A Multi-Source Data Fusion Decision-Making Method for Disease and Pest Detection of Grape Foliage Based on ShuffleNet V2
Abstract
:1. Introduction
2. Materials and Methods
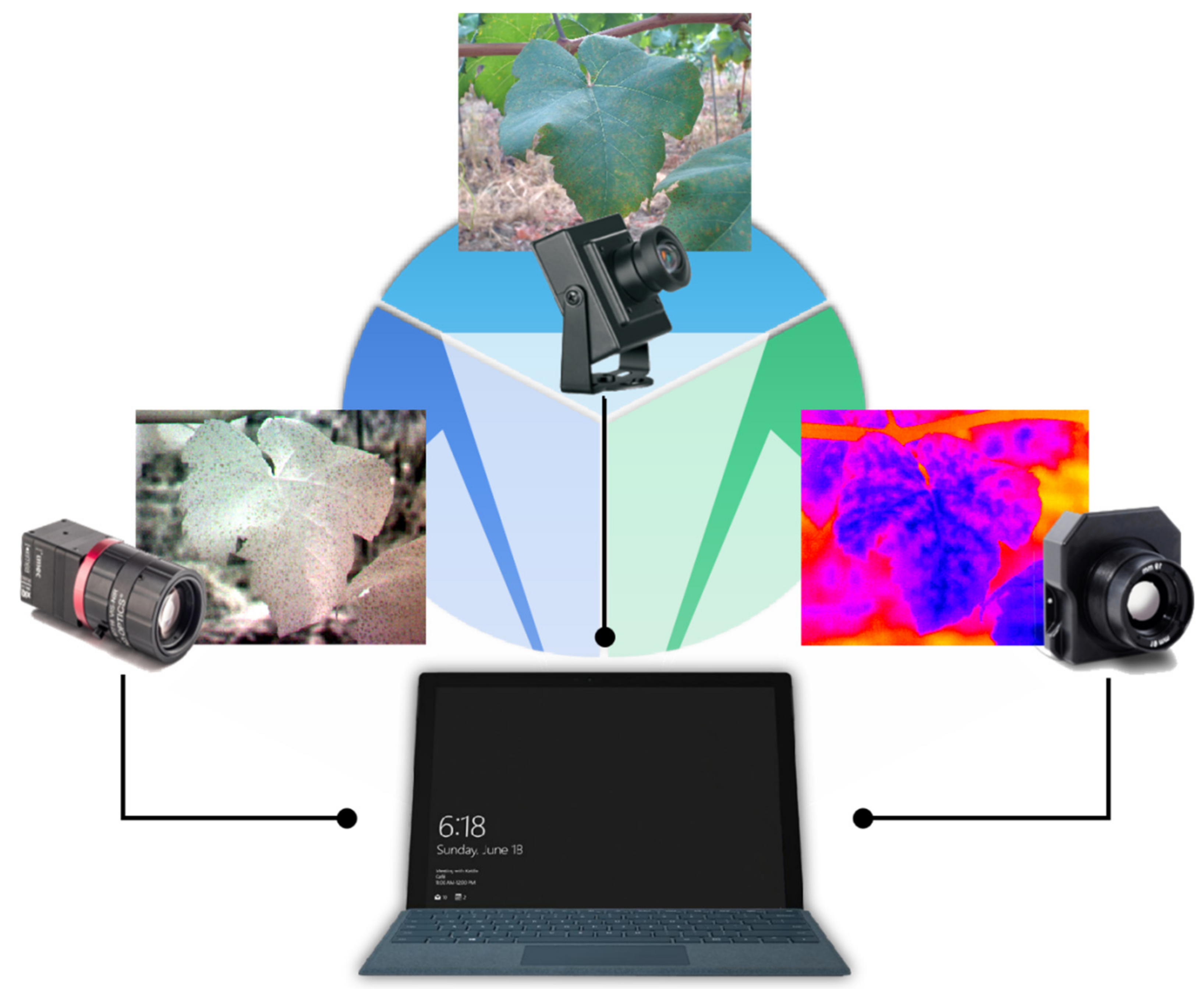
2.1. Data Acquisition
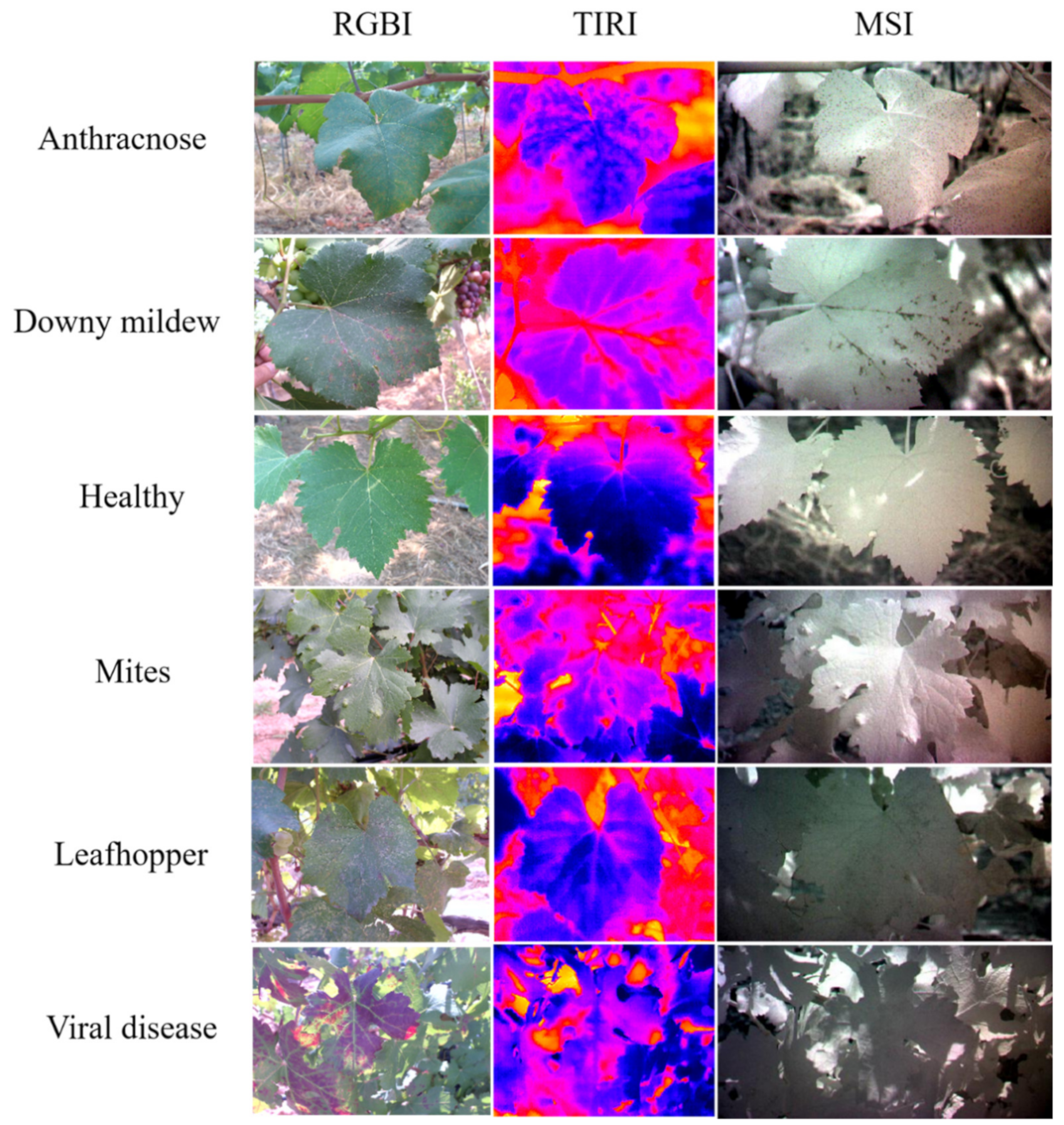
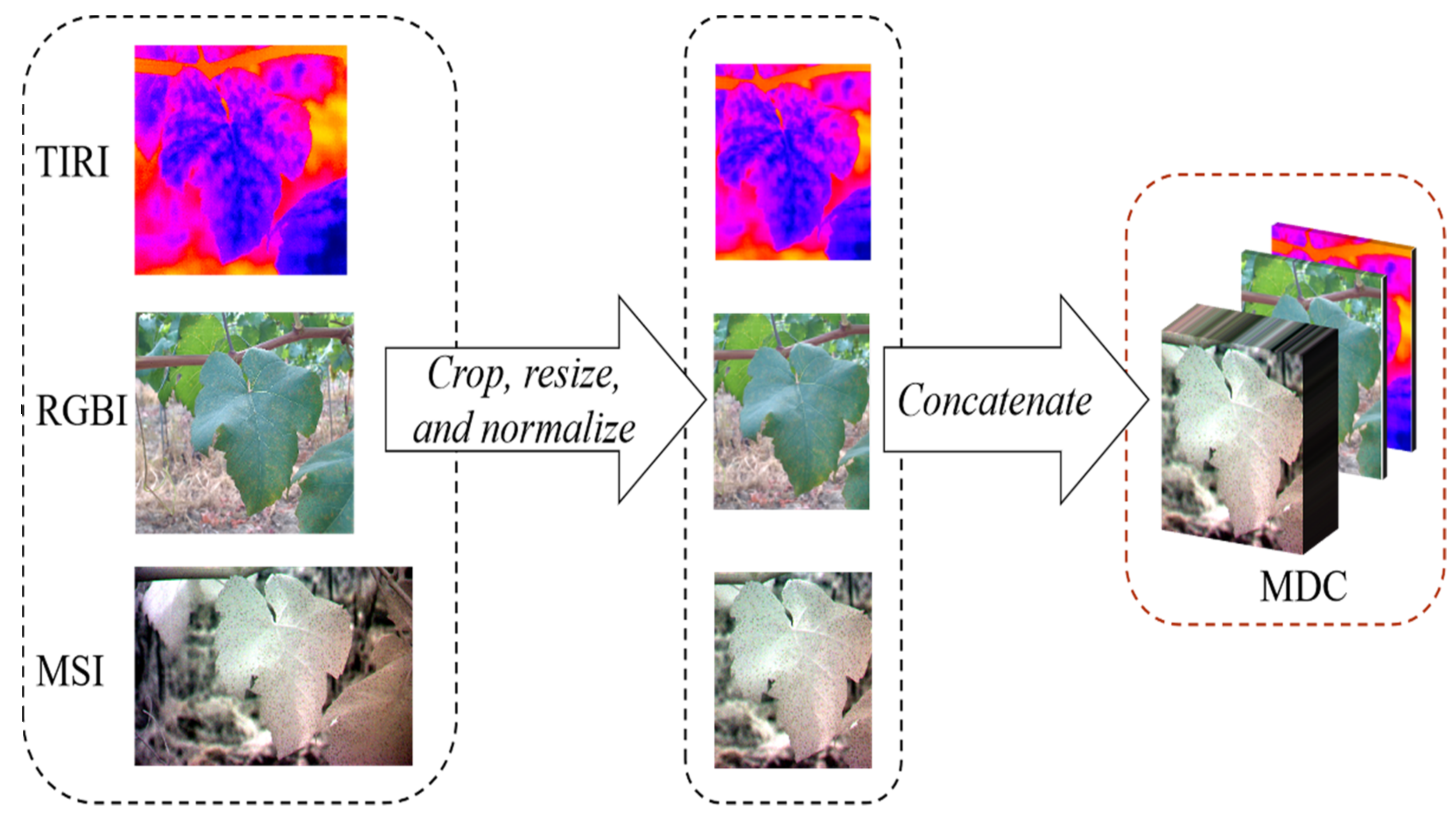
2.2. Data Preprocessing
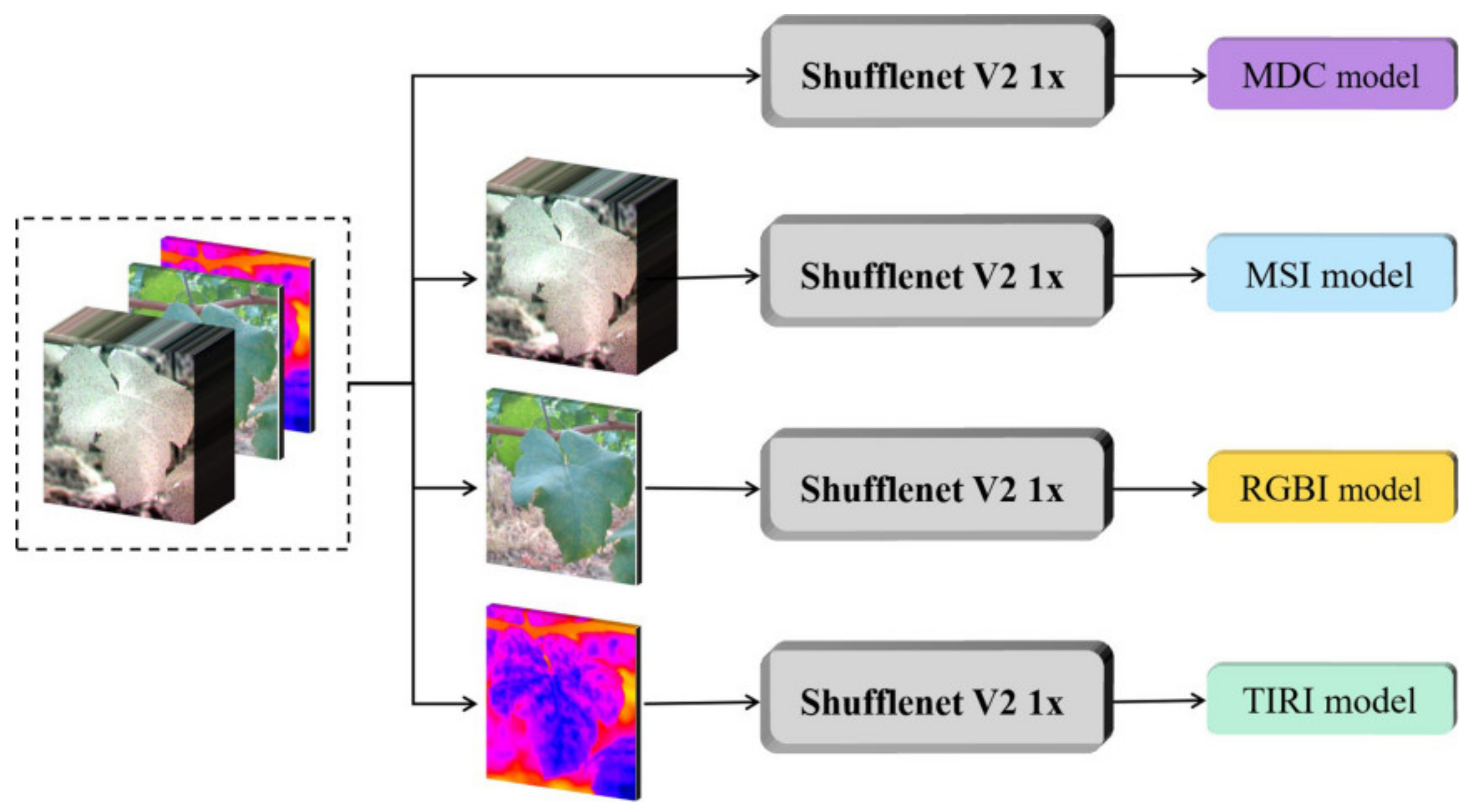
2.3. Detection Model
2.4. Modeling Setup
3. Results
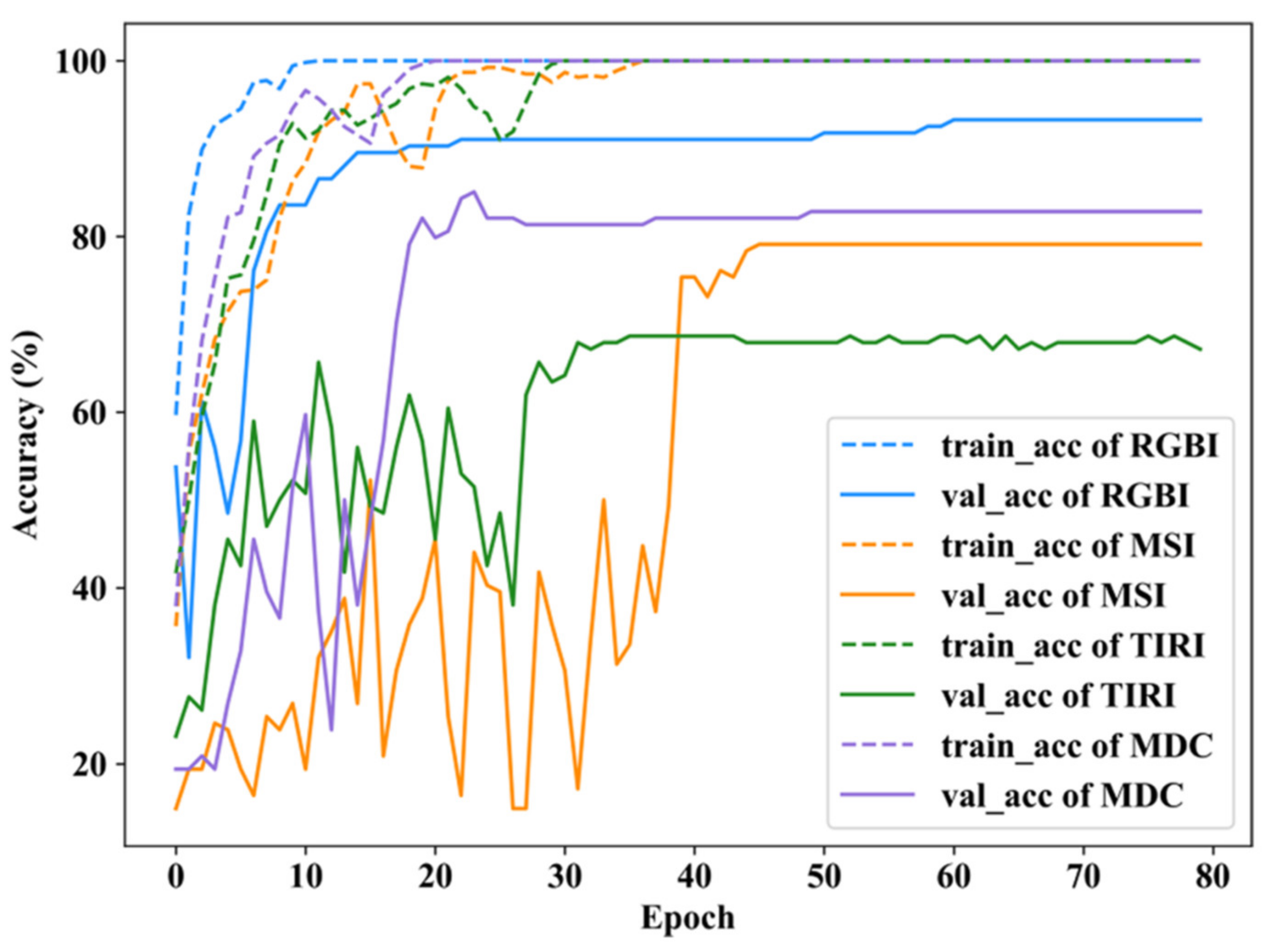
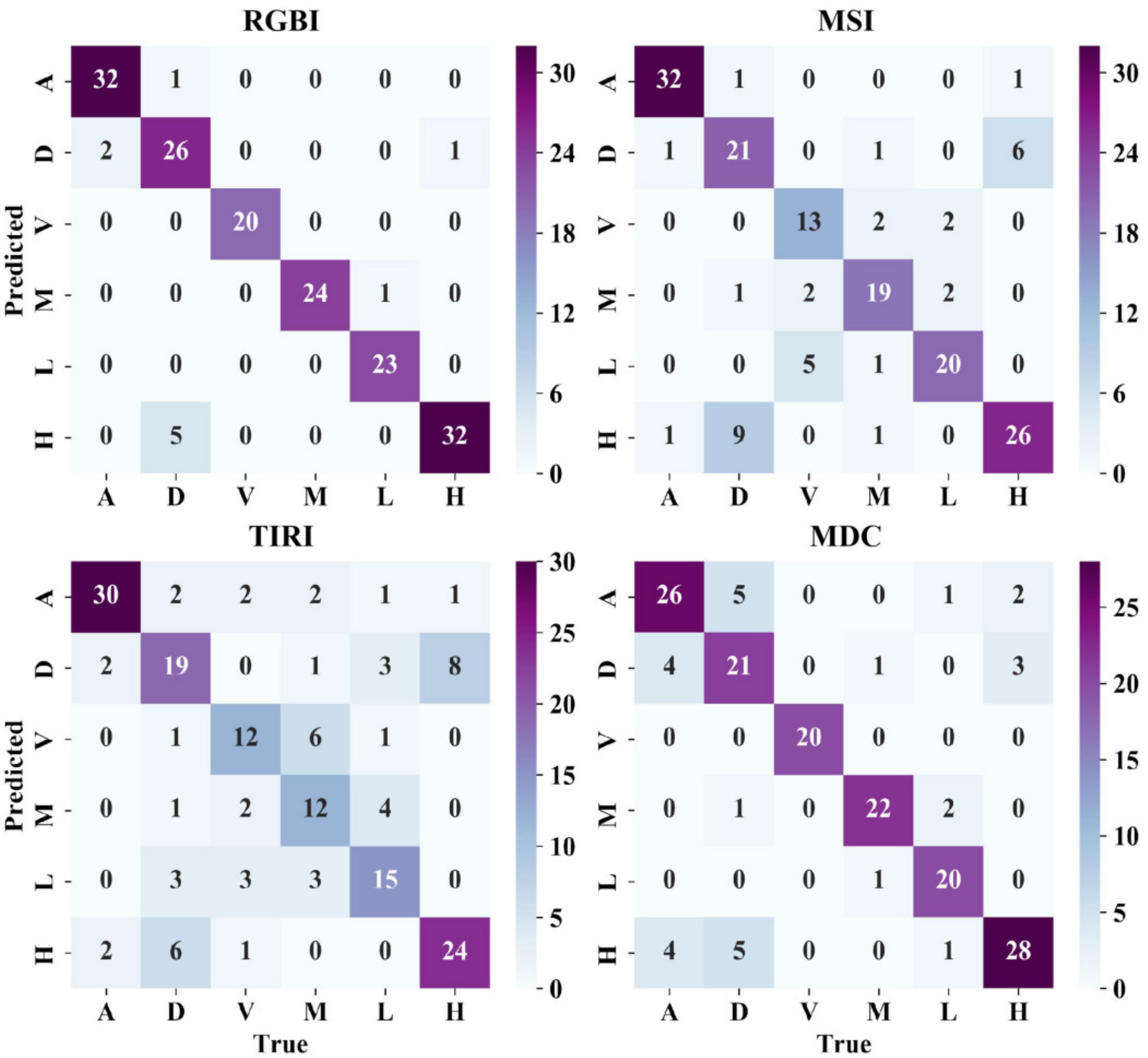
3.1. Results of RGBI, MSI, TIRI, and MDC Models
3.2. MDF Decision-Making Method
3.2.1. Outputs of RGBI, MSI, and TIRI Models
3.2.2. Outputs of RGBI, MSI, and TIRI Models with Label Smoothing
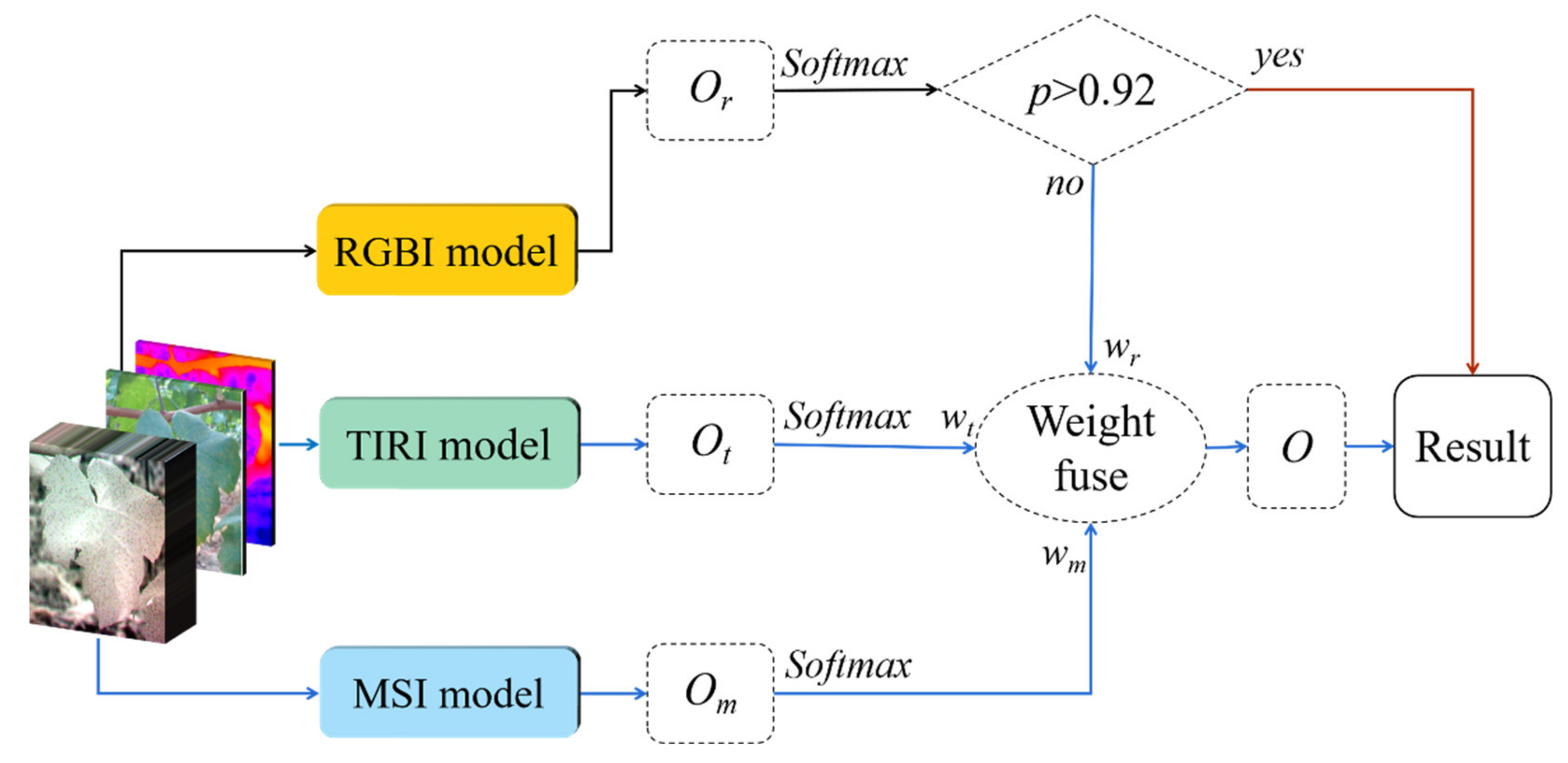
3.2.3. MDF Model
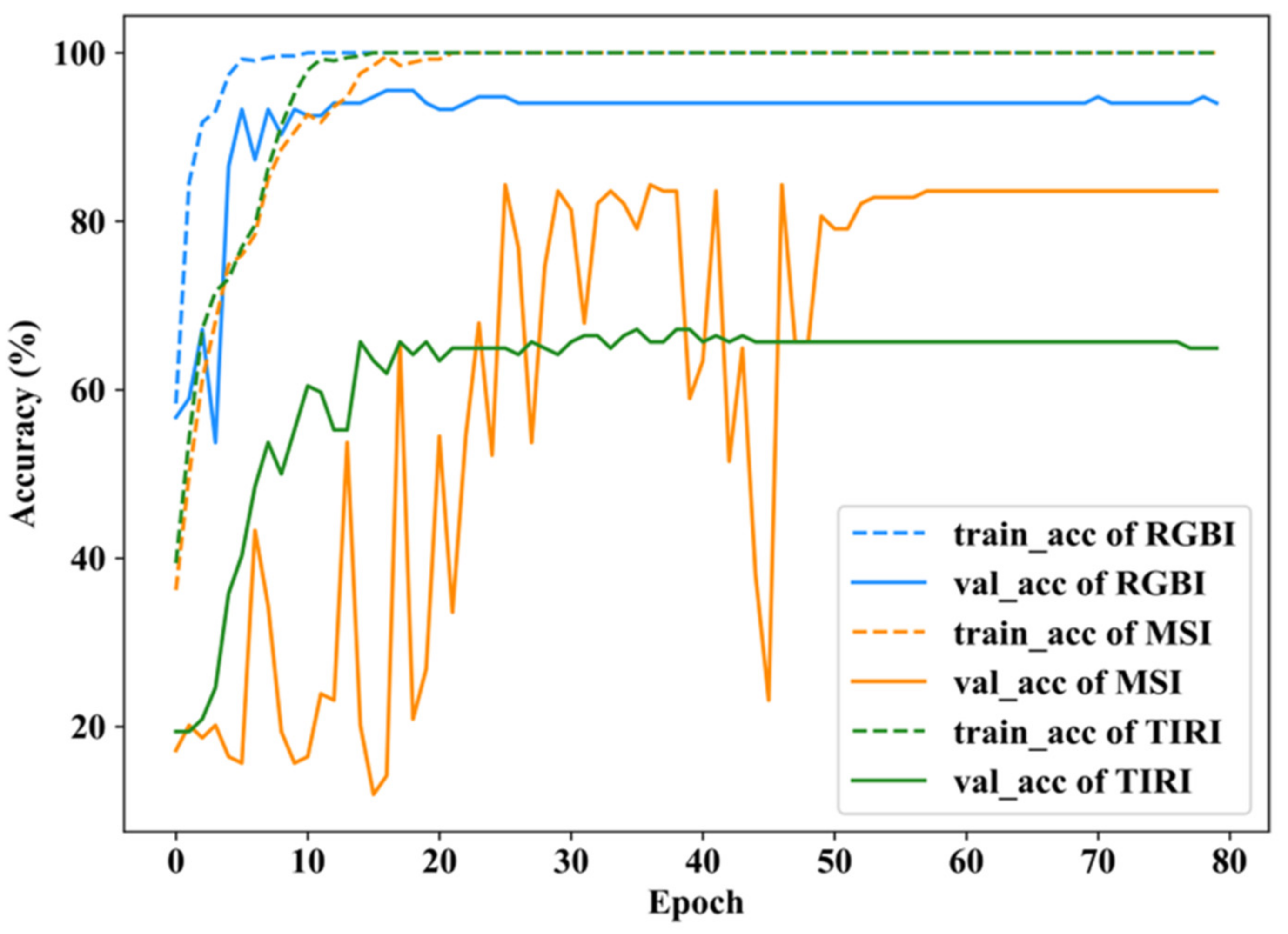
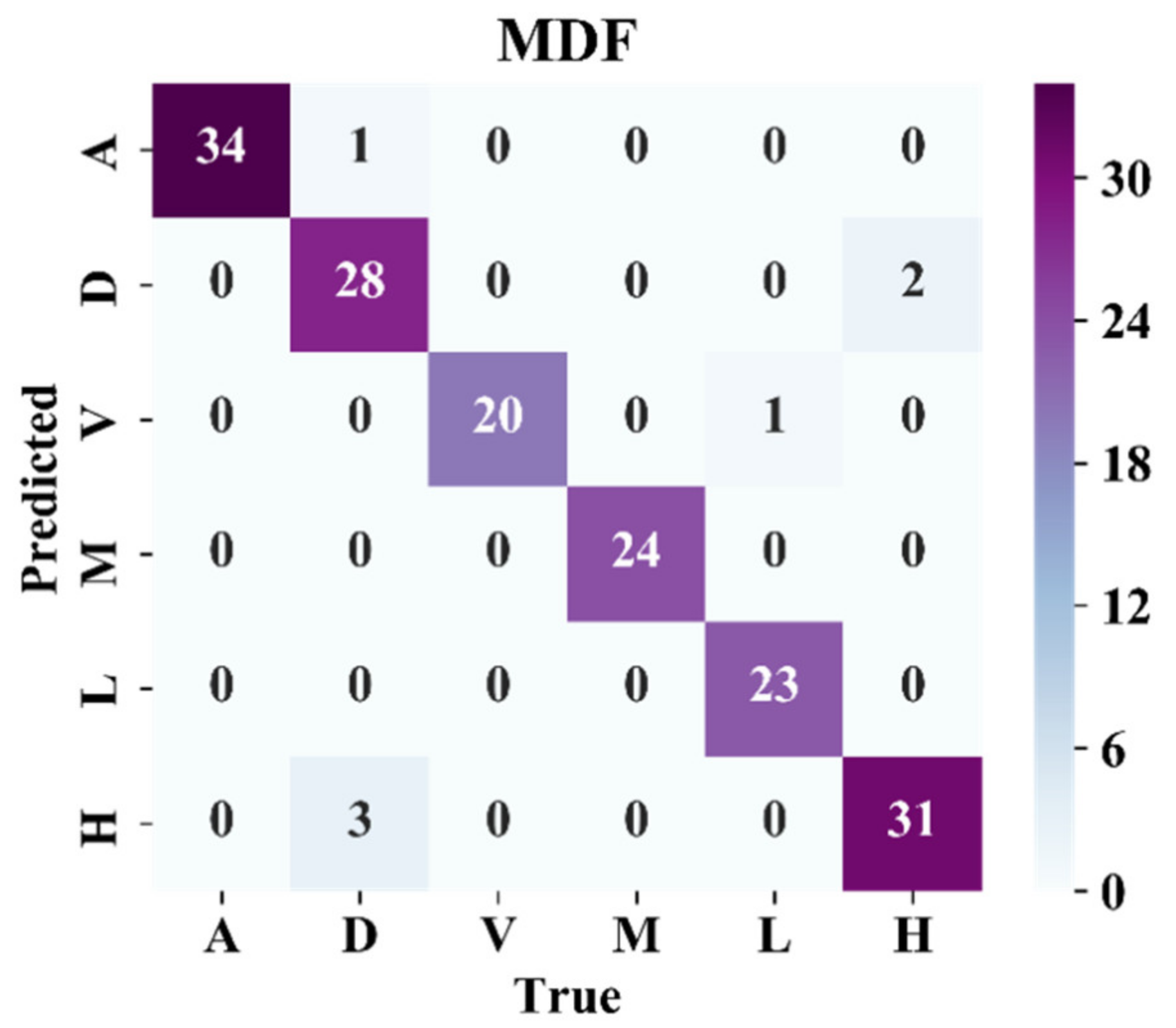
3.2.4. Result of MDF Model
3.3. Lightweight of MDF Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaisakthi, S.M.; Mirunalini, P.; Thenmozhi, D.; Vatsala. Grape Leaf Disease Identification Using Machine Learning Techniques. In Proceedings of the 2019 International Conference on Computational Intelligence in Data Science (ICCIDS), Chennai, India, 21–23 February 2019; pp. 1–6. [Google Scholar]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A Critical Review of Plant Protection Tools for Reducing Pesticide Use on Grapevine and New Perspectives for the Implementation of IPM in Viticulture. Crop Prot. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- Nalam, V.; Louis, J.; Shah, J. Plant Defense against Aphids, the Pest Extraordinaire. Plant Sci. 2019, 279, 96–107. [Google Scholar] [CrossRef]
- Wu, A.; Zhu, J.; He, Y. Computer Vision Method Applied for Detecting Diseases in Grape Leaf System. In Cognitive Internet of Things: Frameworks, Tools and Applications; Lu, H., Ed.; Studies in Computational Intelligence; Springer International Publishing: Cham, Switzerland, 2020; pp. 367–376. [Google Scholar]
- Meunkaewjinda, A.; Kumsawat, P.; Attakitmongcol, K.; Srikaew, A. Grape Leaf Disease Detection from Color Imagery Using Hybrid Intelligent System. In Proceedings of the 2008 5th International Conference on Electrical Engineering/Electronics, Computer, Telecommunications and Information Technology, Krabi, Thailand, 14–17 May 2008; pp. 513–516. [Google Scholar]
- Sannakki, S.S.; Rajpurohit, V.S.; Nargund, V.B.; Kulkarni, P. Diagnosis and Classification of Grape Leaf Diseases Using Neural Networks. In Proceedings of the 2013 Fourth International Conference on Computing, Communications and Networking Technologies (ICCCNT), Tiruchengode, India, 4–6 July 2013; pp. 1–5. [Google Scholar]
- Khirade, S.D.; Patil, A.B. Plant Disease Detection Using Image Processing. In Proceedings of the 2015 International Conference on Computing Communication Control and Automation, Pune, India, 26–27 February 2015; pp. 768–771. [Google Scholar]
- Padol, P.B.; Yadav, A.A. SVM Classifier Based Grape Leaf Disease Detection. In Proceedings of the 2016 Conference on Advances in Signal Processing (CASP), Pune, India, 9–11 June 2016; pp. 175–179. [Google Scholar]
- Padol, P.B.; Sawant, S.D. Fusion Classification Technique Used to Detect Downy and Powdery Mildew Grape Leaf Diseases. In Proceedings of the 2016 International Conference on Global Trends in Signal Processing, Information Computing and Communication (ICGTSPICC), Jalgaon, India, 22–24 December 2016; pp. 298–301. [Google Scholar]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep Learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Liu, B.; Ding, Z.; Tian, L.; He, D.; Li, S.; Wang, H. Grape Leaf Disease Identification Using Improved Deep Convolutional Neural Networks. Front. Plant Sci. 2020, 11, 1082. [Google Scholar] [CrossRef]
- Ji, M.; Zhang, L.; Wu, Q. Automatic Grape Leaf Diseases Identification Via UnitedModel Based on Multiple Convolutional Neural Networks. Inf. Process. Agric. 2020, 7, 418–426. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Lin, M.; Sun, J. Shufflenet: An Extremely Efficient Convolutional Neural Network for Mobile Devices. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 6848–6856. [Google Scholar]
- Iandola, F.N.; Han, S.; Moskewicz, M.W.; Ashraf, K.; Dally, W.J.; Keutzer, K. SqueezeNet: AlexNet-Level Accuracy with 50x Fewer Parameters and <0.5 mb Model Size. arXiv 2016, arXiv:1602.07360. [Google Scholar]
- Howard, A.G.; Zhu, M.; Chen, B.; Kalenichenko, D.; Wang, W.; Weyand, T.; Andreetto, M.; Adam, H. Mobilenets: Efficient Convolutional Neural Networks for Mobile Vision Applications. arXiv 2017, arXiv:1704.04861. [Google Scholar]
- Sandler, M.; Howard, A.; Zhu, M.; Zhmoginov, A.; Chen, L.-C. Mobilenetv2: Inverted Residuals and Linear Bottlenecks. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 4510–4520. [Google Scholar]
- Howard, A.; Sandler, M.; Chu, G.; Chen, L.-C.; Chen, B.; Tan, M.; Wang, W.; Zhu, Y.; Pang, R.; Vasudevan, V. Searching for Mobilenetv3. In Proceedings of the IEEE/CVF International Conference on Computer Vision, Seoul, Korea, 27 October–2 November 2019; pp. 1314–1324. [Google Scholar]
- Tan, M.; Le, Q. Efficientnet: Rethinking Model Scaling for Convolutional Neural Networks. In Proceedings of the 36th International Conference on Machine Learning, Long Beach, CA, USA, 9–15 June 2019; pp. 6105–6114. [Google Scholar]
- Tang, Z.; Yang, J.; Li, Z.; Qi, F. Grape Disease Image Classification Based on Lightweight Convolution Neural Networks and Channelwise Attention. Comput. Electron. Agric. 2020, 178, 105735. [Google Scholar] [CrossRef]
- Chaerle, L.; Van Caeneghem, W.; Messens, E.; Lambers, H.; Van Montagu, M.; Van Der Straeten, D. Presymptomatic Visualization of Plant-Virus Interactions by Thermography. Nat. Biotechnol. 1999, 17, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Farber, C.; Mahnke, M.; Sanchez, L.; Kurouski, D. Advanced Spectroscopic Techniques for Plant Disease Diagnostics. A Review. TrAC Trends Anal. Chem. 2019, 118, 43–49. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, M. Detection of Rice Sheath Blight for In-Season Disease Management Using Multispectral Remote Sensing. Int. J. Appl. Earth Obs. Geoinf. 2005, 7, 115–128. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Steiner, U.; Dehne, H.-W.; Oerke, E.-C. Spectral Signatures of Sugar Beet Leaves for the Detection and Differentiation of Diseases. Precis. Agric. 2010, 11, 413–431. [Google Scholar] [CrossRef]
- Kerkech, M.; Hafiane, A.; Canals, R. VddNet: Vine Disease Detection Network Based on Multispectral Images and Depth Map. Remote Sens. 2020, 12, 3305. [Google Scholar] [CrossRef]
- Fahrentrapp, J.; Ria, F.; Geilhausen, M.; Panassiti, B. Detection of Gray Mold Leaf Infections Prior to Visual Symptom Appearance Using a Five-Band Multispectral Sensor. Front. Plant Sci. 2019, 10, 628. [Google Scholar] [CrossRef] [Green Version]
- Veys, C.; Chatziavgerinos, F.; AlSuwaidi, A.; Hibbert, J.; Hansen, M.; Bernotas, G.; Smith, M.; Yin, H.; Rolfe, S.; Grieve, B. Multispectral Imaging for Presymptomatic Analysis of Light Leaf Spot in Oilseed Rape. Plant Methods 2019, 15, 4. [Google Scholar] [CrossRef]
- Li, Y.; Wu, F.-X.; Ngom, A. A Review on Machine Learning Principles for Multi-View Biological Data Integration. Brief. Bioinform. 2018, 19, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Ouhami, M.; Hafiane, A.; Es-Saady, Y.; El Hajji, M.; Canals, R. Computer Vision, IoT and Data Fusion for Crop Disease Detection Using Machine Learning: A Survey and Ongoing Research. Remote Sens. 2021, 13, 2486. [Google Scholar] [CrossRef]
- Bulanon, D.M.; Burks, T.F.; Alchanatis, V. Image Fusion of Visible and Thermal Images for Fruit Detection. Biosyst. Eng. 2009, 103, 12–22. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Alisaac, E.; Al Masri, A.; Behmann, J.; Dehne, H.-W.; Oerke, E.-C. Comparison and Combination of Thermal, Fluorescence, and Hyperspectral Imaging for Monitoring Fusarium Head Blight of Wheat on Spikelet Scale. Sensors 2019, 19, 2281. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Wu, B.; Zhu, S.; Wang, J.; Su, Z.; Liu, F.; He, Y.; Zhang, C. Investigation on Data Fusion of Multisource Spectral Data for Rice Leaf Diseases Identification Using Machine Learning Methods. Front. Plant Sci. 2020, 11, 577063. [Google Scholar] [CrossRef]
- Prince, G.; Clarkson, J.P.; Rajpoot, N.M. Automatic Detection of Diseased Tomato Plants Using Thermal and Stereo Visible Light Images. PLoS ONE 2015, 10, e0123262. [Google Scholar]
- Maimaitijiang, M.; Ghulam, A.; Sidike, P.; Hartling, S.; Maimaitiyiming, M.; Peterson, K.; Shavers, E.; Fishman, J.; Peterson, J.; Kadam, S.; et al. Unmanned Aerial System (UAS)-Based Phenotyping of Soybean Using Multi-Sensor Data Fusion and Extreme Learning Machine. ISPRS-J. Photogramm. Remote Sens. 2017, 134, 43–58. [Google Scholar] [CrossRef]
- Patro, S.; Sahu, K.K. Normalization: A Preprocessing Stage. arXiv 2015, arXiv:1503.06462. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, X.; Zheng, H.-T.; Sun, J. Shufflenet v2: Practical Guidelines for Efficient CNN Architecture Design. In Proceedings of the European Conference on Computer Vision (ECCV), Munich, Germany, 8–14 September 2018; pp. 116–131. [Google Scholar]
- Kingma, D.P.; Ba, J. Adam: A Method for Stochastic Optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar]
- Xu, J.; Yang, J.; Xiong, X.; Li, H.; Huang, J.; Ting, K.C.; Ying, Y.; Lin, T. Towards Interpreting Multi-Temporal Deep Learning Models in Crop Mapping. Remote Sens. Environ. 2021, 264, 112599. [Google Scholar] [CrossRef]
- Liu, W.; Wen, Y.; Yu, Z.; Yang, M. Large-Margin Softmax Loss for Convolutional Neural Networks. In Proceedings of the 33rd International Conference on Machine Learning, New York, NY, USA, 19–24 June 2016; pp. 507–516. [Google Scholar]
- Guo, C.; Pleiss, G.; Sun, Y.; Weinberger, K.Q. On Calibration of Modern Neural Networks. In Proceedings of the 34th International Conference on Machine Learning, Sydney, Australia, 6–11 August 2017; pp. 1321–1330. [Google Scholar]
- Müller, R.; Kornblith, S.; Hinton, G. When Does Label Smoothing Help? arXiv 2019, arXiv:1906.02629. [Google Scholar]
- Zhou, D.; Hou, Q.; Chen, Y.; Feng, J.; Yan, S. Rethinking Bottleneck Structure for Efficient Mobile Network Design. In Proceedings of the 16th European Conference on Computer Vision, Glasgow, UK, 23–28 August 2020; pp. 680–697. [Google Scholar]
- Ma, J.; Yuan, Y. Dimension Reduction of Image Deep Feature Using PCA. J. Vis. Commun. Image Represent. 2019, 63, 102578. [Google Scholar] [CrossRef]
- Yang, N.; Yuan, M.; Wang, P.; Zhang, R.; Sun, J.; Mao, H. Tea Diseases Detection Based on Fast Infrared Thermal Image Processing Technology. J. Sci. Food Agric. 2019, 99, 3459–3466. [Google Scholar] [CrossRef] [PubMed]


| Class | Anthracnose | Downy Mildew | Healthy | Mites | Leafhopper | Viral Disease |
|---|---|---|---|---|---|---|
| Collected region | HZ | HZ | HZ | YC | YC | YC |
| Number of RGBI | 168 | 162 | 162 | 120 | 121 | 101 |
| Number of MSI | 168 | 162 | 162 | 120 | 121 | 101 |
| Number of TIRI | 168 | 162 | 162 | 120 | 121 | 101 |
| Configuration | Value |
|---|---|
| Central processing unit | Intel Corei7-10750H |
| Graphics processor unit | NVIDIA Quadro RTX 5000 with Max-Q Design |
| Operation system | Windows 10 |
| Deep learning framework | PyTorch |
| F1 Score | Overall Accuracy | ||||||
|---|---|---|---|---|---|---|---|
| Anthracnose | Downy Mildew | Viral Disease | Mites | Leafhopper | Healthy | ||
| RGBI | 0.9384 | 0.8711 | 1.0000 | 0.9794 | 0.9654 | 0.9121 | 93.77% |
| MSI | 0.9364 | 0.7030 | 0.7307 | 0.8621 | 0.7946 | 0.7424 | 79.88% |
| TIRI | 0.7731 | 0.5690 | 0.6118 | 0.5964 | 0.6282 | 0.6765 | 64.91% |
| MDC | 0.7879 | 0.7226 | 0.9738 | 0.9098 | 0.8930 | 0.7987 | 83.23% |
| Distribution Proportion | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (0, 0.5] | (0.5, 0.55] | (0.55, 0.6] | (0.6, 0.65] | (0.65, 0.7] | (0.7, 0.75] | (0.75, 0.8] | (0.8, 0.85] | (0.85, 0.9] | (0.9, 0.95] | (0.95, 1] | |
| Right | 1.61% | 0.32% | 0.00% | 0.48% | 0.32% | 0.16% | 1.12% | 1.61% | 1.61% | 3.53% | 89.25% |
| Wrong | 11.36% | 0.00% | 2.27% | 2.27% | 2.27% | 4.55% | 11.36% | 9.09% | 2.27% | 11.36% | 43.18% |
| Distribution Proportion | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (0, 0.5] | (0.5, 0.55] | (0.55, 0.6] | (0.6, 0.65] | (0.65, 0.7] | (0.7, 0.75] | (0.75, 0.8] | (0.8, 0.85] | (0.85, 0.9] | (0.9, 0.95] | (0.95, 1] | |
| Right-LS | 3.44% | 1.56% | 1.09% | 2.34% | 3.13% | 3.75% | 4.69% | 8.91% | 23.13% | 36.25% | 11.72% |
| Change | +1.83% | +1.24% | +1.09% | +1.86% | +2.80% | +3.59% | +3.56% | +7.30% | +21.52% | +32.72% | −77.53% |
| Wrong-LS | 18.52% | 3.70% | 11.11% | 3.70% | 14.81% | 7.41% | 3.70% | 7.41% | 7.41% | 22.22% | 0.00% |
| Change | +7.15% | +3.70% | +8.84% | +1.43% | +12.54% | +2.86% | −7.66% | −1.68% | +5.13% | +10.86% | −43.18% |
| F1 Score | Overall Accuracy | ||||||
|---|---|---|---|---|---|---|---|
| Anthracnose | Downy Mildew | Viral Disease | Mites | Leafhopper | Healthy | ||
| RGBI | 0.9464 | 0.8633 | 1.0000 | 0.9832 | 0.9616 | 0.8930 | 93.41% |
| MSI | 0.9414 | 0.7520 | 0.7684 | 0.8619 | 0.8207 | 0.7784 | 82.40% |
| TIRI | 0.8399 | 0.5610 | 0.7007 | 0.7103 | 0.6163 | 0.6709 | 68.26% |
| MDF | 0.9826 | 0.9233 | 0.9846 | 0.9840 | 0.9783 | 0.9283 | 96.05% |
| Model | Total Params | MAdds |
|---|---|---|
| MobileNet V2 and MobileNeXt | 1.7~6.9 M | 0.059~0.690 G |
| RGBI model | 1.260 M | 0.106 G |
| MSI model | 1.265 M | 0.150 G |
| TIRI model | 1.260 M | 0.106 G |
| MDF model | 3.785 M | 0.362 G |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Lu, X.; Huang, J.; Zhou, J.; Jiao, J.; Liu, Y.; Liu, F.; Su, B.; Gu, P. A Multi-Source Data Fusion Decision-Making Method for Disease and Pest Detection of Grape Foliage Based on ShuffleNet V2. Remote Sens. 2021, 13, 5102. https://doi.org/10.3390/rs13245102
Yang R, Lu X, Huang J, Zhou J, Jiao J, Liu Y, Liu F, Su B, Gu P. A Multi-Source Data Fusion Decision-Making Method for Disease and Pest Detection of Grape Foliage Based on ShuffleNet V2. Remote Sensing. 2021; 13(24):5102. https://doi.org/10.3390/rs13245102
Chicago/Turabian StyleYang, Rui, Xiangyu Lu, Jing Huang, Jun Zhou, Jie Jiao, Yufei Liu, Fei Liu, Baofeng Su, and Peiwen Gu. 2021. "A Multi-Source Data Fusion Decision-Making Method for Disease and Pest Detection of Grape Foliage Based on ShuffleNet V2" Remote Sensing 13, no. 24: 5102. https://doi.org/10.3390/rs13245102
APA StyleYang, R., Lu, X., Huang, J., Zhou, J., Jiao, J., Liu, Y., Liu, F., Su, B., & Gu, P. (2021). A Multi-Source Data Fusion Decision-Making Method for Disease and Pest Detection of Grape Foliage Based on ShuffleNet V2. Remote Sensing, 13(24), 5102. https://doi.org/10.3390/rs13245102









