Stand Structural Characteristics Derived from Combined TLS and Landsat Data Support Predictions of Mushroom Yields in Mediterranean Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design

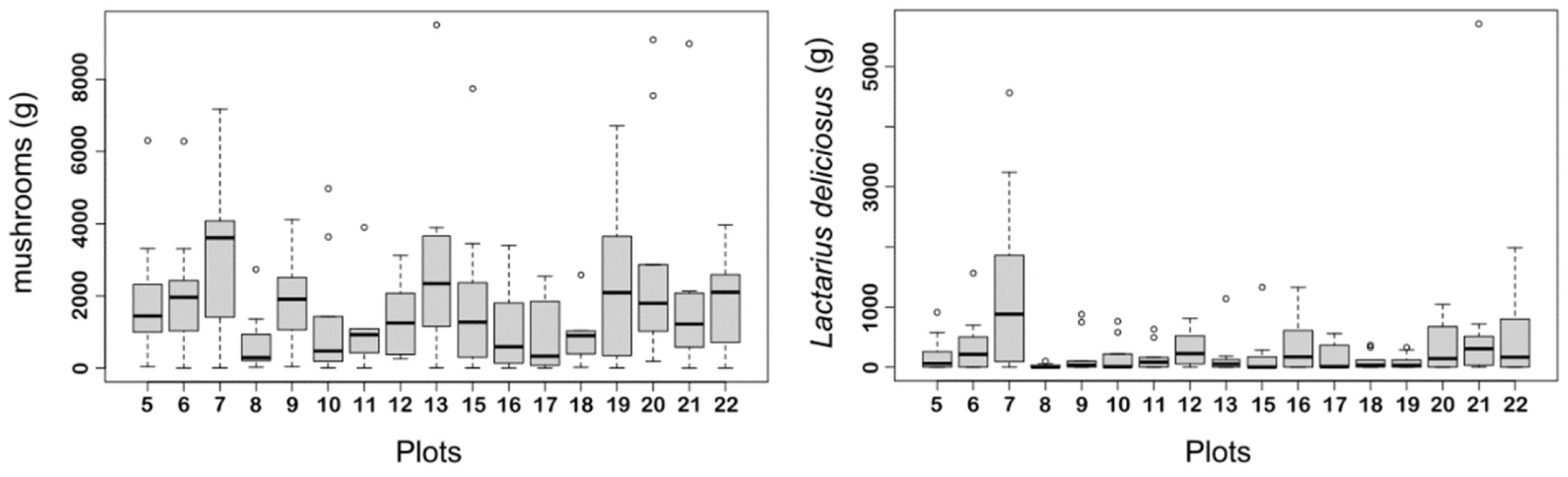
2.2. Mushroom Yield Data
2.3. Climatic Data
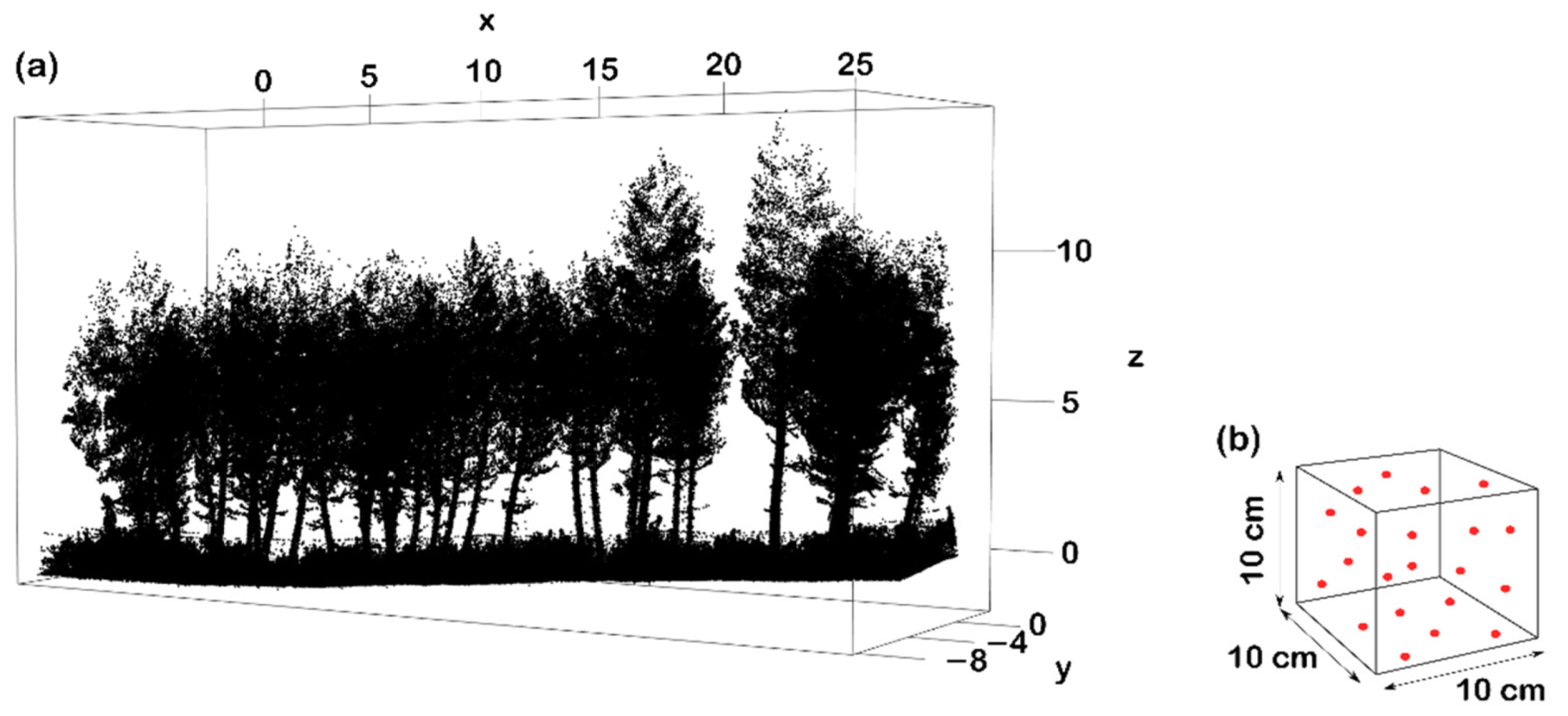
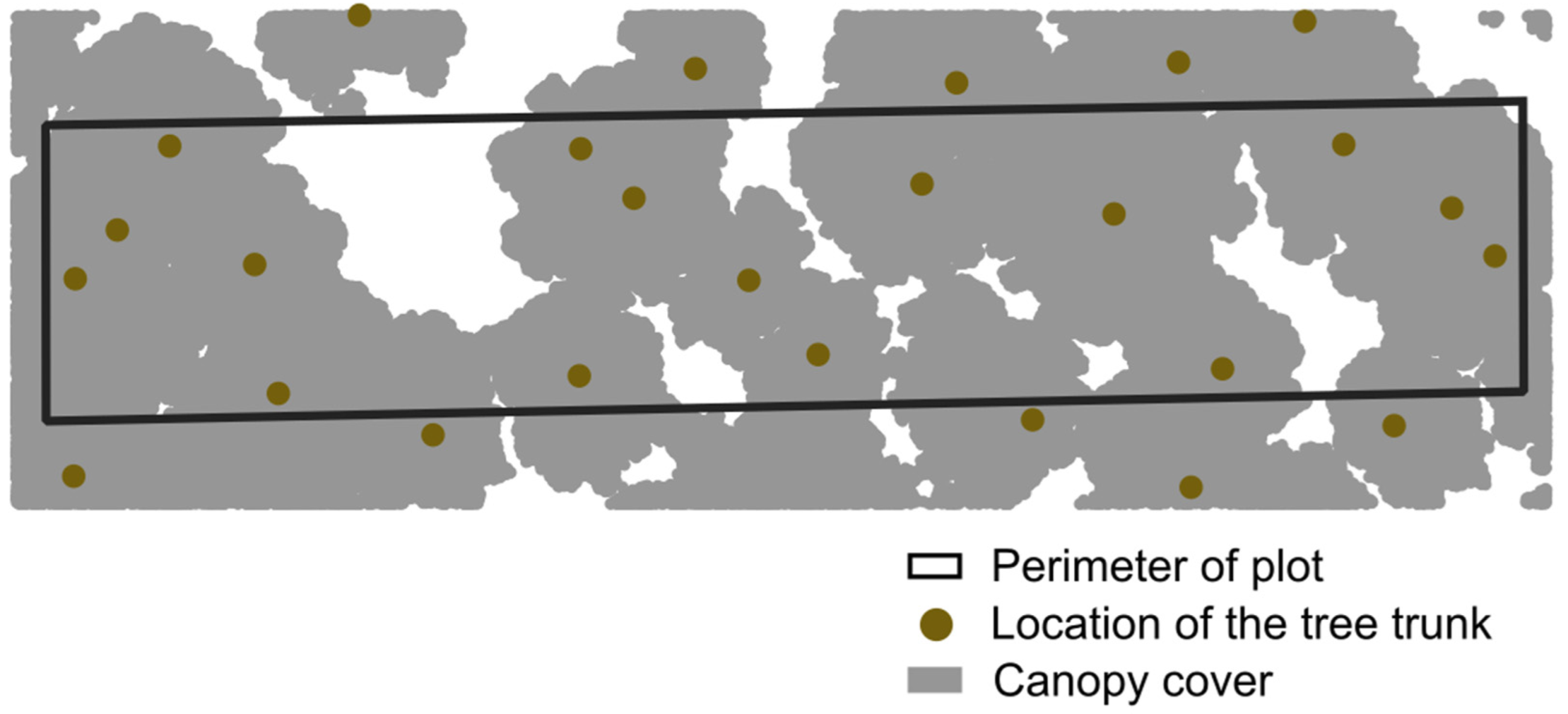
2.4. Forest Structural Measurements
2.5. Landsat Data
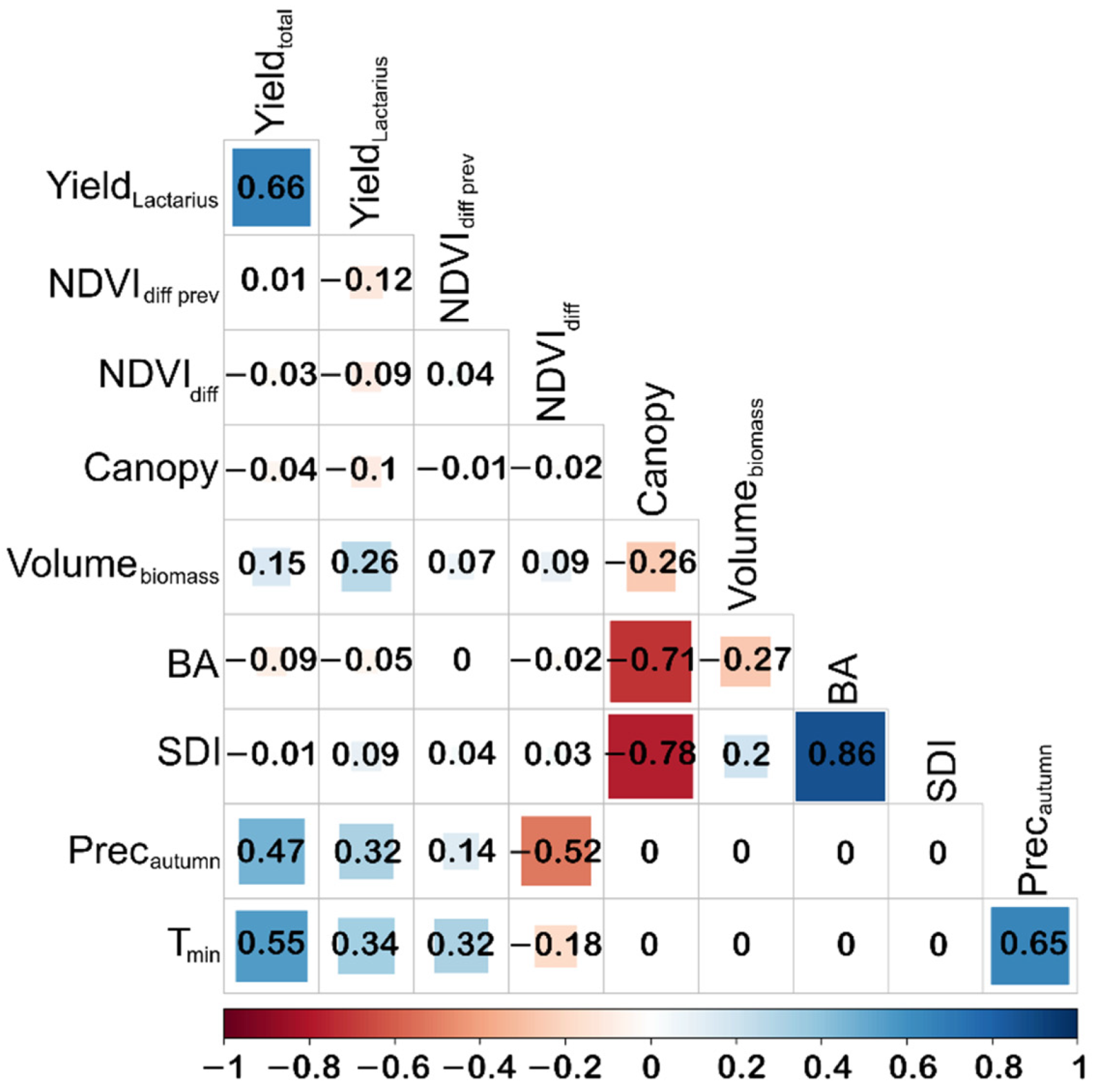
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lechner, A.M.; Foody, G.M.; Boyd, D.S. Applications in Remote Sensing to Forest Ecology and Management. One Earth 2020, 2, 405–412. [Google Scholar] [CrossRef]
- White, J.C.; Coops, N.C.; Wulder, M.A.; Vastaranta, M.; Hilker, T.; Tompalski, P. Remote Sensing Technologies for Enhancing Forest Inventories: A Review. Can. J. Remote Sens. 2016, 42, 619–641. [Google Scholar] [CrossRef]
- Li, J.; Roy, D.P. A global analysis of Sentinel-2a, Sentinel-2b and Landsat-8 data revisit intervals and implications for terrestrial monitoring. Remote Sens. 2017, 9, 902. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-Resolution Global Maps of 21st-century forest cover change. Science 2013, 134, 850–854. [Google Scholar] [CrossRef]
- Kennedy, R.E.; Andréfouët, S.; Cohen, W.B.; Gómez, C.; Griffiths, P.; Hais, M.; Healey, S.P.; Helmer, E.H.; Hostert, P.; Lyons, M.B.; et al. Bringing an ecological view of change to Landsat-based remote sensing. Front. Ecol. Environ. 2014, 12, 339–346. [Google Scholar] [CrossRef]
- Wulder, M.A.; Coops, N.C.; Roy, D.P.; White, J.C.; Hermosilla, T. Land cover 2.0. Int. J. Remote Sens. 2018, 39, 4254–4284. [Google Scholar] [CrossRef]
- Gómez, C.; Alejandro, P.; Hermosilla, T.; Montes, F.; Pascual, C.; Ruiz, L.A.; Álvarez-Taboada, F.; Tanase, M.A.; Valbuena, R. Remote sensing for the Spanish forests in the 21st century: A review of advances, needs, and opportunities. For. Syst. 2019, 28, 1–33. [Google Scholar] [CrossRef]
- Küçüker, D.M.; Baskent, E.Z. Spatial prediction of Lactarius deliciosus and Lactarius salmonicolor mushroom distribution with logistic regression models in the Kızılcasu Planning Unit, Turkey. Mycorrhiza 2014, 25, 1–11. [Google Scholar] [CrossRef]
- Sánchez-González, M.; de-Miguel, S.; Martín-Pinto, P.; Martínez-Peña, F.; Pasalodos-Tato, M.; Oria-de-Rueda, J.A.; de Aragón, J.M.; Cañellas, I.; Bonet, J.A. Yield models for predicting aboveground ectomycorrhizal fungal productivity in Pinus sylvestris and Pinus pinaster stands of northern Spain. For. Ecosyst. 2019, 6, 52. [Google Scholar] [CrossRef]
- Herrero, C.; Berraondo, I.; Bravo, F.; Pando, V.; Ordóñez, C.; Olaizola, J.; Martín-Pinto, P.; de Rueda, J.A.O. Predicting mushroom productivity from long-term field-data series in mediterranean Pinus pinaster ait. forests in the context of climate change. Forests 2019, 10, 206. [Google Scholar] [CrossRef]
- Cockle, K.L.; Martin, K.; Robledo, G. Linking fungi, trees, and hole-using birds in a Neotropical tree-cavity network: Pathways of cavity production and implications for conservation. For. Ecol. Manag. 2012, 264, 210–219. [Google Scholar] [CrossRef]
- Müller, J.; Bütler, R. A review of habitat thresholds for dead wood: A baseline for management recommendations in European forests. Eur. J. For. Res. 2010, 129, 981–992. [Google Scholar] [CrossRef]
- Croitoru, L. Valuing the non-timber forest products in the Mediterranean region. Ecol. Econ. 2007, 63, 768–775. [Google Scholar] [CrossRef]
- Boa, E. Wild Edible Fungi: A Global Overview of Their Use and Importance to People; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2004; Volume 60. [Google Scholar]
- United Nations. The 17 Goals|Sustainable Development; United Nations: New York, NY, USA, 2022; Available online: https://sdgs.un.org/es/goals (accessed on 8 September 2022).
- Alday, J.G.; de Aragón, J.M.; de Miguel, S.; Bonet, J.A. Mushroom biomass and diversity are driven by different spatio-Temporal scales along Mediterranean elevation gradients. Sci. Rep. 2017, 7, 45824. [Google Scholar] [CrossRef]
- Ágreda, T.; Águeda, B.; Olano, J.M.; Sergio, M.; Serrano, V.; Fernández-Toirán, M. Increased evapotranspiration demand in a Mediterranean climate might cause a decline in fungal yields under global warming. Glob. Chang. Biol. 2015, 21, 3499–3510. [Google Scholar] [CrossRef] [PubMed]
- Olano, J.M.; Martínez-Rodrigo, R.; Altelarrea, J.M.; Ágreda, T.; Fernández-Toirán, M.; García-Cervigón, A.I.; Rodríguez-Puerta, F.; Águeda, B. Primary productivity and climate control mushroom yields in Mediterranean pine forests. Agric. For. Meteorol. 2020, 288–289, 108015. [Google Scholar] [CrossRef]
- Morera, A.; de Aragón, J.M.; De Cáceres, M.; Bonet, J.A.; De Miguel, S. Historical and future spatially-explicit climate change impacts on mycorrhizal and saprotrophic macrofungal productivity in Mediterranean pine forests. Agric. For. Meteorol. 2022, 319, 108918. [Google Scholar] [CrossRef]
- Straatsma, G.; Ayer, F.; Egli, S. Species richness, abundance, and phenology of fungal fruit bodies over 21 years in a Swiss forest plot. Mycol. Res. 2001, 105, 515–523. [Google Scholar] [CrossRef]
- Rüehling, Å.; Tyler, G. Soil Factors Influencing the Distribution of Macrofungi in Oak Forests of Southern Sweden. Holarct. Ecol. 1990, 13, 11–18. [Google Scholar] [CrossRef]
- Hagenbo, A.; Alday, J.G.; de Aragón, J.M.; Castaño, C.; De-Miguel, S.; Bonet, J.A. Variations in biomass of fungal guilds are primarily driven by factors related to soil conditions in Mediterranean Pinus pinaster forests. Biol. Fertil. Soils 2022, 58, 487–501. [Google Scholar] [CrossRef]
- Koide, R.T.; Fernandez, C.; Petprakob, K. General principles in the community ecology of ectomycorrhizal fungi. Ann. For. Sci. 2011, 68, 45–55. [Google Scholar] [CrossRef]
- Tomao, A.; Bonet, J.A.; de Aragón, J.M.; de-Miguel, S. Is silviculture able to enhance wild forest mushroom resources? Current knowledge and future perspectives. For. Ecol. Manag. 2017, 402, 102–114. [Google Scholar] [CrossRef]
- Bonet, J.A.; Pukkala, T.; Fischer, C.R.; Palahí, M.; de Aragón, J.M.; Colinas, C. Empirical models for predicting the production of wild mushrooms in Scots pine (Pinus sylvestris L.) forests in the Central Pyrenees. Ann. For. Sci. 2008, 65, 206. [Google Scholar] [CrossRef]
- Bonet, J.A.; Palahí, M.; Colinas, C.; Pukkala, T.; Fischer, C.R.; Miina, J.; de Aragón, J.M. Modelling the production and species richness of wild mushrooms in pine forests of the Central Pyrenees in northeastern Spain. Can. J. For. Res. 2010, 40, 347–356. [Google Scholar] [CrossRef]
- Ágreda, T.; Cisneros, O.; Águeda, B.; Fernández-Toirán, L.M. Age class influence on the yield of edible fungi in a managed Mediterranean forest. Mycorrhiza 2013, 242, 143–152. [Google Scholar] [CrossRef]
- De Miguel, S.; Bonet, J.A.; Pukkala, T.; de Aragón, J.M.M. Impact of forest management intensity on landscape-level mushroom productivity: A regional model-based scenario analysis. For. Ecol. Manag. 2014, 330, 218–227. [Google Scholar] [CrossRef]
- Bonet, J.A.; de Miguel, S.; de Aragón, J.M.; Pukkala, T.; Palahí, M. Immediate effect of thinning on the yield of Lactarius group deliciosus in Pinus pinaster forests in Northeastern Spain. For. Ecol. Manag. 2012, 265, 211–217. [Google Scholar] [CrossRef]
- Küçüker, D.M.; Başkent, E.Z. Sustaining the joint production of timber and Lactarius mushroom: A case study of a forest management planning unit in Northwestern Turkey. Sustainability 2017, 9, 92. [Google Scholar] [CrossRef]
- Lefsky, M.A.; Cohen, W.B.; Parker, G.G.; Harding, D.J. Lidar Remote Sensing for Ecosystem Studies. Sciences 2002, 52, 19–30. [Google Scholar] [CrossRef]
- Beland, M.; Parker, G.; Sparrow, B.; Harding, D.; Chasmer, L.; Phinn, S.; Antonarakis, A.; Strahler, A. On promoting the use of lidar systems in forest ecosystem research. For. Ecol. Manag. 2019, 450, 117484. [Google Scholar] [CrossRef]
- Åkerblom, M.; Kaitaniemi, P. Terrestrial laser scanning: A new standard of forest measuring and modelling? Ann. Bot. 2021, 128, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Thers, H.; Brunbjer, A.K.; Læssøe, T.; Ejrnæs, R.; Bøcher, P.K.; Svenning, J.C. Lidar-derived variables as a proxy for fungal species richness and composition in temperate Northern Europe. Remote Sens. Environ. 2017, 200, 102–113. [Google Scholar] [CrossRef]
- Peura, M.; González, R.S.; Müller, J.; Heurich, M.; Vierling, L.A.; Mönkkönen, M.; Bässler, C. Mapping a ‘cryptic kingdom’: Performance of lidar derived environmental variables in modelling the occurrence of forest fungi. Remote Sens. Environ. 2016, 186, 428–438. [Google Scholar] [CrossRef]
- Oria de Rueda, J.A.; Hernández-Rodríguez, M.; Marín-Pinto, P.; Pando, V.; Olaizola, J. Could artificial reforestations provide as much production and diversity of fungal species as natural forest stands in marginal Mediterranean areas? For. Ecol. Manag. 2010, 260, 171–180. [Google Scholar] [CrossRef]
- Vásquez, P.; de Rueda, J.O.; Martín-Pinto, P. P. Pinaster under extreme ecological conditions provides high fungal production and diversity. For. Ecol. Manag. 2015, 337, 161–173. [Google Scholar] [CrossRef]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological maps for the main European woody species. Data Brief 2017, 12, 662–666. [Google Scholar] [CrossRef]
- Lecigne, B.; Delagrange, S.; Messier, C. Exploring trees in three dimensions: VoxR, a novel voxel-based R package dedicated to analysing the complex arrangement of tree crowns. Ann. Bot. 2018, 121, 589–601. [Google Scholar] [CrossRef]
- Reineke, L.H. Perfecting a stand-density index for even-aged forest. J. Agric. Res. 1933, 46, 627–638. [Google Scholar]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the great plains with ERTS-1. In Proceedings of the Third ERTS Symposium, College Station, TX, USA, 1 January 1974; pp. 309–317. [Google Scholar]
- Huang, S.; Tang, L.; Hupy, J.P.; Wang, Y.; Shao, G. A commentary review on the use of normalized difference vegetation index (NDVI) in the era of popular remote sensing. J. For. Res. 2021, 32, 1–6. [Google Scholar] [CrossRef]
- Mennis, J. Exploring relationships between ENSO and vegetation vigour in the South- east USA using AVHRR data. Remote Sens. 2001, 22, 3077–3092. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Camarero, J.J.; Olano, J.M.; Martín-Hernández, N.; Peña-Gallardo, M.; Tomás-Burguera, M.; Gazol, A.; Azorin-Molina, C.; Bhuyan, U.; El Kenawy, A. Diverse relationships between forest growth and the Normalized Difference Vegetation Index at a global scale. Remote Sens. Environ. 2016, 187, 14–29. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle. In Selected Papers of Hirotugu Akaike; Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer: New York, NY, USA, 1998; pp. 199–213. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.r-project.org (accessed on 8 September 2022).
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman and Hall/CRC: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Biber, P.; Seifert, S.; Zaplata, M.K.; Schaaf, W.; Pretzsch, H.; Fischer, A. Relationships between substrate, surface characteristics, and vegetation in an initial ecosystem. Biogeosciences 2013, 10, 8283–8303. [Google Scholar] [CrossRef]
- Hunsicker, M.E.; Kappel, C.V.; Selkoe, K.A.; Halpern, B.S.; Scarborough, C.; Mease, L.; Amrhein, A. Characterizing driver–response relationships in marine pelagic ecosystems for improved ocean management. Ecol. Appl. 2016, 26, 651–663. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Indicators ODS; United Nations: New York, NY, USA, 2022; Available online: https://unstats.un.org/sdgs/report/2022/Goal-15/ (accessed on 8 September 2022).
- van der Heijden MG, A.; Sanders, I.R. Mycorrhizal Ecology; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Anthony, M.A.; Crowther, T.W.; Van der Linde, S.; Suz, L.M.; Bidartondo, M.L.; Cox, F.; Schaub, M.; Rautio, P.; Ferreti, M.; Vesterdal, L.; et al. Forest tree growth is linked to mycorrhizal fungal composition and function across Europe. ISME J. 2022, 16, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Steidinger, B.S.; Crowther, T.W.; Liang, J.; Van Nuland, M.E.; Werner, G.D.A.; Reich, P.B.; Nabuurs, G.J.; De Miguel, S.; Zhou, M.; Picard, N.; et al. Climatic controls of decomposition drive the global biogeography of forest-tree symbioses. Nature 2019, 569, 404–408. [Google Scholar] [CrossRef]
- Miina, J.; Bonet, J.A.; De Miguel, S.; de Aragón, J.M.; Kurttila, M.; Salo, K.; Tahvanainen, V. Promoting wild mushroom yields by forest management. Tech. Rep. 2016. [Google Scholar] [CrossRef]
- Tucker, C.J.; Sellers, P.J. Satellite remote sensing of primary production. Int. J. Remote Sens. 1986, 7, 1395–1416. [Google Scholar] [CrossRef]
- Song, C.; Dannenberg, M.P.; Hwang, T. Optical remote sensing of terrestrial ecosystem primary productivity. Prog. Phys. Geogr. 2013, 37, 834–854. [Google Scholar] [CrossRef]
- Kuikka, K.; Härmä, E.; Markkola, A.; Rautio, P.; Roitto, M.; Saikkonen, K.; Ahonen-Jonnarth, U.; Finlay, R.; Tuomi, J. Severe defoliation of Scots pine reduces reproductive investment by ectomycorrhizal symbionts. Ecology 2003, 84, 2051–2061. [Google Scholar] [CrossRef]
- Calama, R.; Tomé, M.; Sánchez-González, M.; Miina, J.; Spanos, K.; Palahí, M. Modelling Non-Wood Forest Products in Europe: A review Introduction: Importance. For. Syst. 2010, 19, 69–85. [Google Scholar] [CrossRef]
- Collado, E.; Bonet, J.A.; Alday, J.G.; de Aragón, J.M.; De Miguel, S. Impact of forest thinning on aboveground macrofungal community composition and diversity in Mediterranean pine stands. Ecol. Indic. 2021, 133, 108340. [Google Scholar] [CrossRef]
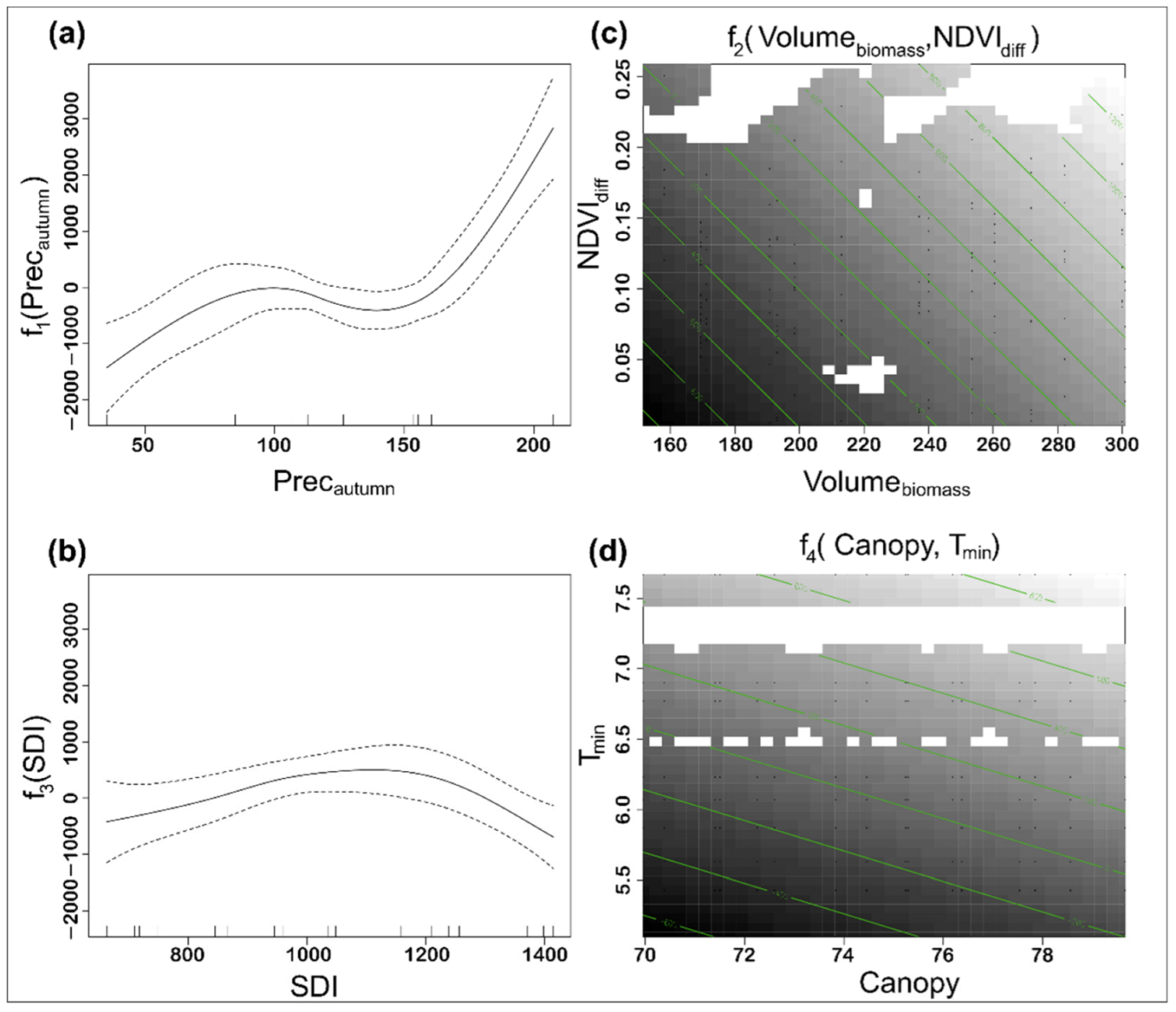

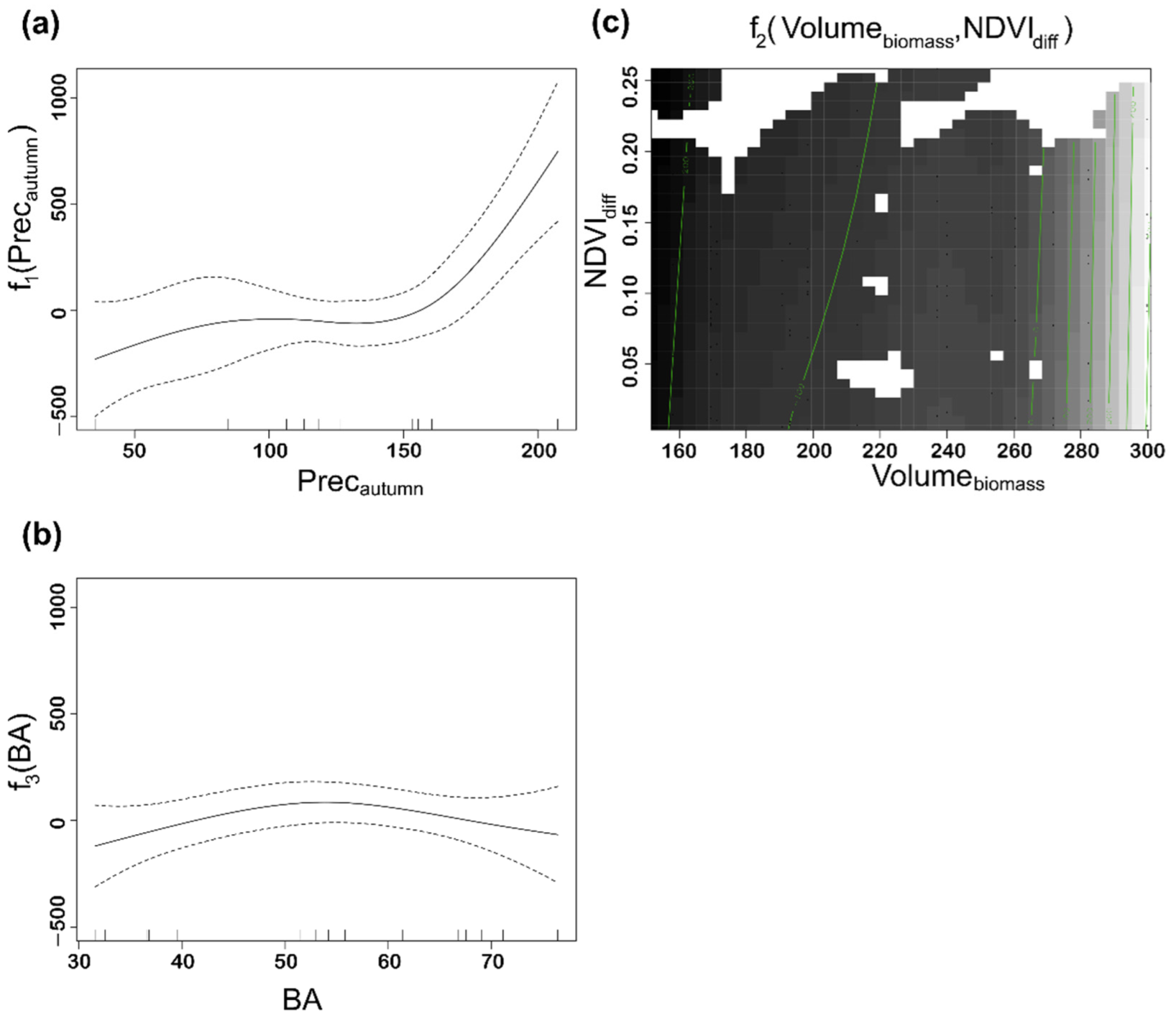
| Variable | Description | Max | Min | Mean | Stedv |
|---|---|---|---|---|---|
| Yieldtotal | Total yield of mushrooms (g) | 9504 | 0 | 1746 | 1910.27 |
| YieldLactarius | Total yield of Lactarius deliciosus (g) | 5706.50 | 0 | 67 | 690.08 |
| NDVIdiff | Difference between winter and summer NDVI (absolute value) | 0.26 | 0.003 | 0.10 | 0.0057 |
| NDVIdiffprev | Difference between winter and summer NDVI of the previous year (absolute value) | 0.26 | 0.002 | 0.10 | 0.0054 |
| Canopy | Canopy cover (%) | 79.66 | 69.96 | 74.37 | 2.94 |
| Volumebiomass | Volume of total aboveground biomass in the plot (m3 ha−1) | 301.00 | 151.50 | 221.60 | 49.16 |
| BA | Basal area of the plot (m2 ha−1) | 76.40 | 31.60 | 54.16 | 14.08 |
| SDI | Stand Density Index | 1414.30 | 662.18 | 1034.8 | 247.79 |
| Precautumn | Accumulated autumn rainfall (mm) | 207.40 | 35.20 | 126.10 | 47.12 |
| Tmin | Average of the autumn months’ minimum temperature (°C) | 7.67 | 5.10 | 6.11 | 0.80 |
| Edf | p-Value | Significance | |
|---|---|---|---|
| f1(Precautumn) | 3.682 | <0.0000 | *** |
| f2(Volumebiomass, NDVIdiff) | 2.000 | 0.0001 | *** |
| f3(SDI) | 2.658 | 0.0112 | * |
| f4(Canopy, Tmin) | 2.000 | 0.0959 | · |
| Edf | p-Value | Significance | |
|---|---|---|---|
| f1(Precautumn) | 3.318 | <0.0000 | *** |
| f2(Volumebiomass, NDVIdiff) | 3.741 | 0.0002 | *** |
| f3(BA) | 2.035 | 0.0694 | · |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Rodrigo, R.; Gómez, C.; Toraño-Caicoya, A.; Bohnhorst, L.; Uhl, E.; Águeda, B. Stand Structural Characteristics Derived from Combined TLS and Landsat Data Support Predictions of Mushroom Yields in Mediterranean Forest. Remote Sens. 2022, 14, 5025. https://doi.org/10.3390/rs14195025
Martínez-Rodrigo R, Gómez C, Toraño-Caicoya A, Bohnhorst L, Uhl E, Águeda B. Stand Structural Characteristics Derived from Combined TLS and Landsat Data Support Predictions of Mushroom Yields in Mediterranean Forest. Remote Sensing. 2022; 14(19):5025. https://doi.org/10.3390/rs14195025
Chicago/Turabian StyleMartínez-Rodrigo, Raquel, Cristina Gómez, Astor Toraño-Caicoya, Luke Bohnhorst, Enno Uhl, and Beatriz Águeda. 2022. "Stand Structural Characteristics Derived from Combined TLS and Landsat Data Support Predictions of Mushroom Yields in Mediterranean Forest" Remote Sensing 14, no. 19: 5025. https://doi.org/10.3390/rs14195025
APA StyleMartínez-Rodrigo, R., Gómez, C., Toraño-Caicoya, A., Bohnhorst, L., Uhl, E., & Águeda, B. (2022). Stand Structural Characteristics Derived from Combined TLS and Landsat Data Support Predictions of Mushroom Yields in Mediterranean Forest. Remote Sensing, 14(19), 5025. https://doi.org/10.3390/rs14195025







