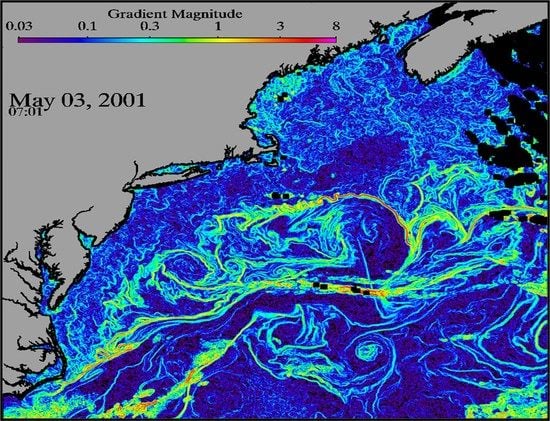
Remote Sensing of Ocean Fronts in Marine Ecology and Fisheries
Abstract
1. Introduction
- Section 2 covers satellite missions and sensors, summing up the most important Earth observation satellite missions and sensors relevant to marine ecology and fisheries;
- Section 3 covers remote sensing in marine fisheries, providing a retrospective of remote sensing applications in marine fisheries and ecology;
- Section 4 covers the ecological role of fronts and eddies, providing a concise review of ecological studies of links between marine animals and major oceanic circulation and structural features, namely fronts and eddies;
- Section 5 covers front detection in satellite imagery, presenting five front detection, visualization, and characterization algorithms and methods that have a history of successful applications in marine ecology and fisheries research;
- Section 6 covers a feature-based approach to remote sensing in marine ecology and fisheries, which is the central part of this review, as it makes a case for feature detection and application of feature-linked remote sensing data;
- Section 7 covers the Discussion, which points out a few scientific and technical problems, suggests a few solutions, draws preliminary conclusions, and outlines perspectives;
- Section 8 provides the Summary, which sums up the major takeaway messages.
2. Satellite Missions and Sensors
3. Remote Sensing in Marine Fisheries
4. Ecological Roles of Fronts and Eddies
5. Front Detection in Satellite Imagery
6. Feature-Based Approach to Remote Sensing in Marine Ecology and Fisheries
7. Discussion
8. Summary
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belkin, I.M. Front. In Interdisciplinary Encyclopedia of Marine Sciences; Nybakken, J.W., Broenkow, W.W., Vallier, T.L., Eds.; Grolier Academic Reference: Danbury, CT, USA, 2002; Volume 1, pp. 433–436. [Google Scholar]
- Belkin, I.M.; Cornillon, P.C.; Sherman, K. Fronts in Large Marine Ecosystems. Prog. Oceanogr. 2009, 81, 223–236. [Google Scholar] [CrossRef]
- Laurs, R.M.; Lynn, R.J. Seasonal migration of North Pacific albacore, Thunnus alalunga, into North American coastal waters: Distribution, relative abundance, and association with Transition Zone waters. Fish. Bull. 1977, 75, 795–822. [Google Scholar]
- Holligan, P.M. Biological implications of fronts on the northwest European continental shelf. Philos. Trans. R. Soc. London Ser. A Math. Phys. Sci. 1981, 302, 547–562. [Google Scholar] [CrossRef]
- Krause, G.; Budeus, G.; Gerdes, D.; Schaumann, K.; Hesse, K. Frontal systems in the German Bight and their physical and biological effects. In Marine Interfaces Ecohydrodynamics; Nihoul, J.C.J., Ed.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 119–140. [Google Scholar] [CrossRef]
- Legeckis, R. A survey of worldwide sea surface temperature fronts detected by environmental satellites. J. Geophys. Res. Oceans 1978, 83, 4501–4522. [Google Scholar] [CrossRef]
- Le Fèvre, J. Aspects of the biology of frontal systems. Adv. Marine Biol. 1987, 23, 163–299. [Google Scholar] [CrossRef]
- Wolanski, E.; Hamner, W.M. Topographically controlled fronts in the ocean and their biological influence. Science 1988, 241, 177–181. [Google Scholar] [CrossRef]
- Olson, D.B.; Hitchcock, G.L.; Mariano, A.J.; Ashjian, C.J.; Peng, G.; Nero, R.W.; Podestá, G. Life on the edge: Marine life and fronts. Oceanography 1994, 7, 52–60. Available online: https://www.jstor.org/stable/43924679 (accessed on 18 February 2021). [CrossRef]
- Acha, E.M.; Mianzan, H.W.; Guerrero, R.A.; Favero, M.; Bava, J. Marine fronts at the continental shelves of austral South America: Physical and ecological processes. J. Mar. Syst. 2004, 44, 83–105. [Google Scholar] [CrossRef]
- Bakun, A. Fronts and eddies as key structures in the habitat of marine fish larvae: Opportunity, adaptive response and competitive advantage. Sci. Mar. 2006, 70, 105–122. [Google Scholar] [CrossRef]
- Bost, C.A.; Cotté, C.; Bailleul, F.; Cherel, Y.; Charrassin, J.B.; Guinet, C.; Ainley, D.G.; Weimerskirch, H. The importance of oceanographic fronts to marine birds and mammals of the southern oceans. J. Mar. Syst. 2009, 78, 363–376. [Google Scholar] [CrossRef]
- Belkin, I.M.; Hunt, G.L.; Hazen, E.L.; Zamon, J.E.; Schick, R.S.; Prieto, R.; Brodziak, J.; Teo, S.L.H.; Thorne, L.; Bailey, H.; et al. Fronts, fish, and predators (editorial). Deep Sea Res. Part II 2014, 107, 1–2. [Google Scholar] [CrossRef]
- Woodson, C.B.; Litvin, S.Y. Ocean fronts drive marine fishery production and biogeochemical cycling. Proc. Natl. Acad. Sci. USA 2015, 112, 1710–1715. [Google Scholar] [CrossRef]
- Snyder, S.; Franks, P.J.; Talley, L.D.; Xu, Y.; Kohin, S. Crossing the line: Tunas actively exploit submesoscale fronts to enhance foraging success. Limnol. Oceanogr. Lett. 2017, 2, 187–194. [Google Scholar] [CrossRef]
- Cox, S.; Embling, C.B.; Hosegood, P.J.; Votier, S.C.; Ingram, S.N. Oceanographic drivers of marine mammal and seabird habitat-use across shelf-seas: A guide to key features and recommendations for future research and conservation management. Estuarine Coast. Shelf Sci. 2018, 212, 294–310. [Google Scholar] [CrossRef]
- Martinetto, P.; Alemany, D.; Botto, F.; Mastrángelo, M.; Falabella, V.; Acha, E.M.; Antón, G.; Bianchi, A.; Campagna, C.; Cañete, G.; et al. Linking the scientific knowledge on marine frontal systems with ecosystem services. Ambio 2020, 49, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Cayula, J.F.; Cornillon, P. Edge detection applied to SST fields. In Digital Image Processing and Visual Communications Technologies in the Earth and Atmospheric Sciences; Janota, P., Ed.; Proceedings of SPIE: Bellingham, WA, USA, 1990; Volume 1301, pp. 13–24. [Google Scholar] [CrossRef]
- Cayula, J.-F.; Cornillon, P. Edge detection algorithm for SST images. J. Atmospheric Ocean. Technol. 1992, 9, 67–80. [Google Scholar] [CrossRef]
- Cayula, J.-F.; Cornillon, P. Multi-image edge detection for SST images. J. Atmospheric Ocean. Technol. 1995, 12, 821–829. [Google Scholar] [CrossRef]
- Belkin, I.M.; O’Reilly, J.E. An algorithm for oceanic front detection in chlorophyll and SST satellite imagery. J. Mar. Syst. 2009, 78, 317–326. [Google Scholar] [CrossRef]
- Minnett, P.J.; Alvera-Azcárate, A.; Chin, T.M.; Corlett, G.K.; Gentemann, C.L.; Karagali, I.; Li, X.; Marsouin, A.; Marullo, S.; Maturi, E.; et al. Half a century of satellite remote sensing of sea-surface temperature. Remote Sens. Environ. 2019, 233, 111366. [Google Scholar] [CrossRef]
- Isoguchi, O.; Ebuchi, N. Detection of current-rips (shiome) using PALSAR/PALSAR-2. J. Remote Sens. Soc. Jpn. 2020, 40, S1–S11. [Google Scholar] [CrossRef]
- Fiedler, P.C.; Smith, G.B.; Laurs, R.M. Fisheries applications of satellite data in the eastern North Pacific. Mar. Fish. Rev. 1984, 46, 1–13. [Google Scholar]
- Laurs, R.M.; Brucks, J.T. Living marine resources applications. Adv. Geophys. 1985, 27, 419–452. [Google Scholar] [CrossRef]
- Cornillon, P.; Hickox, S.; Turton, H. Sea surface temperature charts for the southern New England fishing community. Mar. Technol. Soc. J. 1986, 20, 57–65. [Google Scholar]
- Pettersson, L.H.; Johannessen, O.M.; Kloster, K.; Olaussen, T.I.; Samuel, P. Application of Remote Sensing to Fisheries; Report EUR 12867 EN.; Murray, C.N., Ed.; The Nansen Remote Sensing Center: Solheimsvik, Norway, 1990; Volume 1, 136p, Available online: https://www.google.com/books/edition/Application_of_Remote_Sensing_to_Fisheri/qSMYAQAAIAAJ?hl=en (accessed on 18 February 2021).
- Boehlert, G.W.; Schumacher, J.D. Changing Oceans and Changing Fisheries: Environmental Data for Fishing Research and Management; NOAA Technical Memorandum NMFS. NOAA-TM-NMFS-SWFSC-239; National Oceanic and Atmospheric Administration: Washington, DC, USA, 1997; 155p. Available online: https://repository.library.noaa.gov/view/noaa/3008/noaa_3008_DS1 (accessed on 18 February 2021).
- Santos, A.M.P. Fisheries oceanography using satellite and airborne remote sensing methods: A review. Fish. Res. 2000, 49, 1–20. [Google Scholar] [CrossRef]
- Stuart, V.; Platt, T.; Sathyendranath, S.; Pravin, P. Remote sensing and fisheries: An introduction. ICES J. Mar. Sci. 2011, 68, 639–641. [Google Scholar] [CrossRef]
- Wilson, C. The rocky road from research to operations for satellite ocean-colour data in fishery management. ICES J. Mar. Sci. 2011, 68, 677–686. [Google Scholar] [CrossRef]
- Saitoh, S.-I.; Mugo, R.; Radiarta, I.N.; Asaga, S.; Takahashi, F.; Hirawake, T.; Ishikawa, Y.; Awaji, T.; In, T.; Shima, S. Some operational uses of satellite remote sensing and marine GIS for sustainable fisheries and aquaculture. ICES J. Mar. Sci. 2011, 68, 687–695. [Google Scholar] [CrossRef]
- Klemas, V. Remote sensing of environmental indicators of potential fish aggregation: An overview. Baltica 2012, 25, 99–112. [Google Scholar] [CrossRef]
- Klemas, V. Fishing applications of remote sensing: An overview. Fish. Res. 2013, 148, 124–136. [Google Scholar] [CrossRef]
- Laurs, R.M.; Fiedler, P.C.; Montgomery, D.R. Albacore tuna catch distributions relative to environmental features observed from satellites. Deep Sea Res. 1984, 31, 1085–1099. [Google Scholar] [CrossRef]
- Chassot, E.; Bonhommeau, S.; Reygondeau, G.; Nieto, K.; Polovina, J.J.; Huret, M.; Dulvy, N.K.; Demarcq, H. Satellite remote sensing for an ecosystem approach to fisheries management. ICES J. Mar. Sci. 2011, 68, 651–666. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Brewin, R.J.W.; Brockmann, C.; Brotas, V.; Calton, B.; Chuprin, A.; Cipollini, P.; Couto, A.B.; Dingle, J.; Doerffer, R.; et al. An Ocean-Colour Time Series for Use in Climate Studies: The Experience of the Ocean-Colour Climate Change Initiative (OC-CCI). Sensors 2019, 19, 4285. [Google Scholar] [CrossRef]
- Asch, R.G.; Checkley, D.M., Jr. Dynamic height: A key variable for identifying the spawning habitat of small pelagic fishes. Deep Sea Res. Part I 2013, 71, 79–91. [Google Scholar] [CrossRef]
- Liao, C.H.; Lan, K.W.; Ho, H.Y.; Wang, K.Y.; Wu, Y.L. Variation in the catch rate and distribution of swordtip squid Uroteuthis edulis associated with factors of the oceanic environment in the southern East China Sea. Mar. Coast. Fish. 2018, 10, 452–464. [Google Scholar] [CrossRef]
- Byrne, M.E.; Vaudo, J.J.; Harvey, G.C.M.; Johnston, M.W.; Wetherbee, B.M.; Shivji, M. Behavioral response of a mobile marine predator to environmental variables differs across ecoregions. Ecography 2019, 42, 1569–1578. [Google Scholar] [CrossRef]
- Kiørboe, T.; Munk, P.; Richardson, K.; Christensen, V.; Paulsen, H. Plankton dynamics and larval herring growth, drift and survival in a frontal area. Mar. Ecol. Prog. Ser. 1988, 44, 205–219. [Google Scholar] [CrossRef]
- Polovina, J.J.; Kobayashi, D.R.; Parker, D.M.; Seki, M.P.; Balazs, G.H. Turtles on the edge: Movement of loggerhead turtles (Caretta caretta) along oceanic fronts, spanning longline fishing grounds in the central North Pacific, 1997–1998. Fish. Oceanogr. 2000, 9, 71–82. [Google Scholar] [CrossRef]
- Polovina, J.J.; Howell, E.; Kobayashi, D.R.; Seki, M.P. The transition zone chlorophyll front, a dynamic global feature defining migration and forage habitat for marine resources. Prog. Oceanogr. 2001, 49, 469–483. [Google Scholar] [CrossRef]
- Palacios, D.M.; Bograd, S.J.; Foley, D.G.; Schwing, F.B. Oceanographic characteristics of biological hot spots in the North Pacific: A remote sensing perspective. Deep Sea Res. Part II 2006, 53, 250–269. [Google Scholar] [CrossRef]
- Dunn, D.C.; Ardron, J.; Ban, N.; Bax, N.; Bernal, P.; Bograd, S.; Corrigan, C.; Dunstan, P.; Game, E.; Gjerde, K.; et al. Ecologically or Biologically Significant Areas in the Pelagic Realm: Examples & Guidelines—Workshop Report; IUCN: Gland, Switzerland, 2011; 44p. [Google Scholar]
- Scales, K.L.; Miller, P.I.; Embling, C.B.; Ingram, S.N.; Pirotta, E.; Votier, S.C. Mesoscale fronts as foraging habitats: Composite front mapping reveals oceanographic drivers of habitat use for a pelagic seabird. J. R. Soc. Interface 2014, 11, 20140679. [Google Scholar] [CrossRef]
- Watanuki, Y.; Suryan, R.M.; Sasaki, H.; Yamamoto, T.; Hazen, E.L.; Renner, M.; Santora, J.A.; O’Hara, P.D.; Sydeman, W.J. (Eds.) Spatial Ecology of Marine Top Predators in the North Pacific: Tools for Integrating across Datasets and Identifying High Use Areas; PICES Scientific Report No. 50; North Pacific Marine Science Organization: Sidney, BC, Canada, 2016; 55p. [Google Scholar]
- Sarma, V.V.S.S.; Desai, D.V.; Patil, J.S.; Khandeparker, L.; Aparna, S.G.; Shankar, D.; D’Souza, S.; Dalabehera, H.B.; Mukherjee, J.; Sudharani, P.; et al. Ecosystem response in temperature fronts in the northeastern Arabian Sea. Prog. Oceanogr. 2018, 165, 317–331. [Google Scholar] [CrossRef]
- Etnoyer, P.; Canny, D.; Mate, B.R.; Morgan, L.E.; Ortega-Ortiz, J.G.; Nichols, W.J. Sea-surface temperature gradients across blue whale and sea turtle foraging trajectories off the Baja California Peninsula, Mexico. Deep Sea Res. Part II 2006, 53, 340–358. [Google Scholar] [CrossRef]
- Galarza, J.A.; Carreras-Carbonell, J.; Macpherson, E.; Pascual, M.; Roques, S.; Turner, G.F.; Rico, C. The influence of oceanographic fronts and early-life-history traits on connectivity among littoral fish species. Proc. Natl. Acad. Sci. USA 2009, 106, 1473–1478. [Google Scholar] [CrossRef]
- Druon, J.N.; Fromentin, J.M.; Aulanier, F.; Heikkonen, J. Potential feeding and spawning habitats of Atlantic bluefin tuna in the Mediterranean Sea. Mar. Ecol. Prog. Ser. 2011, 439, 223–240. [Google Scholar] [CrossRef]
- Woodson, C.B.; McManus, M.A.; Tyburczy, J.A.; Barth, J.A.; Washburn, L.; Caselle, J.E.; Carr, M.H.; Malone, D.P.; Raimondi, P.T.; Menge, B.A.; et al. Coastal fronts set recruitment and connectivity patterns across multiple taxa. Limnol. Oceanogr. 2012, 57, 582–596. [Google Scholar] [CrossRef]
- Sabarros, P.S.; Grémillet, D.; Demarcq, H.; Moseley, C.; Pichegru, L.; Mullers, R.H.; Stenseth, N.C.; Machu, E. Fine-scale recognition and use of mesoscale fronts by foraging Cape gannets in the Benguela upwelling region. Deep Sea Res. Part II 2014, 107, 77–84. [Google Scholar] [CrossRef]
- Scales, K.L.; Miller, P.I.; Ingram, S.N.; Hazen, E.L.; Bograd, S.J.; Phillips, R.A. Identifying predictable foraging habitats for a wide-ranging marine predator using ensemble ecological niche models. Divers. Distrib. 2016, 22, 212–224. [Google Scholar] [CrossRef]
- Luo, J.; Ault, J.S.; Shay, L.K.; Hoolihan, J.P.; Prince, E.D.; Brown, C.A.; Rooker, J.R. Ocean heat content reveals secrets of fish migrations. PLoS ONE 2015, 10, e0141101. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.L.; Miller, P.I.; Embling, C.B.; Scales, K.L.; Bicknell, A.W.J.; Hosegood, P.J.; Morgan, G.; Ingram, S.N.; Votier, S.C. Seabird diving behaviour reveals the functional significance of shelf-sea fronts as foraging hotspots. R. Soc. Open Sci. 2016, 3, 160317. [Google Scholar] [CrossRef]
- Chambault, P.; Roquet, F.; Benhamou, S.; Baudena, A.; Pauthenet, E.; De Thoisy, B.; Bonola, M.; Dos Reis, V.; Crasson, R.; Brucker, M.; et al. The Gulf Stream frontal system: A key oceanographic feature in the habitat selection of the leatherback turtle? Deep Sea Res. Part I 2017, 123, 35–47. [Google Scholar] [CrossRef]
- Chambault, P.; Albertsen, C.M.; Patterson, T.A.; Hansen, R.G.; Tervo, O.; Laidre, K.L.; Heide-Jørgensen, M.P. Sea surface temperature predicts the movements of an Arctic cetacean: The bowhead whale. Sci. Rep. 2018, 8, 9658. [Google Scholar] [CrossRef] [PubMed]
- Braun, C.D. Movements and Oceanographic Associations of Large Pelagic Fishes in the North Atlantic Ocean. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, Woods Hole, MA, USA, 2018; 154p. [Google Scholar] [CrossRef]
- Jakubas, D.; Wojczulanis-Jakubas, K.; Iliszko, L.M.; Kidawa, D.; Boehnke, R.; Błachowiak-Samołyk, K.; Stempniewicz, L. Flexibility of little auks foraging in various oceanographic features in a changing Arctic. Sci. Rep. 2020, 10, 8283. [Google Scholar] [CrossRef]
- Sabal, M.C.; Hazen, E.L.; Bograd, S.J.; MacFarlane, R.B.; Schroeder, I.D.; Hayes, S.A.; Harding, J.A.; Scales, K.L.; Miller, P.I.; Ammann, A.J.; et al. California Current seascape influences juvenile salmon foraging ecology at multiple scales. Mar. Ecol. Prog. Ser. 2020, 634, 159–173. [Google Scholar] [CrossRef]
- Svendsen, G.M.; Reinaldo, M.O.; Romero, M.A.; Williams, G.; Magurran, A.; Luque, S.; González, R.A. Drivers of diversity gradients of a highly mobile marine assemblage in a mesoscale seascape. Mar. Ecol. Prog. Ser. 2020, 638, 149–164. [Google Scholar] [CrossRef]
- Podesta, G.P.; Browder, J.A.; Hoey, J.J. Exploring the association between swordfish catch rates and thermal fronts on US longline grounds in the western North Atlantic. Cont. Shelf Res. 1993, 13, 253–277. [Google Scholar] [CrossRef]
- Dalla Rosa, L.; Ford, J.K.; Trites, A.W. Distribution and relative abundance of humpback whales in relation to environmental variables in coastal British Columbia and adjacent waters. Cont. Shelf Res. 2012, 36, 89–104. [Google Scholar] [CrossRef]
- Tseng, C.-T.; Sun, C.-L.; Belkin, I.M.; Yeh, S.-Z.; Kuo, C.-L.; Liu, D.-C. Sea surface temperature fronts affect distribution of Pacific saury (Cololabis saira) in the Northwestern Pacific Ocean. Deep Sea Res. Part II 2014, 107, 15–21. [Google Scholar] [CrossRef]
- Alemany, D.; Iribarne, O.O.; Acha, E.M. Mar. fronts as preferred habitats for young Patagonian hoki Macruronus magellanicus on the southern Patagonian shelf. Mar. Ecol. Prog. Ser. 2018, 588, 191–200. [Google Scholar] [CrossRef]
- Santiago, J.; Uranga, J.; Quincoces, I.; Orue, B.; Grande, M.; Murua, H.; Merino, G.; Urtizberea, A.; Pascual, P.; Boyra, G. A Novel Index of Abundance of Juvenile Yellowfin Tuna in the Indian Ocean Derived from Echosounder Buoys; IOTC-2019-WPTT21-45; FAO: Rome, Italy, 2019; 21p, Available online: https://www.iotc.org/documents/WPTT/21/45 (accessed on 18 February 2021).
- Santiago, J.; Uranga, J.; Quincoces, I.; Orue, B.; Grande, M.; Murua, H.; Merino, G.; Urtizberea, A.; Pascual, P.; Boyra, G. A novel index of abundance of juvenile yellowfin tuna in the Atlantic Ocean derived from echosounder buoys. Collect. Vol. Sci. Pap. ICCAT 2020, 76, 321–343. Available online: https://www.iccat.int/Documents/CVSP/CV076_2019/n_6/CV076060321 (accessed on 18 February 2021).
- Marra, J.; Houghton, R.W.; Garside, C. Phytoplankton growth at the shelf-break front in the Middle Atlantic Bight. J. Mar. Res. 1990, 48, 851–868. [Google Scholar] [CrossRef]
- Guo, L.; Xiu, P.; Chai, F.; Xue, H.J.; Wang, D.X.; Sun, J. Enhanced chlorophyll concentrations induced by Kuroshio intrusion fronts in the northern South China Sea. Geophys. Res. Lett. 2017, 44, 11565–11572. [Google Scholar] [CrossRef]
- Alemany, D.; Acha, E.M.; Iribarne, O. The relationship between marine fronts and fish diversity in the Patagonian Shelf Large Marine Ecosystem. J. Biogeogr. 2009, 36, 2111–2124. [Google Scholar] [CrossRef]
- Kurlansky, M. Cod: A Biography of the Fish that Changed the World; Penguin Books: New York, NY, USA, 1998; 304p. [Google Scholar]
- Uda, M. Researches on “Siome” or current rip in the seas and oceans. Geophys. Mag. 1938, 11, 307–372. Available online: https://lib.s.kaiyodai.ac.jp/library/maincollection/uda-bunko/resources/pdfs/gyouseki/099.pdf (accessed on 18 February 2021).
- Alemany, D.; Acha, E.M.; Iribarne, O.O. Marine fronts are important fishing areas for demersal species at the Argentine Sea (Southwest Atlantic Ocean). J. Sea Res. 2014, 87, 56–67. [Google Scholar] [CrossRef]
- Bogazzi, E.; Baldoni, A.N.A.; Rivas, A.; Martos, P.; Reta, R.A.U.L.; Orensanz, J.M.; Lasta, M.; Dell’Arciprete, P.; Werner, F. Spatial correspondence between areas of concentration of Patagonian scallop (Zygochlamys patagonica) and frontal systems in the southwestern Atlantic. Fish. Oceanogr. 2005, 14, 359–376. [Google Scholar] [CrossRef]
- Mauna, A.C.; Franco, B.C.; Baldoni, A.; Acha, E.M.; Lasta, M.L.; Iribarne, O.O. Cross-front variations in adult abundance and recruitment of Patagonian scallop (Zygochlamys patagonica) at the SW Atlantic Shelf Break Front. ICES J. Mar. Sci. 2008, 65, 1184–1190. [Google Scholar] [CrossRef]
- Herron, R.C.; Leming, T.D.; Li, J.L. Satellite-detected fronts and butterfish aggregations in the northeastern Gulf of Mexico. Continental Shelf Res. 1989, 9, 569–588. [Google Scholar] [CrossRef]
- Bigelow, K.A.; Boggs, C.H.; He, X.I. Environmental effects on swordfish and blue shark catch rates in the US North Pacific longline fishery. Fish. Oceanogr. 1999, 8, 178–198. [Google Scholar] [CrossRef]
- Chelton, D.B.; Schlax, M.G.; Samelson, R.M. Global observations of nonlinear mesoscale eddies. Prog. Oceanogr. 2011, 91, 167–216. [Google Scholar] [CrossRef]
- Lian, Z.; Sun, B.N.; Wei, Z.X.; Wang, Y.G.; Wang, X.Y. Comparison of eight detection algorithms for the quantification and characterization of mesoscale eddies in the South China Sea. J. Atmos. Ocean. Technol. 2019, 36, 1361–1380. [Google Scholar] [CrossRef]
- Braun, C.D.; Gaube, P.; Sinclair-Taylor, T.H.; Skomal, G.B.; Thorrold, S.R. Mesoscale eddies release pelagic sharks from thermal constraints to foraging in the ocean twilight zone. Proc. Natl. Acad. Sci. USA 2019, 116, 17187–17192. [Google Scholar] [CrossRef]
- Gaube, P.; Barceló, C.; McGillicuddy, D.J., Jr.; Domingo, A.; Miller, P.; Giffoni, B.; Marcovaldi, N.; Swimmer, Y. The use of mesoscale eddies by juvenile loggerhead sea turtles (Caretta caretta) in the southwestern Atlantic. PLoS ONE 2017, 12, e0172839. [Google Scholar] [CrossRef]
- Godø, O.R.; Samuelsen, A.; Macaulay, G.J.; Patel, R.; Hjøllo, S.S.; Horne, J.; Kaartvedt, S.; Johannessen, J.A. Mesoscale eddies are oases for higher trophic marine life. PLoS ONE 2012, 7, e30161. [Google Scholar] [CrossRef]
- Arur, A.; Krishnan, P.; Kiruba-Sankar, R.; Suryavanshi, A.; Lohith Kumar, K.; Kantharajan, G.; Choudhury, S.B.; Manjulatha, C.; Babu, D.E. Feasibility of targeted fishing in mesoscale oceanic eddies: A study from commercial fishing grounds of Andaman and Nicobar Islands, India. Int. J. Remote Sens. 2020, 41, 5011–5045. [Google Scholar] [CrossRef]
- Chambault, P.; Baudena, A.; Bjorndal, K.A.; Santos, M.A.R.; Bolten, A.B.; Vandeperre, F. Swirling in the ocean: Immature loggerhead turtles seasonally target old anticyclonic eddies at the fringe of the North Atlantic gyre. Prog. Oceanogr. 2019, 175, 345–358. [Google Scholar] [CrossRef]
- Tew Kai, E.; Marsac, F. Influence of mesoscale eddies on spatial structuring of top predators’ communities in the Mozambique Channel. Prog. Oceanogr. 2010, 86, 214–223. [Google Scholar] [CrossRef]
- Arur, A.; Krishnan, P.; George, G.; Goutham Bharathi, M.P.; Kaliyamoorthy, M.; Hareef Baba Shaeb, K.; Suryavanshi, A.S.; Kumar, T.S.; Joshi, A.K. The influence of mesoscale eddies on a commercial fishery in the coastal waters of the Andaman and Nicobar Islands, India. Int. J. Remote Sens. 2014, 35, 6418–6443. [Google Scholar] [CrossRef][Green Version]
- Ye, H.J.; Kalhoro, M.A.; Morozov, E.; Tang, D.L.; Wang, S.F.; Thies, P.R. Increased chlorophyll-a concentration in the South China Sea caused by occasional sea surface temperature fronts at peripheries of eddies. Int. J. Remote Sens. 2018, 39, 4360–4375. [Google Scholar] [CrossRef]
- Canny, J. A computational approach to edge detection. IEEE Trans. Pattern Anal. Mach. Intell. 1986, 8, 679–698. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Sakaida, F.; Kawamura, H.; Okumura, T. Application of an edge detection method to satellite images for distinguishing sea surface temperature fronts near the Japanese coast. Remote Sens. Environ. 2005, 98, 21–34. [Google Scholar] [CrossRef]
- Miller, P.I. Composite front maps for improved visibility of dynamic sea-surface features on cloudy SeaWiFS and AVHRR data. J. Mar. Syst. 2009, 78, 327–336. [Google Scholar] [CrossRef]
- Nieto, K.; Demarcq, H.; McClatchie, S. Mesoscale frontal structures in the Canary Upwelling System: New front and filament detection algorithms applied to spatial and temporal patterns. Remote Sens. Environ. 2012, 123, 339–346. [Google Scholar] [CrossRef]
- Castelao, R.M.; Barth, J.A.; Mavor, T.P. Flow-topography interactions in the northern California Current System observed from geostationary satellite data. Geophys. Res. Lett. 2005, 32, L24612. [Google Scholar] [CrossRef]
- Castelao, R.M.; Mavor, T.P.; Barth, J.A.; Breaker, L.C. Sea surface temperature fronts in the California Current System from geostationary satellite observations. J. Geophys. Res. Oceans 2006, 111, C09026. [Google Scholar] [CrossRef]
- Castelao, R.M.; Wang, Y.T. Wind-driven variability in sea surface temperature front distribution in the California Current System. J. Geophys. Res. Oceans 2014, 119, 1861–1875. [Google Scholar] [CrossRef]
- Wang, Y.T.; Castelao, R.M.; Yuan, Y.P. Seasonal variability of alongshore winds and sea surface temperature fronts in Eastern Boundary Current Systems. J. Geophys. Res. Oceans 2015, 120, 2385–2400. [Google Scholar] [CrossRef]
- Hu, J.W.; Shi, M.C.; Zhang, T.L.; Chen, S.G.; Wu, L.Y. Evolution of surface cold patches in the North Yellow Sea based on satellite SST data. J. Ocean Univ. China 2016, 15, 936–946. [Google Scholar] [CrossRef]
- Chen, H.H.; Qi, Y.Q.; Wang, Y.T.; Chai, F. Seasonal variability of SST fronts and winds on the southeastern continental shelf of Brazil. Ocean Dyn. 2019, 69, 1387–1399. [Google Scholar] [CrossRef]
- Chen, H.H.; Tang, R.; Zhang, H.R.; Yu, Y.; Wang, Y.T. Investigating the relationship between sea surface chlorophyll and major features of the South China Sea with satellite information. J. Vis. Exp. 2020, e61172. [Google Scholar] [CrossRef] [PubMed]
- Saldías, G.S.; Lara, C. Satellite-derived sea surface temperature fronts in a river-influenced coastal upwelling area off central–southern Chile. Reg. Stud. Mar. Sci. 2020, 37, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.T.; Yu, Y.; Zhang, Y.; Zhang, H.-R.; Chai, F. Distribution and variability of sea surface temperature fronts in the South China Sea. Estuarine Coast. Shelf Sci. 2020, 240, 106793. [Google Scholar] [CrossRef]
- Wall, C.C.; Muller-Karger, F.E.; Roffer, M.A.; Hu, C.M.; Yao, W.S.; Luther, M.E. Satellite remote Sens. of surface oceanic fronts in coastal waters off west-central Florida. Remote Sens. Environ. 2008, 112, 2963–2976. [Google Scholar] [CrossRef]
- Chakraborty, K.; Maity, S.; Lotliker, A.A.; Samanta, A.; Ghosh, J.; Masuluri, N.K.; Swetha, N.; Bright, R.P. Modelling of marine ecosystem in regional scale for short term prediction of satellite-aided operational fishery advisories. J. Oper. Oceanogr. 2019, 12, S157–S175. [Google Scholar] [CrossRef]
- Jones, C.T.; Sikora, T.D.; Vachon, P.W.; Wolfe, J. Toward automated identification of sea surface temperature front signatures in Radarsat-2 images. J. Atmos. Ocean. Technol. 2012, 29, 89–102. [Google Scholar] [CrossRef]
- Jones, C.T.; Sikora, T.D.; Vachon, P.W.; Wolfe, J.; DeTracey, B. Automated discrimination of certain brightness fronts in Radarsat-2 images of the ocean surface. J. Atmos. Ocean. Technol. 2013, 30, 2203–2215. [Google Scholar] [CrossRef]
- Belkin, I.M.; Cornillon, P.C. Fronts in the World Ocean’s Large Marine Ecosystems; ICES CM 2007/D:21; International Council for the Exploration of the Sea: Copenhagen, Denmark, 2007; 33p, Available online: https://www.ices.dk/sites/pub/CM%20Doccuments/CM-2007/D/D2107 (accessed on 18 February 2021).
- Kahru, M.; Håkansson, B.; Rud, O. Distributions of the sea-surface temperature fronts in the Baltic Sea as derived from satellite imagery. Cont. Shelf Res. 1995, 15, 663–679. [Google Scholar] [CrossRef]
- Ullman, D.S.; Cornillon, P.C. Satellite-derived sea surface temperature fronts on the continental shelf of the northeast U.S. coast. J. Geophys. Res. 1999, 104, 23459–23478. [Google Scholar] [CrossRef]
- Ullman, D.S.; Cornillon, P.C. Continental shelf surface thermal fronts in winter off the northeast US coast. Cont. Shelf Res. 2001, 21, 1139–1156. [Google Scholar] [CrossRef]
- Stegmann, P.M.; Ullman, D.S. Variability in chlorophyll and sea surface temperature fronts in the Long Island Sound outflow region from satellite observations. J. Geophys. Res. C Oceans 2004, 109, C07S03. [Google Scholar] [CrossRef]
- Mavor, T.P.; Bisagni, J.J. Seasonal variability of sea-surface temperature fronts on Georges Bank. Deep Sea Res. Part II 2001, 48, 215–243. [Google Scholar] [CrossRef]
- Schick, R.S.; Goldstein, J.; Lutcavage, M.E. Bluefin tuna (Thunnus thynnus) distribution in relation to sea surface temperature fronts in the Gulf of Maine (1994-96). Fish. Oceanogr. 2004, 13, 225–238. [Google Scholar] [CrossRef]
- Peliz, Á.; Dubert, J.; Santos, A.M.P.; Oliveira, P.B.; Le Cann, B. Winter upper ocean circulation in the Western Iberian Basin—Fronts, eddies and poleward flows: An overview. Deep Sea Res. Part I 2005, 52, 621–646. [Google Scholar] [CrossRef]
- Otero, P.; Ruiz-Villarreal, M.; Peliz, Á. River plume fronts off NW Iberia from satellite observations and model data. ICES J. Mar. Sci. 2009, 66, 1853–1864. [Google Scholar] [CrossRef]
- Mantas, V.M.; Pereira, A.J.S.C.; Marques, J.C. Partitioning the ocean using dense time series of Earth Observation data. Regions and natural boundaries in the Western Iberian Peninsula. Ecol. Indic. 2019, 103, 9–21. [Google Scholar] [CrossRef]
- Ullman, D.S.; Cornillon, P.C.; Shan, Z. On the characteristics of subtropical fronts in the North Atlantic. J. Geophys. Res. Oceans 2007, 112, C01010. [Google Scholar] [CrossRef]
- Cyr, F.; Larouche, P. Thermal Fronts Atlas of Canadian Coastal Waters. Atmos. Ocean 2015, 53, 212–236. [Google Scholar] [CrossRef]
- Miller, P.I.; Read, J.F.; Dale, A.C. Thermal front variability along the North Atlantic Current observed using microwave and infrared satellite data. Deep Sea Res. Part II 2013, 98, 244–256. [Google Scholar] [CrossRef]
- Belkin, I.M.; Cornillon, P.C. SST fronts of the Pacific coastal and marginal seas. Pacific Oceanogr. 2003, 1, 90–113. [Google Scholar]
- Belkin, I.M.; Cornillon, P.C.; Ullman, D.S. Ocean fronts around Alaska from satellite SST data. In Proceedings of the American Meteorological Society’s 7th Conference on the Polar Meteorology and Oceanography and Joint Symposium on High-Latitude Climate Variations, Hyannis, MA, USA, 12–16 May 2003; Paper 12.7. Available online: https://ams.confex.com/ams/7POLAR/techprogram/paper_61548.htm (accessed on 18 February 2021).
- Belkin, I.M.; Cornillon, P.C. Bering Sea thermal fronts from Pathfinder data: Seasonal and interannual variability. Pac. Oceanogr. 2005, 3, 6–20. [Google Scholar]
- Belkin, I.M. Comparative assessment of the West Bering Sea and East Bering Sea Large Marine Ecosystems. Environ. Dev. 2016, 17, 145–156. [Google Scholar] [CrossRef]
- Belkin, I.M.; Cornillon, P.C. Surface thermal fronts of the Okhotsk Sea. Pac. Oceanogr. 2004, 2, 6–19. [Google Scholar]
- Kahru, M.; Di Lorenzo, E.; Manzano-Sarabia, M.; Mitchell, B.G. Spatial and temporal statistics of sea surface temperature and chlorophyll fronts in the California Current. J. Plankton Res. 2012, 34, 749–760. [Google Scholar] [CrossRef]
- Kahru, M.; Jacox, M.; Ohman, M.D. CCE1: Decrease in the frequency of oceanic fronts and surface chlorophyll concentration in the California Current System during the 2014-2016 northeast Pacific warm anomalies. Deep Sea Res. Part I 2018, 140, 4–13. [Google Scholar] [CrossRef]
- Hickox, R.; Belkin, I.; Cornillon, P.; Shan, Z. Climatology and seasonal variability of ocean fronts in the East China, Yellow and Bohai Seas from satellite SST data. Geophys. Res. Lett. 2000, 27, 2945–2948. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Qi, Y.; Shi, P. Seasonal variability of thermal fronts in the northern South China Sea from satellite data. Geophys. Res. Lett. 2001, 28, 3963–3966. [Google Scholar] [CrossRef]
- Yao, J.L.; Belkin, I.; Chen, J.; Wang, D.X. Thermal fronts of the southern South China Sea from satellite and in situ data. Int. J. Remote Sens. 2012, 33, 7458–7468. [Google Scholar] [CrossRef]
- Hobday, A.J.; Hartog, J.R. Derived ocean features for dynamic ocean management. Oceanography 2014, 27, 134–145. [Google Scholar] [CrossRef]
- Bedriñana-Romano, L.; Hucke-Gaete, R.; Viddi, F.A.; Morales, J.; Williams, R.; Ashe, E.; Garcés-Vargas, J.; Torres-Florez, J.P.; Ruiz, J. Integrating multiple data sources for assessing blue whale abundance and distribution in Chilean Northern Patagonia. Divers. Distrib. 2018, 24, 991–1004. [Google Scholar] [CrossRef]
- Eladawy, A.; Nadaoka, K.; Negm, A.; Abdel-Fattah, S.; Hanafy, M.; Shaltout, M. Characterization of the northern Red Sea’s oceanic features with remote sensing data and outputs from a global circulation model. Oceanologia 2017, 59, 213–237. [Google Scholar] [CrossRef]
- Mohanty, P.C.; Mahendra, R.S.; Nayak, R.K.; Nimit, K.N.; Srinivasa Kumar, T.; Dwivedi, R.M. Persistence of productive surface thermal fronts in the northeast Arabian Sea. Reg. Stud. Mar. Sci. 2017, 16, 216–224. [Google Scholar] [CrossRef]
- Sarkar, K.; Aparna, S.G.; Dora, S.; Shankar, D. Seasonal variability of sea-surface temperature fronts associated with large marine ecosystems in the north Indian Ocean. J. Earth Syst. Sci. 2019, 128, 20. [Google Scholar] [CrossRef]
- Mustapha, S.B.; Larouche, P.; Dubois, J.-M. Spatial and temporal variability of sea-surface temperature fronts in the coastal Beaufort Sea. Cont. Shelf Res. 2016, 124, 134–141. [Google Scholar] [CrossRef]
- Bontempi, P.S.; Yoder, J.A. Spatial variability in SeaWiFS imagery of the South Atlantic bight as evidenced by gradients (fronts) in chlorophyll a and water-leaving radiance. Deep Sea Res. Part II 2004, 51, 1019–1032. [Google Scholar] [CrossRef]
- Roberts, J.J.; Best, B.D.; Dunn, D.C.; Treml, E.A.; Halpin, P.N. Marine Geospatial Ecology Tools: An integrated framework for ecological geoprocessing with ArcGIS, Python, R, MATLAB, and C++. Environ. Model. Softw. 2010, 25, 1197–1207. [Google Scholar] [CrossRef]
- Miller, P. Multi-spectral front maps for automatic detection of ocean colour features from SeaWiFS. Int. J. Remote Sens. 2004, 25, 1437–1442. [Google Scholar] [CrossRef]
- Miller, P.I.; Xu, W.; Carruthers, M. Seasonal shelf-sea front mapping using satellite ocean colour and temperature to support development of a marine protected area network. Deep Sea Res. Part II 2015, 119, 3–19. [Google Scholar] [CrossRef]
- Suberg, L.A.; Miller, P.I.; Wynn, R.B. On the use of satellite-derived frontal metrics in time series analyses of shelf-sea fronts, a study of the Celtic Sea. Deep Sea Res. Part I 2019, 149, 103033. [Google Scholar] [CrossRef]
- Nieblas, A.E.; Demarcq, H.; Drushka, K.; Sloyan, B.; Bonhommeau, S. Front variability and surface ocean features of the presumed southern bluefin tuna spawning grounds in the tropical southeast Indian Ocean. Deep Sea Res. Part II 2014, 107, 64–76. [Google Scholar] [CrossRef]
- Roa-Pascuali, L.; Demarcq, H.; Nieblas, A.-E. Detection of mesoscale thermal fronts from 4 km data using smoothing techniques: Gradient-based fronts classification and basin scale application. Remote Sens. Environ. 2015, 164, 225–237. [Google Scholar] [CrossRef]
- Nieto, K.; Xu, Y.; Teo, S.L.; McClatchie, S.; Holmes, J. How important are coastal fronts to albacore tuna (Thunnus alalunga) habitat in the Northeast Pacific Ocean? Prog. Oceanogr. 2017, 150, 62–71. [Google Scholar] [CrossRef]
- Xu, Y.; Nieto, K.; Teo, S.L.; McClatchie, S.; Holmes, J. Influence of fronts on the spatial distribution of albacore tuna (Thunnus alalunga) in the Northeast Pacific over the past 30 years (1982–2011). Prog. Oceanogr. 2017, 150, 72–78. [Google Scholar] [CrossRef]
- Vazquez, D.P.; Atae-Allah, C.; Luque-Escamilla, P.L. Entropic approach to edge detection for SST images. J. Atmos. Ocean. Technol. 1999, 16, 970–979. [Google Scholar] [CrossRef]
- Barranco-López, V.-L.; Luque-Escamilla, P.-E.; Martίnez-Aroza, J.-A.; Román-Roldán, R.-R. Entropic texture-edge detection for image segmentation. Electron. Lett. 1995, 31, 867–869. [Google Scholar] [CrossRef]
- Lin, J.H. Divergence measures based on the Shannon entropy. IEEE Trans. Inf. Theory 1991, 37, 145–151. [Google Scholar] [CrossRef]
- Liu, D.Y.; Wang, Y.N.; Wang, Y.Q.; Keesing, J.K. Ocean fronts construct spatial zonation in microfossil assemblages. Global Ecol. Biogeogr. 2018, 27, 1225–1237. [Google Scholar] [CrossRef]
- Sun, J.C.; Yang, D.Z.; Yin, B.S.; Chen, H.Y.; Feng, X.R. Onshore warm tongue and offshore cold tongue in the western Yellow Sea in winter: The evidence. J. Oceanol. Limnol. 2018, 36, 1475–1483. [Google Scholar] [CrossRef]
- Lin, L.; Liu, D.Y.; Luo, C.X.; Xie, L. Double fronts in the Yellow Sea in summertime identified using sea surface temperature data of multi-scale ultra-high resolution analysis. Cont. Shelf Res. 2019, 175, 76–86. [Google Scholar] [CrossRef]
- Zeng, X.Z.; Belkin, I.M.; Peng, S.Q.; Li, Y.N. East Hainan upwelling fronts detected by remote sensing and modelled in summer. Int. J. Remote Sens. 2014, 35, 4441–4451. [Google Scholar] [CrossRef]
- Liu, Z.; Hou, Y.J. Kuroshio front in the East China Sea from satellite SST and remote Sens. data. IEEE GeoSci. Remote Sens. Lett. 2012, 9, 517–520. [Google Scholar] [CrossRef]
- Scales, K.L.; Miller, P.I.; Hawkes, L.A.; Ingram, S.N.; Sims, D.W.; Votier, S.C. On the front line: Frontal zones as priority at-sea conservation areas for mobile marine vertebrates. J. Appl. Ecol. 2014, 51, 1575–1583. [Google Scholar] [CrossRef]
- Hazen, E.L.; Scales, K.L.; Maxwell, S.M.; Briscoe, D.K.; Welch, H.; Bograd, S.J.; Bailey, H.; Benson, S.R.; Eguchi, T.; Dewar, H.; et al. A dynamic ocean management tool to reduce bycatch and support sustainable fisheries. Sci. Adv. 2018, 4, eaar3001. [Google Scholar] [CrossRef]
- Abdullah, M.; Malik, S.; Siddiqui, M.D.; Khan, A.A. Satellite derived sea surface temperature fronts in relation with tuna catch in EEZ of Pakistan. Pak. J. Eng. Technol. Sci. 2017, 7, 19–31. [Google Scholar] [CrossRef][Green Version]
- Austin, R.A.; Hawkes, L.A.; Doherty, P.D.; Henderson, S.M.; Inger, R.; Johnson, L.; Pikesley, S.K.; Solandt, J.L.; Speedie, C.; Witt, M.J. Predicting habitat suitability for basking sharks (Cetorhinus maximus) in UK waters using ensemble ecological niche modelling. J. Sea Res. 2019, 153, 101767. [Google Scholar] [CrossRef]
- Brigolin, D.; Girardi, P.; Miller, P.I.; Xu, W.; Nachite, D.; Zucchetta, M.; Pranovi, F. Using remote sensing indicators to investigate the association of landings with fronts: Application. to the Alboran Sea (western Mediterranean Sea). Fish. Oceanogr. 2018, 27, 408–416. [Google Scholar] [CrossRef]
- Brodie, S.; Hobday, A.J.; Smith, J.A.; Everett, J.D.; Taylor, M.D.; Gray, C.A.; Suthers, I.M. Modelling the oceanic habitats of two pelagic species using recreational fisheries data. Fish. Oceanogr. 2015, 24, 463–477. [Google Scholar] [CrossRef]
- Camacho, D. Influence of Ocean Fronts on Cetacean Habitat Selection and Diversity within the CalCOFI Study Area; University of California San Diego Capstone Papers; UC San Diego: Center for Marine Biodiversity and Conservation: San Diego, CA, USA, 2010; 34p, Available online: https://escholarship.org/uc/item/2d39303q (accessed on 18 February 2021).
- Chen, X.J.; Tian, S.Q.; Guan, W.J. Variations of oceanic fronts and their influence on the fishing grounds of Ommastrephes bartramii in the Northwest Pacific. Acta Oceanol. Sin. 2014, 33, 45–54. [Google Scholar] [CrossRef]
- Dell, J.; Wilcox, C.; Hobday, A.J. Estimation of yellowfin tuna (Thunnus albacares) habitat in waters adjacent to Australia’s East Coast: Making the most of commercial catch data. Fish. Oceanogr. 2011, 20, 383–396. [Google Scholar] [CrossRef]
- Dodge, K.L.; Galuardi, B.; Miller, T.J.; Lutcavage, M.E. Leatherback turtle movements, dive behavior, and habitat characteristics in ecoregions of the Northwest Atlantic Ocean. PLoS ONE 2014, 9, e91726. [Google Scholar] [CrossRef]
- Druon, J.N. Ocean Productivity Index for Fish in the Arctic: First Assessment from Satellite-Derived Plankton-to-Fish Favourable Habitats; EUR 29006 EN.; Publications Office of the European Union: Luxembourg, 2017; ISBN 978-92-79-77299-3. JRC109947. [Google Scholar] [CrossRef]
- Ebango Ngando, N.; Song, L.M.; Cui, H.X.; Xu, S.Q. Relationship between the spatiotemporal distribution of dominant small pelagic fishes and environmental factors in Mauritanian waters. J. Ocean Univ. China 2020, 19, 393–408. [Google Scholar] [CrossRef]
- Francis, P.A.; Jithin, A.K.; Effy, J.B.; Chatterjee, A.; Chakraborty, K.; Paul, A.; Balaji, B.; Shenoi, S.S.C.; Biswamoy, P.; Mukherjee, A.; et al. High-resolution operational ocean forecast and reanalysis system for the Indian ocean. Bull. Am. Meteorol. Soc. 2020, 101, E1340–E1356. [Google Scholar] [CrossRef]
- Friedland, K.D.; Langan, J.A.; Large, S.I.; Selden, R.L.; Link, J.S.; Watson, R.A.; Collie, J.S. Changes in higher trophic level productivity, diversity and niche space in a rapidly warming continental shelf ecosystem. Sci. Total Environ. 2020, 704, 135270. [Google Scholar] [CrossRef] [PubMed]
- Glembocki, N.G.; Williams, G.N.; Góngora, M.E.; Gagliardini, D.A.; Orensanz, J.M.L. Synoptic oceanography of San Jorge Gulf (Argentina): A template for Patagonian red shrimp (Pleoticus muelleri) spatial dynamics. J. Sea Res. 2015, 95, 22–35. [Google Scholar] [CrossRef]
- Haberlin, D.; Raine, R.; McAllen, R.; Doyle, T.K. Distinct gelatinous zooplankton communities across a dynamic shelf sea. Limnol. Oceanogr. 2019, 64, 1802–1818. [Google Scholar] [CrossRef]
- Hidayat, R.; Zainuddin, M.; Putri, A.R.S.; Safruddin, S. Skipjack tuna (Katsuwonus pelamis) catches in relation to chlorophyll-a front in Bone Gulf during the southeast monsoon. Aquac. Aquar. Conserv. Legis. 2019, 12, 209–218. Available online: http://www.bioflux.com.ro/aacl (accessed on 18 February 2021).
- Hsu, T.Y.; Chang, Y. Modeling the habitat suitability index of skipjack tuna (Katsuwonus pelamis) in the Western and Central Pacific Ocean. In Proceedings of the 2017 IEEE International GeoScience and Remote Sensing Symposium (IGARSS), Fort Worth, TX, USA, 23–28 July 2017; pp. 2950–2953. [Google Scholar] [CrossRef]
- Hua, C.X.; Li, F.; Zhu, Q.C.; Zhua, G.P.; Meng, L.W. Habitat suitability of Pacific saury (Cololabis saira) based on a yield-density model and weighted analysis. Fish. Res. 2020, 221, 105408. [Google Scholar] [CrossRef]
- Jishad, M.; Sarangi, R.K.; Ratheesh, S.; Ali, S.M.; Sharma, R. Tracking fishing ground parameters in cloudy region using ocean colour and satellite-derived surface flow estimates: A study in the Bay of Bengal. J. Oper. Oceanogr. 2019. [Google Scholar] [CrossRef]
- Kulik, V.V.; Baitaliuk, A.A.; Katugin, O.N.; Ustinova, E.I. Modeling distribution of saury catches in relation with environmental factors. Izv. TINRO 2019, 199, 193–213, (in Russian, with English abstract and captions). [Google Scholar] [CrossRef]
- Lan, K.W.; Kawamura, H.; Lee, M.A.; Lu, H.J.; Shimada, T.; Hosoda, K.; Sakaida, F. Relationship between albacore (Thunnus alalunga) fishing grounds in the Indian Ocean and the thermal environment revealed by cloud-free microwave sea surface temperature. Fish. Res. 2012, 113, 1–7. [Google Scholar] [CrossRef]
- Lennert-Cody, C.E.; Roberts, J.J.; Stephenson, R.J. Effects of gear characteristics on the presence of bigeye tuna (Thunnus obesus) in the catches of the purse-seine fishery of the eastern Pacific Ocean. ICES J. Mar. Sci. 2008, 65, 970–978. [Google Scholar] [CrossRef]
- Liu, S.H.; Liu, Y.; Alabia, I.D.; Tian, Y.; Ye, Z.; Yu, H.; Li, J.; Cheng, J. Impact of climate change on wintering ground of Japanese anchovy (Engraulis japonicus) using marine geospatial statistics. Front. Mar. Sci. 2020, 7, 604. [Google Scholar] [CrossRef]
- Louzao, M.; Bécares, J.; Rodríguez, B.; Hyrenbach, K.D.; Ruiz, A.; Arcos, J.M. Combining vessel-based surveys and tracking data to identify key Mar. areas for seabirds. Mar. Ecol. Prog. Ser. 2009, 391, 183–197. [Google Scholar] [CrossRef]
- Mazur, M.D.; Friedland, K.D.; McManus, M.C.; Goode, A.G. Dynamic changes in American lobster suitable habitat distribution on the Northeast US Shelf linked to oceanographic conditions. Fish. Oceanogr. 2020, 29, 349–365. [Google Scholar] [CrossRef]
- Miller, P.I.; Scales, K.L.; Ingram, S.N.; Southall, E.J.; Sims, D.W. Basking sharks and oceanographic fronts: Quantifying associations in the north-east Atlantic. Funct. Ecol. 2015, 29, 1099–1109. [Google Scholar] [CrossRef]
- Mitchell, J.D.; Collins, K.J.; Miller, P.I.; Suberg, L.A. Quantifying the impact of environmental variables upon catch per unit effort of the blue shark Prionace glauca in the western English Channel. J. Fish Biol. 2014, 85, 657–670. [Google Scholar] [CrossRef]
- Mugo, R.M.; Saitoh, S.I.; Takahashi, F.; Nihira, A.; Kuroyama, T. Evaluating the role of fronts in habitat overlaps between cold and warm water species in the western North Pacific: A proof of concept. Deep Sea Res. Part II 2014, 107, 29–39. [Google Scholar] [CrossRef]
- Nishizawa, B.; Ochi, D.; Minami, H.; Yokawa, K.; Saitoh, S.I.; Watanuki, Y. Habitats of two albatross species during the non-breeding season in the North Pacific Transition Zone. Mar. Biol. 2015, 162, 743–752. [Google Scholar] [CrossRef]
- Oh, Y.J.; Kim, D.W.; Jo, Y.H.; Hwang, J.D.; Chung, C.Y. Spatial variability of fishing grounds in response to oceanic front changes detected by multiple satellite measurements in the East (Japan) Sea. Int. J. Remote Sens. 2020, 41, 5884–5904. [Google Scholar] [CrossRef]
- Pikesley, S.K.; Maxwell, S.M.; Pendoley, K.; Costa, D.P.; Coyne, M.S.; Formia, A.; Godley, B.J.; Klein, W.; Makanga-Bahouna, J.; Maruca, S.; et al. On the front line: Integrated habitat mapping for olive ridley sea turtles in the southeast Atlantic. Divers. Distrib. 2013, 19, 1518–1530. [Google Scholar] [CrossRef]
- Pikesley, S.K.; Broderick, A.C.; Cejudo, D.; Coyne, M.S.; Godfrey, M.H.; Godley, B.J.; Lopez, P.; López-Jurado, L.F.; Elsy Merino, S.; Varo-Cruz, N.; et al. Modelling the niche for a marine vertebrate: A case study incorporating behavioural plasticity, proximate threats and climate change. Ecography 2015, 38, 803–812. [Google Scholar] [CrossRef]
- Reese, D.C.; O’Malley, R.T.; Brodeur, R.D.; Churnside, J.H. Epipelagic fish distributions in relation to thermal fronts in a coastal upwelling system using high-resolution remote-sensing techniques. ICES J. Mar. Sci. 2011, 68, 1865–1874. [Google Scholar] [CrossRef]
- Retana, M.V.; Lewis, M.N. Suitable habitat for marine mammals during austral summer in San Jorge Gulf, Argentina. Rev. Biol. Mar. Oceanogr. 2017, 52, 275–288. [Google Scholar] [CrossRef]
- Royer, F.; Fromentin, J.M.; Gaspar, P. Association between bluefin tuna schools and oceanic features in the western Mediterranean. Mar. Ecol. Prog. Ser. 2004, 269, 249–263. [Google Scholar] [CrossRef]
- Sagarminaga, Y.; Arrizabalaga, H. Relationship of Northeast Atlantic albacore juveniles with surface thermal and chlorophyll-a fronts. Deep Sea Res. Part II 2014, 107, 54–63. [Google Scholar] [CrossRef]
- Scales, K.L.; Miller, P.I.; Varo-Cruz, N.; Hodgson, D.J.; Hawkes, L.A.; Godley, B.J. Oceanic loggerhead turtles Caretta caretta associate with thermal fronts: Evidence from the Canary Current Large Mar. Ecosystem. Mar. Ecol. Prog. Ser. 2015, 519, 195–207. [Google Scholar] [CrossRef]
- Soldatini, C.; Albores-Barajas, Y.V.; Ramos-Rodriguez, A.; Munguia-Vega, A.; González+Rodríguez, E.; Catoni, C.; Dell’Omo, G. Tracking reveals behavioural coordination driven by environmental constraints in the black-vented shearwater Puffinus opisthomelas. Popul. Ecol. 2019, 61, 227–239. [Google Scholar] [CrossRef]
- Sousa, L.L.; López-Castejón, F.; Gilabert, J.; Relvas, P.; Couto, A.; Queiroz, N.; Caldas, R.; Dias, P.S.; Dias, H.; Faria, M.; et al. Integrated monitoring of Mola mola behaviour in space and time. PLoS ONE 2016, 11, e0160404. [Google Scholar] [CrossRef] [PubMed]
- Suhadha, A.G.; Ibrahim, A. Association study between thermal front phenomena and Bali sardinella fishing areas in Bali Strait. Indones. J. Geogr. 2020, 52, 154–162. [Google Scholar] [CrossRef]
- Swetha, N.; Nimit, K.; Nayak, J.; Maity, S.; Preethi, M.; Bright, R.P.; Immaneni, S. Automated Identification of Oceanic Fronts for Operational Generation of Potential Fishing Zone (PFZ) Advisories; ESSO/INCOIS/ASG/TR/02(2017); Indian National Centre for Ocean Information Services: Hyderabad, India, 2017. Available online: http://moeseprints.incois.gov.in/id/eprint/4506 (accessed on 18 February 2021).
- Thorne, L.H.; Baird, R.W.; Webster, D.L.; Stepanuk, J.E.; Read, A.J. Predicting fisheries bycatch: A case study and field test for pilot whales in a pelagic longline fishery. Divers. Distrib 2019, 25, 909–923. [Google Scholar] [CrossRef]
- Trew, B.T.; Grantham, H.S.; Barrientos, C.; Collins, T.; Doherty, P.D.; Formia, A.; Godley, B.J.; Maxwell, S.M.; Parnell, R.J.; Pikesley, S.K.; et al. Using cumulative impact mapping to prioritise marine conservation efforts in Equatorial Guinea. Front. Mar. Sci. 2019, 6, 717. [Google Scholar] [CrossRef]
- Varo-Cruz, N.; Bermejo, J.A.; Calabuig, P.; Cejudo, D.; Godley, B.J.; López-Jurado, L.F.; Pikesley, S.K.; Witt, M.J.; Hawkes, L.A. New findings about the spatial and temporal use of the Eastern Atlantic Ocean by large juvenile loggerhead turtles. Divers. Distrib. 2016, 22, 481–492. [Google Scholar] [CrossRef]
- Wall, C.C.; Muller-Karger, F.E.; Roffer, M.A. Linkages between environmental conditions and recreational king mackerel (Scomberomorus cavalla) catch off west-central Florida. Fish. Oceanogr. 2009, 18, 185–199. [Google Scholar] [CrossRef]
- Wang, J.; Pierce, G.J.; Sacau, M.; Portela, J.; Santos, M.B.; Cardoso, X.; Bellido, J.M. Remotely sensed local oceanic thermal features and their influence on the distribution of hake (Merluccius hubbsi) at the Patagonian shelf edge in the SW Atlantic. Fish. Res. 2007, 83, 133–144. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chen, W.Y.; Chang, Y.; Lee, M.A. Ichthyoplankton community associated with oceanic fronts in early winter on the continental shelf of the southern East China Sea. J. Mar. Sci. Technol. 2013, 21, 65–76. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chan, J.W.; Lan, Y.C.; Yang, W.C.; Lee, M.A. Satellite observation of the winter variation of sea surface temperature fronts in relation to the spatial distribution of ichthyoplankton in the continental shelf of the southern East China Sea. Int. J. Remote Sens. 2018, 39, 4550–4564. [Google Scholar] [CrossRef]
- White, T.P.; Veit, R.R. Spatial Ecology of long-tailed ducks and white-winged scoters wintering on Nantucket Shoals. Ecosphere 2020, 11, e03002. [Google Scholar] [CrossRef]
- Zainuddin, M.; Mallawa, A.; Safruddin, S.; Hidayat, R.; Putri, A.R.S.; Ridwan, M. Spatio-temporal thermal fronts distribution during January-December 2018 in the Makassar Strait: An important implication for pelagic fisheries. J. Ilmu Kelaut. J. Mar. Sci. SPERMONDE 2020, 6, 11–15. [Google Scholar] [CrossRef]
- Zhou, C.; He, P.G.; Xu, L.X.; Bach, P.; Wang, X.F.; Wan, R.; Tang, H.; Zhang, Y. The effects of mesoscale oceanographic structures and ambient conditions on the catch of albacore tuna in the South Pacific longline fishery. Fish. Oceanogr. 2020, 29, 238–251. [Google Scholar] [CrossRef]
| Reference (First Author, Ref. No.) | Region | Front Detection Algorithm/Technique | Species |
|---|---|---|---|
| Abdullah [154] | Arabian Sea | CCA-SIED | Tuna |
| Austin [155] | NE Atlantic off UK | CCA-SIED-MGET; Miller | Basking shark |
| Bedriñana-Romano [130] | Chilean Patagonia | CCA-SIED-MGET | Blue whale |
| Bigelow [78] | North Pacific | Gradient | Swordfish, blue shark |
| Bogazzi [75] | Patagonian Shelf | Gradient | Patagonian scallops |
| Braun [59] | North Atlantic | BOA-RG | Sharks |
| Brigolin [156] | Alboran Sea | CCA-SIED; Miller | Various fishes |
| Brodie [157] | Tasman Sea | CCA | Dolphinfish, kingfish |
| Byrne [40] | North Atlantic | BOA-RG | Shortfin mako shark |
| Camacho [158] | California Current | CCA-SIED | Whales and dolphins |
| Chakraborty [103] | North Indian Ocean | CCA-SIED; Canny | Various species |
| Chambault [57] | Gulf Stream | Gradient | Leatherback turtle |
| Chambault [58] | Baffin Bay, Hudson Bay | Gradient | Bowhead whale |
| Chen X.J. [159] | NW Pacific | Gradient | Neon flying squid |
| Cox [56] | Celtic Sea | CCA-SIED; Miller | Gannets |
| Dalla Rosa [64] | NE Pacific | CCA-SIED-MGET | Humpback whale |
| Dell [160] | Tasman Sea | CCA-SIED | Yellowfin tuna |
| Dodge [161] | Northwest Atlantic | BOA-RG | Leatherback turtle |
| Druon [51] | Mediterranean Sea | Gradient | Atlantic bluefin tuna |
| Druon [162] | Arctic Ocean | Gradient | Various species |
| Ebango Ngando [163] | Mauritanian upwelling | CCA-SIED | Mackerel, sardinella |
| Etnoyer [49] | NE Subtropical Pacific | Gradient | Blue whales, turtles |
| Francis [164] | Indian Ocean | CCA-SIED; Canny | Potential fishing zone |
| Friedland [165] | US Northeast Shelf | Gradient | Various species |
| Glembocki [166] | Patagonian Shelf | Gradient | Patagonian red shrimp |
| Haberlin [167] | Celtic Sea | CCA-SIED-MGET | Gelatinous zooplankton |
| Herron [77] | Gulf of Mexico | Gradient | Butterfish |
| Hidayat [168] | Bone Gulf, Indonesia | CCA-SIED | Skipjack tuna |
| Hsu [169] | West & Central Pacific | BOA | Skipjack tuna |
| Hua [170] | NW Pacific | Gradient | Pacific saury |
| Jakubas [60] | Svalbard | CCA-SIED | Little auks |
| Jishad [171] | Bay of Bengal | Gradient | Various species |
| Kulik [172] | Northwest Pacific | BOA-RG | Pacific saury |
| Lan [173] | South Indian Ocean | Shimada | Albacore tuna |
| Lennert-Cody [174] | Eastern Tropical Pacific | CCA-SIED | Bigeye tuna |
| Liao [39] | East China Sea | Shimada | Swordtip squid |
| Liu [175] | East China Seas | CCA-SIED-MGET | Anchovy |
| Louzao [176] | Western Mediterranean | CCA-SIED-MGET | Cory’s shearwater |
| Luo [55] | Western North Atlantic | BOA | Tuna, marlin, sailfish |
| Mauna [76] | Patagonian Shelf | Gradient | Patagonian scallop |
| Mazur [177] | US Northeast Shelf | CCA | American lobster |
| Miller [178] | NE Atlantic | CCA-SIED; Miller | Basking shark |
| Mitchell [179] | English Channel | CCA-SIED | Blue shark |
| Mugo [180] | NW Pacific | CCA-SIED-MGET | Tuna, squid, saury |
| Nieblas [140] | SE Tropical Indian Ocean | CCA-SIED-Nieto; Canny | Southern bluefin tuna |
| Nieto [142] | NE Pacific | CCA-SIED-Nieto | Albacore tuna |
| Nishizawa [181] | North Pacific | CCA-SIED-MGET | Albatrosses |
| Oh [182] | Japan Sea | BOA | Japanese flying squid |
| Pikesley [183] | Gabon-Angola | CCA-SIED-MGET | Olive ridley turtle |
| Pikesley [184] | Cape Verde | CCA-SIED-MGET | Loggerhead turtle |
| Podesta [63] | Mid-Atlantic Bight | CCA | Swordfish |
| Reese [185] | California Current | CCA-SIED | Sardine, anchovy, herring |
| Retana [186] | San Jorge Gulf, Arg. | CCA-SIED-MGET | Marine mammals |
| Royer [187] | Gulf of Lions, Med. Sea | Canny | Bluefin tuna |
| Sabal [61] | California Current | CCA-SIED | Salmon |
| Sabarros [53] | Benguela Upwelling | CCA-SIED-Nieto | Cape gannet |
| Sagarminaga [188] | NE Atlantic | BOA | Albacore tuna |
| Santiago [67] | Indian Ocean | BOA-RG | Yellowfin tuna |
| Santiago [68] | Atlantic Ocean | BOA-RG | Yellowfin tuna |
| Sarma [48] | Arabian Sea | CCA-SIED | Zooplankton |
| Scales [152] | Celtic Sea | CCA-SIED; Miller | Gannet |
| Scales [189] | Canary Current | CCA-SIED; Miller | Loggerhead turtle |
| Scales [54] | South Atlantic | CCA-SIED; Miller | Grey-headed albatross |
| Schick [112] | Gulf of Maine | CCA | Bluefin tuna |
| Soldatini [190] | Baja California Peninsula | BOA | Black-vented shearwater |
| Sousa [191] | Gulf of Cadiz | CCA-SIED-MGET | Sunfish (mola mola) |
| Suhadha [192] | Bali Strait, Indonesia | CCA-SIED-MGET | Bali sardinella |
| Svendsen [62] | San Matias Gulf, Arg. | CCA-SIED-MGET | Various species |
| Swetha [193] | Northern Indian Ocean | CCA-SIED-MGET | Potential Fishing Zone |
| Thorne [194] | NW Atlantic | CCA-SIED-MGET | Pilot whale |
| Trew [195] | Gulf of Guinea | CCA-SIED-MGET | Mammals, turtles |
| Tseng [65] | Northwest Pacific | CCA-SIED | Pacific saury |
| Varo-Cruz [196] | Eastern North Atlantic | CCA-SIED-MGET | Loggerhead turtle |
| Wall [197] | West Florida Shelf | CCA-SIED; Canny | King mackerel |
| Wang J [198] | Patagonian Shelf | Gradient | Hake |
| Wang YC [199] | South China Sea | Shimada | Ichthyoplankton |
| Wang YC [200] | East China Sea | Shimada | Ichthyoplankton |
| White [201] | Nantucket Shoals | BOA | Ducks, scoters |
| Woodson [52] | California Current | BOA | Rockfishes, invertebrates |
| Xu [143] | NE Pacific | CCA-SIED-Nieto | Albacore tuna |
| Zainuddin [202] | Makassar Strait | CCA-SIED | Skipjack tuna |
| Zhou [203] | South Pacific | BOA; CCA-SIED-Nieto | Albacore tuna |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belkin, I.M. Remote Sensing of Ocean Fronts in Marine Ecology and Fisheries. Remote Sens. 2021, 13, 883. https://doi.org/10.3390/rs13050883
Belkin IM. Remote Sensing of Ocean Fronts in Marine Ecology and Fisheries. Remote Sensing. 2021; 13(5):883. https://doi.org/10.3390/rs13050883
Chicago/Turabian StyleBelkin, Igor M. 2021. "Remote Sensing of Ocean Fronts in Marine Ecology and Fisheries" Remote Sensing 13, no. 5: 883. https://doi.org/10.3390/rs13050883
APA StyleBelkin, I. M. (2021). Remote Sensing of Ocean Fronts in Marine Ecology and Fisheries. Remote Sensing, 13(5), 883. https://doi.org/10.3390/rs13050883