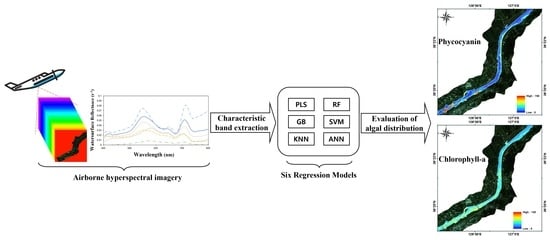
Optimal Band Selection for Airborne Hyperspectral Imagery to Retrieve a Wide Range of Cyanobacterial Pigment Concentration Using a Data-Driven Approach
Abstract
:1. Introduction
2. Materials and Methods
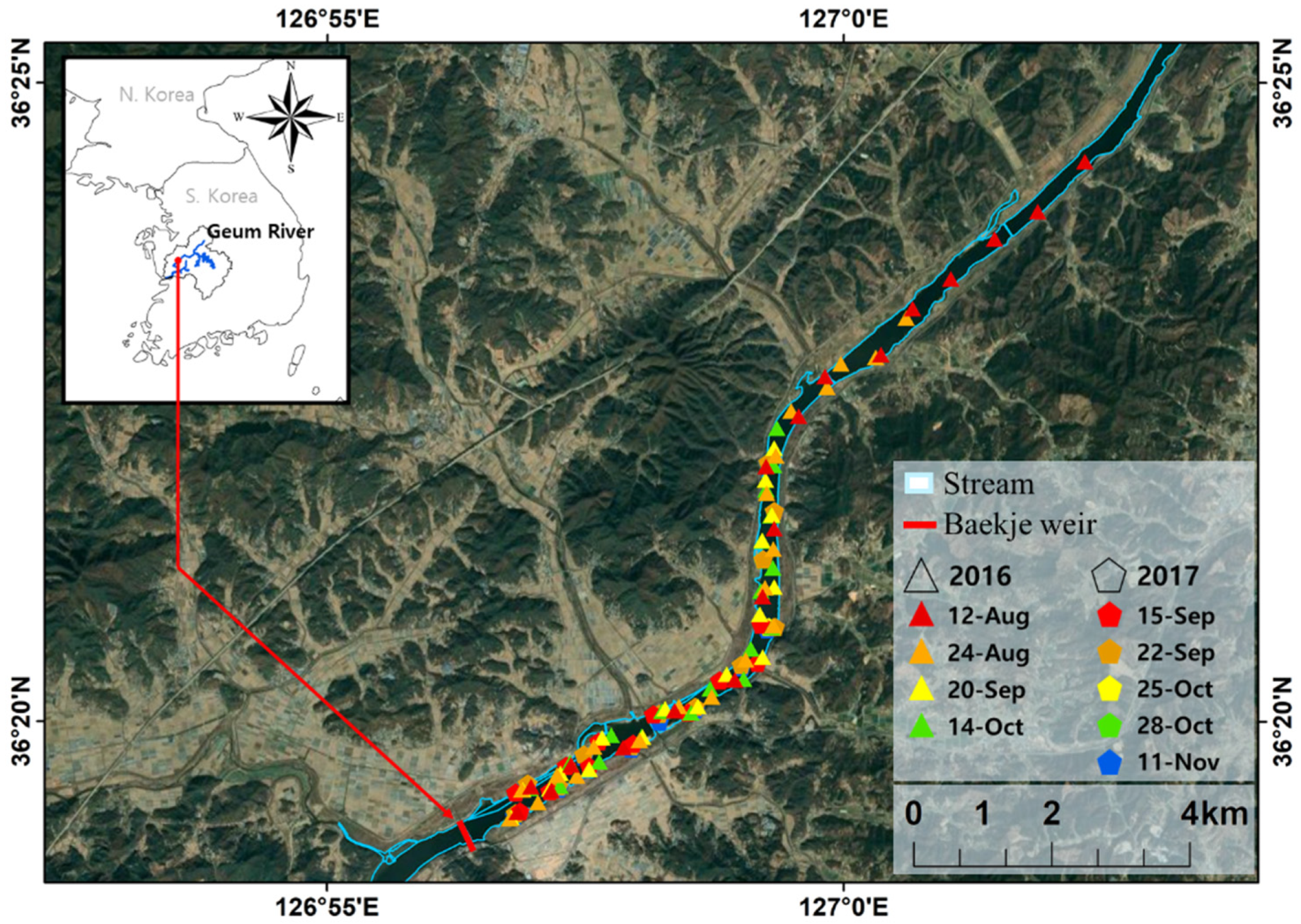
2.1. Study Area
2.2. Data Acquisition
2.2.1. Hyperspectral Imagery
2.2.2. Chl-a and PC Sampling
2.3. Selection of Input Bands
2.4. Development of Optical Models to Retrieve Pigments
2.4.1. Partial Least Squares
2.4.2. Tree-Based Ensemble Regression
2.4.3. K-Nearest Neighbors Regression
2.4.4. Support Vector Machine
2.4.5. Artificial Neural Network
2.4.6. Regression Model Optimization
2.5. Performance Evaluation Parameters
3. Results
3.1. Band Selection
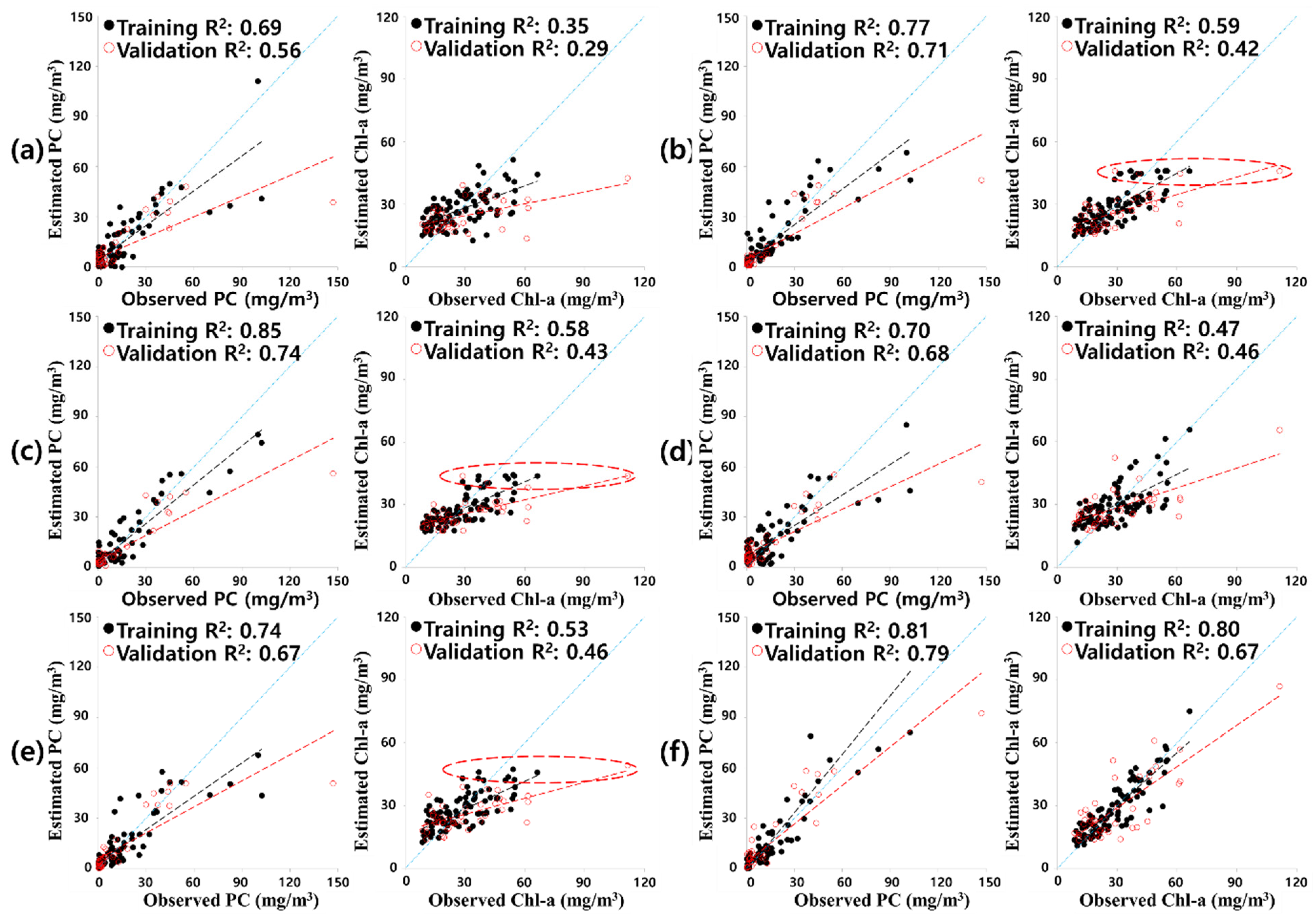
3.2. Model Development
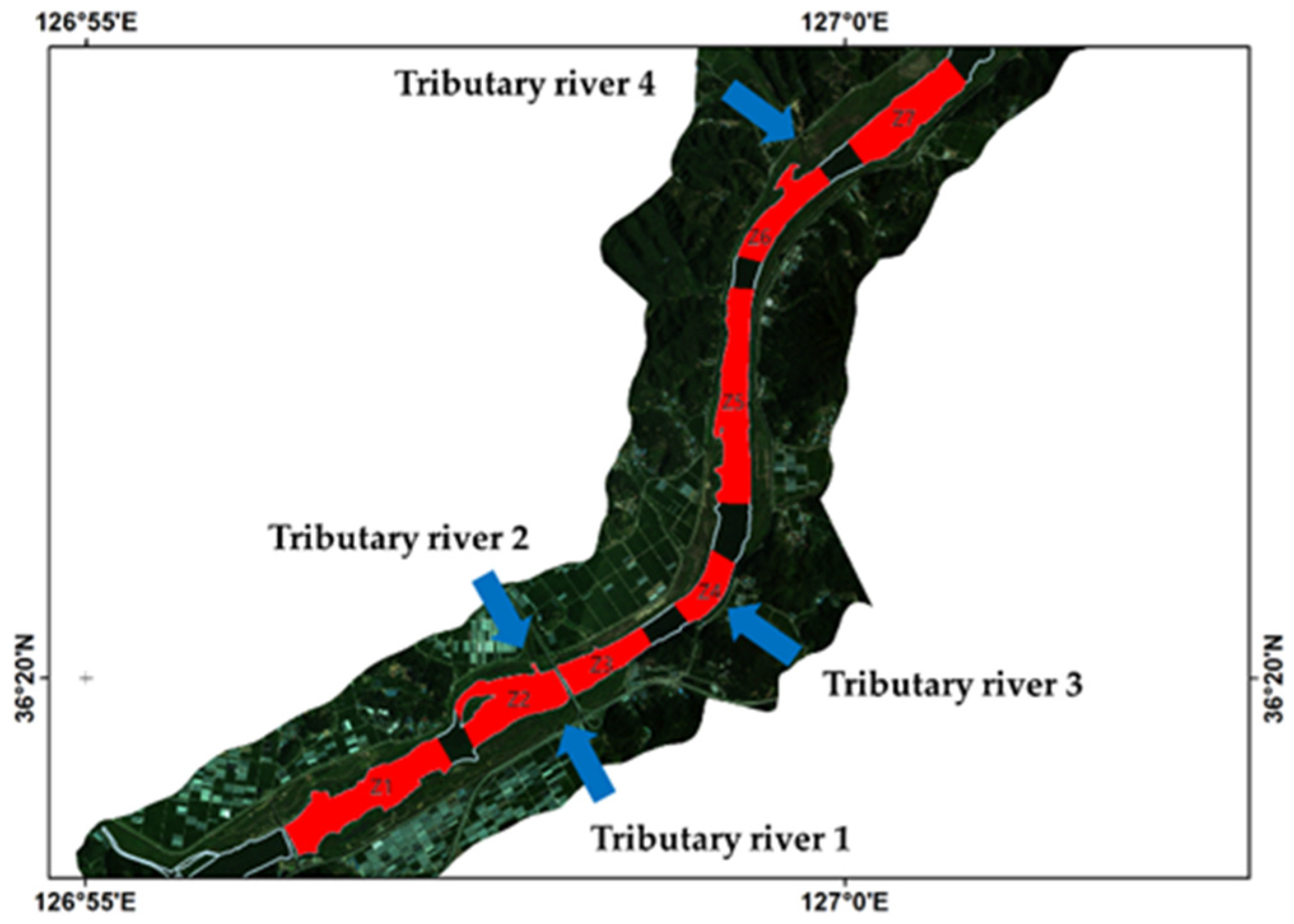
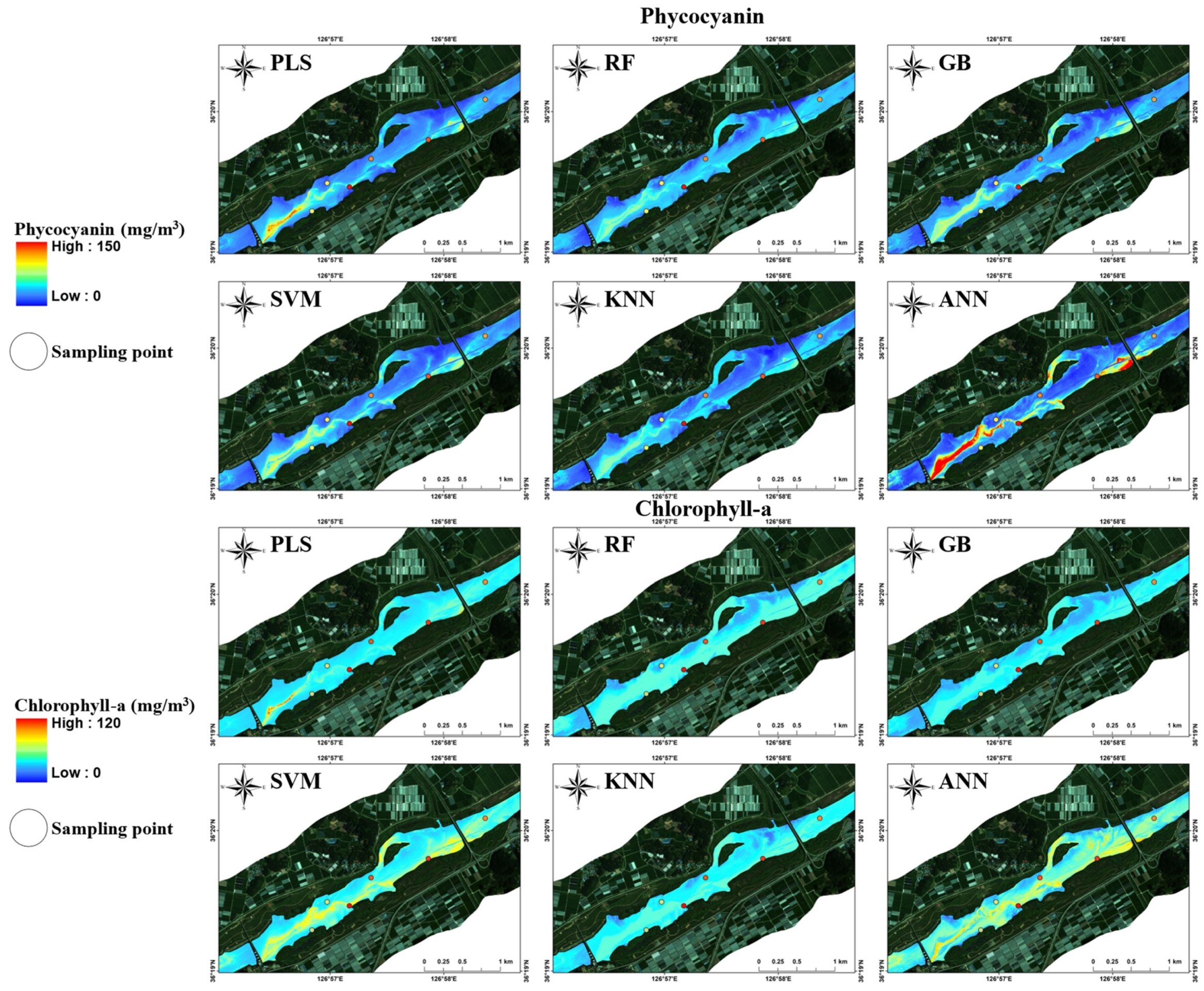
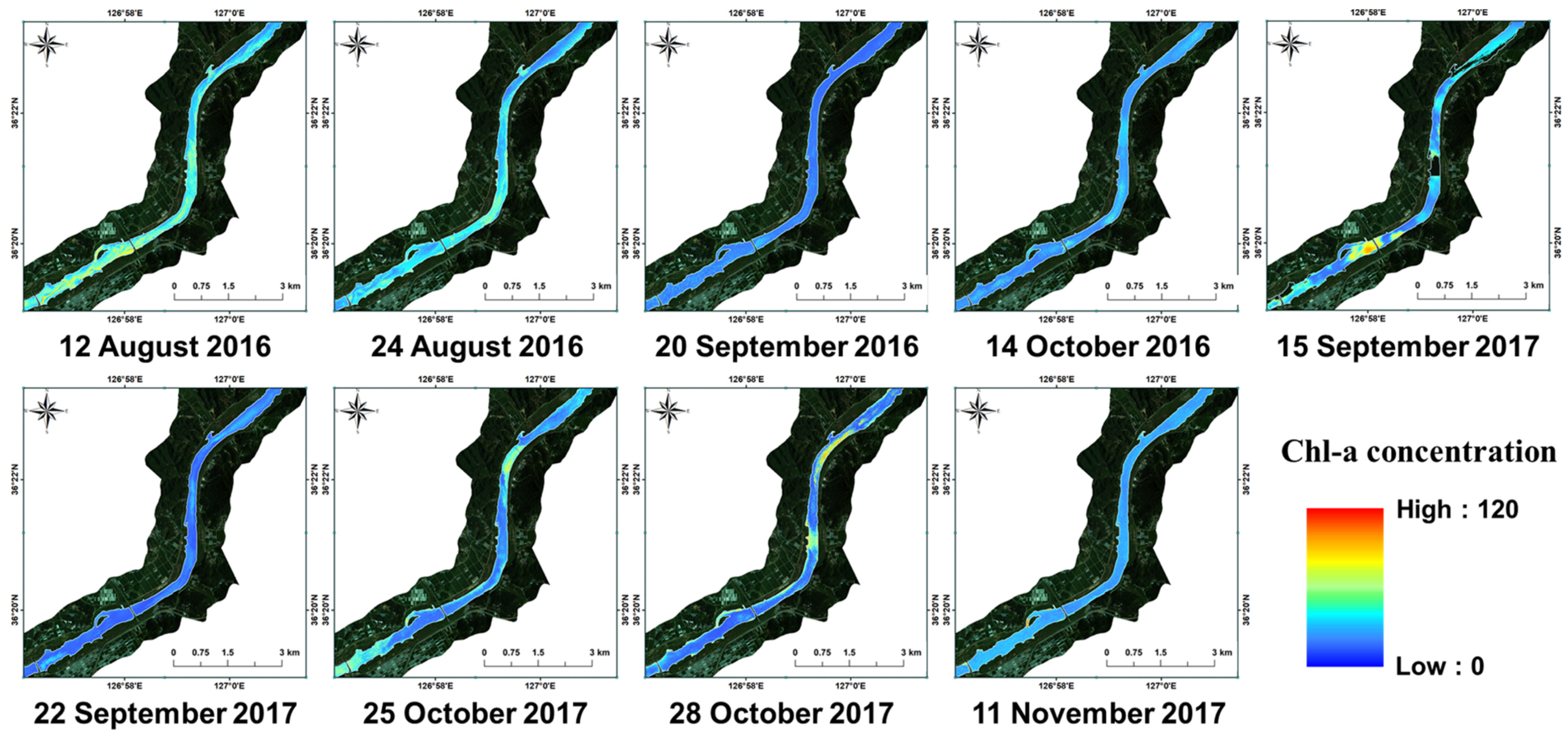
3.3. Baekje Weir Algae Spatial Distribution Generation
4. Discussion
4.1. Band Selection for Inland Cyanobacteria Pigments
4.2. Cyanobacteria Optical Algorithm Specialized for BJW
4.3. Spatio-Temporal Distribution Characteristics of Cyanobacteria in BJW
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Statistics of Phycocyanin and Chlorophyll-a Spatial Concentration
| Pigment | Date | Zone | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Overall | |||||||||
| MEAN | STD | MEAN | STD | MEAN | STD | MEAN | STD | MEAN | STD | MEAN | STD | MEAN | STD | Mean | ||
| PC | 12 August 2016 | 43.80 | 34.70 | 34.11 | 30.16 | 30.88 | 20.30 | 22.10 | 12.85 | 37.20 | 26.53 | 33.24 | 23.46 | 25.79 | 18.40 | 32.45 |
| 24 August 2016 | 36.13 | 24.68 | 49.92 | 15.46 | 49.21 | 24.11 | 39.82 | 13.87 | 33.08 | 16.23 | 20.78 | 5.98 | 11.84 | 3.52 | 34.40 | |
| 20 September 2016 | 4.54 | 4.57 | 3.82 | 3.93 | 3.67 | 3.34 | 3.28 | 3.74 | 4.57 | 3.69 | 5.96 | 4.15 | 6.49 | 4.83 | 4.62 | |
| 14 October 2016 | 4.51 | 4.43 | 4.26 | 5.35 | 5.46 | 7.19 | 7.45 | 7.34 | 3.61 | 2.81 | 3.76 | 2.54 | 4.48 | 4.32 | 4.79 | |
| 15 September 2017 | 1.22 | 2.01 | 8.49 | 7.78 | 9.28 | 5.89 | 10.13 | 4.52 | 3.09 | 3.13 | 3.74 | 3.70 | 1.32 | 1.76 | 5.32 | |
| 22 September 2017 | 11.99 | 9.25 | 11.45 | 8.82 | 8.63 | 7.22 | 2.51 | 2.46 | 2.49 | 3.05 | 3.63 | 3.80 | 5.93 | 4.69 | 6.66 | |
| 25 October 2017 | 11.07 | 9.51 | 12.73 | 10.73 | 6.21 | 8.54 | 14.91 | 8.65 | 11.16 | 8.10 | 12.66 | 8.33 | 5.37 | 5.14 | 10.59 | |
| 28 October 2017 | 19.87 | 10.72 | 15.26 | 13.80 | 12.32 | 14.42 | 10.12 | 9.82 | 12.81 | 9.59 | 12.97 | 7.41 | 10.08 | 7.87 | 13.35 | |
| 11 November 2017 | 3.03 | 4.92 | 2.78 | 4.18 | 2.27 | 3.15 | 2.42 | 2.78 | 1.59 | 2.45 | 2.04 | 2.86 | 2.91 | 3.86 | 2.43 | |
| Avg. | 15.13 | 11.64 | 15.87 | 11.13 | 14.21 | 10.46 | 12.53 | 7.34 | 12.18 | 8.40 | 10.97 | 6.92 | 8.25 | 6.04 | 12.73 | |
| Chl-a | 12 August 2016 | 48.35 | 15.29 | 50.45 | 15.05 | 47.14 | 11.81 | 45.59 | 13.02 | 41.87 | 11.15 | 37.71 | 12.13 | 33.92 | 9.77 | 43.57 |
| 24 August 2016 | 36.34 | 10.29 | 35.12 | 8.18 | 39.68 | 9.01 | 47.14 | 9.11 | 37.10 | 10.55 | 30.82 | 11.60 | 28.51 | 9.47 | 36.39 | |
| 20 September 2016 | 22.36 | 9.43 | 21.99 | 7.01 | 23.05 | 6.81 | 20.44 | 4.65 | 19.09 | 3.99 | 17.31 | 2.94 | 17.02 | 3.22 | 20.18 | |
| 14 October 2016 | 24.03 | 8.01 | 23.78 | 6.99 | 25.06 | 8.33 | 29.55 | 8.29 | 27.09 | 4.69 | 24.28 | 3.51 | 26.61 | 5.26 | 25.77 | |
| 15 September 2017 | 33.09 | 12.83 | 52.56 | 24.65 | 40.87 | 24.36 | 27.29 | 8.68 | 30.42 | 12.14 | 30.03 | 9.68 | 35.70 | 5.20 | 35.71 | |
| 22 September 2017 | 18.69 | 6.15 | 16.16 | 3.36 | 15.67 | 4.75 | 18.04 | 3.87 | 17.81 | 5.35 | 18.41 | 5.91 | 21.55 | 5.08 | 18.05 | |
| 25 October 2017 | 37.85 | 13.87 | 23.54 | 12.51 | 24.65 | 9.95 | 26.32 | 11.76 | 25.42 | 10.43 | 37.51 | 16.99 | 26.86 | 7.73 | 28.88 | |
| 28 October 2017 | 23.61 | 13.43 | 23.45 | 16.44 | 24.88 | 15.78 | 24.15 | 12.98 | 32.52 | 21.71 | 47.05 | 29.11 | 26.27 | 18.20 | 28.85 | |
| 11 November 2017 | 30.03 | 7.10 | 29.93 | 13.58 | 25.67 | 5.78 | 25.27 | 3.68 | 26.19 | 4.52 | 27.80 | 7.37 | 26.65 | 4.82 | 27.36 | |
| Avg. | 30.48 | 10.71 | 30.77 | 11.98 | 29.63 | 10.73 | 29.31 | 8.45 | 28.61 | 9.39 | 30.10 | 11.03 | 27.01 | 7.64 | 29.42 | |
Appendix B. July to November Weather and Water Quality Features in Baekje Weir

References
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Brooks, B.W.; Lazorchak, J.M.; Howard, M.D.A.; Johnson, M.V.V.; Morton, S.L.; Perkins, D.A.K.; Reavie, E.D.; Scott, G.I.; Smith, S.A.; Steevens, J.A. Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems? Environ. Toxicol. Chem. 2016, 35, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Hudnell, H.K. The state of U.S. freshwater harmful algal blooms assessments, policy and legislation. Toxicon 2010, 55, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Chapra, S.C.; Canale, R.P.; Amy, G.L. Empirical Models for Disinfection By-Products in Lakes and Reservoirs. J. Environ. Eng. 1997, 123, 1–12. [Google Scholar] [CrossRef]
- Matthews, M.W.; Bernard, S.; Robertson, L. An algorithm for detecting trophic status (chlorophyll-a), cyanobacterial-dominance, surface scums and floating vegetation in inland and coastal waters. Remote Sens. Environ. 2012, 124, 637–652. [Google Scholar] [CrossRef]
- Matthews, M.W.; Odermatt, D. Improved algorithm for routine monitoring of cyanobacteria and eutrophication in inland and near-coastal waters. Remote Sens. Environ. 2015, 156, 374–382. [Google Scholar] [CrossRef]
- Su, T.C.; Chou, H.T. Application of multispectral sensors carried on unmanned aerial vehicle (UAV) to trophic state mapping of small reservoirs: A case study of Tain-Pu reservoir in Kinmen, Taiwan. Remote Sens. 2015, 7, 10078–10097. [Google Scholar] [CrossRef] [Green Version]
- Binding, C.E.; Greenberg, T.A.; Jerome, J.H.; Bukata, R.P.; Letourneau, G. An assessment of MERIS algal products during an intense bloom in Lake of the Woods. J. Plankton Res. 2011, 33, 793–806. [Google Scholar] [CrossRef] [Green Version]
- Duan, H.; Ma, R.; Zhang, Y.; Loiselle, S.A.; Xu, J.; Zhao, C.; Zhou, L.; Shang, L. A new three-band algorithm for estimating chlorophyll concentrations in turbid inland lakes. Environ. Res. Lett. 2010, 5, 044009. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, Y.; Qin, B.; Zhou, B. Remote sensing of cyanobacterial blooms in inland waters: Present knowledge and future challenges. Sci. Bull. 2019, 64, 1540–1556. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Im, J.; Ha, H.K.; Choi, J.K.; Ha, S. Machine learning approaches to coastal water quality monitoring using GOCI satellite data. GISci. Remote Sens. 2014, 51, 158–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Pulliainen, J.; Koponen, S.; Hallikainen, M. Application of an empirical neural network to surface water quality estimation in the Gulf of Finland using combined optical data and microwave data. Remote Sens. Environ. 2002, 81, 327–336. [Google Scholar] [CrossRef]
- Kutser, T.; Herlevi, A.; Kallio, K.; Arst, H. A hyperspectral model for interpretation of passive optical remote sensing data from turbid lakes. Sci. Total Environ. 2001, 268, 47–58. [Google Scholar] [CrossRef]
- Lunetta, R.S.; Knight, J.F.; Paerl, H.W.; Streicher, J.J.; Peierls, B.L.; Gallo, T.; Lyon, J.G.; Mace, T.H.; Buzzelli, C.P. Measurement of water colour using AVIRIS imagery to assess the potential for an operational monitoring capability in the Pamlico Sound Estuary, USA. Int. J. Remote Sens. 2009, 30, 3291–3314. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sengpiel, R.E.; Pascual, D.L.; Tedesco, L.P.; Wilson, J.S.; Soyeux, A. Using hyperspectral remote sensing to estimate chlorophyll-a and phycocyanin in a mesotrophic reservoir. Int. J. Remote Sens. 2010, 31, 4147–4162. [Google Scholar] [CrossRef]
- Pyo, J.C.; Duan, H.; Baek, S.; Kim, M.S.; Jeon, T.; Kwon, Y.S.; Lee, H.; Cho, K.H. A convolutional neural network regression for quantifying cyanobacteria using hyperspectral imagery. Remote Sens. Environ. 2019, 233, 111350. [Google Scholar] [CrossRef]
- Pyo, J.C.; Duan, H.; Ligaray, M.; Kim, M.; Baek, S.; Kwon, Y.S.; Lee, H.; Kang, T.; Kim, K.; Cha, Y.K.; et al. An integrative remote sensing application of stacked autoencoder for atmospheric correction and cyanobacteria estimation using hyperspectral imagery. Remote Sens. 2020, 12, 1073. [Google Scholar] [CrossRef] [Green Version]
- Keller, S.; Maier, P.M.; Riese, F.M.; Norra, S.; Holbach, A.; Börsig, N.; Wilhelms, A.; Moldaenke, C.; Zaake, A.; Hinz, S. Hyperspectral data and machine learning for estimating CDOM, chlorophyll a, diatoms, green algae and turbidity. Int. J. Environ. Res. Public Health 2018, 15, 1881. [Google Scholar] [CrossRef] [Green Version]
- Šindelář, R.; Babuška, R. Input selection for nonlinear regression models. IEEE Trans. Fuzzy Syst. 2004, 12, 688–696. [Google Scholar] [CrossRef]
- Dekker, A.G. Detection of Optical Water Quality Parameters for Eutrophic Waters by High Resolution Remote Sensing; Institute for Environmental Studies: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Mishra, S.; Mishra, D.R.; Schluchter, W.M. A novel algorithm for predicting phycocyanin concentrations in cyanobacteria: A proximal hyperspectral remote sensing approach. Remote Sens. 2009, 1, 758–775. [Google Scholar] [CrossRef] [Green Version]
- Simis, S.G.H.; Peters, S.W.M.; Gons, H.J. Remote sensing of the cyanobacterial pigment phycocyanin in turbid inland water. Limnol. Oceanogr. 2005, 50, 237–245. [Google Scholar] [CrossRef]
- Kallio, K.; Kutser, T.; Hannonen, T.; Koponen, S.; Pulliainen, J.; Vepsäläinen, J.; Pyhälahti, T. Retrieval of water quality from airborne imaging spectrometry of various lake types in different seasons. Sci. Total Environ. 2001, 268, 59–77. [Google Scholar] [CrossRef]
- Shafique, N.A.; Fulk, F.; Autrey, B.C.; Flotemersch, J. Water area extraction using geocoded high resolution imagery of TerraSAR-X radar satellite in cloud prone Brahmaputra River valley. J. Geomat. 2009, 3, 9–12. [Google Scholar]
- Ogashawara, I.; Mishra, D.R.; Mishra, S.; Curtarelli, M.P.; Stech, J.L. A performance review of reflectance based algorithms for predicting phycocyanin concentrations in inland waters. Remote Sens. 2013, 5, 4774–4798. [Google Scholar] [CrossRef] [Green Version]
- Schalles, J.F.; Yacobi, Y.Z. Remote detection and seasonal patterns of phycocyanin, carotenoid and chlorophyll pigments in eutrophic waters. Ergeb. Limnol. 2000, 55, 153–168. [Google Scholar]
- Soja-Woźniak, M.; Darecki, M.; Wojtasiewicz, B.; Bradtke, K. Laboratory measurements of remote sensing reflectance of selected phytoplankton species from the Baltic Sea. Oceanologia 2018, 60, 86–96. [Google Scholar] [CrossRef]
- Hestir, E.L.; Brando, V.E.; Bresciani, M.; Giardino, C.; Matta, E.; Villa, P.; Dekker, A.G. Measuring freshwater aquatic ecosystems: The need for a hyperspectral global mapping satellite mission. Remote Sens. Environ. 2015, 167, 181–195. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Bao, Z.; Shao, J. Phycocyanin concentration retrieval in inland waters: A comparative review of the remote sensing techniques and algorithms. J. Great Lakes Res. 2018, 44, 748–755. [Google Scholar] [CrossRef]
- Pyo, J.C.; Ligaray, M.; Kwon, Y.S.; Ahn, M.H.; Kim, K.; Lee, H.; Kang, T.; Cho, S.B.; Park, Y.; Cho, K.H. High-spatial resolution monitoring of phycocyanin and chlorophyll-a using airborne hyperspectral imagery. Remote Sens. 2018, 10, 1180. [Google Scholar] [CrossRef] [Green Version]
- Berk, A.; Conforti, P.; Kennett, R.; Perkins, T.; Hawes, F.; Van Den Bosch, J. MODTRAN® 6: A major upgrade of the MODTRAN® radiative transfer code. In Proceedings of the Workshop on Hyperspectral Image and Signal Processing, Evolution in Remote Sensing, Lausanne, Switzerland, 24–27 June 2014; pp. 1–4. [Google Scholar]
- Pyo, J.; Ha, S.; Pachepsky, Y.A.; Lee, H.; Ha, R.; Nam, G.; Kim, M.S.; Im, J.; Cho, K.H. Chlorophyll- a concentration estimation using three difference bio-optical algorithms, including a correction for the low-concentration range: The case of the Yiam reservoir, Korea. Remote Sens. Lett. 2016, 7, 407–416. [Google Scholar] [CrossRef]
- Pyo, J.C.; Pachepsky, Y.; Baek, S.S.; Kwon, Y.S.; Kim, M.J.; Lee, H.; Park, S.; Cha, Y.K.; Ha, R.; Nam, G.; et al. Optimizing semi-analytical algorithms for estimating chlorophyll-a and phycocyanin concentrations in inland waters in Korea. Remote Sens. 2017, 9, 542. [Google Scholar] [CrossRef] [Green Version]
- Sarada, R.; Pillai, M.G.; Ravishankar, G.A. Phycocyanin from Spirulina sp.: Influence of processing of biomass on phycocyanin yield, analysis of efficacy of extraction methods and stability studies on phycocyanin. Process Biochem. 1999, 34, 795–801. [Google Scholar] [CrossRef]
- Le Bris, A.; Chehata, N.; Briottet, X.; Paparoditis, N. A random forest class memberships based wrapper band selection criterion: Application to hyperspectral. Int. Geosci. Remote Sens. Symp. 2015, 2015, 1112–1115. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, J.K.; Samikannu, R. Application of Random Forest Algorithm on Feature Subset Selection and Classification and Regression. In Proceedings of the 2017 World Congress on Computing and Communication Technologies (WCCCT), Tiruchirappalli, India, 2–4 February 2017; pp. 65–68. [Google Scholar] [CrossRef]
- Reinart, A.; Kutser, T. Comparison of different satellite sensors in detecting cyanobacterial bloom events in the Baltic Sea. Remote Sens. Environ. 2006, 102, 74–85. [Google Scholar] [CrossRef]
- Menken, K.D.; Brezonik, P.L.; Bauer, M.E. Influence of chlorophyll and colored dissolved organic matter (CDOM) on lake reflectance spectra: Implications for measuring lake properties by remote sensing. Lake Reserv. Manag. 2006, 22, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Brezonik, P.L.; Olmanson, L.G.; Finlay, J.C.; Bauer, M.E. Factors affecting the measurement of CDOM by remote sensing of optically complex inland waters. Remote Sens. Environ. 2015, 157, 199–215. [Google Scholar] [CrossRef]
- Ha, N.T.T.; Thao, N.T.P.; Koike, K.; Nhuan, M.T. Selecting the best band ratio to estimate chlorophyll-a concentration in a tropical freshwater lake using sentinel 2A images from a case study of Lake Ba Be (Northern Vietnam). ISPRS Int. J. Geo-Inf. 2017, 6, 290. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Schalles, J.F.; Rundquist, D.C.; Schiebe, F.R.; Yacobi, Y.Z. Comparative reflectance properties of algal cultures with manipulated densities. J. Appl. Phycol. 1999, 11, 345–354. [Google Scholar] [CrossRef]
- Quibell, G. The effect of suspended sediment on reflectance from freshwater algae. Int. J. Remote Sens. 1991, 12, 177–182. [Google Scholar] [CrossRef]
- Geladi, P.; Kowalski, B.R. Partial least-squares regression: A tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Abdi, H. Partial least square regression (PLS regression). Encycl. Res. Methods Soc. Sci. 2003, 6, 792–795. [Google Scholar] [CrossRef]
- Guo, L.; Chehata, N.; Mallet, C.; Boukir, S. Relevance of airborne lidar and multispectral image data for urban scene classification using Random Forests. ISPRS J. Photogramm. Remote Sens. 2011, 66, 56–66. [Google Scholar] [CrossRef]
- Rodriguez-Galiano, V.; Sanchez-Castillo, M.; Chica-Olmo, M.; Chica-Rivas, M. Machine learning predictive models for mineral prospectivity: An evaluation of neural networks, random forest, regression trees and support vector machines. Ore Geol. Rev. 2015, 71, 804–818. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Khazaee Poul, A.; Shourian, M.; Ebrahimi, H. A Comparative Study of MLR, KNN, ANN and ANFIS Models with Wavelet Transform in Monthly Stream Flow Prediction. Water Resour. Manag. 2019, 33, 2907–2923. [Google Scholar] [CrossRef]
- Vapnik, V.; Guyon, I.; Hastie, T.; Rosset, S.; Zhu, J.; Tibshirani, R. Support vector machines. Mach. Learn 1995, 20, 273–297. [Google Scholar]
- Coulibaly, M.S.K.P. Application of support vector machine in power system. Study Dyn. Syst. 2006, 11, 199–205. [Google Scholar] [CrossRef]
- Mohammadpour, R.; Shaharuddin, S.; Chang, C.K.; Zakaria, N.A.; Ghani, A.A.; Chan, N.W. Prediction of water quality index in constructed wetlands using support vector machine. Environ. Sci. Pollut. Res. 2015, 22, 6208–6219. [Google Scholar] [CrossRef]
- Zou, J.; Han, Y.; So, S.S. Overview of artificial neural networks. Methods Mol. Biol. 2008, 458, 15–23. [Google Scholar] [CrossRef]
- Palani, S.; Liong, S.Y.; Tkalich, P. An ANN application for water quality forecasting. Mar. Pollut. Bull. 2008, 56, 1586–1597. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Duchesnay, E. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Santhi, C.; Arnold, J.G.; Williams, J.R.; Dugas, W.A.; Srinivasan, R.; Hauck, L.M. Validation of the swat model on a large rwer basin with point and nonpoint sources 1. JAWRA J. Am. Water Resour. Assoc. 2001, 37, 1169–1188. [Google Scholar] [CrossRef]
- Moriasi, D.N.; Arnold, J.G.; Van Liew, M.W.; Bingner, R.L.; Harmel, R.D.; Veith, T.L. Model evaluation guidelines for systematic quantification of accuracy in watershed simulations. Trans. ASABE 2007, 50, 885–900. [Google Scholar] [CrossRef]
- Nash, J.E.; Sutcliffe, J.V. River flow forecasting through conceptual models part I—A discussion of principles. J. Hydrol. 1970, 10, 282–290. [Google Scholar] [CrossRef]
- Singh, J.; Knapp, H.V.; Arnold, J.G.; Demissie, M. Hydrological modeling of the Iroquois River watershed using HSPF and SWAT. J. Am. Water Resour. Assoc. 2005, 41, 343–360. [Google Scholar] [CrossRef]
- Oki, K. Why is the ratio of reflectivity effective for chlorophyll estimation in the lake water? Remote Sens. 2010, 2, 1722–1730. [Google Scholar] [CrossRef] [Green Version]
- Vincent, R.K.; Qin, X.; McKay, R.M.L.; Miner, J.; Czajkowski, K.; Savino, J.; Bridgeman, T. Phycocyanin detection from LANDSAT TM data for mapping cyanobacterial blooms in Lake Erie. Remote Sens. Environ. 2004, 89, 381–392. [Google Scholar] [CrossRef]
- Yacobi, Y.Z.; Moses, W.J.; Kaganovsky, S.; Sulimani, B.; Leavitt, B.C.; Gitelson, A.A. NIR-red reflectance-based algorithms for chlorophyll-a estimation in mesotrophic inland and coastal waters: Lake Kinneret case study. Water Res. 2011, 45, 2428–2436. [Google Scholar] [CrossRef] [Green Version]
- Augusto-Silva, P.B.; Ogashawara, I.; Barbosa, C.C.F.; de Carvalho, L.A.S.; Jorge, D.S.F.; Fornari, C.I.; Stech, J.L. Analysis of MERIS reflectance algorithms for estimating chlorophyll-a concentration in a Brazilian reservoir. Remote Sens. 2014, 6, 11689–11707. [Google Scholar] [CrossRef] [Green Version]
- Rundquist, D.C.; Han, L.; Schalles, J.F.; Peake, J.S. Remote measurement of algal chlorophyll in surface waters: The case for the first derivative of reflectance near 690 nm. Photogramm. Eng. Remote Sens. 1996, 62, 195–200. [Google Scholar]
- Schalles, J.F.; Rundquist, D.C.; Schiebe, F.R. The influence of suspended clays on phytoplankton reflectance signatures and the remote estimation of chlorophyll. SIL Proc. 2001, 27, 3619–3625. [Google Scholar] [CrossRef]
- Sagan, V.; Peterson, K.T.; Maimaitijiang, M.; Sidike, P.; Sloan, J.; Greeling, B.A.; Maalouf, S.; Adams, C. Monitoring inland water quality using remote sensing: Potential and limitations of spectral indices, bio-optical simulations, machine learning, and cloud computing. Earth-Sci. Rev. 2020, 205, 103187. [Google Scholar] [CrossRef]
- Song, K.; Li, L.; Tedesco, L.P.; Li, S.; Hall, B.E.; Du, J. Remote quantification of phycocyanin in potable water sources through an adaptive model. ISPRS J. Photogramm. Remote Sens. 2014, 95, 68–80. [Google Scholar] [CrossRef]
- Chang, N.-B.; Vannah, B. Intercomparisons between empirical models with data fusion techniques for monitoring water quality in a large lake. In Proceedings of the 2013 10th IEEE International Conference on Networking, Sensing and Control (ICNSC 2013), Evry, France, 10–12 April 2013; pp. 258–263. [Google Scholar] [CrossRef]
- He, J.; Chen, Y.; Wu, J.; Stow, D.A.; Christakos, G. Space-time chlorophyll-a retrieval in optically complex waters that accounts for remote sensing and modeling uncertainties and improves remote estimation accuracy. Water Res. 2020, 171, 115403. [Google Scholar] [CrossRef]
- Zhou, L.; Ma, W.; Zhang, H.; Li, L.; Tang, L. Developing a PCA–ANN model for predicting chlorophyll a concentration from field hyperspectral measurements in dianshan lake, China. Water Qual. Expo. Health 2015, 7, 591–602. [Google Scholar] [CrossRef]
- Kim, S.; Chung, S.; Park, H.; Cho, Y.; Lee, H. Analysis of environmental factors associated with cyanobacterial dominance after river weir installation. Water 2019, 11, 1163. [Google Scholar] [CrossRef] [Green Version]
- Konopka, A.; Brock, T.D. Effect of temperature on blue-green algae (Cyanobacteria) in Lake Mendota. Appl. Environ. Microbiol. 1978, 36, 572–576. [Google Scholar] [CrossRef] [Green Version]
- Lürling, M.; Eshetu, F.; Faassen, E.J.; Kosten, S.; Huszar, V.L.M. Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshw. Biol. 2013, 58, 552–559. [Google Scholar] [CrossRef]
- Nalley, J.O.; O’Donnell, D.R.; Litchman, E. Temperature effects on growth rates and fatty acid content in freshwater algae and cyanobacteria. Algal Res. 2018, 35, 500–507. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef]
- Berg, M.; Sutula, M. Factors Affecting Growth of Cyanobacteria. Monaldi Arch. Chest Dis. Pulm. Ser. 2015, 59, 103–107. [Google Scholar]
- Dev, P.J.; Sukenik, A.; Mishra, D.R.; Ostrovsky, I. Cyanobacterial pigment concentrations in inland waters: Novel semi-analytical algorithms for multi- and hyperspectral remote sensing data. Sci. Total Environ. 2022, 805, 150423. [Google Scholar] [CrossRef]
- Park, Y.; Pyo, J.C.; Kwon, Y.S.; Cha, Y.K.; Lee, H.; Kang, T.; Cho, K.H. Evaluating physico-chemical influences on cyanobacterial blooms using hyperspectral images in inland water, Korea. Water Res. 2017, 126, 319–328. [Google Scholar] [CrossRef]
- Ha, K.; Cho, E.A.; Kim, H.W.; Joo, G.J. Microcystis bloom formation in the lower Nakdong River, South Korea: Importance of hydrodynamics and nutrient loading. Mar. Freshw. Res. 1999, 50, 89–94. [Google Scholar] [CrossRef]
- Oliver, R.L.; Ganf, G.G. Freshwater blooms. In The Ecology of Cyanobacteria; Springer: Berlin/Heidelberg, Germany, 2000; pp. 149–194. [Google Scholar]


| Date | Number of Samples | PC | Chlorophyll-a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| * Avg. | * Std. | Min | Max | Avg. | Std. | Min | Max | ||
| 12 August 2016 | 18 | 35.46 | 36.10 | 6.04 | 146.99 | 40.65 | 23.38 | 14.19 | 111.40 |
| 24 August 2016 | 20 | 38.07 | 23.58 | 12.25 | 100.00 | 37.24 | 8.02 | 25.95 | 61.44 |
| 20 September 2016 | 17 | 1.23 | 0.27 | 0.83 | 1.64 | 25.51 | 11.32 | 11.85 | 60.88 |
| 14 October 2016 | 20 | 0.33 | 0.17 | 0.19 | 0.88 | 28.21 | 9.38 | 13.74 | 46.17 |
| 15 September 2017 | 12 | 8.34 | 0.66 | 7.41 | 9.66 | 47.28 | 8.54 | 30.24 | 61.52 |
| 22 September 2017 | 12 | 12.63 | 3.96 | 7.64 | 21.69 | 17.57 | 3.80 | 14.08 | 27.89 |
| 25 October 2017 | 12 | 3.51 | 0.67 | 2.64 | 4.56 | 13.18 | 2.85 | 10.56 | 20.92 |
| 28 October 2017 | 12 | 4.35 | 4.52 | 1.18 | 14.77 | 10.54 | 2.28 | 8.45 | 16.73 |
| 11 November 2017 | 11 | 0.35 | 0.14 | 0.23 | 0.71 | 22.00 | 6.76 | 12.76 | 38.43 |
| Pigment | Concentration (mg/L) | No. of Bands Selected | Band (nm) |
|---|---|---|---|
| PC | 0–3 | 14 | 452, 470, 484, 604, 674, 684, 708, 712, 717, 727, 784, 789, 794, 799 |
| 3–15 | 14 | 466, 525, 679, 741, 746, 751, 755, 760, 765, 770, 775, 779, 784, 789 | |
| 15–147 | 20 | 457, 461, 470, 475, 488, 497, 502, 507, 511, 516, 520, 525, 529, 543, 665, 670, 674, 693, 717, 784 | |
| Chl-a | 0–20 | 21 | 566, 571, 580, 585, 590, 604, 646, 651, 655, 660, 665, 670, 674, 698, 703, 708, 717, 722, 727, 784, 789 |
| 20–35 | 25 | 452, 466, 470, 488, 507, 511, 516, 520, 525, 529, 539, 543, 552, 674, 689, 722, 736, 751, 755, 760, 770, 775, 784, 789, 794 | |
| 35–111 | 22 | 488, 548, 590, 599, 604, 627, 646, 651, 655, 689, 698, 703, 708, 712, 717, 731, 736, 741, 755, 779, 789, 794 |
| Pigment | Type | MIN | MAX | ||
|---|---|---|---|---|---|
| PC | peak | 641.18 | ~ | 655.35 | Rpp |
| * abs. | 603.60 | ~ | 631.76 | Rpa | |
| Chl-a | peak | 698.04 | ~ | 712.33 | Rcp |
| abs. | 664.81 | ~ | 679.03 | Rca | |
| Green | peak | 465.74 | ~ | 589.58 | Rgp |
| Water | abs. | 731.42 | ~ | 784.11 | Rwa |
| Case * | Pigment | Training | Validation | |||||
|---|---|---|---|---|---|---|---|---|
| Method | R2 | NSE | RMSE | R2 | NSE | RMSE | ||
| * Case 1 | PC | PLS | 0.60 | 0.60 | 14.69 | 0.34 | 0.33 | 9.46 |
| RF | 0.73 | 0.70 | 11.55 | 0.51 | 0.43 | 12.84 | ||
| GB | 0.79 | 0.73 | 10.55 | 0.59 | 0.54 | 12.13 | ||
| SVM | 0.68 | 0.81 | 12.50 | 0.71 | 0.43 | 19.58 | ||
| KNN | 0.69 | 0.69 | 13.40 | 0.51 | 0.34 | 8.06 | ||
| ANN | 0.80 | 0.76 | 11.41 | 0.68 | 0.49 | 9.99 | ||
| Avg. | 0.71 | 0.72 | 12.35 | 0.56 | 0.43 | 12.01 | ||
| * Case 2 | Chl-a | PLS | 0.29 | −1.44 | 13.73 | 0.28 | −6.77 | 10.98 |
| RF | 0.46 | −1.02 | 11.37 | 0.35 | −4.10 | 15.97 | ||
| GB | 0.67 | 0.18 | 9.62 | 0.30 | 0.11 | 14.00 | ||
| SVM | 0.48 | −0.46 | 11.77 | 0.34 | 0.06 | 11.14 | ||
| KNN | 0.38 | −1.18 | 11.09 | 0.30 | −4.75 | 18.05 | ||
| ANN | 0.52 | 0.48 | 11.60 | 0.43 | 0.05 | 12.09 | ||
| Avg. | 0.47 | −0.57 | 11.53 | 0.33 | −2.57 | 13.70 | ||
| * Case 3 | PC | PLS | 0.69 | 0.69 | 10.98 | 0.56 | 0.64 | 18.09 |
| RF | 0.77 | 0.76 | 9.63 | 0.71 | 0.74 | 15.38 | ||
| GB | 0.85 | 0.84 | 7.78 | 0.74 | 0.74 | 15.32 | ||
| SVM | 0.70 | 0.69 | 11.14 | 0.68 | 0.70 | 16.35 | ||
| KNN | 0.74 | 0.73 | 10.39 | 0.67 | 0.73 | 15.61 | ||
| ANN | 0.81 | 0.65 | 11.72 | 0.79 | 0.84 | 11.92 | ||
| Avg. | 0.76 | 0.73 | 10.27 | 0.69 | 0.73 | 15.45 | ||
| Chl-a | PLS | 0.35 | 0.35 | 10.81 | 0.29 | 0.79 | 17.79 | |
| RF | 0.59 | 0.58 | 8.71 | 0.42 | 0.83 | 15.97 | ||
| GB | 0.58 | 0.53 | 9.16 | 0.43 | 0.82 | 16.63 | ||
| SVM | 0.47 | 0.45 | 9.94 | 0.46 | 0.85 | 15.08 | ||
| KNN | 0.53 | 0.51 | 9.39 | 0.46 | 0.83 | 16.12 | ||
| ANN | 0.80 | 0.79 | 6.09 | 0.67 | 0.92 | 11.38 | ||
| Avg. | 0.55 | 0.54 | 9.02 | 0.46 | 0.84 | 15.50 | ||
| Pigment | Case | Training | Validation | ||||
|---|---|---|---|---|---|---|---|
| R2 | NSE | RMSE | R2 | NSE | RMSE | ||
| PC | A | 0.805 | 0.799 | 9.447 | 0.719 | 0.188 | 14.881 |
| B | 0.869 | 0.848 | 7.176 | 0.717 | 0.328 | 14.438 | |
| C | 0.795 | 0.666 | 9.974 | 0.597 | 0.216 | 16.813 | |
| Chl-a | A | 0.544 | 0.288 | 9.360 | 0.604 | 0.030 | 12.898 |
| B | 0.564 | 0.220 | 8.934 | 0.587 | −0.992 | 14.075 | |
| C | 0.565 | 0.272 | 9.094 | 0.480 | −1.763 | 14.995 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, W.; Park, Y.; Pyo, J.; Park, S.; Kim, J.; Kim, J.H.; Cho, K.H.; Shin, J.-K.; Kim, S. Optimal Band Selection for Airborne Hyperspectral Imagery to Retrieve a Wide Range of Cyanobacterial Pigment Concentration Using a Data-Driven Approach. Remote Sens. 2022, 14, 1754. https://doi.org/10.3390/rs14071754
Jang W, Park Y, Pyo J, Park S, Kim J, Kim JH, Cho KH, Shin J-K, Kim S. Optimal Band Selection for Airborne Hyperspectral Imagery to Retrieve a Wide Range of Cyanobacterial Pigment Concentration Using a Data-Driven Approach. Remote Sensing. 2022; 14(7):1754. https://doi.org/10.3390/rs14071754
Chicago/Turabian StyleJang, Wonjin, Yongeun Park, JongCheol Pyo, Sanghyun Park, Jinuk Kim, Jin Hwi Kim, Kyung Hwa Cho, Jae-Ki Shin, and Seongjoon Kim. 2022. "Optimal Band Selection for Airborne Hyperspectral Imagery to Retrieve a Wide Range of Cyanobacterial Pigment Concentration Using a Data-Driven Approach" Remote Sensing 14, no. 7: 1754. https://doi.org/10.3390/rs14071754
APA StyleJang, W., Park, Y., Pyo, J., Park, S., Kim, J., Kim, J. H., Cho, K. H., Shin, J.-K., & Kim, S. (2022). Optimal Band Selection for Airborne Hyperspectral Imagery to Retrieve a Wide Range of Cyanobacterial Pigment Concentration Using a Data-Driven Approach. Remote Sensing, 14(7), 1754. https://doi.org/10.3390/rs14071754