3.1. Assessment of Normalized Water-Leaving Radiance
Since the reliability of remote sensing monitoring of algal bloom mainly depends on the degree of the quantification of satellite derived
Rrs, the
Rrs at bands with lowest uncertainty and best degree of quantification in the study area should be preferred for constructing the algorithm. Validating the accuracy and spectral characteristics of VIIRS retrieved
Lwn(λ) or the equivalent
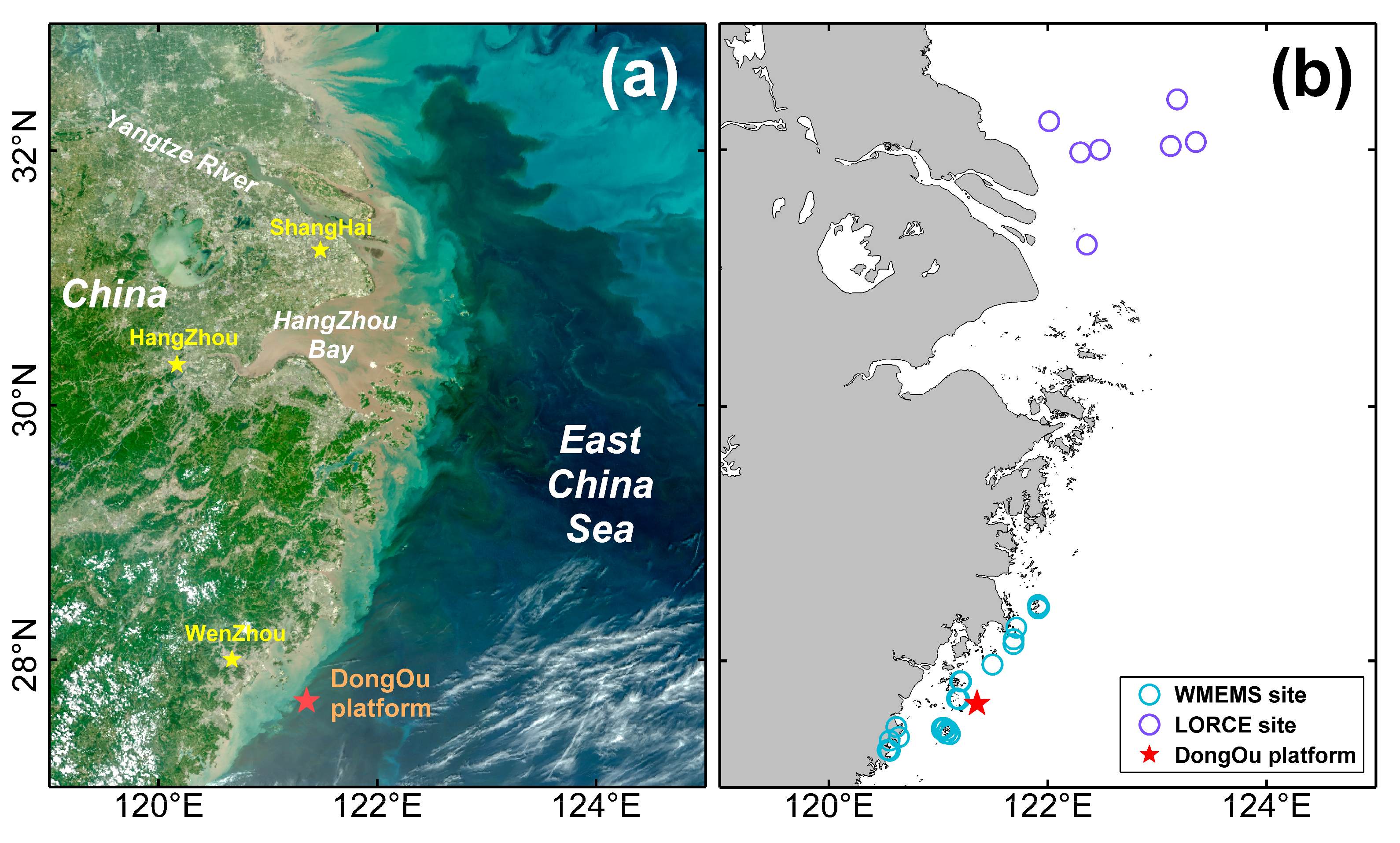
Rrs(λ) is, thus, an important prerequisite for carrying out algal bloom monitoring. A first step when evaluating the quality of the satellite data is to examine the spectral consistency of the water-leaving radiances from the VIIRS and MODIS missions based on the in situ data collected from the Dongou site. The Dongou site is frequently characterized by a large variability in bio-optical quantities because of its position in a transitional region between open sea and coastal waters (
Figure 3a,e,i). Thus, the
Lwn spectra from VIIRS and MODIS (
Figure 3b,f,j) also show high variability. It can be found that the spectra exhibiting large variations show minima at 410 and 671 nm, with the majority of values below 20 W/m
2/nm/sr and unique maxima at 551 nm with values in the range of 4.0–50 W/m
2/nm/sr, and suggest the presence of seawater that is moderately dominated by sediments, as well as the derived water type provides a further confirmation with an occurrence of roughly 70% Case 2 water (of which MODISA, VIIRS-SNPP, and VIIRS-NA20 spectral data accounted for 75%, 83%, and 79% of this type, respectively) [
42]. The comparison of
Lwn spectra indicates that the spectral variation ranges of MODISA, VIIRS/SNPP, and VIIRS/NA20 are very consistent to each other. Furthermore, the matchup
Lwn spectra from SeaPRISM was selected (
Figure 3c,g,k); matchups of the overall average
Lwn spectra were also calculated from all available spectral data of satellites and SeaPRISM, as seen in the fourth column of
Figure 3d,h,l. These matchup comparisons indicate that a general spectral concordance exists between satellite and in situ data. However, in the average
Lwn spectra match-up comparisons, a spectral discrepancy also can be observed at the shorter wavelength bands (410 and 443 nm).
Thus, additional insight from the qualitative matchup analysis of satellite
Lwn(λ) data against in situ SeaPRISM for each individual spectral band is presented in
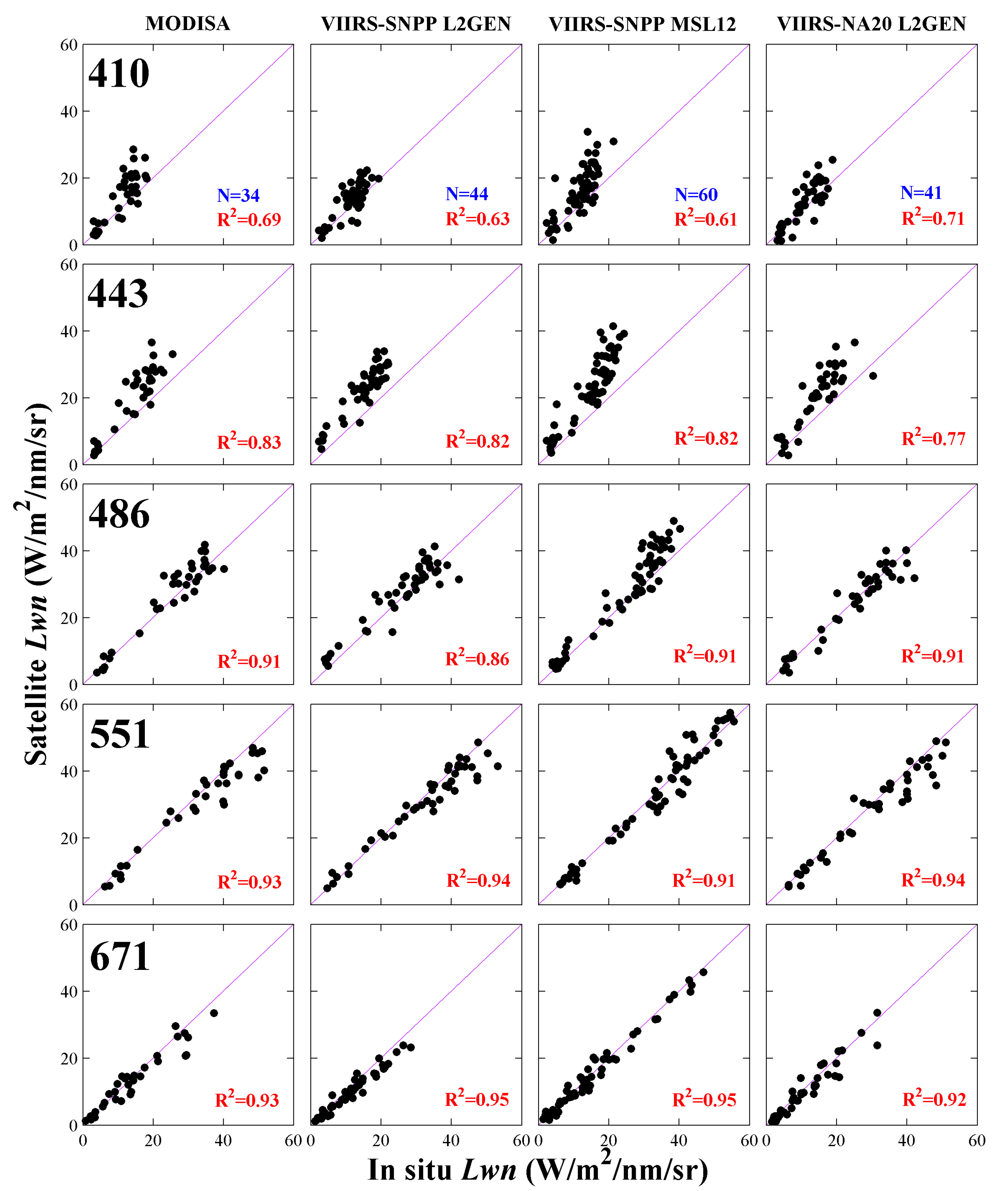
Figure 4 and
Table 4. After the quality control methods were applied, a total of 135 in situ
Lwn spectra matched up with at least one satellite dataset, in which there are 34, 60, and 41 matchups available for MODIS/Aqua, VIIRS/SNPP MSL12, and VIIRS/NA20, respectively. It is noticeable that only 44 matchups were available for VIIRS-SNPP L2GEN, less than that for VIIRS- SNPP MSL12, because much tighter restrictions in the flag conditions applied in the filtering cloud and bright pixels were implemented in the L2GEN procedure. Additionally, the number of matchups of MODSA was less than the other three VIIRS sources, probably because the coastal water of the ECS is dominated by sediments (as seen in
Figure 3a,e,i), which will more easily result in saturation of MODIS 862 nm and thus makes fewer matchups with
Lwn spectra available.
Overall, the correlation coefficients (
R2) between the SeaPRISM and satellite datasets at each wavelength were relatively high, and all comparisons, particularly at 488 nm, 551 nm, and 671 nm, were also very close to a 1:1 line (
Figure 4). Notably, the MODISA data performed quite well even though this MODIS sensor has been in operation during orbit for more than 17 years and has long passed its expected lifetime. At individual wavelengths, correlations for the four satellites were very close to each other, which indicate good consistency between MODIS and the two VIIRS sensors; in addition, the variations in the water-leaving radiance data for the Dongou location were well captured by all satellite sensors. Nevertheless, the resulting
R2 values showed strong spectral dependencies exhibiting the tendency of displaying larger differences in the shorter end of the spectrum. For example, VIIRS-SNPP MSL12 achieved stronger
R2 values of 0.91, 0.91, and 0.95 at 486 nm, 551 nm, and 671 nm, respectively, while at 410 nm and 443 nm only moderate correlations were attained with
R2 values equal to 0.61 and 0.82, respectively. Similar spectral behavior and values of
R2 can be found in MODISA, VIIRS-SNPP L2GEN, and VIIRS-NA20 L2GEN comparisons. This degradation in the correlation for 410 nm and 443 nm agrees with more recent findings from the Long Island Sound coasts, USA, which was considered to have originated from the data processing procedure [
10].
In addition, percent differences (APD and RPD) suggest good qualities of
Lwn retrievals from satellites at 488 nm, 551 nm, and 671 nm, although high uncertainties and bias still exist at 410 nm and 443 nm (
Table 4). The highest APD values were observed at 443 nm (with 39.48%, 61.55%, 59.89%, and 48.88% for MODISA, VIIRS-NPP L2GEN, VIIRS-NPP MSL12, and VIIRS-N20 L2GEN, respectively), followed by 410 nm with a minimum of 28.14%. The larger uncertainties further confirmed the moderate correlations at the two deep blue bands. In the individual band match-up comparisons (
Figure 4), it can be found in all the four datasets that had satellite-derived
Lwn at 410 nm and 443 nm as a whole were larger than that from SeaPRISM; a similar trend was shown in comparisons of the average
Lwn spectra (
Figure 3). Although these overestimates could be partially attributed to the uncertainties in the SeaPRISM datasets, limited retrieval accuracies were achieved at the two blue bands in both MODIS and VIIRS, indicating the challenge of using current remote sensing
Lwn at 410 nm and 443 nm to derive bio-optical products for the ECS. Despite these problems, as compared to shorter wavelengths, matchup statistics were much better for the 486 nm, 551 nm, and 671 nm wavelengths.
In order to fully access the VIIRS
Rrs products, the supplementary underway
Rrs measurements in the ECS were used.
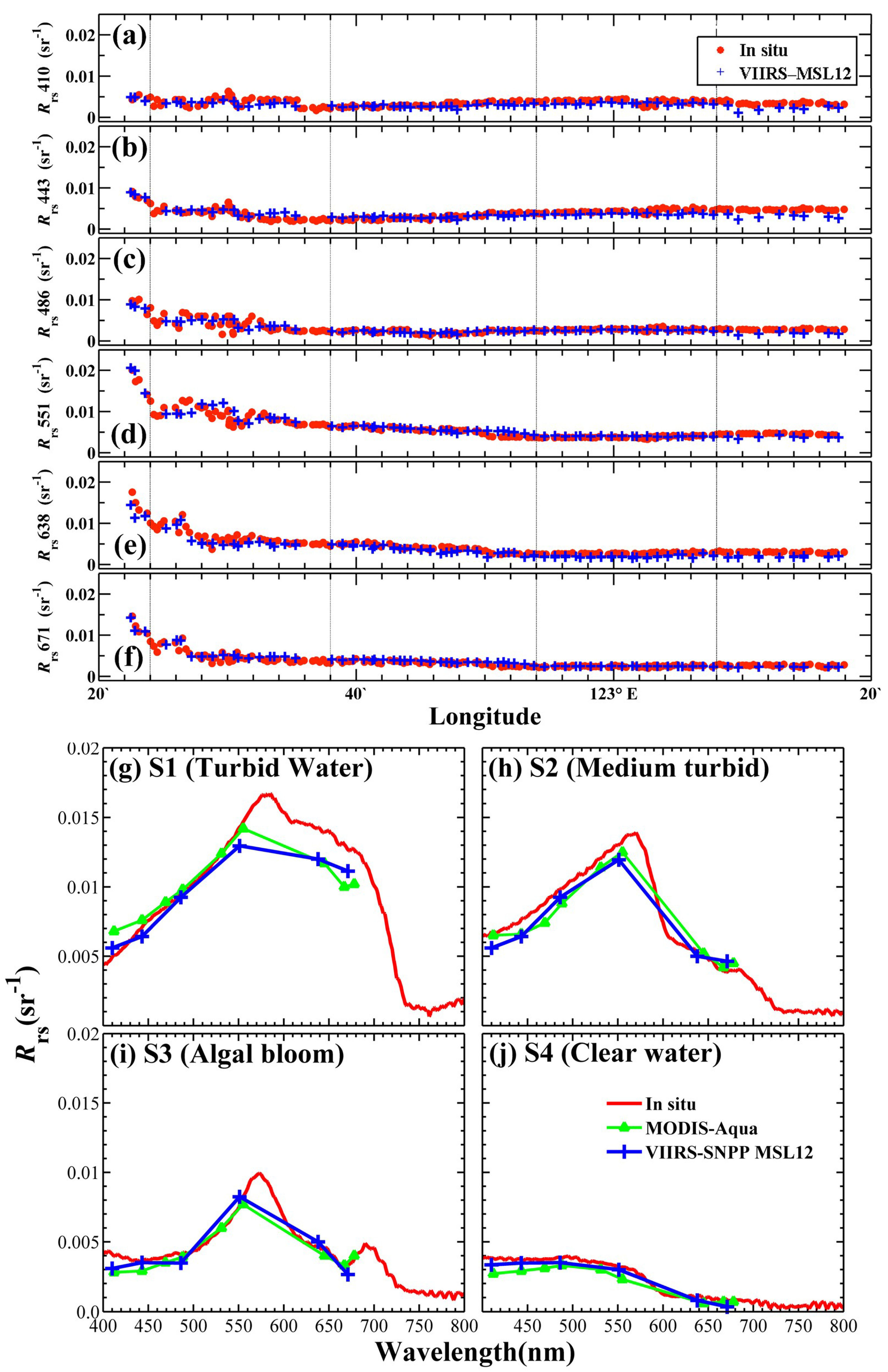
Figure 5 shows that the section of underway
Rrs measurements crossed the coastal area from turbid to clean water bodies; it fortunately covered turbid, moderately turbid, clean, and HAB water bodies at the same time. Although it does not cover extremely turbid water bodies, the HAB events were very infrequent in such turbid waters due to light limitations; the satellite
Rrs data were usually not available.
Figure 5a–f shows the validation results of the section of underway
Rrs observation against the VIIRS MSL12
Rrs data. Similar to the validation results at the Dongou platform (
Figure 4), the VIIRS
Rrs data of 410 nm and 443 nm showed a relatively high bias, while those at the 486 nm, 551 nm, and 671 nm bands were in very good agreement with the underway data. The spectral comparison in the four sites associated with turbid, moderately turbid, HAB, and clean water bodies is also presented in
Figure 5g–j. In terms of magnitude and spectral shape,
Rrs results obtained from VIIRS were very consistent with field measurements.
In the aggregate, these analyses show that as attributing to the most recent calibration efforts (and associated atmospheric correction routines) and reprocessing efforts of both NOAA and NASA, the MODISA, VIIRS/SNPP, and VIIRS/NA20 datasets from either NASA or NOAA yield reliable performance and consistent environmental data records, showing the band data of 486 nm, 551 nm, and 671 nm had better potential for the long-term monitoring in coasts of the ECS.
3.2. HAB Algorithm Development and Validation
Comparative analysis of various VIIRS algorithms was carried out to assess advantages and limitations in the application of these techniques for the detection of algal blooms in the sediment-dominated coasts of the ECS. Recently, different forms of HAB algorithms have become available, which specially include reflectance band-ratios and spectral band difference models [
47]. As seen in
Table 2, the two band-ratio algorithms, Algal Bloom Ratio (RAB) [
28] and RGCI, and the two spectral band difference ones, Red Tide index (RI) [
46] and normalized spectral shape at 488 nm (SS(488)), were selected for the comparison. Due to the similar band configuration of VIIRS with MODIS, most of these MODIS based algorithms can be directly applied to VIIRS. However, as mentioned above, VIIRS lacks the 531 nm band so that the 488 nm and 551 nm bands were used instead of MODIS 531 and 555 nm bands in the RAB algorithm.
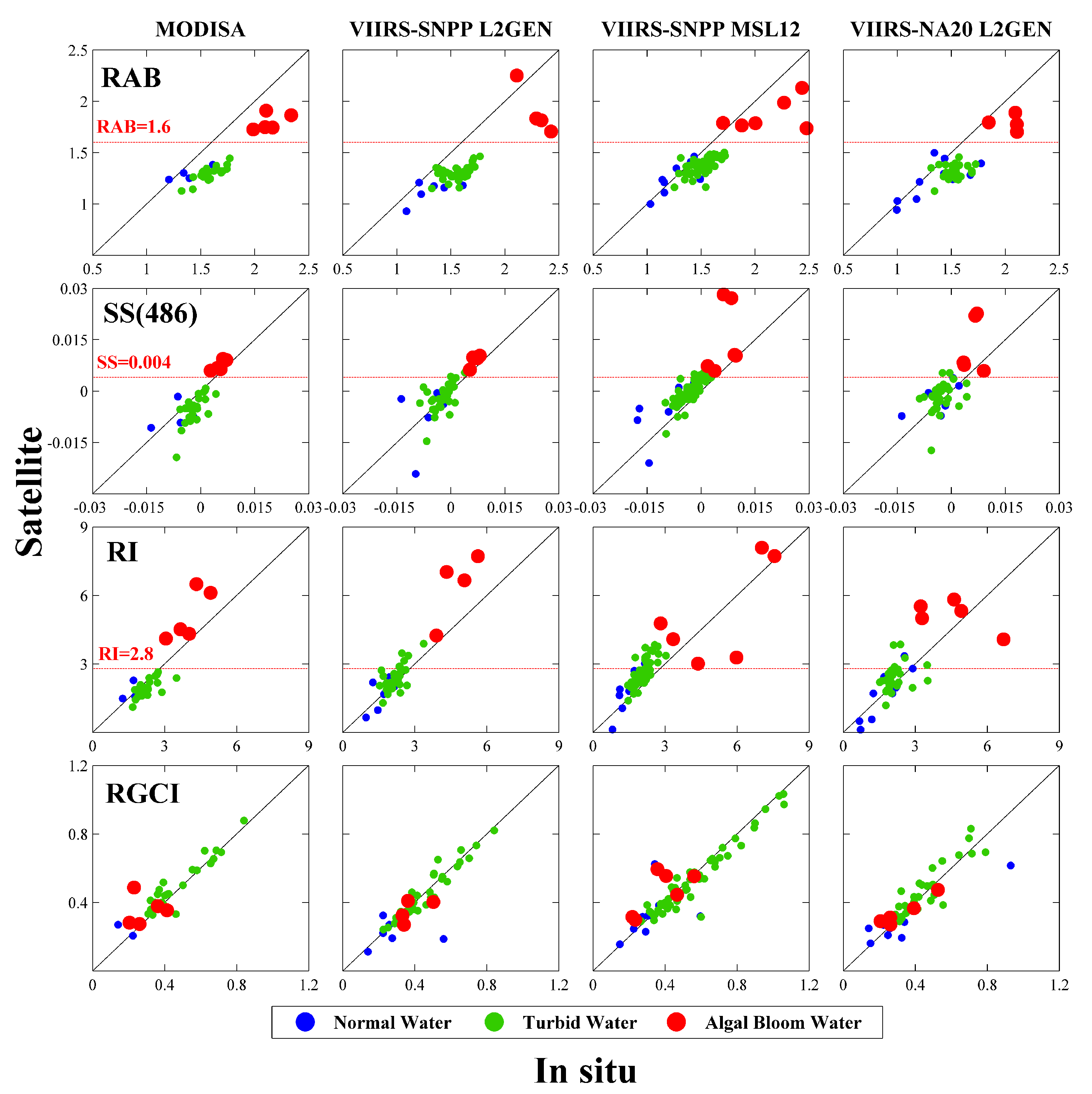
Figure 6 shows scatterplots of in situ/satellite matchups for the four HAB indices, in which the algal bloom observations confirmed by field measurements at the Dongou site are plotted as red circles, as well as green circles indicating turbid waters with
Rrs(551) greater than 0.014 sr
−1, and blue circles were associated with clear ones with
Rrs(551) lower than 0.014 sr
−1. General consistency between satellite data and the SeaPRISM data can be found for all the four indices, but a difference exists in the capacity of the separation of bloom waters from normal ones. However, for sediment-dominated waters, the optical properties in the red bands were determined by both phytoplankton and sediment, and thus the separation of bloom areas from turbid waters by RGCI was not satisfactory. Although the performance of RI and SS(486) seems to be better, the separation in the two methods makes both not clear enough, probably because the blue band of 443 nm with relative high uncertainties was used in both of them. Thus, it can be clearly found that the RAB method probably yielded the best discrimination and the threshold value of 1.6 seems suitable for both MODIS and VIIRS for the identification of algal blooms. This success can be attributed not only to good quality of 488 nm and 551 nm data (
Figure 4), but also to the much shearer slope between 488 nm and 551 nm of phytoplankton absorption [
28].
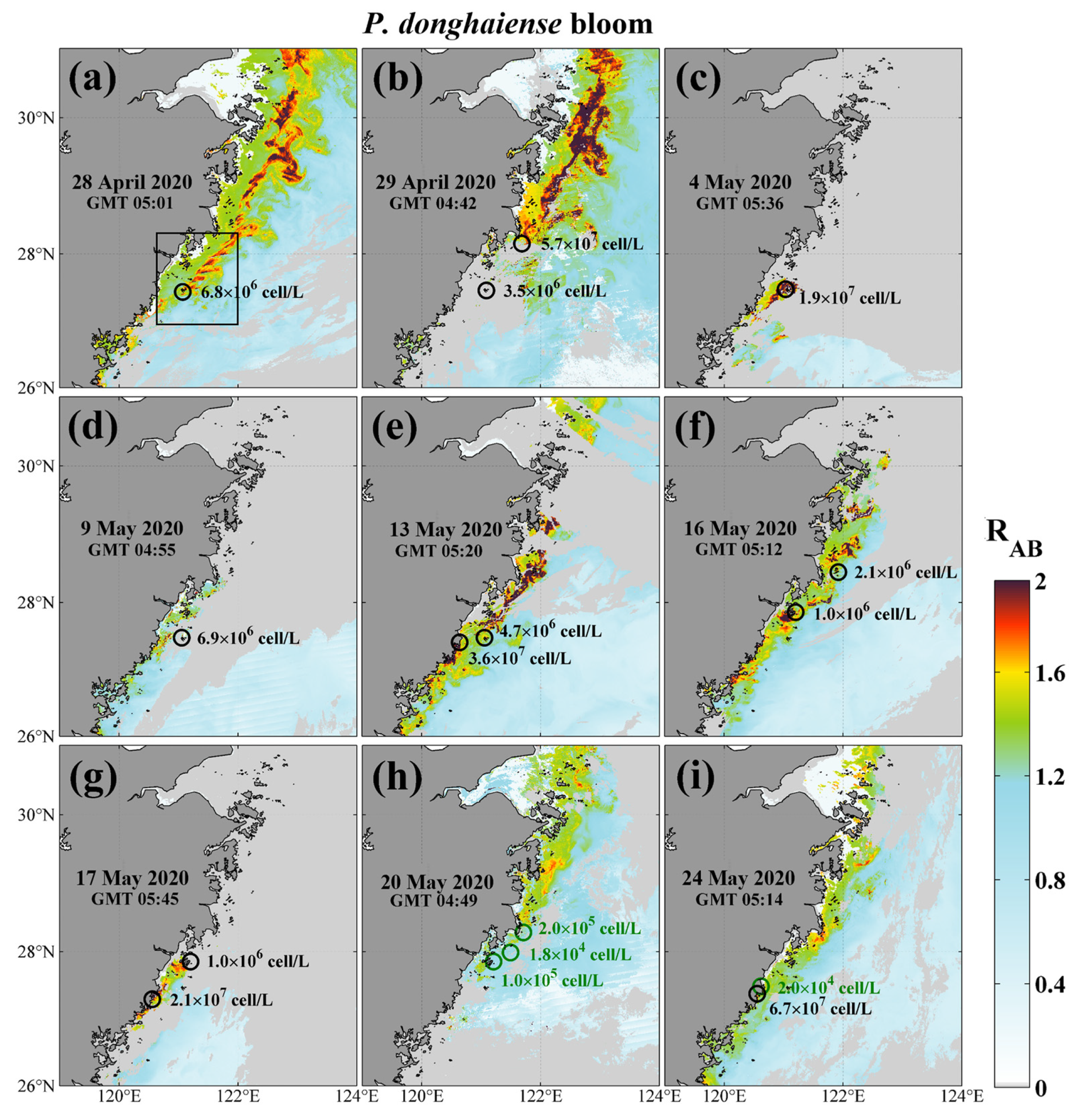
To further access the performance of the simple VIIRS RAB method in identifying HAB in the ECS, image series and near-concurrent field surveys for large-scale
P. donghaiense blooms in April to May 2020 were used for method validation (
Figure 7). These blooms were massive, extending over thousands of square kilometers, and persisted for nearly one month. The field measurements of cell counts from WMEMC confirmed that the RAB method can successfully differentiate bloom and non-bloom waters. Particularly, even in a very near-shore region, as seen in
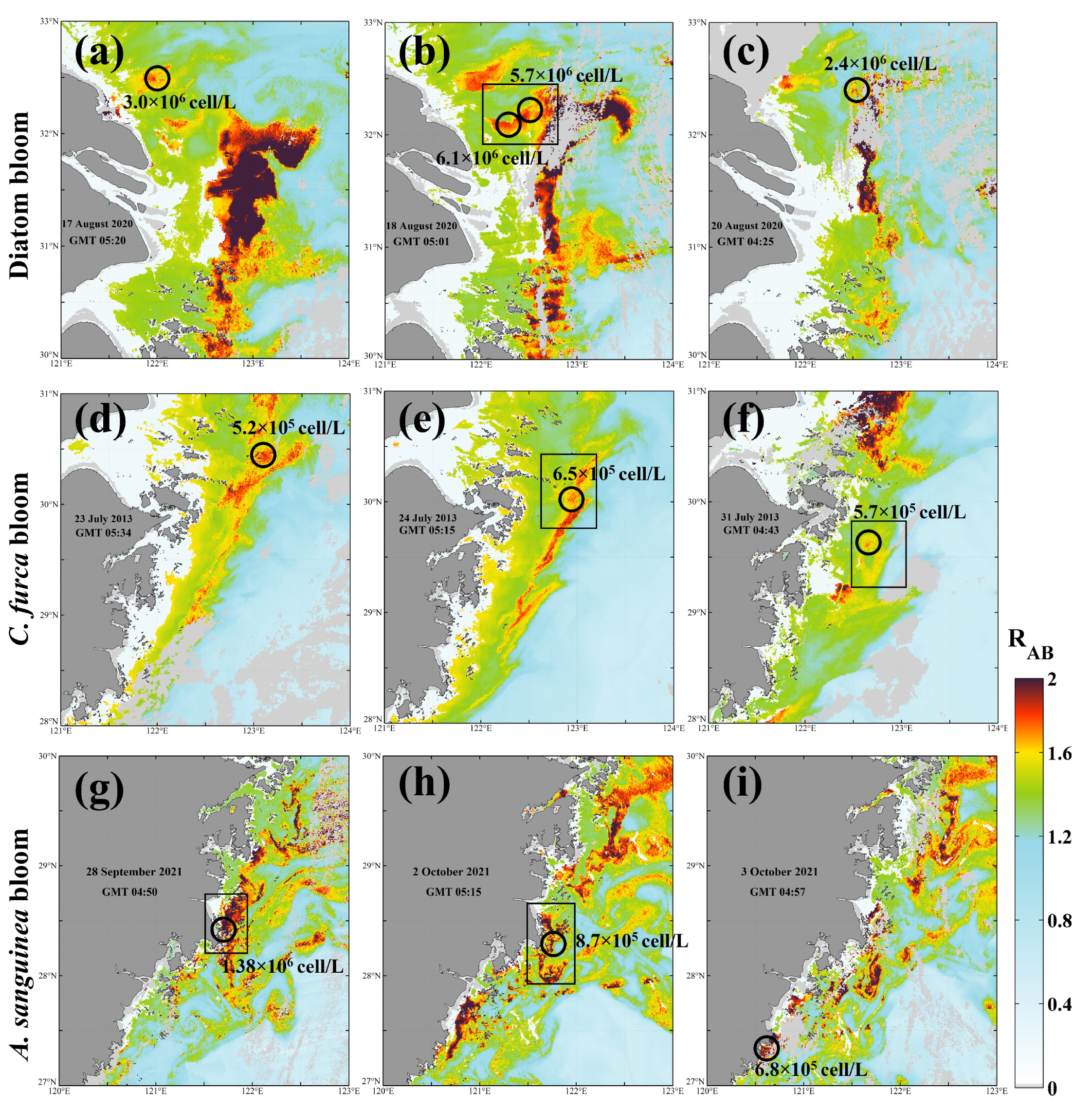
Figure 7e,f, small-area bloom events can also be captured in the RAB images. Similar success was achieved in the diatom bloom event occurring near the YRE in August 2020 (
Figure 8a–c), indicating that RAB is also suitable for diatom bloom detection. In addition, RAB can also identify blooms dominated by other algal species, such as
C. furca and
A. sanguinea, validation results of which are shown in
Figure 8d,e,g–i, respectively. Although there is an hourly error in the matchups of VIIRS derived RAB data and field surveys, these results are still sufficient to confirm the ability of VIIRS to detect HABs in the ECS.
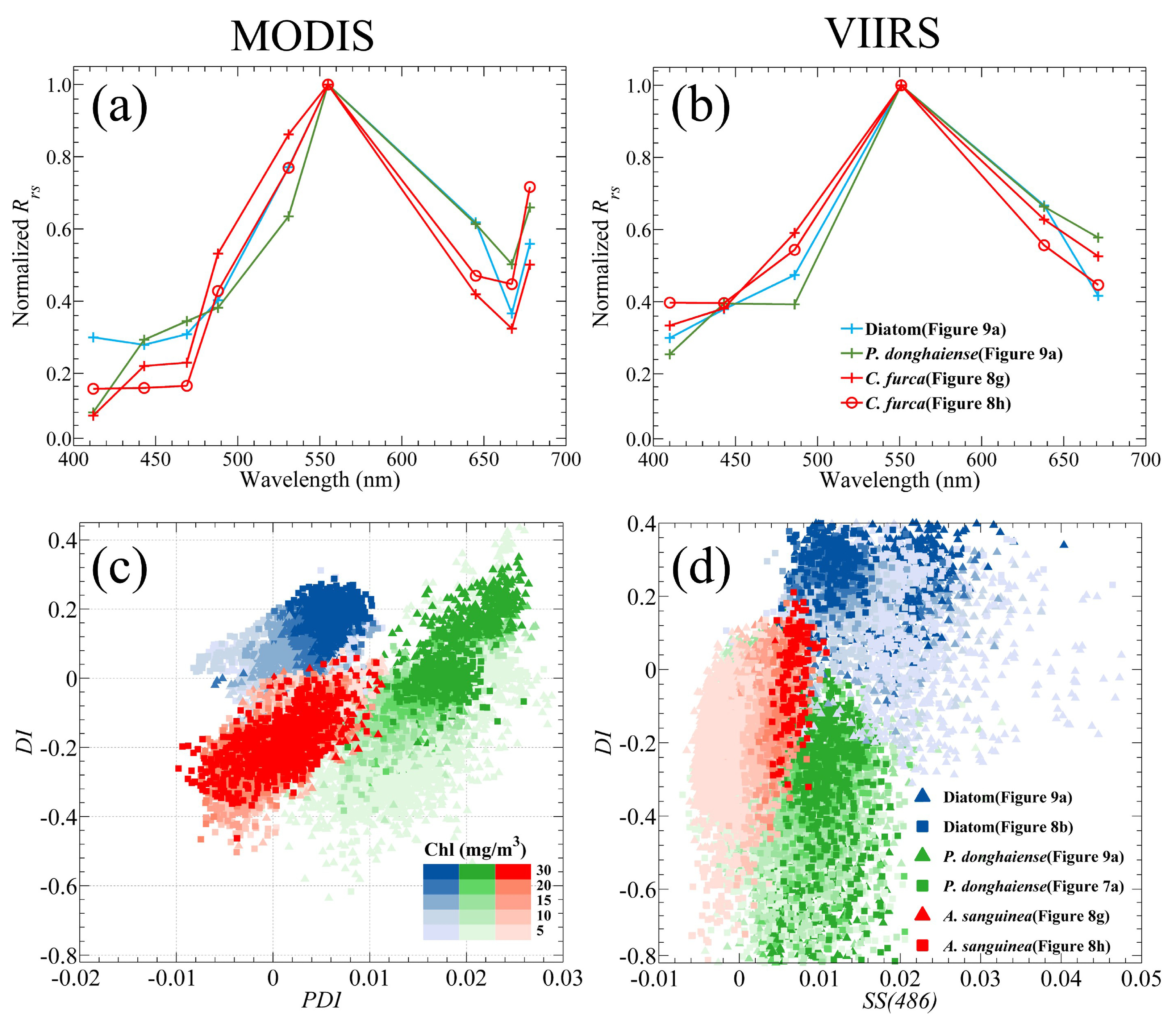
3.3. Capability in Bloom Type Discrimination
Although the reduction in the number of VIIRS bands has little impact on the identification of HABs in the ECS, it was found that it has a significant impact on the discrimination of bloom types or dominant species.
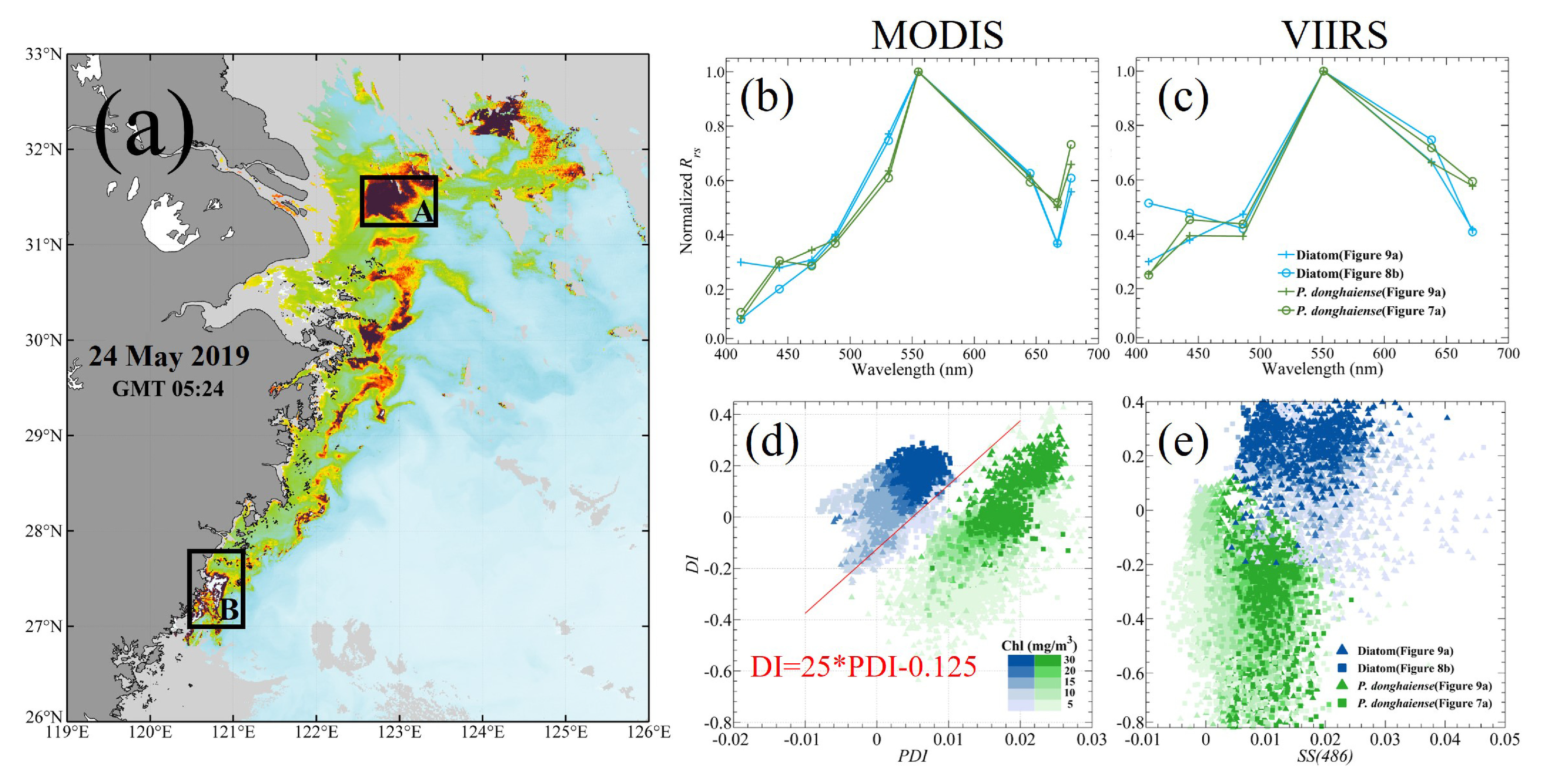
Figure 9a shows the distribution of HAB events on 24 May 2019; note that the dominant algal species in region A was identified as
P. donghaiense, while the one of region B in the other end of the stretch was caused by diatoms. In the above two regions, the typical
Rrs spectra of
P. donghaiense and diatom blooms from both MODIS and VIIRS were normalized by the maximum value of each spectrum in the green bands (
Figure 9b,c). Other
Rrs spectra of`
P. donghaiense blooms that occurred on 28 April 2020 (
Figure 7a) and a diatom bloom that occurred on 18 August 2020 (
Figure 8b) are also presented. According to the finding of Tao et al. [
28], the MODIS derived spectra of
P. donghaiense and diatoms show a large difference mainly near the 531 nm and 645 nm bands; the
Rrs of diatoms has a high shoulder peak at the 645 nm while that of
P. donghaiense has a more obvious trough at 531 nm (
Figure 9b). Therefore, the
P. donghaiense bloom can be concluded as having extremely high PDI value and relatively low DI value compared to those of diatoms; then, the two type blooms can be clearly classified in the scatter plot of DI against PDI (
Figure 9d). For VIIRS, due to a lack of a band near 531 nm, the difference between
P. donghaiense and diatoms in their VIIRS
Rrs spectra was only observed at 638 nm (
Figure 9c). After the replacement of the PDI index by SS(486) for VIIRS, the separation of
P. donghaiense and diatoms in the scatter plot of SS(486) against DI is not clear enough when compared with that in MODIS (
Figure 9e). In contrast to PDI, SS(486) did not have obvious data that could be used to distinguish between
P. donghaiense and diatoms; nevertheless, owing to the presence of the DI index, a clear trend still exists that can be used to separate the two types of blooms. Although VIIRS still has the ability to distinguish between
P. donghaiense and diatoms in the ECS to some extent, the lack of a 531 nm band greatly reduces its ability to discriminate other types of algal blooms.
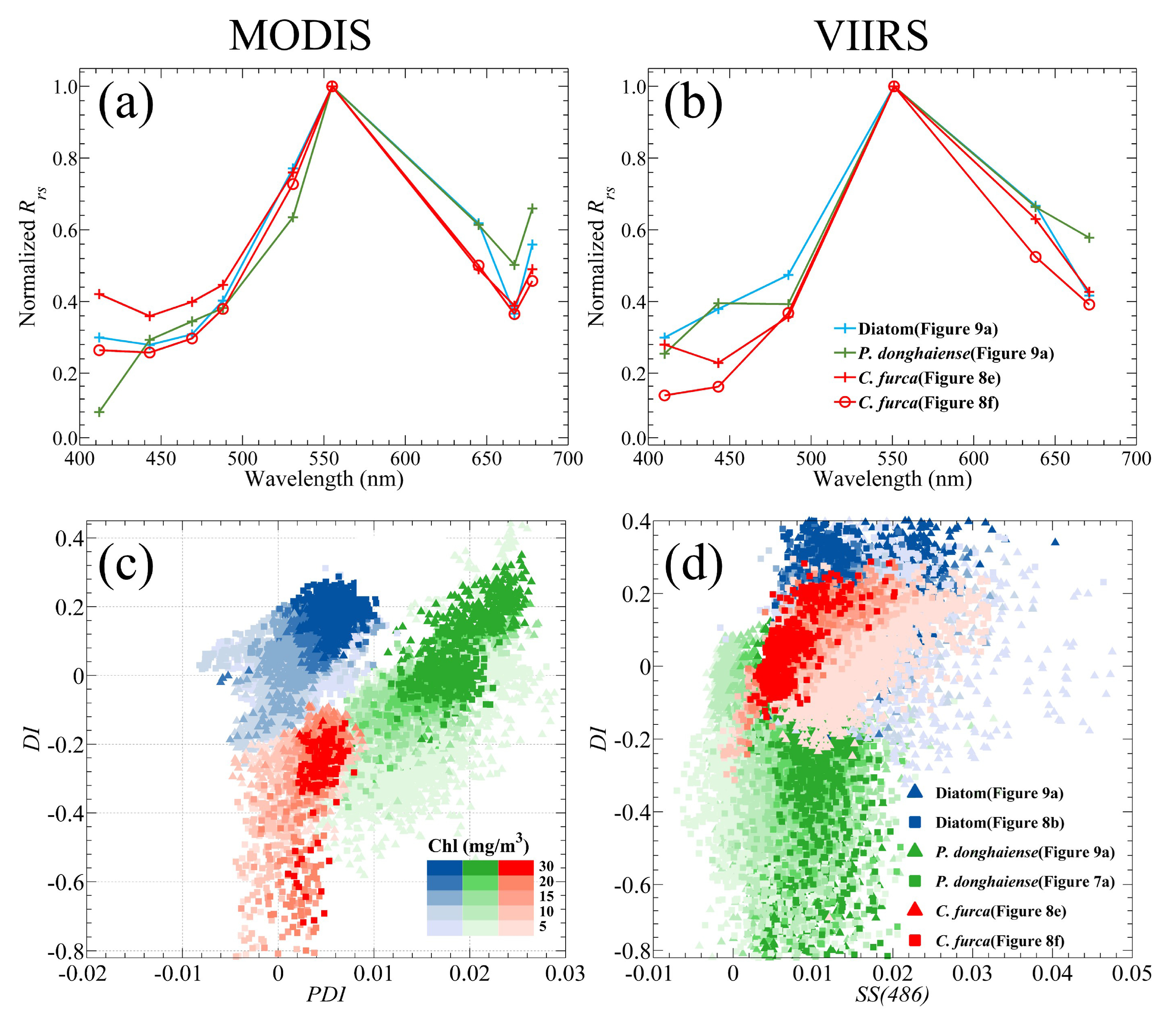
Based on the observation of the
C. furca blooms in July 2013, its
Rrs spectra derived from MODIS showed some distinguishing spectral features that can be used to separate them from
P. donghaiense and diatom blooms (
Figure 10a). Compared with diatoms, a
C. furca bloom does not have a prominent shoulder peak at 645 nm, while compared with
P. donghaiense, it has no reflection trough at 531 nm. These features can be directly reflected in the scatter plot of PDI to DI (
Figure 10c). The clusters of
C. furca blooms are roughly located in the lower left quarter of
Figure 10c so that the separation between
C. furca and diatom blooms is very clear, although some points of a
C. furca bloom are not well separated from clusters associated with
P. donghaiense. Nevertheless, the classification of
C. furca,
P. donghaiense, and diatom blooms cannot be achieved based on the VIIRS data, since the VIIRS derived
Rrs spectra between 488 nm and 551 nm of a
C. furca bloom do not yield different features from the other two types of blooms when the 531 nm band is not available (
Figure 10b,d).
For
A. sanguinea blooms, there is another interesting feature found near 488 nm in
Rrs spectra of both MODIS and VIIRS (
Figure 11a,b), which makes both PDI and SS(486) of
A. sanguinea become significantly lower than that of
P. donghaiense. Similar to the classification results of
C. furca, the distribution of
A. sanguinea points in the PDI-DI scatter plot of MODIS (
Figure 11c) is at the lower left of
P. donghaiense and diatom clusters, and more importantly, the distinction between the
A. sanguinea and other two type blooms is more evident. Comparatively, the distribution of
A. sanguinea points in the SS(486)-DI scatter plot of VIIRS partially overlaps with the
P. donghaiense and diatom points (
Figure 11d), but the separations of
A. sanguinea from the
P. donghaiense and diatom blooms is also better than that of
C. furca (
Figure 10d). Although the classification results of VIIRS showed a certain trend of separation, but was still not enough to separate
A. sanguinea from
P. donghaiense and diatoms. Based on the above results, one can conclude that the 531 nm band is essential for the bloom type discrimination, a lack of which significantly limits the capability of VIIRS.