Monitoring the Vertical Distribution of Maize Canopy Chlorophyll Content Based on Multi-Angular Spectral Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
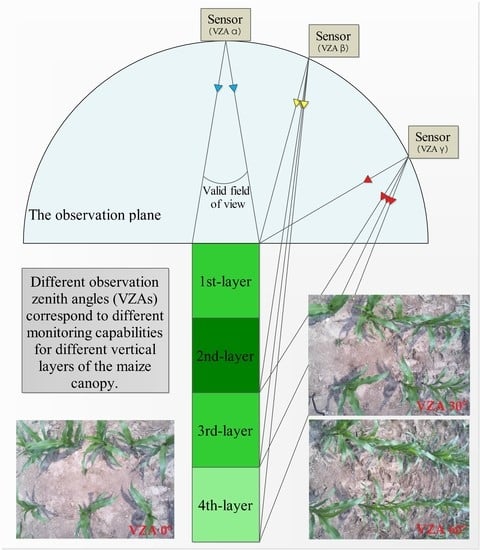
2.2. Multi-Angular Spectral Reflectance Measurements
2.3. Leaf Stratification and SPAD Measurements
2.4. Vegetation Indices and Data Analysis
3. Results
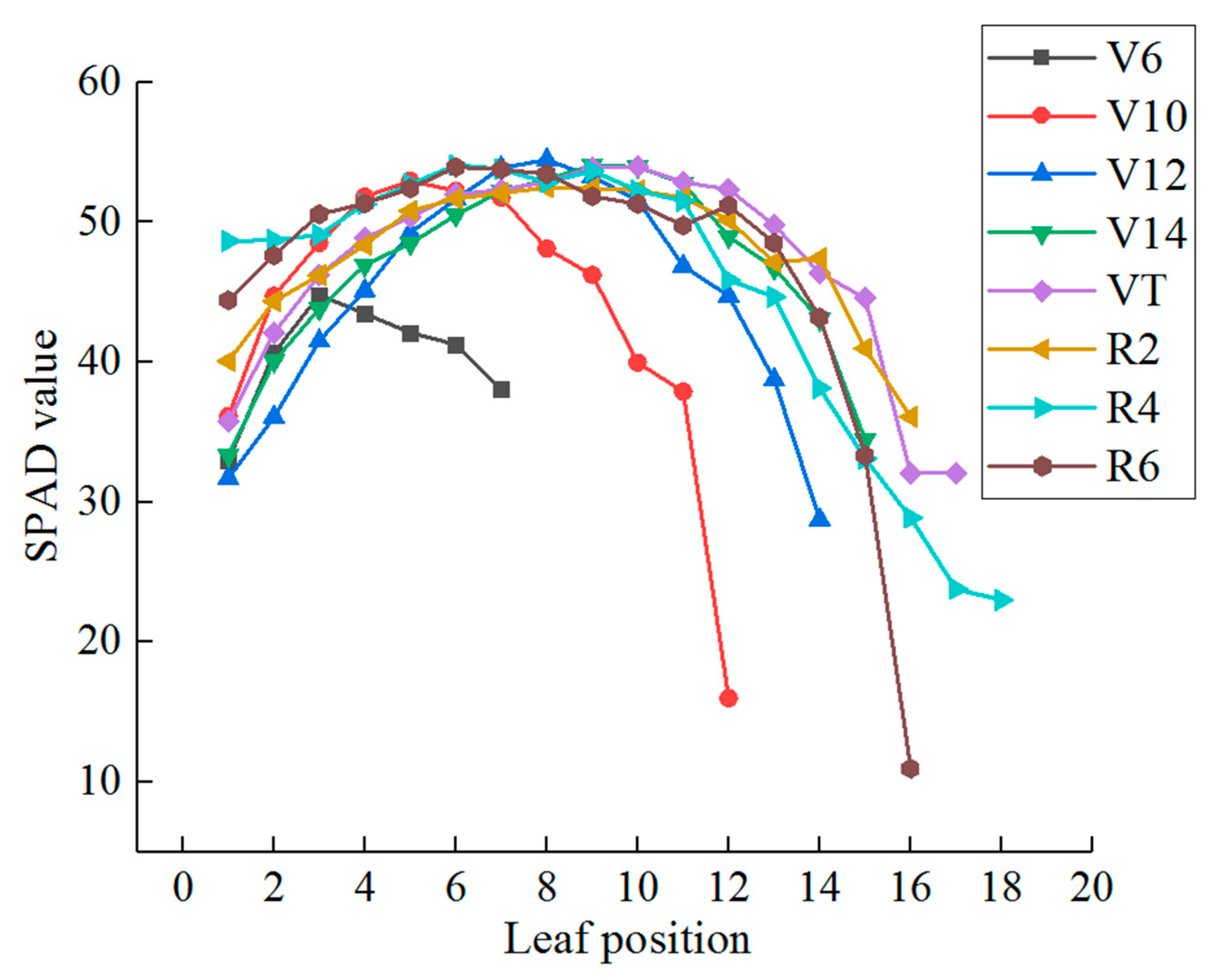
3.1. Temporal and Spatial Distribution of Chlorophyll Content
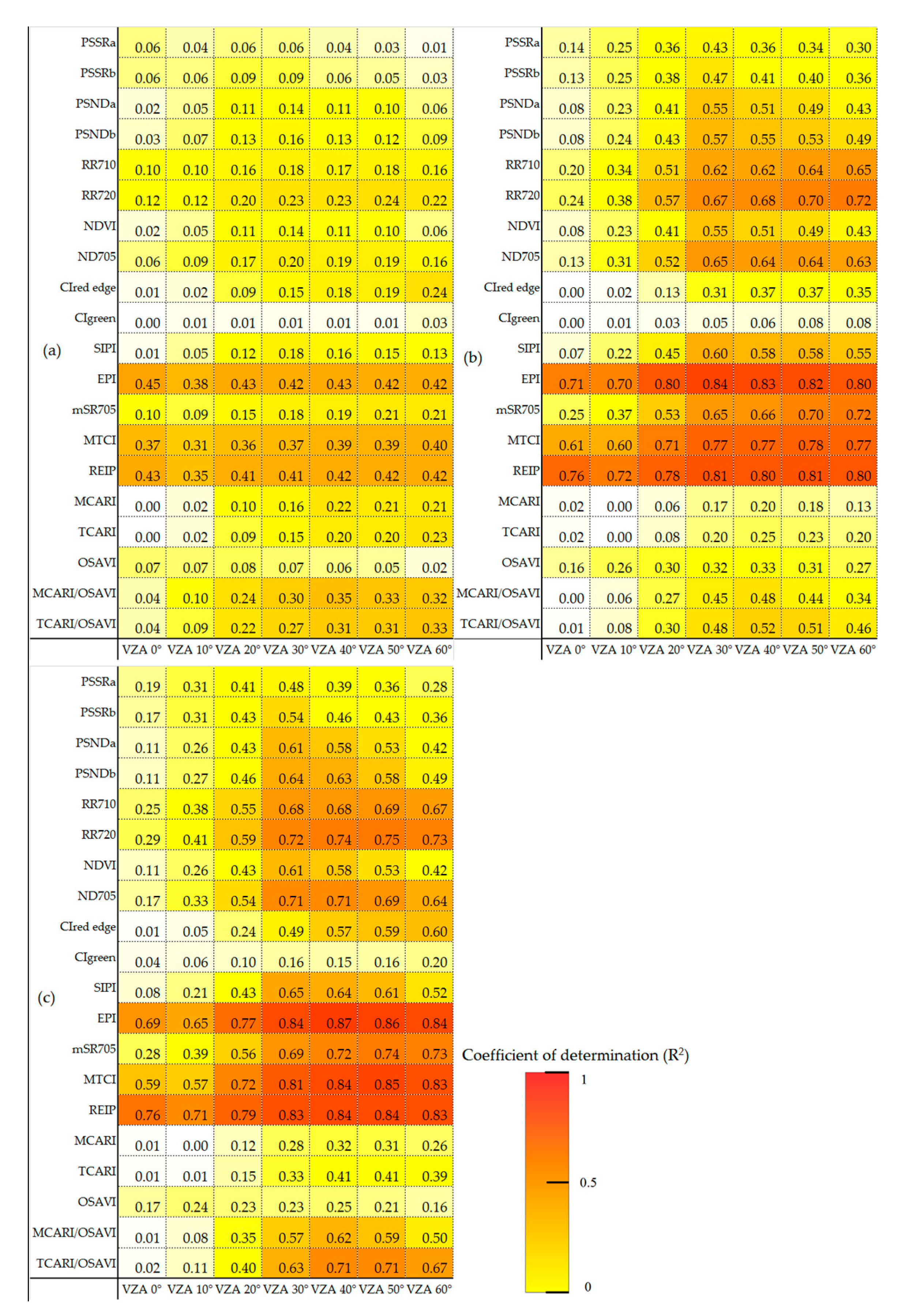
3.2. Correlation Analysis of SPAD Value at Different Vertical Layers and Chlorophyll Sensitivity Indices
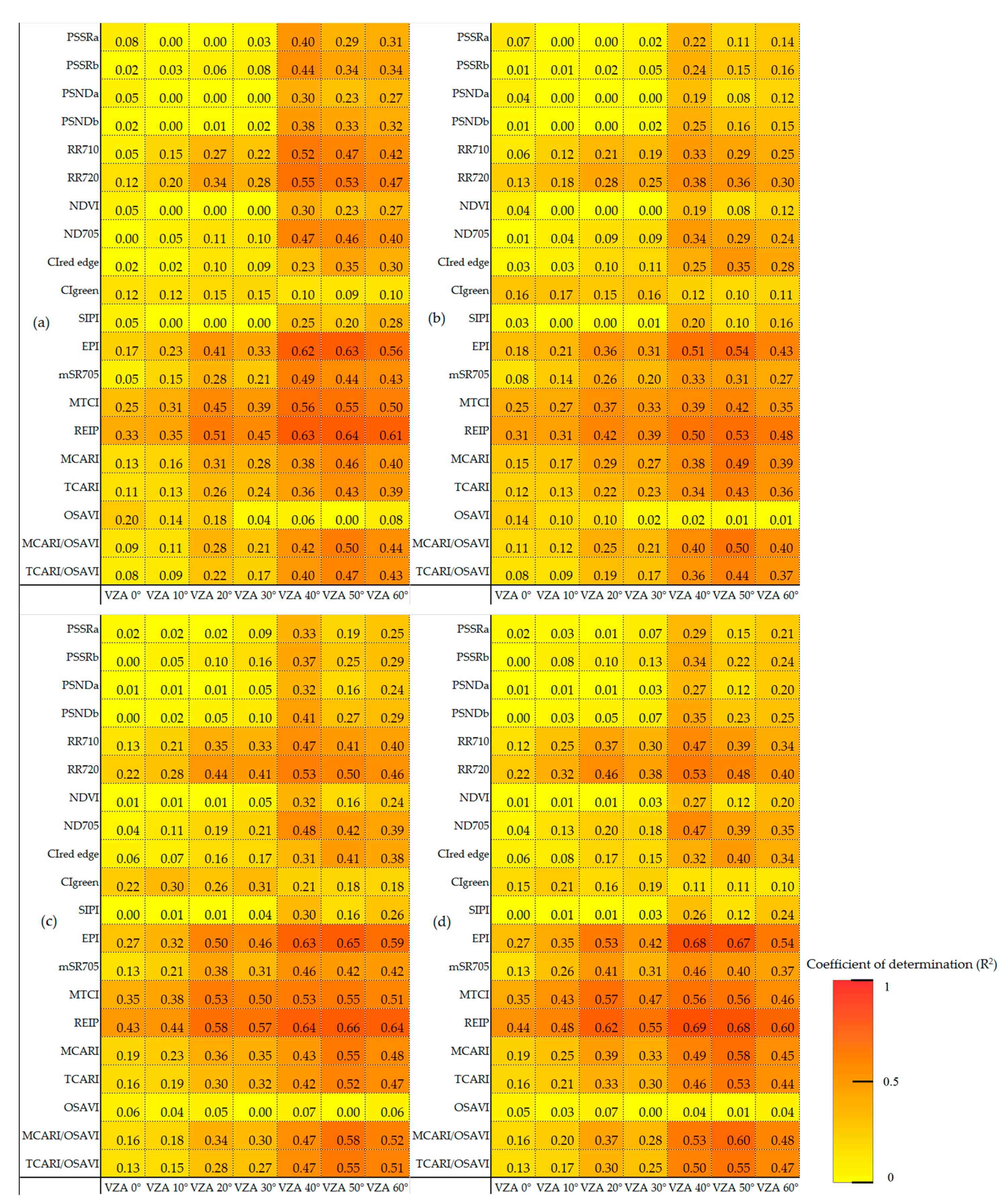
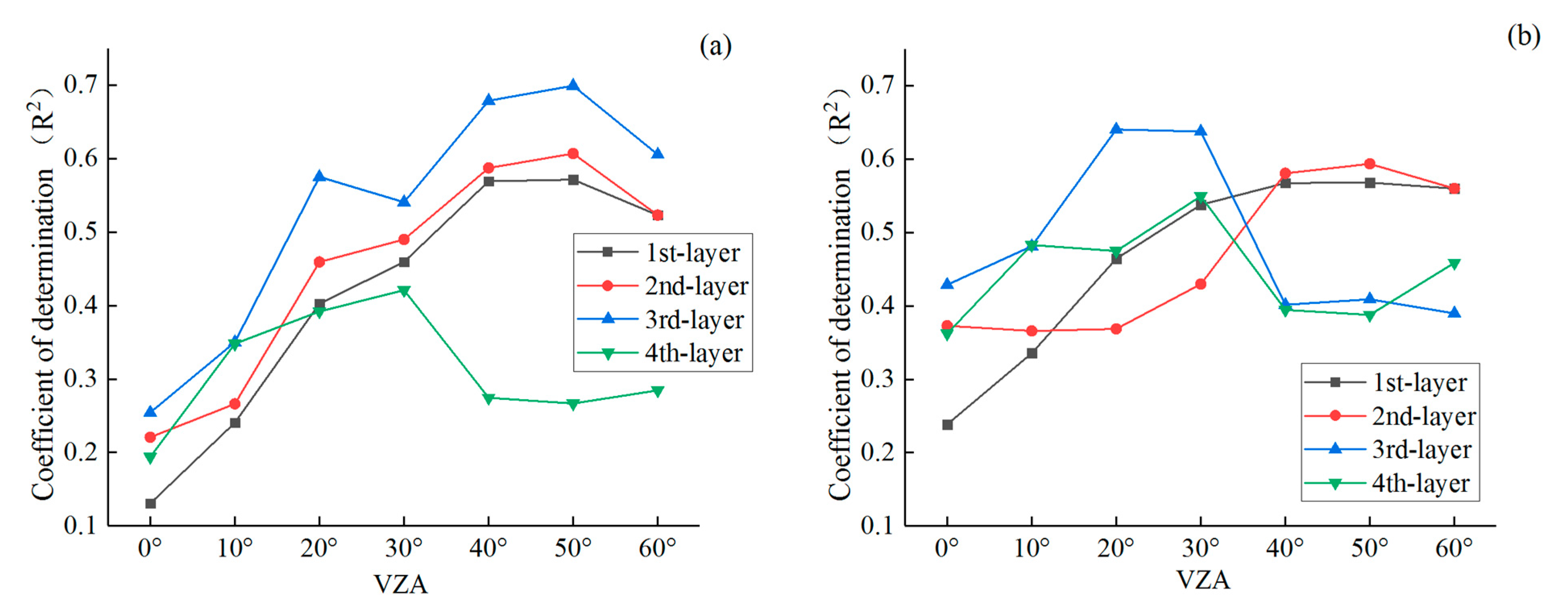
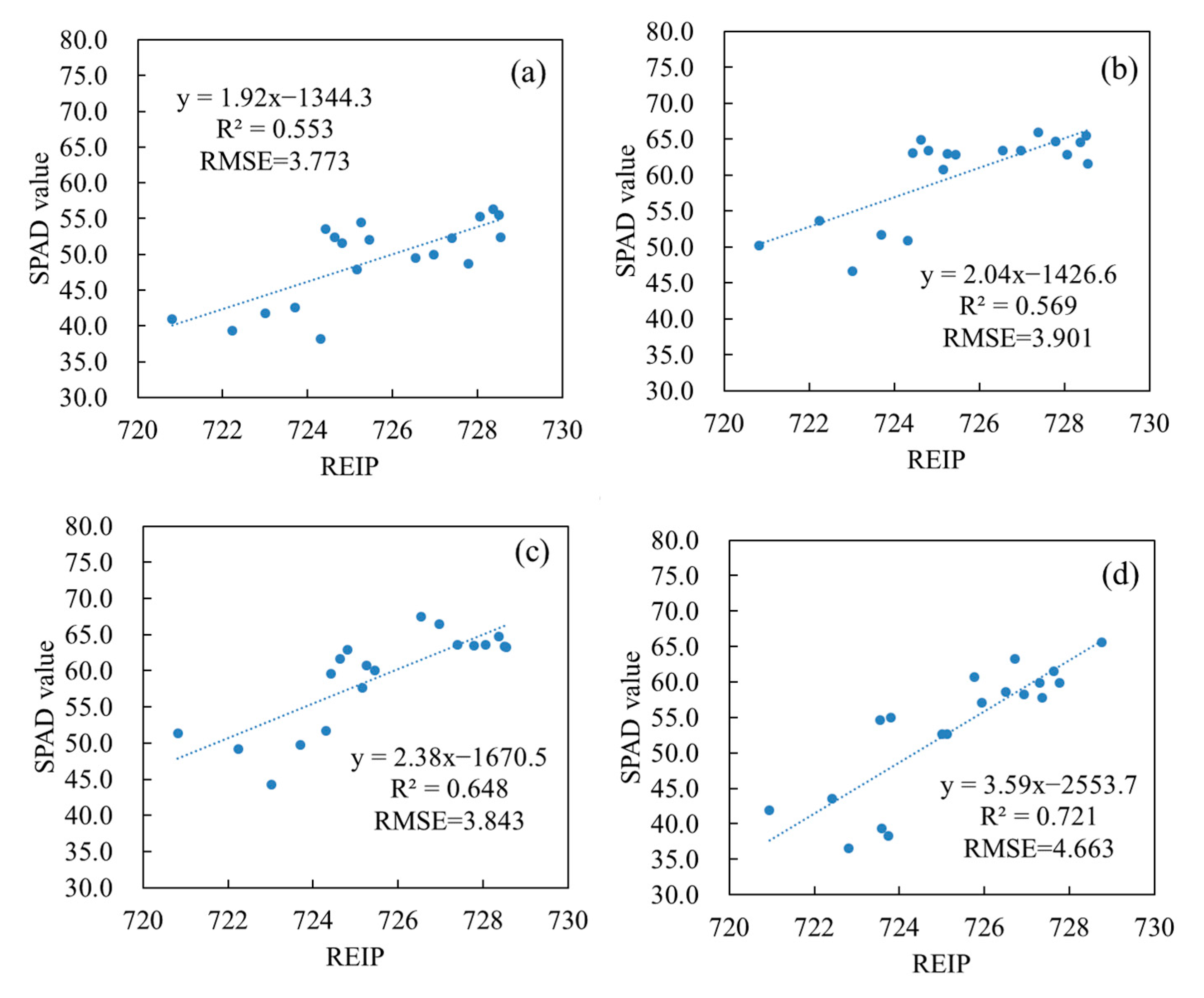
3.3. Optimized Monitoring VZAs for Vertical Leaf SPAD Estimations
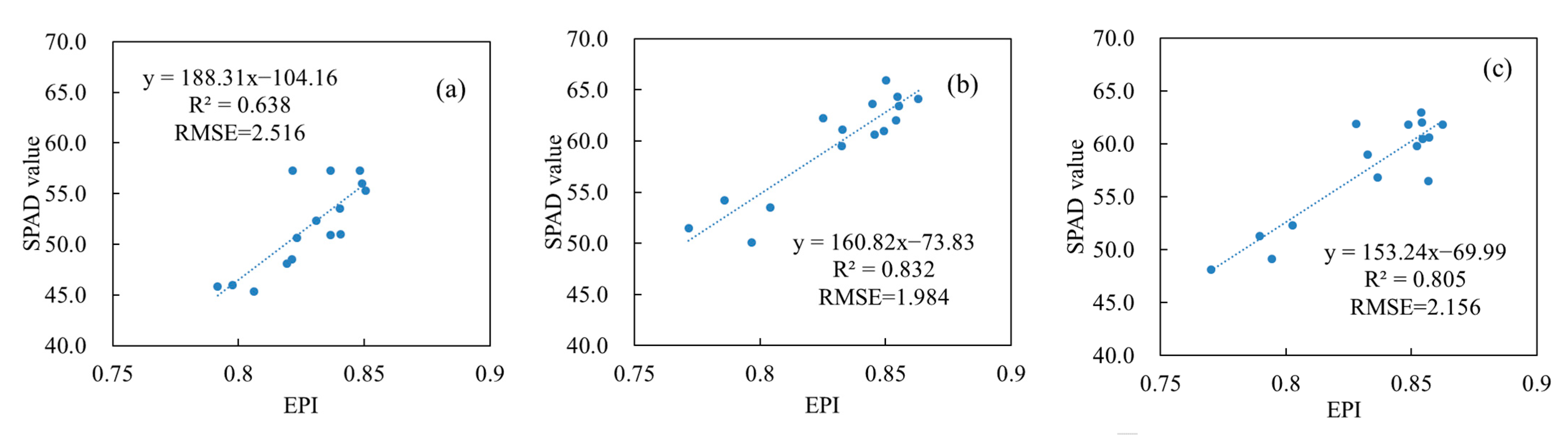
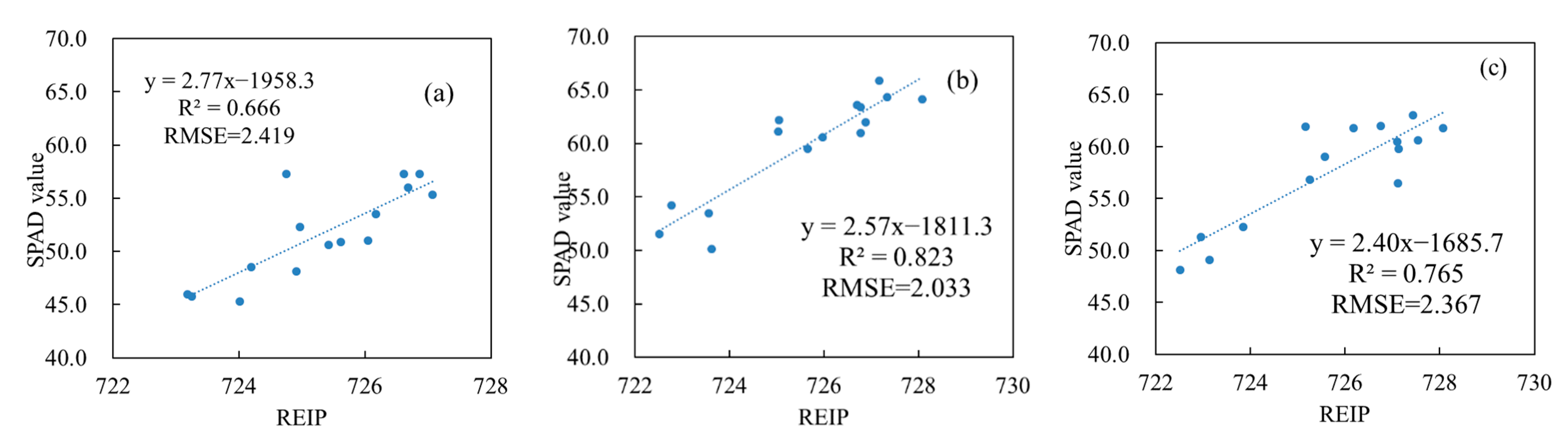
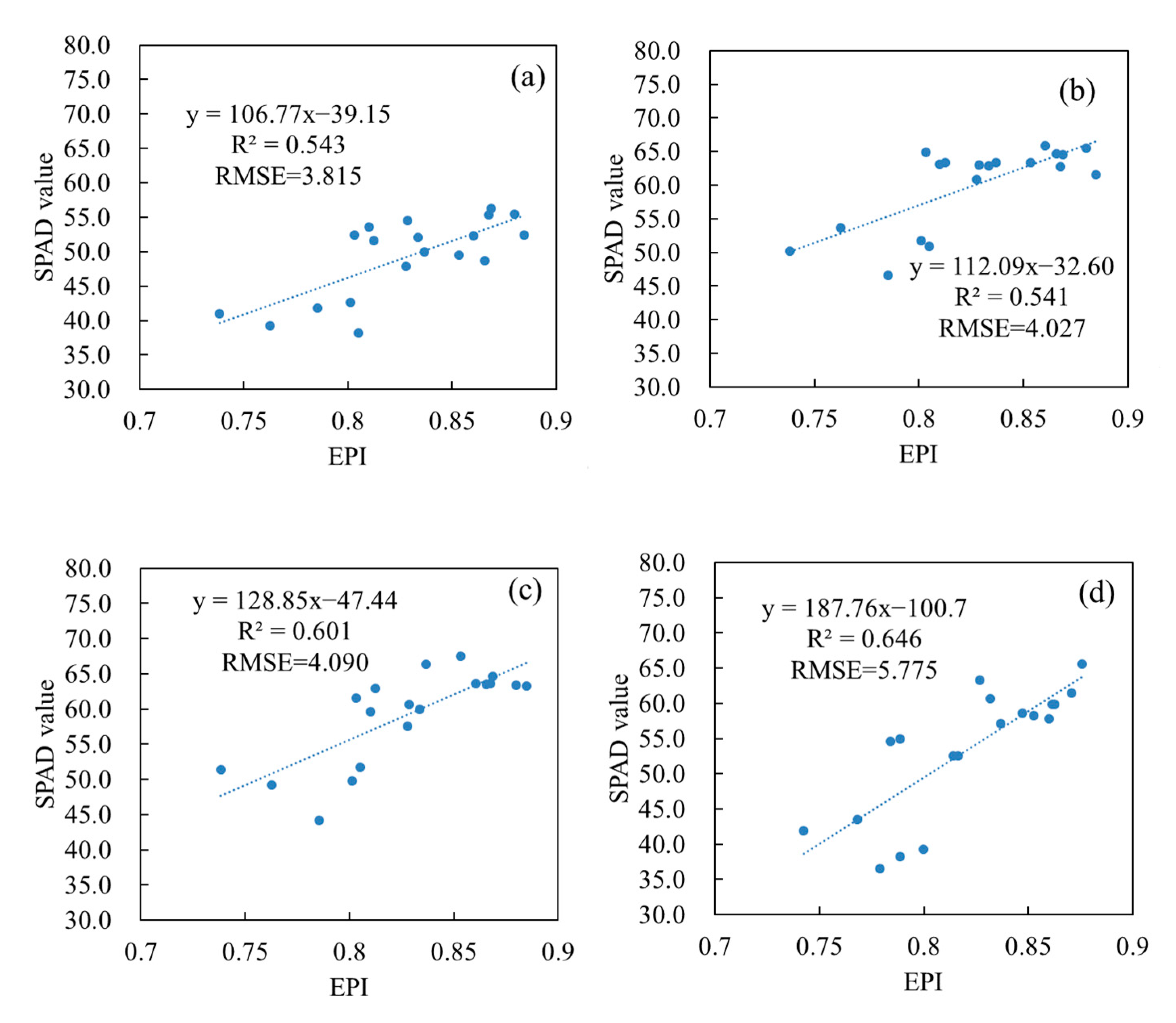
3.4. Verification of Vertical Leaf SPAD Estimation Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, B.; Ata-Ul-Karim, S.T.; Liu, Z.; Zhang, J.; Xiao, J.; Liu, Z.; Qin, A.; Ning, D.; Yang, Q.; Zhang, Y. Simple assessment of nitrogen nutrition index in summer maize by using chlorophyll meter readings. Front. Plant Sci. 2018, 9, 11. [Google Scholar] [CrossRef]
- Hirose, T.; Werger, M.J. Nitrogen use efficiency in instantaneous and daily photosynthesis of leaves in the canopy of a Solidago altissima stand. Physiol. Plant. 1987, 70, 215–222. [Google Scholar] [CrossRef]
- Dreccer, M.; Van Oijen, M.; Schapendonk, A.; Pot, C.; Rabbinge, R. Dynamics of vertical leaf nitrogen distribution in a vegetative wheat canopy. Impact on canopy photosynthesis. Ann. Bot. 2000, 86, 821–831. [Google Scholar] [CrossRef]
- He, J.; Zhang, X.; Guo, W.; Pan, Y.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.; Tian, Y. Estimation of vertical leaf nitrogen distribution within a rice canopy based on hyperspectral data. Front. Plant Sci. 2019, 10, 1802. [Google Scholar] [CrossRef]
- Luo, J.; Ma, R.; Feng, H.; Li, X. Estimating the total nitrogen concentration of reed canopy with hyperspectral measurements considering a non-uniform vertical nitrogen distribution. Remote Sens. 2016, 8, 789. [Google Scholar] [CrossRef]
- Duan, D.; Zhao, C.; Li, Z.; Yang, G.; Yang, W. Estimating total leaf nitrogen concentration in winter wheat by canopy hyperspectral data and nitrogen vertical distribution. J. Integr. Agric. 2019, 18, 1562–1570. [Google Scholar] [CrossRef]
- Li, L.; Jákli, B.; Lu, P.; Ren, T.; Ming, J.; Liu, S.; Wang, S.; Lu, J. Assessing leaf nitrogen concentration of winter oilseed rape with canopy hyperspectral technique considering a non-uniform vertical nitrogen distribution. Ind. Crop. Prod. 2018, 116, 1–14. [Google Scholar] [CrossRef]
- Hirooka, Y.; Homma, K.; Shiraiwa, T. Parameterization of the vertical distribution of leaf area index (LAI) in rice (Oryza sativa L.) using a plant canopy analyzer. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Ye, H.; Huang, W.; Huang, S.; Wu, B.; Dong, Y.; Cui, B. Remote estimation of nitrogen vertical distribution by consideration of maize geometry characteristics. Remote Sens. 2018, 10, 1995. [Google Scholar] [CrossRef]
- Wang, G.; Bronson, K.F.; Thorp, K.R.; Mon, J.; Badaruddin, M. Multiple leaf measurements improve effectiveness of chlorophyll meter for durum wheat nitrogen management. Crop Sci. 2014, 54, 817–826. [Google Scholar] [CrossRef]
- Ali, A.M.; Thind, H.S.; Sharma, S.; Singh, Y. Site-specific nitrogen management in dry direct-seeded rice using chlorophyll meter and leaf colour chart. Pedosphere 2015, 25, 72–81. [Google Scholar] [CrossRef]
- Wu, J.; Wang, D.; Rosen, C.J.; Bauer, M.E. Comparison of petiole nitrate concentrations, SPAD chlorophyll readings, and QuickBird satellite imagery in detecting nitrogen status of potato canopies. Field Crop. Res. 2007, 101, 96–103. [Google Scholar] [CrossRef]
- Dordas, C.A. Chlorophyll meter readings, N leaf concentration and their relationship with N use efficiency in oregano. J. Plant Nutr. 2017, 40, 391–403. [Google Scholar] [CrossRef]
- Yuan, Z.; Ata-Ul-Karim, S.T.; Cao, Q.; Lu, Z.; Cao, W.; Zhu, Y.; Liu, X. Indicators for diagnosing nitrogen status of rice based on chlorophyll meter readings. Field Crop. Res. 2016, 185, 12–20. [Google Scholar] [CrossRef]
- Ciganda, V.S.; Gitelson, A.A.; Schepers, J. How deep does a remote sensor sense? Expression of chlorophyll content in a maize canopy. Remote Sens. Environ. 2012, 126, 240–247. [Google Scholar] [CrossRef]
- Wang, J.; Huang, W.; Lao, C.; Zhang, L.; Luo, C.; Wang, T.; Liu, L.; Song, X.; Ma, Z. Inversion of winter wheat foliage vertical distribution based on canopy reflected spectrum by partial least squares regression method. Guang Pu Xue Yu Guang Pu Fen Xi 2007, 27, 1319–1322. [Google Scholar]
- Wang, Z.; Wang, J.; Liu, L.; Huang, W.; Zhao, C.; Lu, Y. Estimation of Nitrogen Status in Middle and Bottom Layers of Winter Wheat Canopy by Using Ground-Measured Canopy Reflectance. Commun. Soil Sci. Plant Anal. 2005, 36, 2289–2302. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Z.; Huang, L.; Lamb, D.W.; Ma, Z.; Zhang, J.; Wang, J.; Zhao, C. Estimation of vertical distribution of chlorophyll concentration by bi-directional canopy reflectance spectra in winter wheat. Precis. Agric. 2011, 12, 165–178. [Google Scholar] [CrossRef]
- Keating, B.; Wafula, B. Modelling the fully expanded area of maize leaves. Field Crop. Res. 1992, 29, 163–176. [Google Scholar] [CrossRef]
- Valentinuz, O.R.; Tollenaar, M. Vertical profile of leaf senescence during the grain-filling period in older and newer maize hybrids. Crop Sci. 2004, 44, 827–834. [Google Scholar]
- Ciganda, V.; Gitelson, A.; Schepers, J. Vertical profile and temporal variation of chlorophyll in maize canopy: Quantitative “crop vigor” indicator by means of reflectance-based techniques. Agron. J. 2008, 100, 1409–1417. [Google Scholar] [CrossRef]
- Kong, W.; Huang, W.; Zhou, X.; Ye, H.; Dong, Y.; Casa, R. Off-nadir hyperspectral sensing for estimation of vertical profile of leaf chlorophyll content within wheat canopies. Sensors 2017, 17, 2711. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, G.A. Quantifying chlorophylls and caroteniods at leaf and canopy scales: An evaluation of some hyperspectral approaches. Remote Sens. Environ. 1998, 66, 273–285. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Noland, T.L.; Mohammed, G.H.; Sampson, P.H. Scaling-up and model inversion methods with narrowband optical indices for chlorophyll content estimation in closed forest canopies with hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1491–1507. [Google Scholar] [CrossRef]
- Vogelmann, J.; Rock, B.; Moss, D. Red edge spectral measurements from sugar maple leaves. Remote Sens. 1993, 14, 1563–1575. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Gamon, J.; Serrano, L.; Surfus, J. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys. Res. Lett. 2006, 33, 11. [Google Scholar] [CrossRef]
- Penuelas, J.; Baret, F.; Filella, I. Semi-empirical indices to assess carotenoids/chlorophyll a ratio from leaf spectral reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Datt, B. Remote sensing of water content in Eucalyptus leaves. Aust. J. Bot. 1999, 47, 909–923. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P. The MERIS Terrestrial Chlorophyll Index. Int. J. Remote Sens. 2004, 25, 5403–5413. [Google Scholar] [CrossRef]
- Daughtry, C.; Walthall, C.; Kim, M.; De Colstoun, E.B.; McMurtrey, J., III. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Main, R.; Cho, M.A.; Mathieu, R.; O’Kennedy, M.M.; Ramoelo, A.; Koch, S. An investigation into robust spectral indices for leaf chlorophyll estimation. ISPRS J. Photogramm. Remote Sens. 2011, 66, 751–761. [Google Scholar] [CrossRef]
- Lötscher, M.; Stroh, K.; Schnyder, H. Vertical leaf nitrogen distribution in relation to nitrogen status in grassland plants. Ann. Bot. 2003, 92, 679–688. [Google Scholar] [CrossRef]
- Bertheloot, J.; Martre, P.; Andrieu, B. Dynamics of light and nitrogen distribution during grain filling within wheat canopy. Plant Physiol. 2008, 148, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Drouet, J.; Bonhomme, R. Do variations in local leaf irradiance explain changes to leaf nitrogen within row maize canopies? Ann. Bot. 1999, 84, 61–69. [Google Scholar] [CrossRef]
- Shiratsuchi, H.; Yamagishi, T.; Ishii, R. Leaf nitrogen distribution to maximize the canopy photosynthesis in rice. Field Crop. Res. 2006, 95, 291–304. [Google Scholar] [CrossRef]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Estimating chlorophyll content from hyperspectral vegetation indices: Modeling and validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Huang, W.; Yang, G. Non-uniform vertical nitrogen distribution within plant canopy and its estimation by remote sensing: A review. Field Crop. Res. 2013, 142, 75–84. [Google Scholar] [CrossRef]
- Trawczynski, C. Assessment of the nutrition of potato plants with nitrogen according to the NNI test and SPAD indicator. J. Elem. 2019, 24, 2. [Google Scholar] [CrossRef]
- Lambert, R.; Johnson, R. Leaf angle, tassel morphology, and the performance of maize hybrids 1. Crop Sci. 1978, 18, 499–502. [Google Scholar] [CrossRef]
- Azumi, Y.; Watanabe, A. Evidence for a senescence-associated gene induced by darkness. Plant Physiol. 1991, 95, 577–583. [Google Scholar] [CrossRef]
- Maddonni, G.A.; Otegui, M.E.; Cirilo, A.G. Plant population density, row spacing and hybrid effects on maize canopy architecture and light attenuation. Field Crop. Res. 2001, 71, 183–193. [Google Scholar] [CrossRef]
- Monsi, M.; Saeki, T. On the factor light in plant communities and its importance for matter production. Ann. Bot. 2005, 95, 549. [Google Scholar] [CrossRef]
- Ma, D.; Xie, R.; Niu, X.; Li, S.; Long, H.; Liu, Y. Changes in the morphological traits of maize genotypes in China between the 1950s and 2000s. Eur. J. Agron. 2014, 58, 1–10. [Google Scholar] [CrossRef]
- Schlemmer, M.; Gitelson, A.; Schepers, J.; Ferguson, R.; Peng, Y.; Shanahan, J.; Rundquist, D. Remote estimation of nitrogen and chlorophyll contents in maize at leaf and canopy levels. Int. J. Appl. Earth Obs. Geoinf. 2013, 25, 47–54. [Google Scholar] [CrossRef]
- Feng, W.; Qi, S.; Heng, Y.; Zhou, Y.; Wu, Y.; Liu, W.; He, L.; Li, X. Canopy vegetation indices from in situ hyperspectral data to assess plant water status of winter wheat under powdery mildew stress. Front. Plant Sci. 2017, 8, 1219. [Google Scholar] [CrossRef] [PubMed]

| Short Name | Name | Formula | Applications | Reference |
|---|---|---|---|---|
| PSSRa | Pigment specific simple ratio | R800/R680 | Vegetation; Chlorophyll | [23] |
| PSSRb | Pigment specific simple ratio | R800/R635 | Vegetation; Chlorophyll | [23] |
| RR710 | Red edge reflectance ratio | R750/R710 | Vegetation; Chlorophyll | [24] |
| RR720 | Red edge reflectance ratio | R740/R720 | Vegetation; Chlorophyll | [25] |
| PSNDa | Pigment specific normalized difference | (R800 − R680)/(R800 + R680) | Chlorophyll; LAI; PAR; Crop yield; et al. | [23] |
| PSNDb | Pigment specific normalized difference | (R800 − R635)/(R800 + R635) | Vegetation; Chlorophyll | [23] |
| NDVI | Normalized difference vegetation index | (R800 − R670)/(R800 + R670) | Chlorophyll; LAI; Crop yield; et al. | [26] |
| ND705 | Normalized difference red edge | (R750 − R705)/(R750 + R705) | Vegetation; Chlorophyll | [26] |
| PRI | Photochemical reflectance index | (R531 − R570)/(R750 + R705) | Vegetation; Chlorophyll | [27] |
| CIred edge | Chlorophyll index red edge | (R750−1 − R700−1)/R700 | Vegetation; Chlorophyll | [28] |
| CIgreen | Chlorophyll index green | (R750−1 − R550−1)/R550 | Vegetation; Chlorophyll | [28] |
| SIPI | Structure insensitive pigment index | (R800 − R445)/(R800 − R680) | Vegetation; Chlorophyll | [29] |
| EPI | Eucalyptus pigment index | (R850 − R710)/(R850 − R680) | Vegetation; Chlorophyll | [30] |
| mSR705 | Modified simple ratio | (R750 − R445)/(R705 − R445) | Vegetation; Chlorophyll | [31] |
| MTCI | MERIS terrestrial chlorophyll index | (R750 − R710)/(R710 − R680) | Chlorophyll | [32] |
| REIP | Red edge inflection point | R700+40[(R670+R780)/2) − R700]/(R740 − R700) | Vegetation; Chlorophyll; Red-edge position | [25] |
| MCARI | Modified CARI | [(R700-R670) − 0.2(R700 − R550)]*(R700/R670) | Vegetation; Chlorophyll | [33] |
| TCARI | Transformed CARI | 3[(R700-R670) − 0.2(R700 − R550)*(R700/R670)] | Chlorophyll | [34] |
| OSAVI | Optimized SAVI | (1 + 0.16)*(R800 − R680)/(R800 + R680 + 0.16) | Vegetation; Soil | [35] |
| MCARI/OSAVI | MCARI/OSAVI | MCARI/OSAVI | - | - |
| TCARI/OSAVI | TCARI/OSAVI | TCARI/OSAVI | - | - |
| Layer | n | Max | Min | Mean | SD + | CV ++ (%) |
|---|---|---|---|---|---|---|
| Early growth stage | ||||||
| 1st-layer | 24 | 57.30 | 45.30 | 51.97 | 3.78 | 7.27 |
| 2nd-layer | 24 | 65.90 | 50.10 | 61.34 | 4.45 | 7.25 |
| 3rd-layer | 24 | 65.50 | 48.10 | 59.90 | 5.02 | 8.38 |
| Late growth stage | ||||||
| 1st-layer | 31 | 62.10 | 38.20 | 51.65 | 5.87 | 11.36 |
| 2nd-layer | 31 | 66.40 | 46.60 | 61.44 | 5.22 | 8.50 |
| 3rd-layer | 31 | 67.50 | 44.20 | 61.21 | 5.97 | 9.75 |
| 4th-layer | 31 | 65.80 | 36.50 | 56.57 | 8.21 | 14.52 |
| All stages | ||||||
| 1st-layer | 55 | 62.10 | 38.20 | 51.79 | 5.02 | 9.70 |
| 2nd-layer | 55 | 66.40 | 46.60 | 61.40 | 4.86 | 7.91 |
| 3rd-layer | 55 | 67.50 | 44.20 | 60.64 | 5.56 | 9.17 |
| 4th-layer | 31 | 65.80 | 36.50 | 56.57 | 8.21 | 14.52 |
| Layer | 1st-Layer | 2nd-Layer | 3rd-Layer | 4th-Layer |
|---|---|---|---|---|
| 1st-layer | 1 | - | - | - |
| 2nd-layer | 0.47 | 1 | - | - |
| 3rd-layer | 0.32 | 0.94 * | 1 | - |
| 4th-layer | 0.12 | 0.66 | 0.85 * | 1 |
| Early Growth Stage | Late Growth Stage | |||
|---|---|---|---|---|
| Layer | EPI | REIP | EPI | REIP |
| 1st-layer | VZA 0° | VZA 0° | VZA 50° | VZA 50° |
| 2nd-layer | VZA 30° | VZA 30° | VZA 50° | VZA 50° |
| 3rd-layer | VZA 40° | VZA 40° | VZA 50° | VZA 50° |
| 4th-layer | — | — | VZA 40° | VZA 40° |
| EPI | REIP | |||||
|---|---|---|---|---|---|---|
| Layer | R2 | RMSE | MRE (%) | R2 | RMSE | MRE (%) |
| Early growth stage | ||||||
| 1st-layer | 0.32 | 4.14 | 6.90 | 0.34 | 4.61 | 7.96 |
| 2nd-layer | 0.33 | 1.71 | 2.25 | 0.12 | 2.31 | 3.13 |
| 3rd-layer | 0.71 | 1.11 | 1.45 | 0.57 | 1.00 | 1.27 |
| Late growth stage | ||||||
| 1st-layer | 0.22 | 2.97 | 4.28 | 0.28 | 3.16 | 5.22 |
| 2nd-layer | 0.62 | 3.50 | 5.07 | 0.62 | 5.02 | 7.46 |
| 3rd-layer | 0.57 | 2.80 | 3.57 | 0.63 | 4.55 | 6.41 |
| 4th-layer | 0.49 | 4.80 | 6.78 | 0.38 | 7.85 | 11.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Ye, H.; Huang, W.; Wang, H.; Luo, P.; Ren, Y.; Kong, W. Monitoring the Vertical Distribution of Maize Canopy Chlorophyll Content Based on Multi-Angular Spectral Data. Remote Sens. 2021, 13, 987. https://doi.org/10.3390/rs13050987
Wu B, Ye H, Huang W, Wang H, Luo P, Ren Y, Kong W. Monitoring the Vertical Distribution of Maize Canopy Chlorophyll Content Based on Multi-Angular Spectral Data. Remote Sensing. 2021; 13(5):987. https://doi.org/10.3390/rs13050987
Chicago/Turabian StyleWu, Bin, Huichun Ye, Wenjiang Huang, Hongye Wang, Peilei Luo, Yu Ren, and Weiping Kong. 2021. "Monitoring the Vertical Distribution of Maize Canopy Chlorophyll Content Based on Multi-Angular Spectral Data" Remote Sensing 13, no. 5: 987. https://doi.org/10.3390/rs13050987
APA StyleWu, B., Ye, H., Huang, W., Wang, H., Luo, P., Ren, Y., & Kong, W. (2021). Monitoring the Vertical Distribution of Maize Canopy Chlorophyll Content Based on Multi-Angular Spectral Data. Remote Sensing, 13(5), 987. https://doi.org/10.3390/rs13050987