Estimation of Potato Above-Ground Biomass Based on Vegetation Indices and Green-Edge Parameters Obtained from UAVs
Abstract
1. Introduction
2. Experiment and Methods
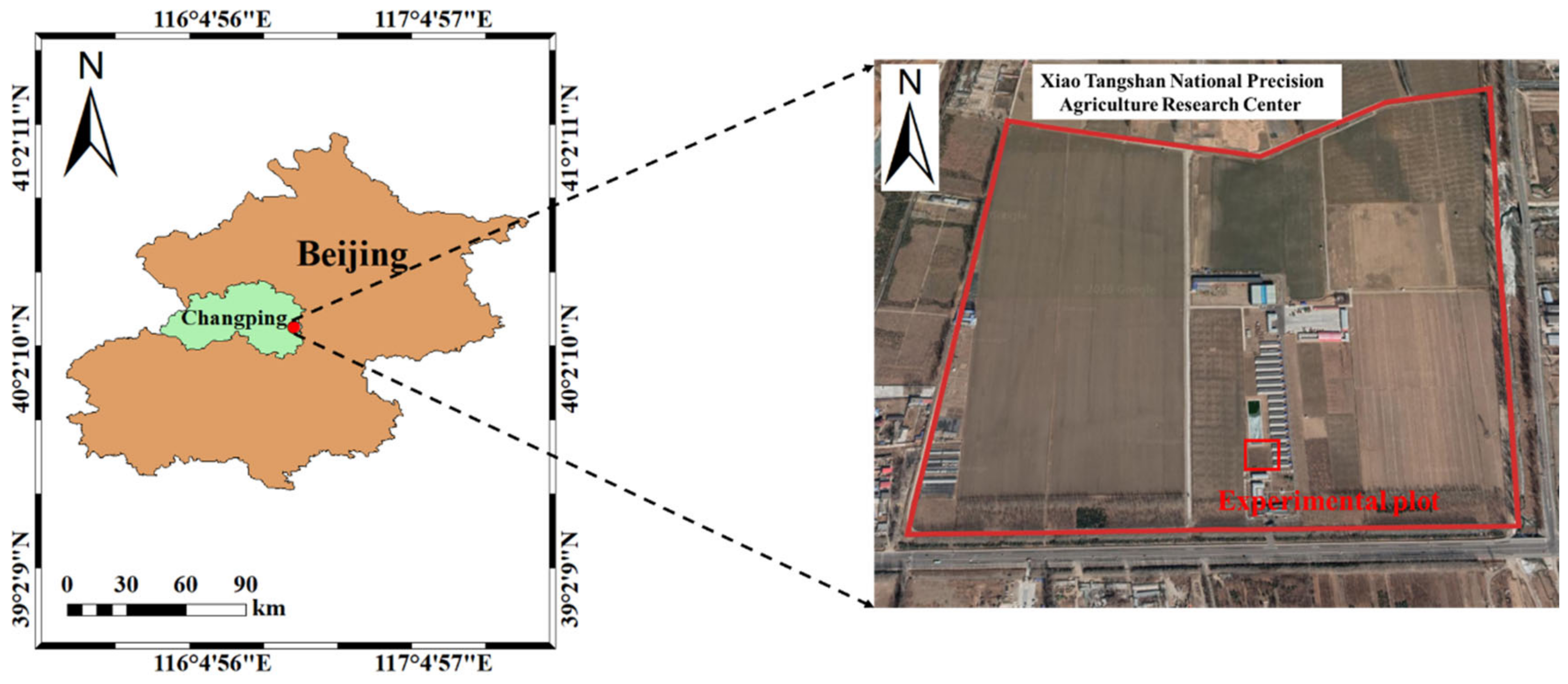
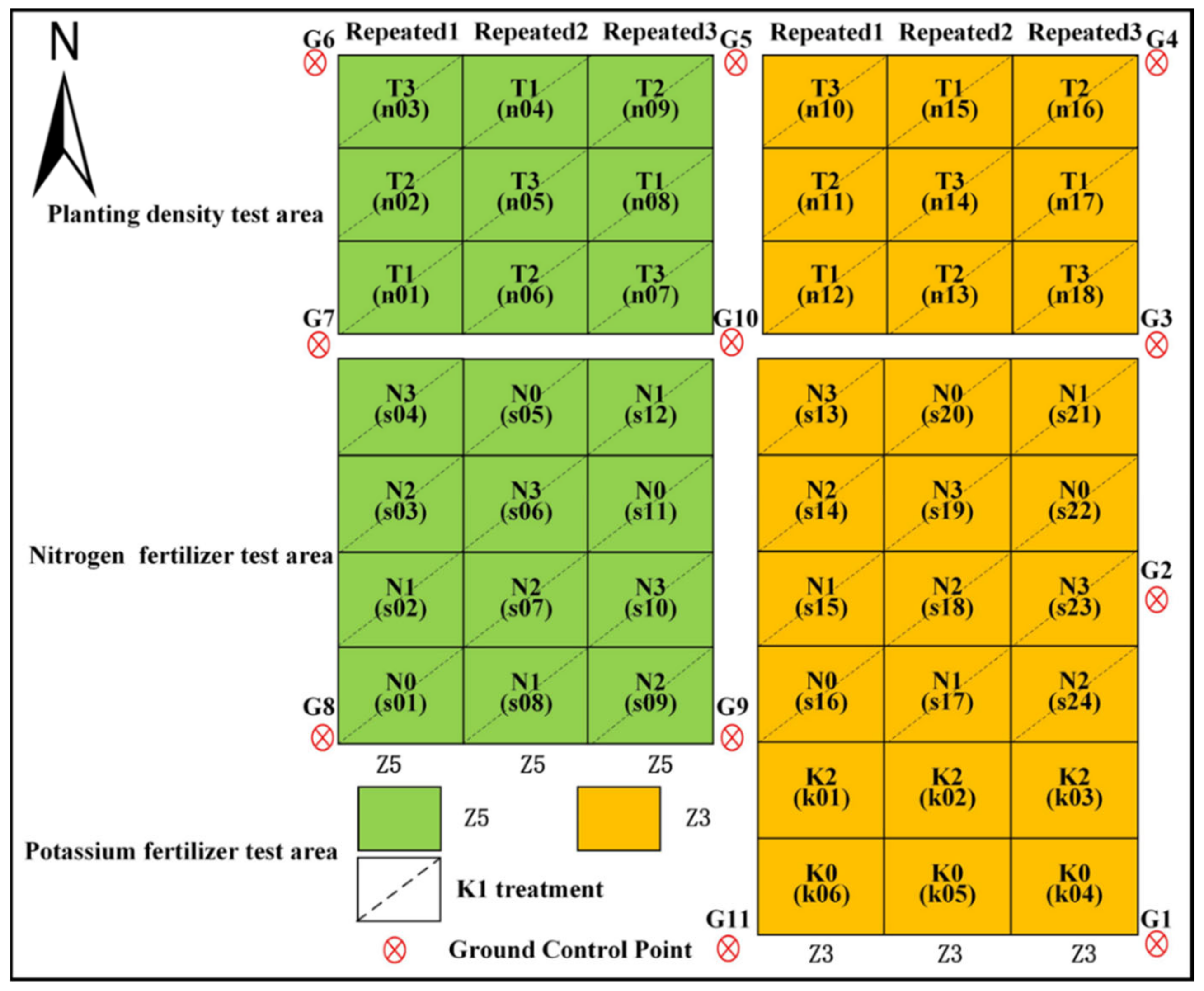
2.1. Overview of Study Area and Experimental Design
2.2. Ground Data Acquisition and Processing
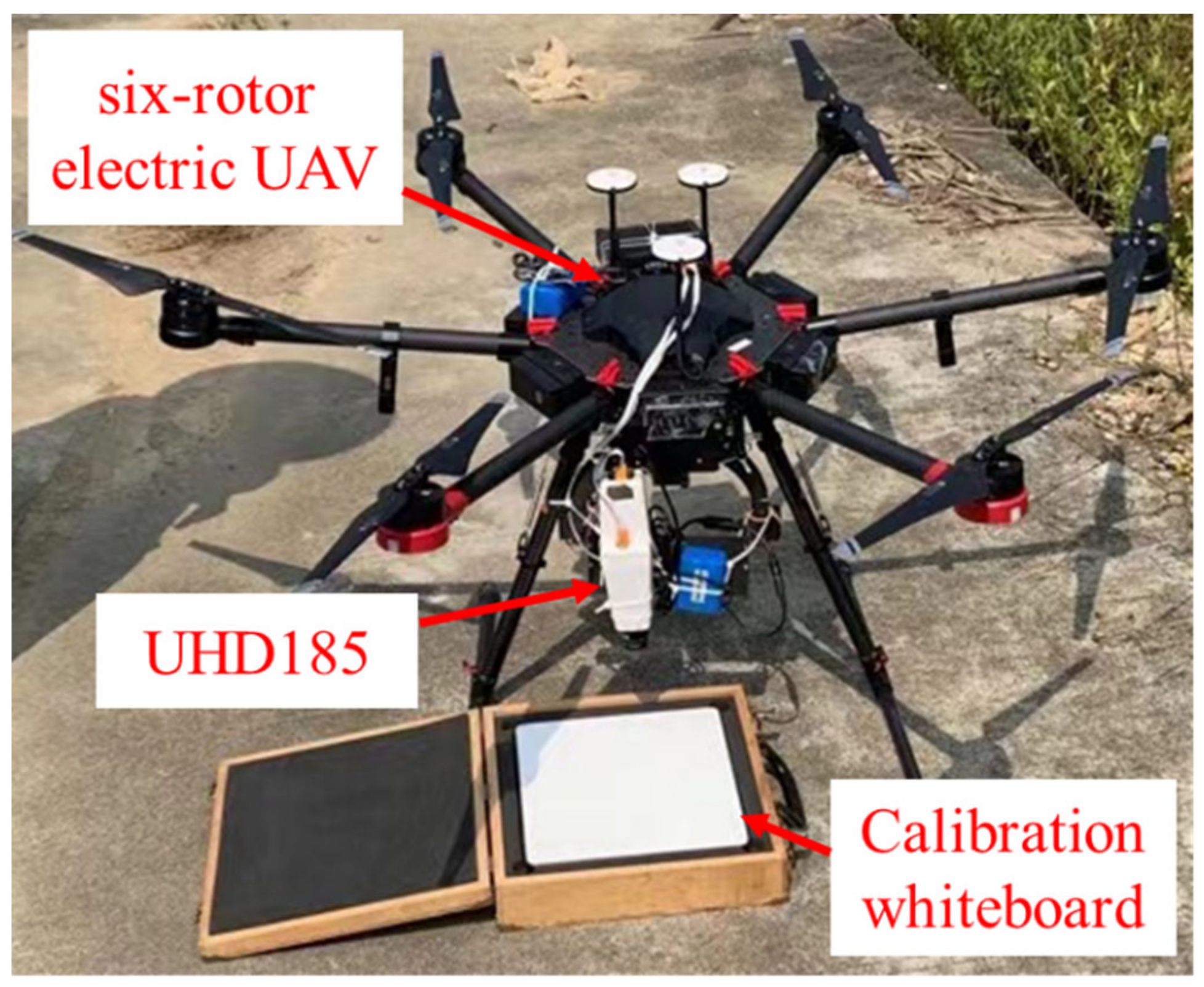
2.3. UAV Hyperspectral Data Acquisition and Processing
2.4. Selection of Vegetation Indices
2.5. Selection of Green-Edge Parameters
2.6. Analysis Methods
3. Results and Analysis
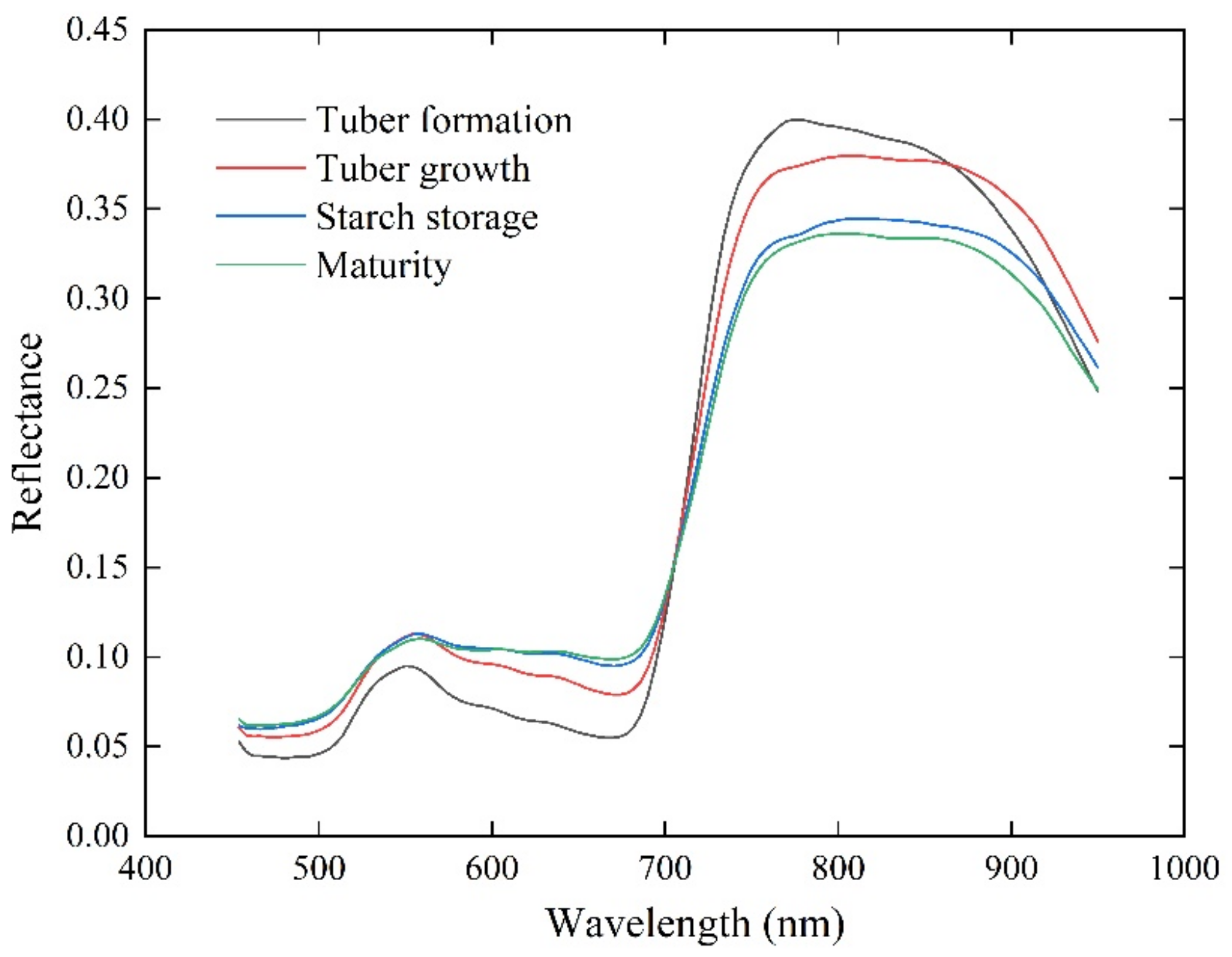
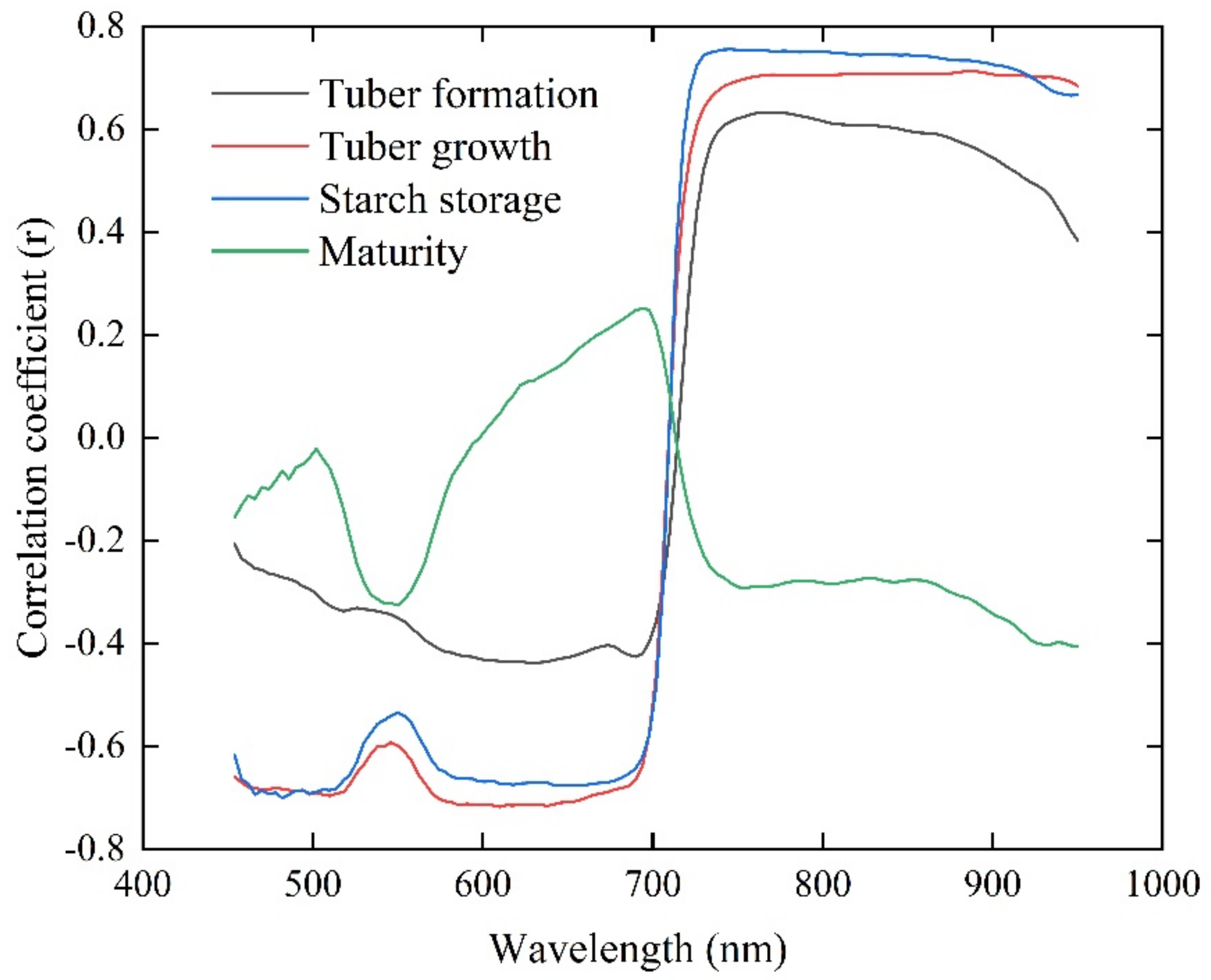
3.1. Correlation between VIs, GEPs, and AGB
3.2. Relationship of Optimal VIs and GEPs with AGB
3.2.1. Relationship between Optimal GEPs and AGB
3.2.2. Relationship between Optimal VIs and AGB
3.2.3. Relationship of Optimal VIs and Optimal GEPs with AGB
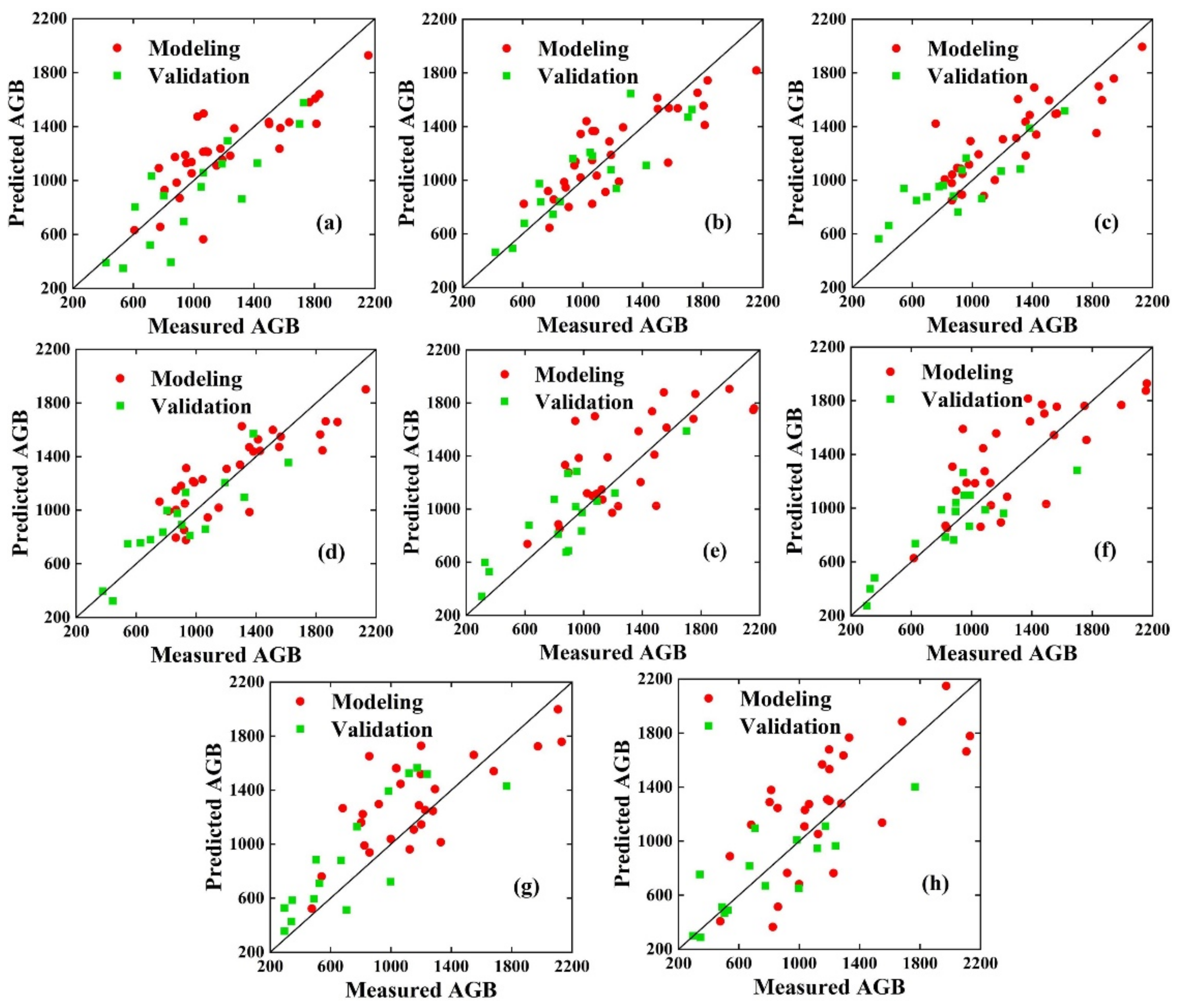
3.3. Estimation of AGB Using VIs, Optimal GEPs Combined with PLSR and RF Methods
3.4. Spatial Distribution of AGB
4. Discussion
4.1. AGB Estimation Based on Spectral Parameters
4.2. AGB Estimation Based on Regression Technique
4.3. Spatial Distribution of AGB
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Feng, H.; Yue, J.; Li, Z.; Yang, G.; Song, X.; Yang, X.; Zhao, Y. Remote-sensing estimation of potato above-ground biomass based on spectral and spatial features extracted from high-definition digital camera images. Comput. Electron. Agric. 2022, 198, 107089. [Google Scholar] [CrossRef]
- Brocks, S.; Bareth, G. Estimating barley biomass with crop surface models from Oblique RGB imagery. Remote Sens. 2018, 10, 268. [Google Scholar] [CrossRef]
- Bendig, J.; Yu, K.; Aasen, H.; Bolten, A.; Bennertz, S.; Broscheit, J.; Gnyp, M.L.; Bareth, G. Combining UAV-based plant height from crop surface models, visible, and near infrared vegetation indices for biomass monitoring in barley. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 79–87. [Google Scholar] [CrossRef]
- Gnyp, M.L.; Miao, Y.; Yuan, F.; Ustin, S.L.; Yu, K.; Yao, Y.; Huang, S.; Bareth, G. Hyperspectral canopy sensing of paddy rice aboveground biomass at different growth stages. Field Crops Res. 2014, 155, 42–55. [Google Scholar] [CrossRef]
- Li, W.; Niu, Z.; Chen, H.; Li, D.; Wu, M.; Zhao, W. Remote estimation of canopy height and aboveground biomass of maize using high-resolution stereo images from a low-cost unmanned aerial vehicle system. Ecol. Indic. 2016, 67, 637–648. [Google Scholar] [CrossRef]
- Zhu, W.; Sun, Z.; Peng, J.; Huang, Y.; Li, J.; Zhang, J.; Yang, B.; Liao, X. Estimating maize above-ground biomass using 3D point clouds of multi-source unmanned aerial vehicle data at multi-spatial scales. Remote Sens. 2019, 11, 2678. [Google Scholar] [CrossRef]
- Walter, J.D.C.; Edwards, J.; McDonald, G.; Kuchel, H. Estimating biomass and canopy height with LiDAR for field crop breeding. Front. Plant Sci. 2019, 10, 1145–1157. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, G.; Wang, J.; Song, X.; Feng, H. Winter wheat biomass estimation based on spectral indices, band depth analysis and partial least squares regression using hyperspectral measurements. Comput. Electron. Agric. 2014, 100, 51–59. [Google Scholar] [CrossRef]
- Yue, J.; Feng, H.; Jin, X.; Yuan, H.; Li, Z.; Zhou, C.; Yang, G.; Tian, Q. A comparison of crop parameters estimation using images from UAV-mounted snapshot hyperspectral sensor and high-definition digital camera. Remote Sens. 2018, 10, 1138. [Google Scholar] [CrossRef]
- Greaves, H.E.; Vierling, L.A.; Eitel, J.U.H.; Boelman, N.T.; Magney, T.S.; Prager, C.M.; Griffin, K.L. Estimating aboveground biomass and leaf area of low-stature Arctic shrubs with terrestrial LiDAR. Remote Sens. Environ. 2015, 164, 26–35. [Google Scholar] [CrossRef]
- Jin, X.; Liu, S.; Baret, F.; Hemerlé, M.; Comar, A. Estimates of plant density of wheat crops at emergence from very low altitude UAV imagery. Remote Sens. Environ. 2017, 198, 105–114. [Google Scholar] [CrossRef]
- Schirrmann, M.; Hamdorf, A.; Garz, A.; Ustyuzhanin, A.; Dammer, K.-H. Estimating wheat biomass by combining image clustering with crop height. Comput. Electron. Agric. 2016, 121, 374–384. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Lu, D.; Li, D. Exploring bamboo forest above ground biomass estimation using sentinel-2 Data. Remote Sens. 2019, 11, 7. [Google Scholar] [CrossRef]
- Duan, B.; Fang, S.; Gong, Y.; Peng, Y.; Wu, X.; Zhu, R. Remote estimation of grain yield based on UAV data in different rice cultivars under contrasting climatic zone. Field Crop. Res. 2021, 267, 108148–108166. [Google Scholar] [CrossRef]
- Tao, H.; Feng, H.; Xu, L.; Miao, M.; Long, H.; Yue, J.; Li, Z.; Yang, G.; Yang, X.; Fan, L. Estimation of crop growth parameters using UAV-based hyperspectral remote sensing data. Sensors 2020, 20, 1296–1317. [Google Scholar] [CrossRef] [PubMed]
- Kross, A.; McNairn, H.; Lapen, D.; Sunohara, M.; Champagne, C. Assessment of RapidEye vegetation indices for estimation of leaf area index and biomass in corn and soybean crops. Int. J. Appl. earth Obs. 2015, 34, 235–248. [Google Scholar] [CrossRef]
- Ma, G.N.; Huang, J.X.; Wu, W.B.; Fan, J.L.; Zou, J.Q.; Wu, S.J. Assimilation of MODIS-LAI into the WOFOST model for forecasting regional winter wheat yield. Math. Comput. Model. 2013, 58, 634–643. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, S.; Shen, S. Assimilating remote sensing information with crop model using Ensemble Kalman Filter for improving LAI monitoring and yield estimation. Ecol. Model. 2013, 270, 30–42. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, X.; Liu, M. Assimilating remote sensing phenological information into the WOFOST model for rice growth simulation. Remote Sens. 2019, 11, 268. [Google Scholar] [CrossRef]
- Montoya, F.; Camargo, D.; Domínguez, A.; Ortega, J.; Córcoles, J. Parametrization of Cropsyst model for the simulation of a potato crop in a Mediterranean environment. Agric. Water Manag. 2018, 203, 297–310. [Google Scholar] [CrossRef]
- Lobell, D.B.; Ortiz-Monasterio, J.I. Regional importance of crop yield constraints: Linking simulation models and geostatistics to interpret spatial patterns. Ecol. Model. 2005, 196, 173–182. [Google Scholar] [CrossRef]
- Chahbi, A.; Zribi, M.; Lili-Chabaane, Z.; Duchemin, B.; Shabou, M.; Mougenot, B.; Boulet, G. Estimation of the dynamics and yields of cereals in a semi-arid area using remote sensing and the SAFY growth model. Int. J. Remote Sens. 2014, 35, 1004–1028. [Google Scholar] [CrossRef]
- Choudhury, M.R.; Das, S.; Christopher, J.; Apan, A.; Chapman, S.; Menzies, N.W.; Dang, Y.P. Improving biomass and grain yield prediction of wheat genotypes on sodic soil using integrated high-resolution multispectral, hyperspectral, 3D point cloud, and machine learning techniques. Remote Sens. 2021, 13, 3482. [Google Scholar] [CrossRef]
- Berger, K.; Atzberger, C.; Danner, M.; D’Urso, G.; Mauser, W.; Vuolo, F.; Hank, T.; Berger, K.; Atzberger, C.; Danner, M.; et al. Evaluation of the PROSAIL model capabilities for future hyperspectral model environments: A review study. Remote Sens. 2018, 10, 85. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, Q.; Braswell, B.; Urbanski, S.; Boles, S.; Wofsy, S.; Moore, B.; Ojima, D. Modeling gross primary production of temperate deciduous broadleaf forest using satellite images and climate data. Remote Sens. Environ. 2004, 91, 256–270. [Google Scholar] [CrossRef]
- Shendryk, Y.; Sofonia, J.; Garrard, R.; Rist, Y.; Skocaj, D.; Thorburn, P. Fine-scale prediction of biomass and leaf nitrogen content in sugarcane using UAV LiDAR and multispectral imaging. Int. J. Appl. Earth Obs. 2020, 92, 102177–102191. [Google Scholar] [CrossRef]
- Mansaray, L.R.; Kanu, A.S.; Yang, L.; Huang, J.; Wang, F. Evaluation of machine learning models for rice dry biomass estimation and mapping using quad-source optical imagery. GISci. Remote Sens. 2020, 57, 785–796. [Google Scholar] [CrossRef]
- Suarez, L.A.; Robson, A.; McPhee, J.; O’Halloran, J.; Van Sprang, C. Accuracy of carrot yield forecasting using proximal hyperspectral and satellite multispectral data. Precis. Agric. 2020, 21, 1304–1326. [Google Scholar] [CrossRef]
- Jin, X.; Li, Z.; Feng, H.; Ren, Z.; Li, S. Deep neural network algorithm for estimating maize biomass based on simulated Sentinel 2A vegetation indices and leaf area index. Crop J. 2020, 8, 87–97. [Google Scholar] [CrossRef]
- Zhang, L.X.; Chen, Y.Q.; Li, Y.X.; Ma, J.C.; Zheng, F.X.; Sun, Z.F. Estimating above ground biomass of winter wheat at early growth stages based on visual spectral. Spectrosc. Spect. Anal. 2018, 39, 2501–2506. [Google Scholar]
- Devia, C.A.; Rojas, J.P.; Petro, E.; Martinez, C.; Mondragon, I.F.; Patino, D.; Rebolledo, M.C.; Colorado, J. High-throughput biomass estimation in rice crops using UAV multispectral imagery. J. Intell. Robot. Syst. 2019, 96, 573–589. [Google Scholar] [CrossRef]
- Han, L.; Yang, G.; Dai, H.; Xu, B.; Yang, H.; Feng, H.; Li, Z.; Yang, X. Modeling maize above-ground biomass based on machine learning approaches using UAV remote-sensing data. Plant Methods 2019, 15, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Asari, N.; Suratman, M.N.; Jaafar, J. Modelling and mapping of above ground biomass (AGB) of oil palm plantations in Malaysia using remotely-sensed data. Int. J. Remote Sens. 2017, 38, 4741–4764. [Google Scholar] [CrossRef]
- Yue, J.; Yang, G.; Li, C.; Li, Z.; Wang, Y.; Feng, H.; Xu, B. Estimation of winter wheat above-ground biomass using unmanned aerial vehicle-based snapshot hyperspectral sensor and crop height improved models. Remote Sens. 2017, 9, 708. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, C.; Yang, H.; Yang, G.; Han, L.; Li, Z.; Feng, H.; Xu, B.; Wu, J.; Lei, L. Estimation of maize above-ground biomass based on stem-leaf separation strategy integrated with LiDAR and optical remote sensing data. PeerJ 2019, 7, 7593–7614. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Lee, B.-W. Assessment of rice leaf growth and nitrogen status by hyperspectral canopy reflectance and partial least square regression. Eur. J. Agron. 2006, 24, 349–356. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, H.; Wang, D.; Li, H.; Mouazen, A.M. A novel approach for estimation of above-ground biomass of sugar beet based on wavelength selection and optimized support vector machine. Remote Sens. 2020, 12, 620–638. [Google Scholar] [CrossRef]
- Yue, J.; Feng, H.; Yang, G.; Li, Z. A Comparison of regression techniques for estimation of above-ground winter wheat biomass using near-surface spectroscopy. Remote Sens. 2018, 10, 66. [Google Scholar] [CrossRef]
- Sun, Q.; Gu, X.; Chen, L.; Xu, X.; Wei, Z.; Pan, Y.; Gao, Y. Monitoring maize canopy chlorophyll density under lodging stress based on UAV hyperspectral imagery. Comput. Electron. Agric. 2022, 193, 106671–106685. [Google Scholar] [CrossRef]
- Yao, X.; Si, H.; Cheng, T.; Jia, M.; Chen, Q.; Tian, Y.; Zhu, Y.; Cao, W.; Chen, C.; Cai, J.; et al. Hyperspectral estimation of canopy leaf biomass phenotype per ground area using a continuous wavelet analysis in wheat. Front. Plant Sci. 2018, 9, 1360–1374. [Google Scholar] [CrossRef]
- Tian, H.; Shi, S.; Wang, H.; Li, F.; Li, Z.; Alva, A.; Zhang, Z. Estimation of sugar beet aboveground biomass by band depth optimization of hyperspectral canopy reflectance. J. Indian Soc. Remote 2017, 45, 795–803. [Google Scholar] [CrossRef]
- Chen, H.Y.H.; Luo, Y.; Reich, P.B.; Searle, E.B.; Biswas, S.R. Climate change—Associated trends in net biomass change are age dependent in western boreal forests of Canada. Ecol. Lett. 2016, 19, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Bendig, J.; Bolten, A.; Bennertz, S.; Broscheit, J.; Eichfuss, S.; Bareth, G. Estimating biomass of barley using crop surface models (CSMs) derived from UAV-based RGB imaging. Remote Sens. 2014, 6, 10395–10412. [Google Scholar] [CrossRef]
- Banerjee, B.; Spangenberg, G.; Kant, S. Fusion of spectral and structural information from aerial images for improved biomass estimation. Remote Sens. 2020, 12, 3164. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Li, J.; Guo, X.; Wang, S.; Lu, J. Estimating biomass of winter oilseed rape using vegetation indices and texture metrics derived from UAV multispectral images. Comput. Electron. Agric. 2019, 166, 105026–105036. [Google Scholar] [CrossRef]
- Yue, J.; Yang, G.; Tian, Q.; Feng, H.; Xu, K.; Zhou, C. Estimate of winter-wheat above-ground biomass based on UAV ultrahigh-ground-resolution image textures and vegetation indices. ISPRS J. Photogramm. Remote Sens. 2019, 150, 226–244. [Google Scholar] [CrossRef]
- Zheng, H.B.; Cheng, T.; Zhou, M.; Li, D.; Yao, X.; Tian, Y.C.; Cao, W.X.; Zhu, Y. Improved estimation of rice aboveground biomass combining textural and spectral analysis of UAV imagery. Precis. Agric. 2019, 20, 611–629. [Google Scholar] [CrossRef]
- Quirós Vargas, J.J.; Zhang, C.; Smitchger, J.A.; McGee, R.J.; Sankaran, S. Phenotyping of plant biomass and performance traits using remote sensing techniques in pea. Sensors 2019, 19, 2031. [Google Scholar] [CrossRef]
- Sankaran, S.; Zhou, J.; Khot, L.R.; Trapp, J.J.; Mndolwa, E.; Miklas, P.N. High-throughput field phenotyping in dry bean using small unmanned aerial vehicle based multispectral imagery. Comput. Electron. Agric. 2018, 151, 84–92. [Google Scholar] [CrossRef]
- Kasim, N.; Shi, Q.D.; Wang, J.Z.; Sawut, R.; Nurmemet, I.; Isak, G. Estimation of spring wheat chlorophyll content based on hyperspectral features and PLSR model. Trans. Chin. Soc. Agric. Eng. 2017, 33, 208–215. [Google Scholar]
- Li, B.; Xu, X.; Zhang, L.; Han, J.; Bian, C.; Li, G.; Liu, J.; Jin, L. Above-ground biomass estimation and yield prediction in potato by using UAV-based RGB and hyperspectral imaging. ISPRS J. Photogramm. Remote Sens. 2020, 162, 161–172. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Bao, Y.; Luo, J.; Jin, X.; Xu, X.; Song, X.; Yang, G. Exploring the best hyperspectral features for LAI estimation using partial least squares regression. Remote Sens. 2014, 6, 6221–6241. [Google Scholar] [CrossRef]


| Vegetation Indices | Definition | References |
|---|---|---|
| MSR (modified simple ratio index) | (R800/R670 − 1)/(R800/R670 + 1)1/2 | [15] |
| MSAVI (modified soil adjusted vegetation index) | 0.5 × [2 × R800 + 1 − ((2×R800 + 1)2 – 8 × (R800 − R670))1/2] | [34] |
| OSAVI (optimizing soil adjusted vegetation index) | 1.16 × (R800 − R670)/(R800 + R670 + 0.16) | [15] |
| PSRI (plant senescence reflectance index) | (R680 − R500)/R750 | [46] |
| RDVI (renormalized difference vegetation index) | (R800 − R670)/(R800 + R670)1/2 | [15] |
| TCARI (transformed chlorophyll absorption ratio index) | 3 × [(R710 − R680) − 0.2 × (R700 − R560)(R710/R680)] | [15] |
| TVI (triangular vegetation index) | 0.5 × [120 × (R750-R550) − 200×(R670 − R550)] | [15] |
| SPVI (spectral polygon vegetation index) | 0.4 × [3.7 × (R800-R670) − 1.2×|R550 − R670|] | [34] |
| DVI (difference vegetation index) | R890-R670 | [15] |
| MCARI (modified chlorophyll absorption ratio index) | ((R700 − R670) − 0.2 × (R700-R550))(R700/R670) | [34] |
| NDVI (normalized difference vegetation index) | (R800 − R680)/(R800 + R680) | [29] |
| PBI (plant biochemical index) | R810/R560 | [38] |
| LCI (linear combination index) | (R850 − R710)/(R850 + R670)1/2 | [38] |
| SRI (simple ratio vegetation index) | R800/R680 | [45] |
| SAVI (soil adjusted vegetation index) | (1 + 0.5) × (R800 − R670)/(R800 + R670 + 0.5) | [45] |
| Spectral Parameters | AGB | ||||
|---|---|---|---|---|---|
| Tuber Form Stage | Tuber Growth Stage | Starch Store Stage | Maturity Stage | ||
| VIs | MSR | 0.636 | 0.733 | 0.724 | −0.237 |
| MSAVI | 0.651 | 0.748 | 0.729 | −0.266 | |
| OSAVI | 0.655 | 0.742 | 0.717 | −0.291 | |
| PSRI | −0.727 | −0.682 | −0.696 | 0.396 | |
| RDVI | 0.642 | 0.746 | 0.731 | −0.270 | |
| TCARI | 0.410 | 0.686 | 0.641 | −0.304 | |
| TVI | 0.659 | 0.741 | 0.714 | −0.314 | |
| SPVI | 0.673 | 0.756 | 0.728 | −0.256 | |
| DVI | 0.650 | 0.742 | 0.733 | −0.302 | |
| MCARI | 0.220 | 0.676 | 0.581 | −0.197 | |
| NDVI | 0.580 | 0.733 | 0.729 | −0.255 | |
| PBI | 0.654 | 0.757 | 0.745 | −0.047 | |
| LCI | 0.661 | 0.725 | 0.751 | −0.284 | |
| SRI | 0.662 | 0.726 | 0.715 | −0.238 | |
| SAVI | 0.639 | 0.746 | 0.731 | −0.270 | |
| GEPs | Rmax | −0.367 | −0.613 | −0.546 | −0.311 |
| Rsum | −0.342 | −0.668 | −0.644 | −0.236 | |
| SDr | 0.023 | 0.471 | 0.462 | −0.447 | |
| Dr | −0.284 | 0.321 | 0.401 | −0.415 | |
| Drmin | −0.545 | −0.509 | −0.382 | 0.196 | |
| Dr/Drmin | 0.122 | 0.581 | 0.491 | −0.260 | |
| Growth Stages | Optimal GEPs | Modeling | Validation | ||||
|---|---|---|---|---|---|---|---|
| R2 | RMSE (kg/hm2) | NRMSE (%) | R2 | RMSE (kg/hm2) | NRMSE (%) | ||
| Tuber form | Drmin | 0.31 | 325.37 | 25.24 | 0.40 | 256.15 | 25.16 |
| Tuber growth | Rsum | 0.37 | 332.83 | 22.94 | 0.52 | 229.96 | 21.39 |
| Starch store | Rsum | 0.23 | 404.96 | 29.11 | 0.38 | 259.51 | 28.57 |
| Maturity | SDr | 0.21 | 428.87 | 35.12 | 0.27 | 279.39 | 32.39 |
| Growth Stages | Optimal VI | Modeling | Validation | ||||
|---|---|---|---|---|---|---|---|
| R2 | RMSE (kg/hm2) | NRMSE (%) | R2 | RMSE (kg/hm2) | NRMSE (%) | ||
| Tuber form | PSRI | 0.43 | 301.88 | 23.42 | 0.59 | 226.59 | 22.24 |
| Tuber growth | PBI | 0.48 | 300.51 | 20.71 | 0.62 | 207.16 | 19.27 |
| Starch store | LCI | 0.38 | 349.76 | 25.14 | 0.46 | 208.98 | 23.01 |
| Maturity | PSRI | 0.26 | 376.33 | 30.82 | 0.36 | 240.49 | 27.88 |
| Growth Stages | Optimal VIs, GEPs | Modeling | Validation | ||||
|---|---|---|---|---|---|---|---|
| R2 | RMSE (kg/hm2) | NRMSE (%) | R2 | RMSE (kg/hm2) | NRMSE (%) | ||
| Tuber form | PSRI, Drmin | 0.48 | 279.46 | 21.68 | 0.62 | 197.43 | 19.39 |
| Tuber growth | PBI, Rsum | 0.54 | 277.03 | 19.09 | 0.65 | 187.70 | 17.46 |
| Starch store | LCI, Rsum | 0.40 | 321.64 | 23.12 | 0.55 | 191.33 | 21.07 |
| Maturity | PSRI, SDr | 0.39 | 331.29 | 27.13 | 0.48 | 221.88 | 25.71 |
| Growth Stages | Data | Modeling | Validation | ||||
|---|---|---|---|---|---|---|---|
| R2 | RMSE (kg/hm2) | NRMSE (%) | R2 | RMSE (kg/hm2) | NRMSE (%) | ||
| Tuber form | VIs | 0.65 | 275.74 | 21.39 | 0.71 | 187.02 | 18.37 |
| VIs, Drmin | 0.68 | 244.01 | 18.93 | 0.75 | 169.93 | 16.68 | |
| Tuber growth | VIs | 0.72 | 254.63 | 17.55 | 0.74 | 162.23 | 15.09 |
| VIs, Rsum | 0.74 | 210.55 | 14.51 | 0.78 | 131.91 | 12.27 | |
| Starch store | VIs | 0.62 | 312.03 | 22.43 | 0.69 | 190.38 | 20.96 |
| VIs, Rsum | 0.66 | 268.65 | 19.31 | 0.72 | 171.37 | 18.87 | |
| Maturity | VIs | 0.58 | 315.55 | 25.84 | 0.67 | 205.12 | 23.78 |
| VIs, SDr | 0.62 | 290.98 | 23.83 | 0.7 | 186.49 | 21.62 | |
| Growth Stages | Data | Modeling | Validation | ||||
|---|---|---|---|---|---|---|---|
| R2 | RMSE (kg/hm2) | NRMSE (%) | R2 | RMSE (kg/hm2) | NRMSE (%) | ||
| Tuber form | VIs | 0.63 | 289.4 | 22.45 | 0.69 | 194.35 | 19.09 |
| VIs, Drmin | 0.66 | 263.86 | 20.47 | 0.72 | 175.95 | 17.27 | |
| Tuber growth | VIs | 0.69 | 265.22 | 18.28 | 0.71 | 192.012 | 17.86 |
| VIs, Rsum | 0.71 | 234.92 | 16.19 | 0.75 | 152.122 | 14.15 | |
| Starch store | VIs | 0.59 | 324.27 | 23.31 | 0.65 | 196.105 | 21.59 |
| VIs, Rsum | 0.64 | 285.9 | 20.55 | 0.68 | 180.37 | 19.86 | |
| Maturity | VIs | 0.55 | 327.15 | 26.79 | 0.62 | 216.853 | 25.14 |
| VIs, SDr | 0.61 | 300.87 | 24.64 | 0.64 | 197.363 | 22.88 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Feng, H.; Yue, J.; Fan, Y.; Jin, X.; Song, X.; Yang, H.; Yang, G. Estimation of Potato Above-Ground Biomass Based on Vegetation Indices and Green-Edge Parameters Obtained from UAVs. Remote Sens. 2022, 14, 5323. https://doi.org/10.3390/rs14215323
Liu Y, Feng H, Yue J, Fan Y, Jin X, Song X, Yang H, Yang G. Estimation of Potato Above-Ground Biomass Based on Vegetation Indices and Green-Edge Parameters Obtained from UAVs. Remote Sensing. 2022; 14(21):5323. https://doi.org/10.3390/rs14215323
Chicago/Turabian StyleLiu, Yang, Haikuan Feng, Jibo Yue, Yiguang Fan, Xiuliang Jin, Xiaoyu Song, Hao Yang, and Guijun Yang. 2022. "Estimation of Potato Above-Ground Biomass Based on Vegetation Indices and Green-Edge Parameters Obtained from UAVs" Remote Sensing 14, no. 21: 5323. https://doi.org/10.3390/rs14215323
APA StyleLiu, Y., Feng, H., Yue, J., Fan, Y., Jin, X., Song, X., Yang, H., & Yang, G. (2022). Estimation of Potato Above-Ground Biomass Based on Vegetation Indices and Green-Edge Parameters Obtained from UAVs. Remote Sensing, 14(21), 5323. https://doi.org/10.3390/rs14215323










