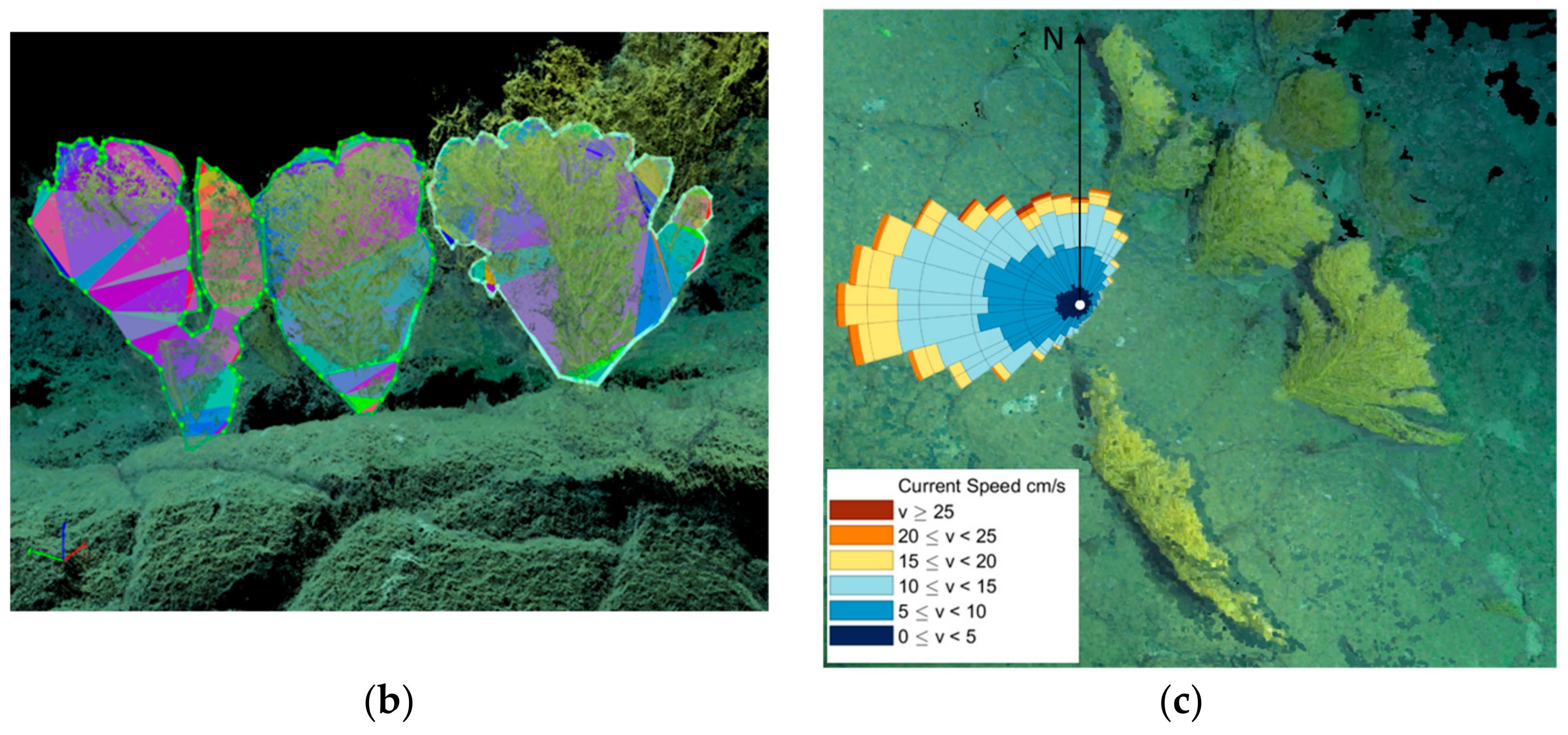
2.2. Placogorgia sp. Colonies Morphometric Characterization
2.3. Image and Oceanographic Data Registration
2.4. Image Processing
2.4.1. Training and Test Dataset
2.4.2. Data Augmentation
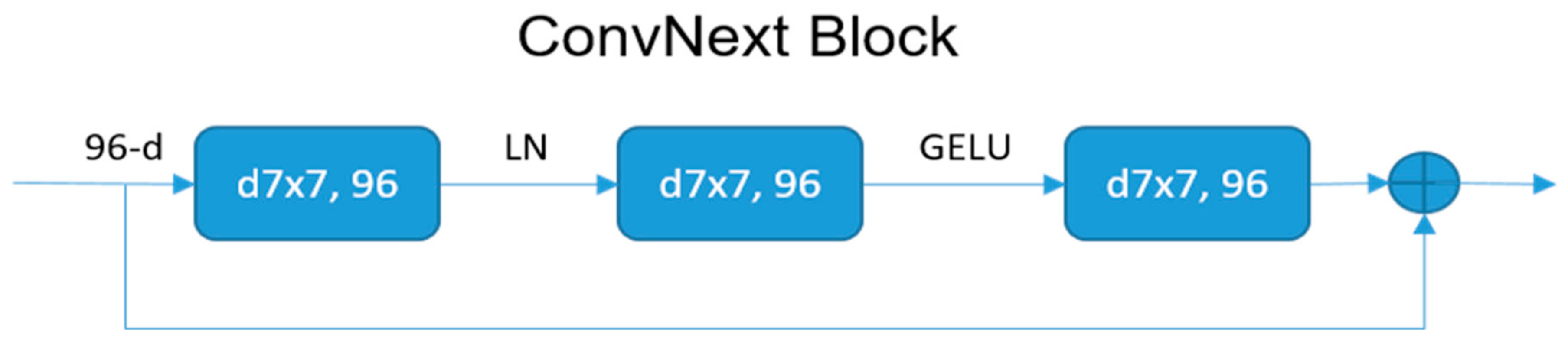
2.4.3. Model
2.5. Temporal Dynamics Analysis
3. Results
3.1. Placogorgia sp. Colonies Morphometric Characterization
3.2. Hydrographic Dynamics
3.3. Semantic Segmentation Model Performance
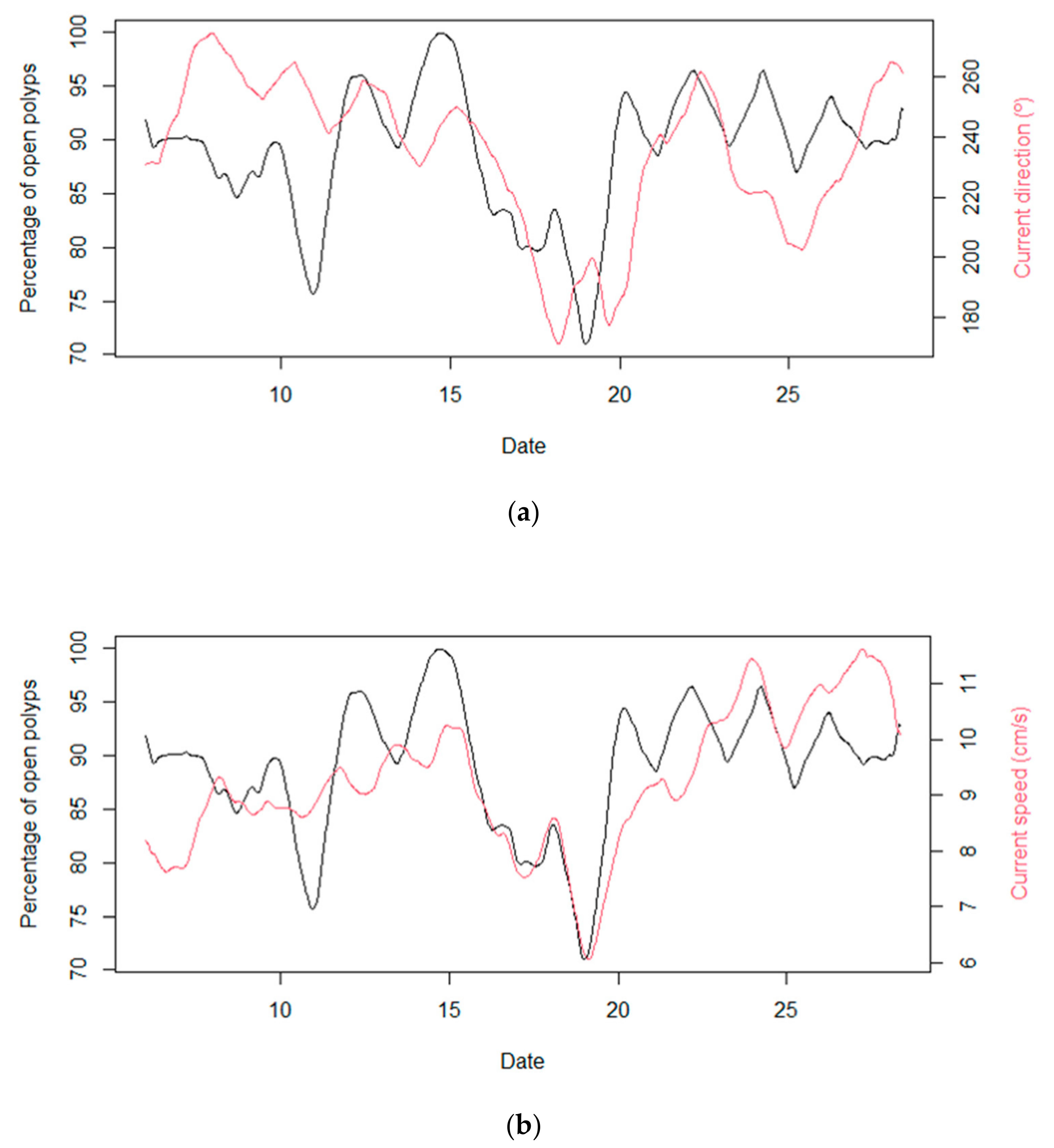
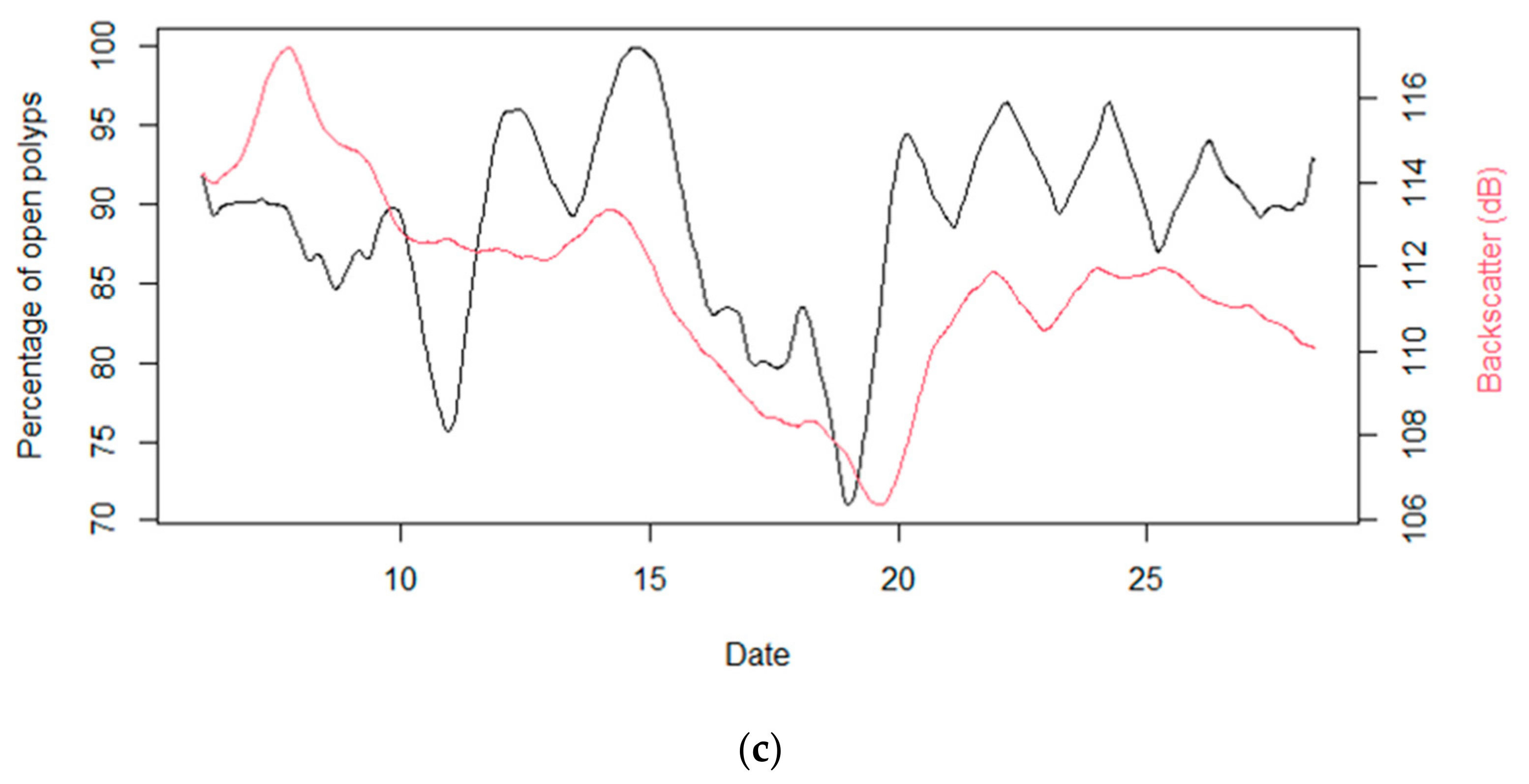
3.4. Temporal Behavior of Polyps and Its Relation with Environmental Dynamics
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossi, S.; Bramanti, L.; Gori, A.; Orejas, C. An overview of the animal forests of the world. In Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots; Rossi, S., Bramanti, L., Gori, A., Orejas, C., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–28. [Google Scholar]
- Buhl-Mortensen, L.; Vanreusel, A.; Gooday, A.J.; Levin, L.A.; Priede, I.G.; Buhl Mortensen, P.; Gheerardyn, H.; King, N.J.; Raes, M. Biological structures as a source of habitat heterogeneity and biodiversity on the deep ocean margins. Mar. Ecol. 2010, 31, 21–50. [Google Scholar] [CrossRef]
- Bongiorni, L.; Mea, M.; Gambi, C.; Pusceddu, A.; Taviani, M. Deep-water corals promote higher diversity in deep-sea meiofaunal assemblages along continental margins. Biol. Conserv. 2010, 143, 1687–1700. [Google Scholar] [CrossRef]
- Rooper, C.N.; Etnoyer, P.J.; Stierhoff, K.L.; Olson, J.V. Effects of fishing gear on deep-sea corals and sponges in U.S. Waters. In The State of DeepSea Coral and Sponge Ecosystems of the United States; Hourigan, T.F., Etnoyer, P.J., Cairns, S.D., Eds.; NOAA Technical Memorandum NMFS-OHC-4; NOAA: Silver Spring, MD, USA, 2017; p. 36. [Google Scholar]
- Betti, F.; Bavestrello, G.; Bo, M.; Ravanetti, G.; Enrichetti, F.; Coppari, M.; Cappanera, V.; Venturini, S.; Cattaneo-Vietti, R. Evidences of fishing impact on the coastal gorgonian forests in-side the Portofino MPA (NW Mediterranean Sea). Ocean. Coast. Manag. 2020, 187, 105105. [Google Scholar] [CrossRef]
- Sherwood, O.A.; Edinger, E.N. Ages and growth rates of some dep-sea gorgonians and antipatharian corals of Newfoundland and Labrador. Can. J. Fish. Aquat. Sci. 2009, 66, 142–152. [Google Scholar] [CrossRef]
- Coppari, M.; Zanella, C.; Rossi, S. The importance of coastal gorgonians in the blue carbon budget. Sci. Rep. 2019, 9, 13550. [Google Scholar] [CrossRef]
- Gili, J.M.; Coma, R. Benthic suspension feeders: Their paramount role in littoral marine food webs. Trends. Ecol. Evol. 1998, 13, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Rizzo, L.; Duchêne, J.C. Polyp expansion of passive suspension feeders: A red coral case study. PeerJ 2019, 7, e7076. [Google Scholar] [CrossRef]
- Coma, R.; Gili, J.M.; Zabala, M.; Riera, T. Feeding and prey capture cycles in the aposymbiontic gorgonian Paramuricea clavata. Mar. Ecol. Prog. Ser. 1994, 115, 257–270. [Google Scholar] [CrossRef]
- Fabricius, K.; Alderslade, P. Soft Corals and Sea Fans—A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea; Australian Institute of Marine Science: Townsville, Australia, 2001. [Google Scholar]
- Orejas, C.; Gili, J.M.; Arntz, W. The role of the small planktonic communities in the diet of two Antarctic octocorals (Primnoisis antarctica and Primnoella sp.). MEPS 2003, 250, 105–116. [Google Scholar] [CrossRef]
- Sebens, K.P.; Vandersall, K.S.; Savina, L.A.; Graham, K.R. Zooplankton capture by two scleractinian corals, Madracis mirabilis and Montastrea cavernosa, in a field enclosure. Mar. Biol. 1996, 127, 303–317. [Google Scholar] [CrossRef]
- Wainwright, S.A.; Dillon, J.R. On the orientation of sea fans (Genus Gorgonia). Biol. Bull. 1969, 136, 136–139. [Google Scholar] [CrossRef]
- Grigg, R.W. Orientation and growth form of sea fans. Limnol. Oceanogr. 1972, 17, 185–192. [Google Scholar] [CrossRef]
- Genin, A.; Dayton, P.; Lonsdale, P.F.; Spiess, F.N. Corals on seamount peaks provide evidence of current acceleration over deep-sea topography. Nature 1986, 322, 59–61. [Google Scholar] [CrossRef]
- Sponaugle, S.; La Barbera, M. Drag-induced deformation: A functional feeding strategy in two species of gorgonians. J. Exp. Mar. Biol. Ecol. 1991, 148, 121–134. [Google Scholar] [CrossRef]
- Sponaugle, S. Flow patterns and velocities around a suspension-feeding gorgonian polyp: Evidence from physical models. J. Exp. Mar. Biol. Ecol. 1991, 148, 135–145. [Google Scholar] [CrossRef]
- Chang-Feng, D.; Ming-Chao, L. The effects of flow on feeding of three gorgonians from southern Taiwan. J. Exp. Mar. Biol. Ecol. 1993, 173, 57–69. [Google Scholar] [CrossRef]
- Liu, H.; Büscher, J.V.; Köser, K.; Greinert, J.; Song, H.; Chen, Y.; Schoening, T. Automated Activity Estimation of the Cold-Water Coral Lophelia pertusa by Multispectral Imaging and Computational Pixel Classification. J. Atmos. Ocean. Technol. 2021, 38, 141–154. [Google Scholar] [CrossRef]
- Osterloff, J.; Nilssen, I.; Järnegren, J.; Buhl-Mortensen, P.; Nattkemper, T.W. Polyp Activity Estimation and Monitoring for Cold Water Corals with a Deep Learning Approach. In Proceedings of the 2016 ICPR 2nd Workshop on Computer Vision for Analysis of Underwater Imagery (CVAUI), Cancun, Mexico, 4 December 2016; pp. 1–6. [Google Scholar]
- Osterloff, J.; Nilssen, I.; Järnegren, J.; Van Engeland, T.; Buhl-Mortensen, P.; Nattkemper, T.W. Computer vision enables short and long-term analysis of Lophelia pertusa polyp behaviour and colour from an underwater observatory. Sci. Rep. 2019, 9, 6578. [Google Scholar] [CrossRef]
- Zuazo, A.; Grinyó, J.; López-Vázquez, V.; Rodríguez, E.; Costa, C.; Ortenzi, L.; Flögel, S.; Valencia, J.; Marini, S.; Zhang, G.; et al. An Automated Pipeline for Image Processing and Data Treatment to Track Activity Rhythms of Paragorgia arborea in Relation to Hydrographic Conditions. Sensors 2020, 20, 6281. [Google Scholar] [CrossRef]
- Girard, F.; Litvin, S.Y.; Sherman, A.; McGill, P.; Gannon, A.; Lovera, C.; DeVogelaere, A.; Burton, E.; Graves, D.; Schnittger, A.; et al. Phenology in the deep sea: Seasonal and tidal feeding rhythms in a keystone octocoral. Proc. R. Soc. B 2022, 289, 20221033. [Google Scholar] [CrossRef]
- Johanson, A.; Flögel, S.; Dullo, W.C.; Linke, P.; Hasselbring, W. Modeling Polyp Activity of Paragorgia arborea Using Supervised Learning. Ecol. Inform. 2017, 39, 109–118. [Google Scholar] [CrossRef]
- Aguzzi, J.; Iveša, N.; Gelli, M.; Costa, C.; Gavrilović, A.; Cukrov, N.; Cukrov, N.; Cukrov, M.; Omanovic, D.; Štifanić, M.; et al. Ecological video monitoring of Marine Protected Areas by underwater cabled surveillance cameras. Mar. Policy 2020, 119, 104052. [Google Scholar] [CrossRef]
- Sánchez, F.; González-Pola, C.; Rodríguez-Basalo, A.; Rodríguez, J.M.; Prado, E.; Módica, L.; Rodríguez-Cabello, C. Faunal behavior in response to near bottom water dynamics in a marine protected area (Cantabrian Sea, southern Bay of Biscay). Estuar. Coast. Shelf Sci. 2022, 277, 108078. [Google Scholar] [CrossRef]
- D’Onghia, G.; Capezzuto, F.; Carlucci, R.; Carluccio, A.; Maiorano, P.; Panza, M.; Ricci, P.; Sion, L.; Tursi, A. Using a benthic lander to explore and monitor vulnerable ecosystems in the Mediterranean Sea. ACTA IMEKO 2018, 7, 45–49. [Google Scholar] [CrossRef]
- Beijbom, O.; Edmunds, P.J.; Kline, D.; Mitchell, B.G.; Kriegman, D. Automated annotation of coral reef survey images. In Proceedings of the 2012 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Providence, RI, USA, 16–21 June 2012; pp. 1170–1177. [Google Scholar]
- Mahmood, A.; Bennamoun, M.; An, S.; Sohel, F.; Boussaid, F.; Hovey, R.; Kendrick, G.; Fisher, R. Automatic annotation of coral reefs using deep learning. In Proceedings of the OCEANS 2016 MTS/IEEE Monterey, Monterey, CA, USA, 19–23 September 2016; pp. 1–5. [Google Scholar]
- Šiaulys, A.; Vaičiukynas, E.; Medelytė, S.; Olenin, S.; Šaškov, A.; Buškus, K.; Verikas, A. A fully-annotated imagery dataset of sublittoral benthic species in Svalbard. Arct. Data Brief 2021, 35, 106823. [Google Scholar] [CrossRef]
- Wicaksono, P.; Aryaguna, P.A.; Lazuardi, W. Benthic Habitat Mapping Model and Cross Validation Using Machine-Learning Classification Algorithms. Remote Sens. 2019, 11, 1279. [Google Scholar] [CrossRef]
- Al-AbdulKader, K.A.; Farrand, W.H.; Blundell, J.S. Marine Habitat Mapping Using High Spatial Resolution Multispectral Satellite Data. In Proceedings of the SPE International Conference on Health, Safety and Environment in Oil and Gas Exploration and Production, Kuala Lumpur, Malaysia, 20–22 March 2002. [Google Scholar]
- Pillay, T.; Cawthra, H.C.; Lombard, A.T.; Sink, K. Benthic habitat mapping from a machine learning perspective on the Cape St Francis inner shelf, Eastern Cape, South Africa. Mar. Geol. 2021, 440, 106595. [Google Scholar] [CrossRef]
- Abad-Uribarren, A.; Prado, E.; Sierra, S.; Cobo, A.; Rodríguez-Basalo, A.; Gómez-Ballesteros, M.; Sánchez, F. Deep learning-assisted high resolution mapping of vulnerable habitats within the Capbreton Canyon System, Bay of Biscay. Estuar. Coast. Shelf Sci. 2022, 75, 107957. [Google Scholar] [CrossRef]
- Liu, F.; Fang, M. Semantic Segmentation of Underwater Images Based on Improved Deeplab. J. Mar. Sci. Eng. 2020, 8, 188. [Google Scholar] [CrossRef]
- Islam, M.J.; Edge, C.; Xiao, Y.; Luo, P.; Mehtaz, M.; Morse, C.; Enan, S.S.; Sattar, J. Semantic Segmentation of Underwater Imagery: Dataset and Benchmark. In Proceedings of the 2020 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Las Vegas, NV, USA, 24 October 2020–24 January 2021; pp. 1769–1776. [Google Scholar]
- King, A.; Bhandarkar, S.M.; Hopkinson, B.M. A Comparison of Deep Learning Methods for Semantic Segmentation of Coral Reef Survey Images. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR) Workshops, Salt Lake City, UT, USA, 18–22 June 2018; pp. 1394–1402. [Google Scholar]
- Purser, A. A Time Series Study of Lophelia pertusa and Reef Megafauna Responses to Drill Cuttings Exposure on the Norwegian Margin. PLoS ONE 2015, 10, e0134076. [Google Scholar] [CrossRef]
- BOE. Real Decreto 1629/2011, de 14 de Noviembre, por el que se Declara Como Área Marina Protegida y como Zona Especial de Conservación el Espacio Marino de El Cachucho, y se Aprueban las Correspondientes Medidas de Conservación; Ministry of the Environment, Rural and Marine Affairs: Madrid, Spain, 2011; pp. 130084–130138.
- González-Pola, C.; Díaz del Río, G.; Ruiz-Villarreal, M.; Sánchez, R.F.; Mohn, C. Circulation patterns at Le Danois Bank, an elongated shelf-adjacent seamount in the Bay of Biscay. Deep Sea Res. I Oceanogr. Res. Pap. 2012, 60, 7–21. [Google Scholar] [CrossRef]
- Sánchez, F.; Serrano, A.; Parra, S.; Ballesteros, M.; Cartes, J.E. Habitat characteristics as determinant of the structure and spatial distribution of epibenthic and demersal communities of Le Danois Bank (Cantabrian Sea, N. Spain). J. Mar. Syst. 2008, 72, 64–86. [Google Scholar] [CrossRef]
- Figueira, W.; Ferrari, R.; Weatherby, E.; Porter, A.; Hawes, S.; Byrne, M. Accuracy and Precision of Habitat Structural Complexity Metrics Derived from Underwater Photogrammetry. Remote Sens. 2015, 7, 16883–16900. [Google Scholar] [CrossRef]
- Price, D.M.; Robert, K.; Callaway, A.; Lo Lacono, C.; Hall, R.A.; Huvenne, V.A. Using 3D photogrammetry from ROV video to quantify cold-water coral reef structural complexity and investigate its influence on biodiversity and community assemblage. Coral Reefs 2019, 38, 1007–1021. [Google Scholar] [CrossRef]
- Fukunaga, A.; Burns, J.H.R.; Pascoe, K.H.; Kosaki, R.K. Associations between benthic cover and habitat complexity metrics obtained from 3d reconstruction of coral reefs at different resolutions. Remote Sens. 2020, 12, 1011. [Google Scholar] [CrossRef]
- Palma, M.; Rivas-Casado, M.; Pantaleo, U.; Pavoni, G.; Pica, D.; Cerrano, C. SfMbased method to assess gorgonian forests (Paramuricea clavata (Cnidaria, octocorallia)). Remote Sens. 2018, 10, 1154. [Google Scholar] [CrossRef]
- Prado, E.; Sánchez, F.; Rodríguez-Basalo, A.; Altuna, A.; Cobo, A. Analysis of the population structure of a gorgonian forest (Placogorgia sp.) using a photogrammetric 3D modeling approach at Le Danois Bank, Cantabrian Sea. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2019, 153, 103124, reprinted in Front. Mar. Sci. 2019, 8, 612613. [Google Scholar] [CrossRef]
- Rossi, P.; Castagnetti, C.; Capra, A.; Brooks, A.J.; Mancin, F. Detecting change in coral reef 3D structure using underwater photogrammetry: Critical issues and performance metrics. Appl. Geomat. 2020, 12 (Suppl. 1), S3–S17. [Google Scholar] [CrossRef]
- Lange, I.D.; Perry, C.T. A quick, easy and non-invasive method to quantify coral growth rates using photogrammetry and 3D model comparisons. Methods Ecol. Evol. 2020, 11, 714–726. [Google Scholar] [CrossRef]
- Bennecke, S.; Kwasnitschka, T.; Metaxas, A.; Dullo, W.C. In situ growth rates of deep-water octocorals determined from 3d photogrammetric reconstructions. Coral Reefs 2016, 35, 1227–1239. [Google Scholar] [CrossRef]
- Ferrari, R.; Figueira, W.F.; Pratchett, M.S.; Boube, T.; Adam, A.; Kobelkowsky-Vidrio, T.; Doo, S.S.; Atwood, T.B.; Byrne, M. 3D photogrammetry quantifies growth and external erosion of individual coral colonies and skeletons. Sci. Rep. 2017, 7, 16737. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.H.J.; Weyenberg, G.; Mandel, T.; Ferreira, S.B.; Gotshalk, D.; Kinoshita, C.K.; Marshall, M.J.; Del Moral, N.A.V.; Murphy, S.J.; Pascoe, K.H.; et al. A Comparison of the Diagnostic Accuracy of in-situ and Digital Image-Based Assessments of Coral Health and Disease. Front. Mar. Sci. 2020, 7, 304. [Google Scholar] [CrossRef]
- Prado, E.; Cristobo, J.; Rodríguez-Basalo, A.; Ríos, P.; Rodríguez-Cabello, C.; Sánchez, F. In situ growth rate assessment of the hexactinellid sponge Asconema setubalense using 3D photogrammetric reconstruction. Front. Mar. Sci. 2021, 8, 612613. [Google Scholar] [CrossRef]
- Gonzalez-Pola, C.; Sánchez, F.; Rodriguez Cobo, L.; Graña, R.; Rodriguez, J.M.; Valdiande-Gutierrez, J.; Hernandez-Urbieta, D.; Aierbe, E. LanderPick, a Remote Operated Trawled Vehicle to cost-effectively deploy and recover lightweight oceanographic landers. In Proceedings of the EGU General Assembly 2022, Vienna, Austria, 23–27 May 2022. EGU22-11921. [Google Scholar]
- Liu, Z.; Mao, H.; Wu, C.Y.; Feichtenhofer, C.; Darrell, T.; Xie, S. A convnet for the 2020s. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, New Orleans, LA, USA, 18–24 June 2022; pp. 11976–11986. [Google Scholar]
- Fletcher, S.; Islam, M.Z. Comparing sets of patterns with the Jaccard index. Australas. J. Inf. Syst. 2018, 22. [Google Scholar] [CrossRef]
- Han, F.; Yao, J.; Zhu, H.; Wang, C. Underwater Image Processing and Object Detection Based on Deep CNN Method. J. Sens. 2020, 2020, 6707328. [Google Scholar] [CrossRef]
- Moreno-Barea, F.J.; Jerez, J.M.; Franco, L. Improving Classification Accuracy Using Data Augmentation on Small Data Sets. Expert Syst. Appl. 2020, 161, 113696. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, B.; Zhu, P.; Li, P.; Zuo, W.; Hu, Q. ECA-Net: Efficient Channel Attention for Deep Convolutional Neural Networks. In Proceedings of the 2020 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Seattle, WA, USA, 13–19 June 2020; pp. 11531–11539. [Google Scholar]
- Xu, Q.; Zhang, M.; Gu, Z.; Pan, G. Overfitting Remedy by Sparsifying Regularization on Fully-Connected Layers of CNNs. Neurocomputing 2019, 328, 69–74. [Google Scholar] [CrossRef]
- Golyandina, N.; Zhigljavsky, A. Singular Spectrum Analysis for Time Series; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Zhigljavsky, A. (Ed.) Statistics and Its Interface, Special Issue on the Singular Spectrum Analysis for Time Series; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Cheng, D. Time Series Decomposition Using Singular Spectrum Analysis. Master’s Thesis, East Tennessee State University, Johnson City, TN, USA, 2014. Paper 2352. [Google Scholar]
- Golyandina, N.; Korobeynikov, A.; Shlemov, A.; Usevich, K. Multivariate and 2D Extensions of Singular Spectrum Analysis with the Rssa Package. J. Stat. Softw. 2015, 67, 1–78. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 13 March 2023).
- Lu, J.; Behbood, V.; Hao, P.; Zuo, H.; Xue, S.; Zhang, G. Transfer learning using computational intelligence: A survey. Knowl. Based Syst. 2015, 80, 14–23. [Google Scholar] [CrossRef]
- Orejas, C.; Gori, A.; Rad-Menéndez, C.; Last, K.S.; Davies, A.J.; Beveridge, C.M.; Sadd, D.; Kiriakoulakis, K.; Witte, U.; Roberts, J.M. The effect of flow speed and food size on the capture efficiency and feeding behaviour of the cold-water coral Lophelia pertusa. J. Exp. Mar. Biol. Ecol. 2016, 481, 34–40. [Google Scholar] [CrossRef]
- Elias-Piera, F.; Rossi, S.; Gili, J.M.; Orejas, C. Trophic ecology of seven Antarctic gorgonian species. MEPS 2013, 477, 93–106. [Google Scholar] [CrossRef]




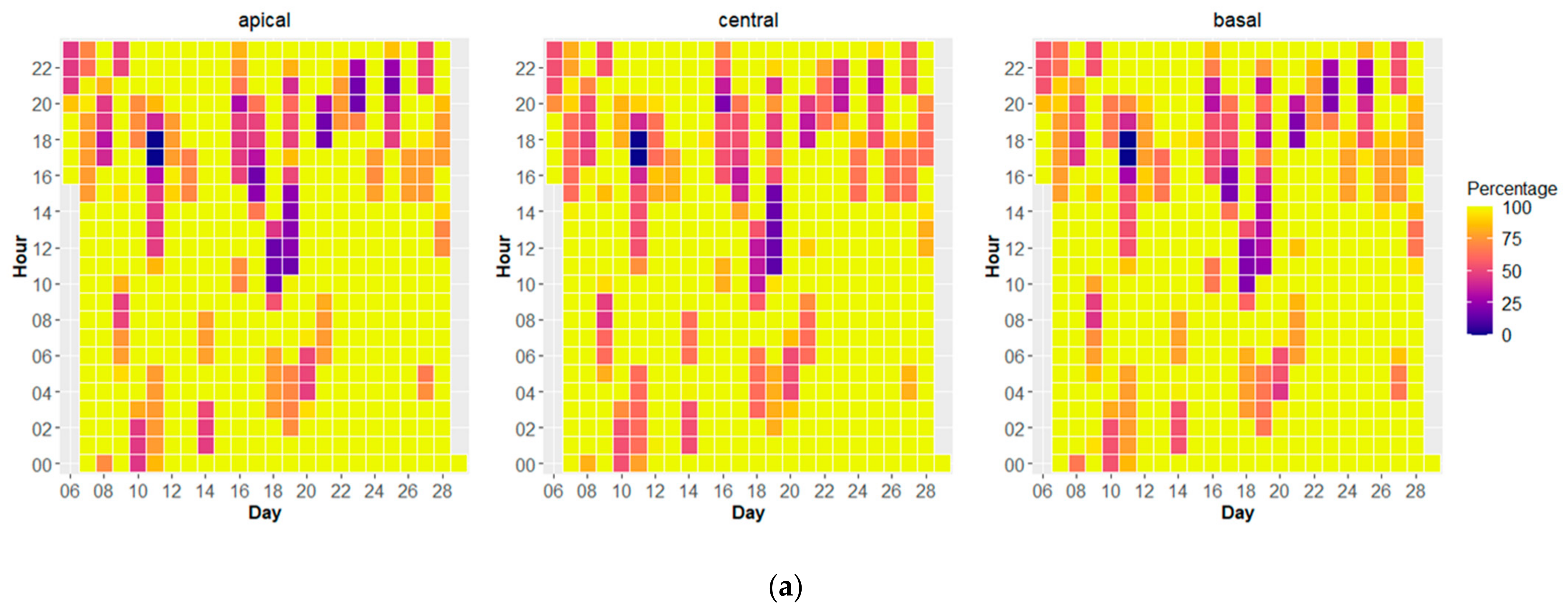
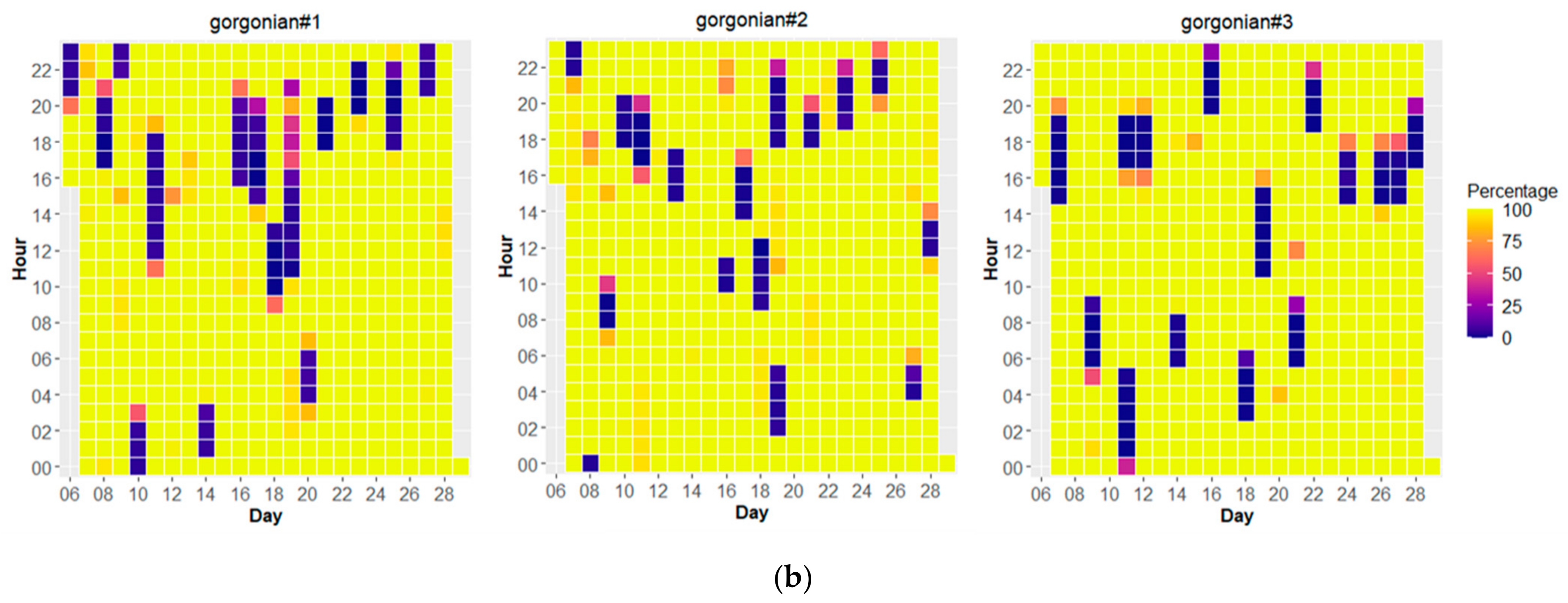
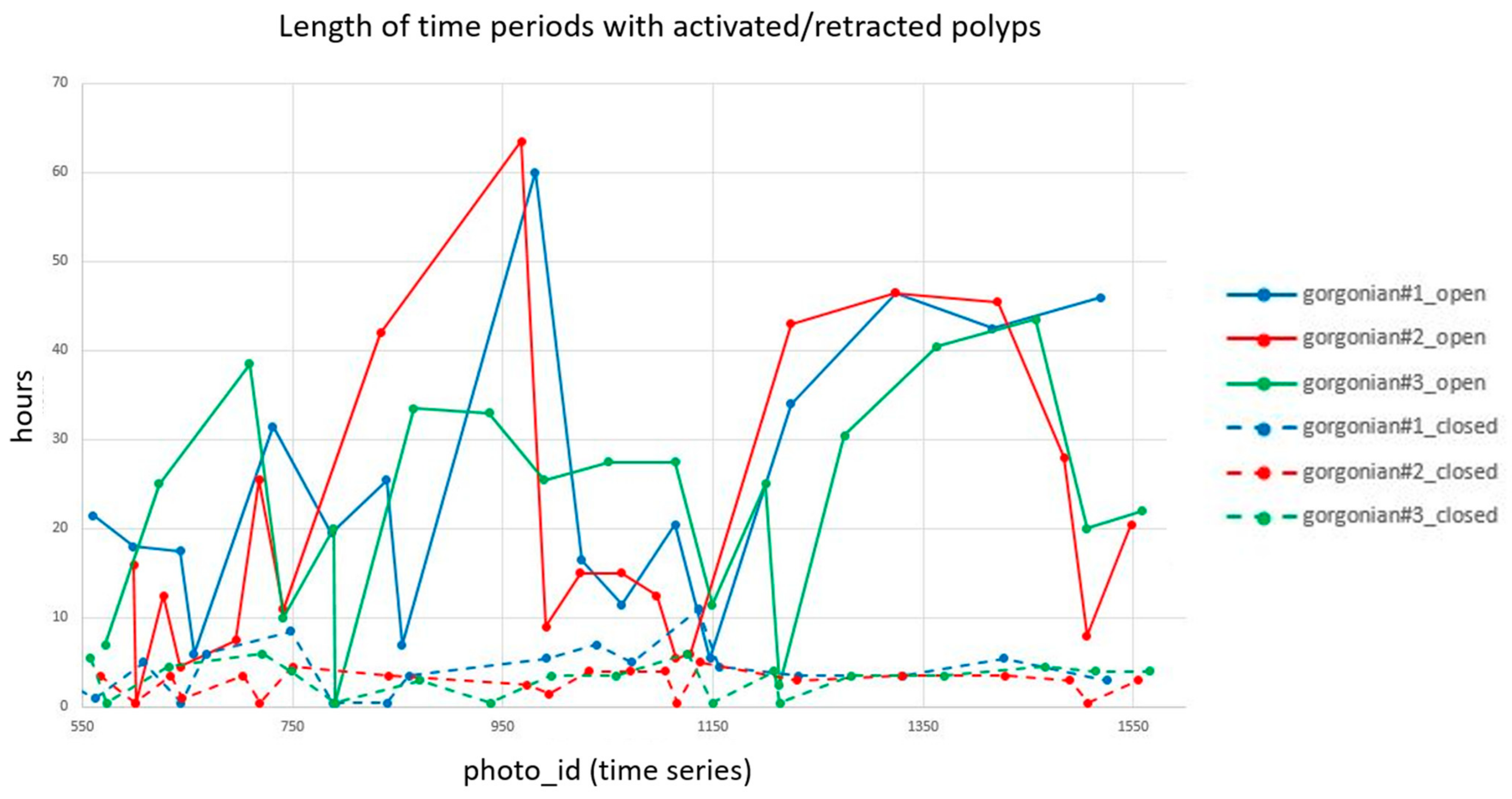
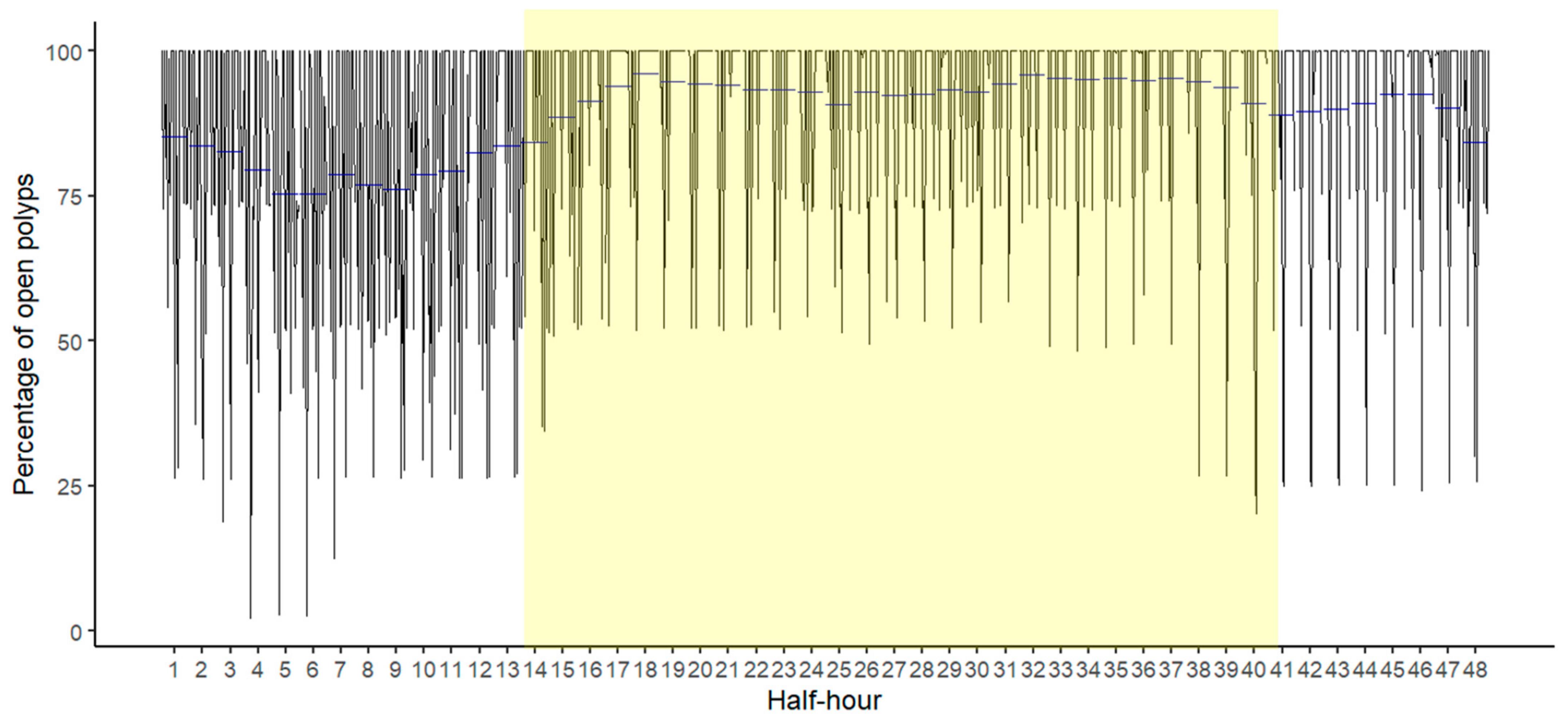

| Average Ground Sampling Distance (GSD) in cm | 0.27 |
|---|---|
| Median of keypoints per image/matches per calibrated image | 9420/2635.9 |
| Number of 2D Keypoint for Bundle Block Adjustment | 2,928,034 |
| Number of 3D Points for Bundle Block Adjustment | 1,032,815 |
| Number of 3D Densified Points/Average Density (per m3) | 15,259,747/159,417 |
| Mean reprojection error | 0.11 |
| Placogorgia sp. Id Code | Height (cm) | Width (cm) | Fan Surface Perimeter (m)/Area (m2) | Fan Orientation (deg) |
|---|---|---|---|---|
| Left | 90.8 | 59.9 | 4.18/0.35 | 139 |
| Center | 81.7 | 57.2 | 2.68/0.16 | 142 |
| Right | 86.4 | 85.2 | 4.35/0.47 | 145 |
| DS_Name | Accuracy | Mean IoU | Recall | F1Score |
|---|---|---|---|---|
| ds0 | 0.99 | 0.964 | 1 | 0.995 |
| Class Name | Accuracy | Mean IoU | Recall | F1Score |
|---|---|---|---|---|
| close | 0.991 | 0.925 | 1 | 0.995 |
| open | 0.984 | 0.98 | 1 | 0.992 |
| Max | Min | Mean | Std. Dv. | |
|---|---|---|---|---|
| Gorgonian#1—left | ||||
| Extended time | 60.0 | 5.50 | 25.3 | 15.9 |
| Retracted time | 11.0 | 0.5 | 4.3 | 2.8 |
| Gorgonian#2—center | ||||
| Extended time | 63.5 | 0.5 | 20.8 | 17.4 |
| Retracted time | 5.0 | 0.5 | 2.8 | 1.4 |
| Gorgonian#3—right | ||||
| Extended time | 43.5 | 0.5 | 23.3 | 12.4 |
| Retracted time | 6.0 | 0.5 | 3.3 | 1.9 |
| Variance Explained (%) | ||||||
|---|---|---|---|---|---|---|
| Trend | Periodicity | |||||
| eigenvectors | 1 | 2 | 3 | 4 | 5 | 6 |
| % of active polyps | 95.7 | 0.83 | 0.82 | 0.59 | 0.47 | 0.4 |
| current speed | 86.09 | 2.79 | 2.54 | 0.97 | 0.88 | 0.87 |
| current direction | 86.71 | 1.75 | 1.62 | 0.72 | 0.68 | 0.49 |
| ADCP intensity | 99.8 | 0.04 | 0.03 | 0.01 | 0.01 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prado, E.; Abad-Uribarren, A.; Ramo, R.; Sierra, S.; González-Pola, C.; Cristobo, J.; Ríos, P.; Graña, R.; Aierbe, E.; Rodríguez, J.M.; et al. Describing Polyps Behavior of a Deep-Sea Gorgonian, Placogorgia sp., Using a Deep-Learning Approach. Remote Sens. 2023, 15, 2777. https://doi.org/10.3390/rs15112777
Prado E, Abad-Uribarren A, Ramo R, Sierra S, González-Pola C, Cristobo J, Ríos P, Graña R, Aierbe E, Rodríguez JM, et al. Describing Polyps Behavior of a Deep-Sea Gorgonian, Placogorgia sp., Using a Deep-Learning Approach. Remote Sensing. 2023; 15(11):2777. https://doi.org/10.3390/rs15112777
Chicago/Turabian StylePrado, Elena, Alberto Abad-Uribarren, Rubén Ramo, Sergio Sierra, César González-Pola, Javier Cristobo, Pilar Ríos, Rocío Graña, Eneko Aierbe, Juan Manuel Rodríguez, and et al. 2023. "Describing Polyps Behavior of a Deep-Sea Gorgonian, Placogorgia sp., Using a Deep-Learning Approach" Remote Sensing 15, no. 11: 2777. https://doi.org/10.3390/rs15112777
APA StylePrado, E., Abad-Uribarren, A., Ramo, R., Sierra, S., González-Pola, C., Cristobo, J., Ríos, P., Graña, R., Aierbe, E., Rodríguez, J. M., Rodríguez-Cabello, C., Modica, L., Rodríguez-Basalo, A., & Sánchez, F. (2023). Describing Polyps Behavior of a Deep-Sea Gorgonian, Placogorgia sp., Using a Deep-Learning Approach. Remote Sensing, 15(11), 2777. https://doi.org/10.3390/rs15112777






