Remotely Piloted Aircraft and Random Forest in the Evaluation of the Spatial Variability of Foliar Nitrogen in Coffee Crop
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
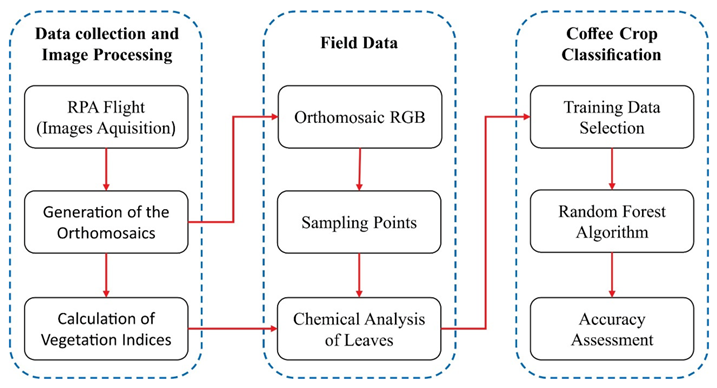
2.2. RPA Image Acquisition and Preprocessing
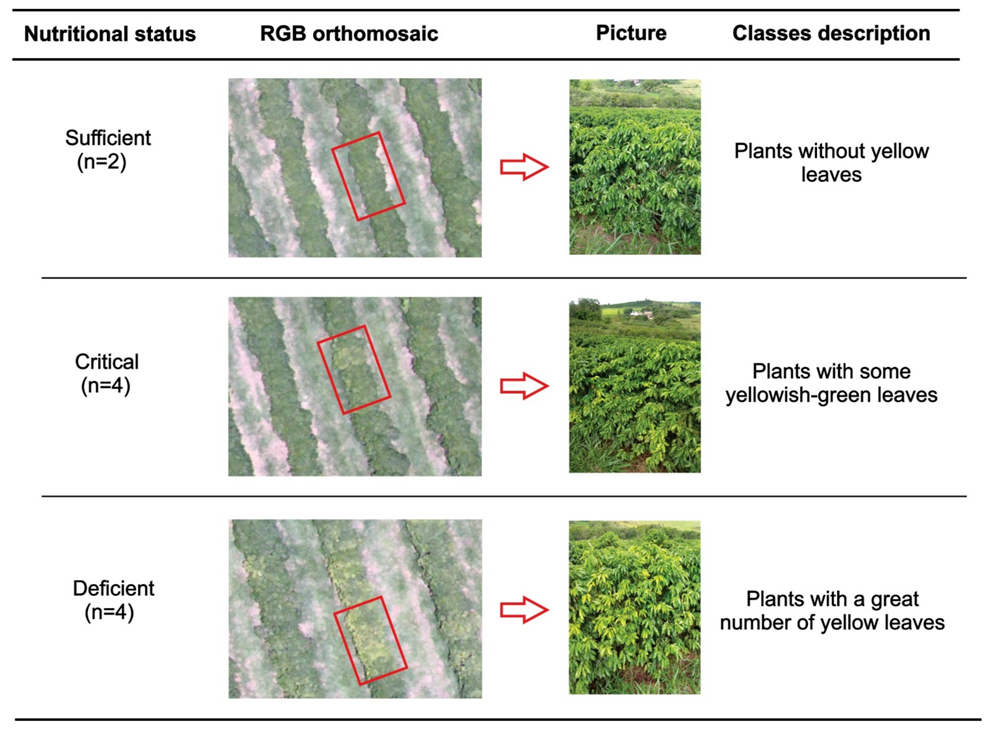
2.3. Determining Leaf Nitrogen
2.4. Vegetation Indices
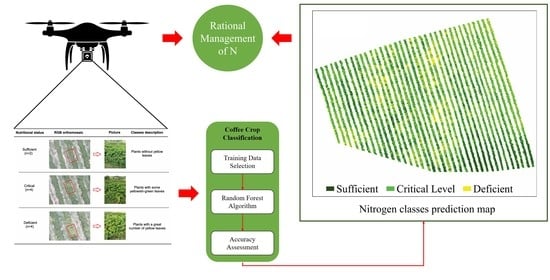
2.5. Random Forest (RF) Classification
2.6. Accuracy Assessment
3. Results and Discussion
3.1. Nitrogen Content in Coffee Leaves
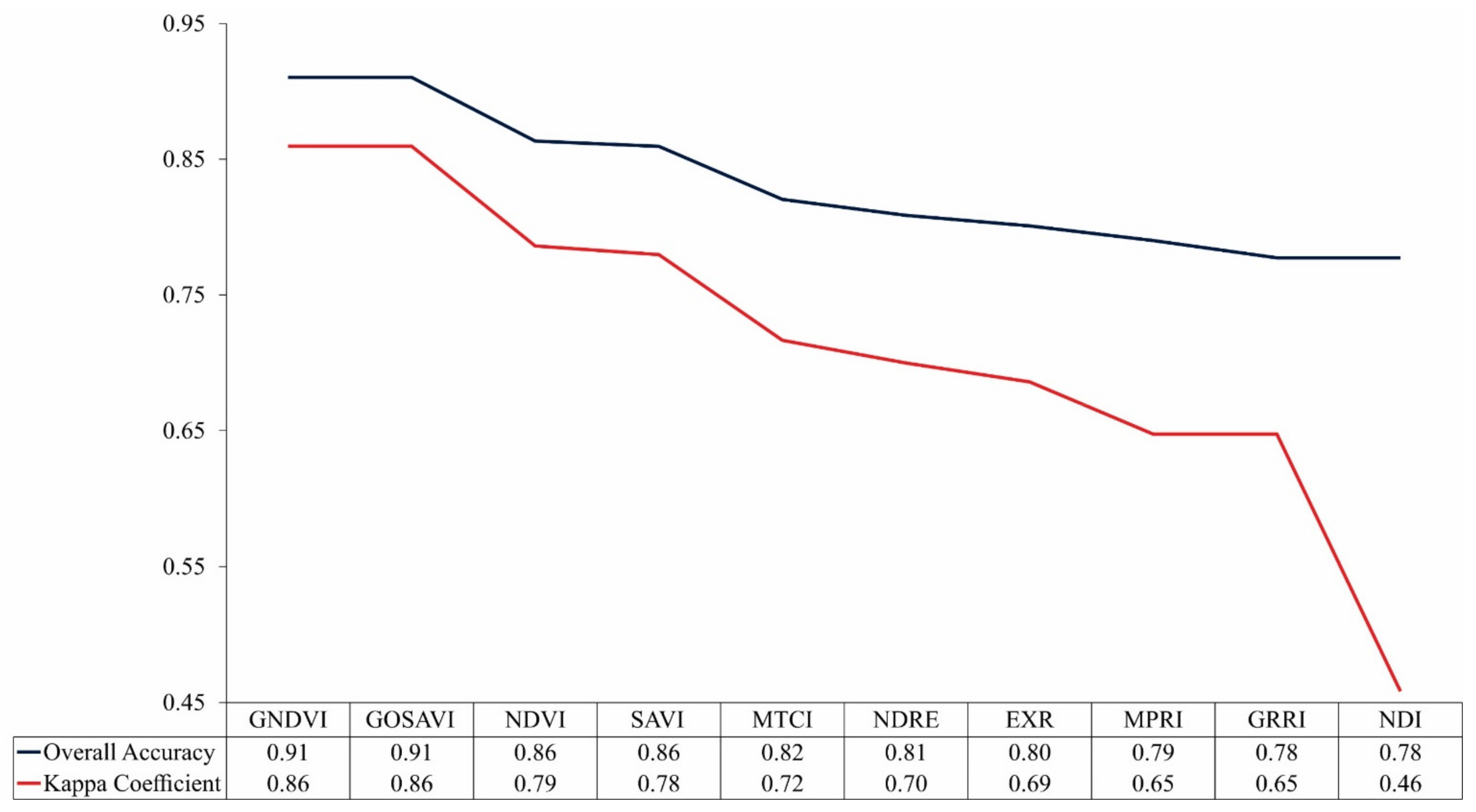
3.2. Overall Accuracy and Kappa Performance
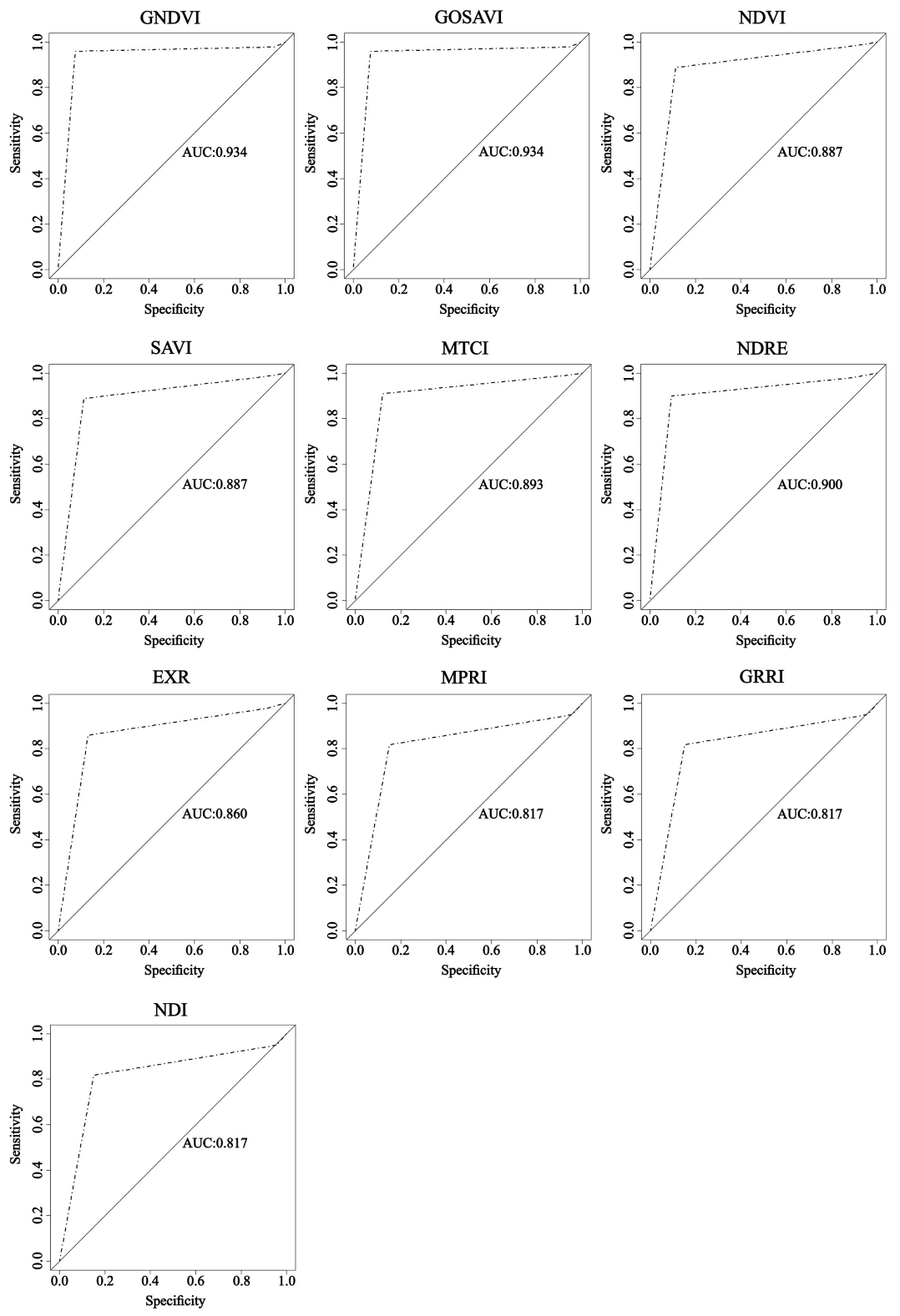
3.3. ROC Curve and AUC
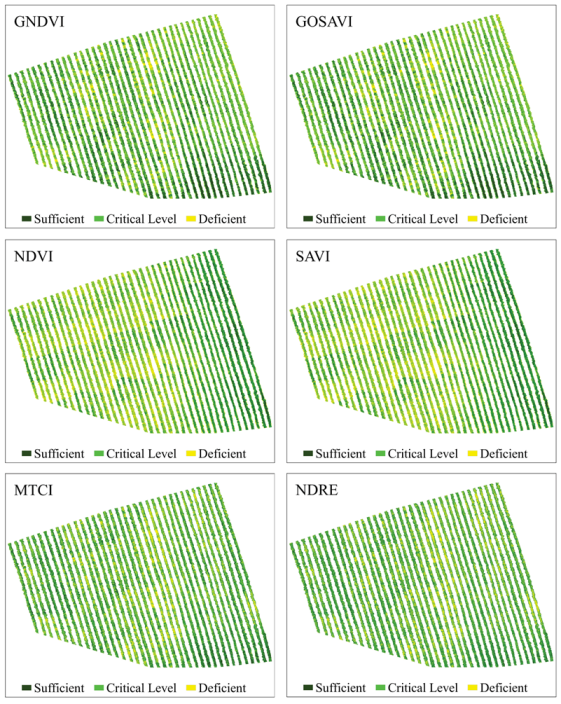
3.4. Mapping and Quantifying N Spatial Distribution in Coffee Leaves
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chemura, A. Modelling Spatial Variability of Coffee (Coffea Arabica L.) Crop Condition with Multispectral Remote Sensing Data. Ph.D. Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2017. [Google Scholar]
- Bote, A.D.; Ayalew, B.; Ocho, F.L.; Anten, N.P.R.; Vos, J. Analysis of coffee (Coffea arabica L.) performance in relation to radiation levels and rates of nitrogen supply I. Vegetative growth, production and distribution of biomass and radiation use efficiency. Eur. J. Agron. 2018, 92, 115–122. [Google Scholar] [CrossRef]
- Nazareno, R.B.; Da Silva Oliveira, C.A.; Sanzonowicz, C.; Ramos Sampaio, J.B.; Pereira Da Silva, J.C.; Guerra, A.F. Crescimento inicial do cafeeiro Rubi em resposta a doses de nitrogênio, fôsforo e potássio e a regimes hídricos. Pesqui. Agropecu. Bras. 2003, 38, 903–910. [Google Scholar] [CrossRef]
- Coste, R. Coffee—The Plant and The Product; MacMillan Press: London, UK, 1992. [Google Scholar]
- Chemura, A.; Mutanga, O.; Odindi, J.; Kutywayo, D. Mapping spatial variability of foliar nitrogen in coffee (Coffea arabica L.) plantations with multispectral Sentinel-2 MSI data. ISPRS J. Photogramm. Remote Sens. 2018, 138, 1–11. [Google Scholar] [CrossRef]
- Putra, B.T.W.; Soni, P.; Morimoto, E.; Pujiyanto, P. Estimating biophysical properties of coffee (Coffea canephora) plants with above-canopy field measurements, using CropSpec. Int. Agrophys. 2018, 32, 183–191. [Google Scholar] [CrossRef]
- Putra, W.B.T.; Soni, P. Enhanced broadband greenness in assessing Chlorophyll a and b, Carotenoid, and Nitrogen in Robusta coffee plantations using a digital camera. Precis. Agric. 2018, 19, 238–256. [Google Scholar] [CrossRef]
- Putra, B.T.W.; Soni, P. Improving nitrogen assessment with an RGB camera across uncertain natural light from above-canopy measurements. Precis. Agric. 2020, 21, 147–159. [Google Scholar] [CrossRef]
- Lima, L.M.; Pozza, E.A.; Torres, H.N.; Pozza, A.A.A.; Salgado, M.; Pfenning, L.H. Relação nitrogênio/potássio com mancha de Phoma e nutrição de mudas de cafeeiro em solução nutritiva. Trop. Plant Pathol. 2010, 35, 223–228. [Google Scholar] [CrossRef]
- Pérez, C.D.P.; Pozza, E.A.; Pozza, A.A.A.; Freitas, A.S.; Da Silva, M.G. Nitrogênio e potássio na intensidade da mancha aureolada do cafeeiro em solução nutritiva. Coffee Sci. 2017, 12, 60–68. [Google Scholar] [CrossRef]
- Martinez, H.E.P.; de Souza, B.P.; Caixeta, E.T.; de Carvalho, F.P.; Clemente, J.M. Water deficit changes nitrate uptake and expression of some nitrogen related genes in coffee-plants (Coffea arabica L.). Sci. Hortic. 2020, 267, 109254. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Loos, R.A.; Silva, E.A.; Loureiro, M.E. Limitations to photosynthesis in Coffea canephora as a result of nitrogen and water availability. J. Plant Physiol. 2002, 159, 975–981. [Google Scholar] [CrossRef]
- Feng, D.; Xu, W.; He, Z.; Zhao, W.; Yang, M. Advances in plant nutrition diagnosis based on remote sensing and computer application. Neural Comput. Appl. 2019, 1–10. [Google Scholar] [CrossRef]
- Ye, X.; Abe, S.; Zhang, S. Estimation and mapping of nitrogen content in apple trees at leaf and canopy levels using hyperspectral imaging. Precis. Agric. 2020, 21, 198–225. [Google Scholar] [CrossRef]
- Mahajan, G.R.; Pandey, R.N.; Sahoo, R.N.; Gupta, V.K.; Datta, S.C.; Kumar, D. Monitoring nitrogen, phosphorus and sulphur in hybrid rice (Oryza sativa L.) using hyperspectral remote sensing. Precis. Agric. 2017, 18, 736–761. [Google Scholar] [CrossRef]
- Fitzgerald, G.; Rodriguez, D.; O’Leary, G. Measuring and predicting canopy nitrogen nutrition in wheat using a spectral index-The canopy chlorophyll content index (CCCI). Field Crop. Res. 2010, 116, 318–324. [Google Scholar] [CrossRef]
- Santos, L.M.; Ferraz, G.A.E.S.; Barbosa, B.D.S.; Andrade, A.D. Use of remotely piloted aircraft in precision agriculture: A review. DYNA 2019, 86, 284–291. [Google Scholar] [CrossRef]
- Yang, G.; Liu, J.; Zhao, C.; Li, Z.; Huang, Y.; Yu, H.; Xu, B.; Yang, X.; Zhu, D.; Zhang, X.; et al. Unmanned aerial vehicle remote sensing for field-based crop phenotyping: Current status and perspectives. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Buchaillot, M.L.; Gracia-Romero, A.; Vergara-Diaz, O.; Zaman-Allah, M.A.; Tarekegne, A.; Cairns, J.E.; Prasanna, B.M.; Araus, J.L.; Kefauver, S.C. Evaluating maize genotype performance under low nitrogen conditions using RGB UAV phenotyping techniques. Sensors 2019, 19, 1815. [Google Scholar] [CrossRef]
- Wang, H.; Mortensen, A.K.; Mao, P.; Boelt, B.; Gislum, R. Estimating the nitrogen nutrition index in grass seed crops using a UAV-mounted multispectral camera. Int. J. Remote Sens. 2019, 40, 2467–2482. [Google Scholar] [CrossRef]
- Hunt, E.R.; Horneck, D.A.; Spinelli, C.B.; Turner, R.W.; Bruce, A.E.; Gadler, D.J.; Brungardt, J.J.; Hamm, P.B. Monitoring nitrogen status of potatoes using small unmanned aerial vehicles. Precis. Agric. 2018, 19, 314–333. [Google Scholar] [CrossRef]
- Zheng, H.; Cheng, T.; Li, D.; Zhou, X.; Yao, X.; Tian, Y.; Cao, W.; Zhu, Y. Evaluation of RGB, color-infrared and multispectral images acquired from unmanned aerial systems for the estimation of nitrogen accumulation in rice. Remote Sens. 2018, 10, 824. [Google Scholar] [CrossRef]
- Osco, L.P.; Paula, A.; Ramos, M.; Pereira, D.R.; Akemi, É.; Moriya, S.; Imai, N.N.; Matsubara, E.T. Predicting Canopy Nitrogen Content in Citrus-Trees Using Random Forest Algorithm Associated to Spectral Vegetation Indices from UAV-Imagery. Remote Sens. 2019, 11, 2925. [Google Scholar] [CrossRef]
- Chlingaryan, A.; Sukkarieh, S.; Whelan, B. Machine learning approaches for crop yield prediction and nitrogen status estimation in precision agriculture: A review. Comput. Electron. Agric. 2018, 151, 61–69. [Google Scholar] [CrossRef]
- Liakos, K.G.; Busato, P.; Moshou, D.; Pearson, S.; Bochtis, D. Machine learning in agriculture: A review. Sensors 2018, 18, 2674. [Google Scholar] [CrossRef]
- Ali, I.; Greifeneder, F.; Stamenkovic, J.; Neumann, M.; Notarnicola, C. Review of machine learning approaches for biomass and soil moisture retrievals from remote sensing data. Remote Sens. 2015, 7, 16398–16421. [Google Scholar] [CrossRef]
- Qiu, J.; Wu, Q.; Ding, G.; Xu, Y.; Feng, S. A survey of machine learning for big data processing. EURASIP J. Adv. Signal Process. 2016, 67, 1–16. [Google Scholar] [CrossRef]
- Osco, L.P.; Junior, J.M.; Ramos, A.P.M.; Furuya, D.E.G.; Santana, D.C.; Teodoro, L.P.R.; Gonçalves, W.N.; Baio, F.H.R.; Pistori, H.; Junior, C.A.D.S.; et al. Leaf nitrogen concentration and plant height prediction for maize using UAV-based multispectral imagery and machine learning techniques. Remote Sens. 2020, 12, 3237. [Google Scholar] [CrossRef]
- Zha, H.; Miao, Y.; Wang, T.; Li, Y.; Zhang, J.; Sun, W.; Feng, Z.; Kusnierek, K. Improving Unmanned Aerial Vehicle Remote Sensing-Based Rice Nitrogen Nutrition Index Prediction with Machine Learning. Remote Sens. 2020, 12, 215. [Google Scholar] [CrossRef]
- Zheng, H.; Li, W.; Jiang, J.; Liu, Y.; Cheng, T.; Tian, Y.; Zhu, Y.; Cao, W.; Zhang, Y.; Yao, X. A comparative assessment of different modeling algorithms for estimating leaf nitrogen content in winter wheat using multispectral images from an unmanned aerial vehicle. Remote Sens. 2018, 10, 2026. [Google Scholar] [CrossRef]
- Parreiras, T.C.; Lense, G.H.E.; Moreira, R.S.; Santana, D.B.; Mincato, R.L. Using unmanned aerial vehicle and machine learning algorithm to monitor leaf nitrogen in coffee. Coffee Sci. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- De Freitas, A.F.; Nadaleti, D.H.S.; Silveira, H.R.D.O.; Carvalho, G.R.; Venturin, R.P.; Silva, V.A. Productivity and beverage sensory quality of arabica coffee intercropped with timber species. Pesqui. Agropecuária Bras. 2020, 55. [Google Scholar] [CrossRef]
- National Aeronautics and Space Administration—NASA. Power Data. Available online: https://power.larc.nasa.gov/data-access-viewer/ (accessed on 29 March 2021).
- Martinez, H.E.P.; Neves, J.C.L.; Shuler, J. Mineral Nutrition and Fertilization. In Coffee: Production, Quality and Chemistry; Farah, A., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 163–201. [Google Scholar]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Deering, D.W.; Schell, J.A.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; Type III Final Report; NASA/GSFC: Greenbelt, MD, USA, 1974.
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P.J. The MERIS terrestrial chlorophyll index. Int. J. Remote Sens. 2004, 25, 5403–5413. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Quantitative estimation of chlorophyll-a using reflectance spectra: Experiments with autumn chestnut and maple leaves. J. Photochem. Photobiol. B Biol. 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Meyer, G.E.; Hindman, T.W.; Lakshmi, K. Machine Vision Detection Parameters for Plant Species Identification. In Proceedings of the Precision Agriculture and Biological Quality, Bellingham, DC, USA, 14 January 1998; pp. 327–335. [Google Scholar] [CrossRef]
- Yang, Z.; Willis, P.; Mueller, R. Impact of band-ratio enhanced AWIFS image to crop classification accuracy. In Proceedings of the 17th William Pecora Memorial Remote Sensing Symposium, Denver, CO, USA, 18–20 November 2008. [Google Scholar]
- Gamon, J.A.; Surfus, J.S. Assessing leaf pigment content and activity with reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef]
- Mao, W.; Wang, Y.; Wang, Y. Real-time detection of between-row weeds using machine vision. In Proceedings of the ASAE Annual Meeting, Las Vegas, NV, USA, 27–30 July 2003; pp. 1–9. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Vienna, Austria. 2013. Available online: http://www.R-project.org/ (accessed on 10 July 2020).
- Liaw, A.; Wiener, M.; Breiman, L.; Cutler, A. Package ‘‘randomForest”. 2018. Available online: https://cran.r-project.org/web/packages/randomForest/randomForest.pdf (accessed on 10 July 2020).
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Son, N.T.; Chen, C.F.; Chen, C.R.; Minh, V.Q. Assessment of Sentinel-1A data for rice crop classification using random forests and support vector machines. Geocarto Int. 2018, 33, 587–601. [Google Scholar] [CrossRef]
- Oliveira, S.; Oehler, F.; San-Miguel-Ayanz, J.; Camia, A.; Pereira, J.M.C. Modeling spatial patterns of fire occurrence in Mediterranean Europe using Multiple Regression and Random Forest. For. Ecol. Manag. 2012, 275, 117–129. [Google Scholar] [CrossRef]
- Rodriguez-Galiano, V.F.; Chica-Olmo, M.; Abarca-Hernandez, F.; Atkinson, P.M.; Jeganathan, C. Random Forest classification of Mediterranean land cover using multi-seasonal imagery and multi-seasonal texture. Remote Sens. Environ. 2012, 121, 93–107. [Google Scholar] [CrossRef]
- Kohavi, R. A Study of Cross-Validation and Bootstrap for Accuracy Estimation and Model Selection. Int. Jt. Conf. Artif. Intell. 1995, 2, 1137–1143. [Google Scholar]
- Foody, G.M. Status of land cover classification accuracy assessment. Remote Sens. Environ. 2002, 80, 185–201. [Google Scholar] [CrossRef]
- Hand, D.J.; Till, R.J. A Simple Generalisation of the Area Under the ROC Curve for Multiple Class Classification Problems. Mach. Learn. 2001, 45, 171–186. [Google Scholar] [CrossRef]
- Hunt, E.R.; Doraiswamy, P.C.; McMurtrey, J.E.; Daughtry, C.S.T.; Perry, E.M.; Akhmedov, B. A visible band index for remote sensing leaf chlorophyll content at the Canopy scale. Int. J. Appl. Earth Obs. Geoinf. 2013, 21, 103–112. [Google Scholar] [CrossRef]
- Hunt, E.R.; Daughtry, C.S.T.; Eitel, J.U.H.; Long, D.S. Remote sensing leaf chlorophyll content using a visible band index. Agron. J. 2011, 103, 1090–1099. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32, 1–4. [Google Scholar] [CrossRef]
- Nigon, T.J.; Mulla, D.J.; Rosen, C.J.; Cohen, Y.; Alchanatis, V.; Rud, R. Evaluation of the nitrogen sufficiency index for use with high resolution, broadband aerial imagery in a commercial potato field. Precis. Agric. 2014, 15, 202–226. [Google Scholar] [CrossRef]
- Bendig, J.; Yu, K.; Aasen, H.; Bolten, A.; Bennertz, S.; Broscheit, J.; Gnyp, M.L.; Bareth, G. Combining UAV-based plant height from crop surface models, visible, and near infrared vegetation indices for biomass monitoring in barley. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 79–87. [Google Scholar] [CrossRef]
- Fernández, P.D.M.; Peña, F.A.G.; Ren, T.I.; Leandro, J.J.G. Fast and robust multiple ColorChecker detection using deep convolutional neural networks. Image Vis. Comput. 2018, 81, 15–24. [Google Scholar] [CrossRef]
- Prati, R.C.; Batista, G.E.D.A.P.A.; Monard, M.C. Evaluating Classifiers Using ROC Curves. IEEE Lat. Am. Trans. 2008, 6, 215–222. [Google Scholar] [CrossRef]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Park, S.H.; Goo, J.M.; Jo, C.H. Receiver operating characteristic (ROC) curve: Practical review for radiologists. Korean J. Radiol. 2004, 5, 11–18. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Li, Y.C. The role of nutrient efficient plants in improving crop yields in the twenty first century. J. Plant Nutr. 2008, 31, 1121–1157. [Google Scholar] [CrossRef]


| Temp (°C) | RH (%) | Pressure (kPa) | WS (m.s−1) | Max Temp (°C) | Min Temp (°C) | RFE (mm) |
|---|---|---|---|---|---|---|
| 19.73 | 73.31 | 91.6 | 1.51 | 25.6 | 13.28 | 0 |
| Vegetation Indices | Equation | Reference |
|---|---|---|
| GNDVI (Green Normalized Difference Vegetation Index) | [35] | |
| GOSAVI (Green Optimal Soil Adjusted Vegetation Index) | [36] | |
| NDVI (Normalized Difference Vegetation Index) | [37] | |
| SAVI (Soil Adjusted Difference Vegetation Index) | [38] | |
| MTCI (MERIS Terrestrial Chlorophyll Index) | [39] | |
| NDRE (Normalized Difference Red Edge) | [40] | |
| EXR (Excessive Red) | [41] | |
| MPRI (Modified Photochemical Reflectance Index) | [42] | |
| GRRI (Green–Red Ratio Index) | [43] | |
| NDI (Normalized Different Index) | [44] |
| Nitrogen Levels | % | |||
|---|---|---|---|---|
| Min | Max | Mean | SD | |
| Sufficient | 3.00 | 3.11 | 3.05 | 0.08 |
| Critical | 2.51 | 2.85 | 2.69 | 0.18 |
| Deficient | 2.13 | 2.44 | 2.31 | 0.13 |
| Vegetation Indices | Area (%) | |||
|---|---|---|---|---|
| Sufficient | Critical | Deficient | Total | |
| GNDVI | 26 | 52 | 22 | 100 |
| GOSAVI | 26 | 52 | 22 | 100 |
| NDVI | 23 | 46 | 31 | 100 |
| SAVI | 23 | 46 | 31 | 100 |
| MTCI | 31 | 47 | 22 | 100 |
| NDRE | 31 | 46 | 22 | 100 |
| EXR | 25 | 45 | 31 | 100 |
| MPRI | 21 | 42 | 37 | 100 |
| GRRI | 21 | 41 | 38 | 100 |
| NDI | 21 | 41 | 38 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, D.B.; Ferraz, G.A.e.S.; Guimarães, P.H.S.; Schwerz, F.; Santana, L.S.; Barbosa, B.D.S.; Barata, R.A.P.; Faria, R.d.O.; Dias, J.E.L.; Conti, L.; et al. Remotely Piloted Aircraft and Random Forest in the Evaluation of the Spatial Variability of Foliar Nitrogen in Coffee Crop. Remote Sens. 2021, 13, 1471. https://doi.org/10.3390/rs13081471
Marin DB, Ferraz GAeS, Guimarães PHS, Schwerz F, Santana LS, Barbosa BDS, Barata RAP, Faria RdO, Dias JEL, Conti L, et al. Remotely Piloted Aircraft and Random Forest in the Evaluation of the Spatial Variability of Foliar Nitrogen in Coffee Crop. Remote Sensing. 2021; 13(8):1471. https://doi.org/10.3390/rs13081471
Chicago/Turabian StyleMarin, Diego Bedin, Gabriel Araújo e Silva Ferraz, Paulo Henrique Sales Guimarães, Felipe Schwerz, Lucas Santos Santana, Brenon Dienevam Souza Barbosa, Rafael Alexandre Pena Barata, Rafael de Oliveira Faria, Jessica Ellen Lima Dias, Leonardo Conti, and et al. 2021. "Remotely Piloted Aircraft and Random Forest in the Evaluation of the Spatial Variability of Foliar Nitrogen in Coffee Crop" Remote Sensing 13, no. 8: 1471. https://doi.org/10.3390/rs13081471
APA StyleMarin, D. B., Ferraz, G. A. e. S., Guimarães, P. H. S., Schwerz, F., Santana, L. S., Barbosa, B. D. S., Barata, R. A. P., Faria, R. d. O., Dias, J. E. L., Conti, L., & Rossi, G. (2021). Remotely Piloted Aircraft and Random Forest in the Evaluation of the Spatial Variability of Foliar Nitrogen in Coffee Crop. Remote Sensing, 13(8), 1471. https://doi.org/10.3390/rs13081471