Phytoplankton Genera Structure Revealed from the Multispectral Vertical Diffuse Attenuation Coefficient
Abstract
1. Introduction
2. Materials and Methods
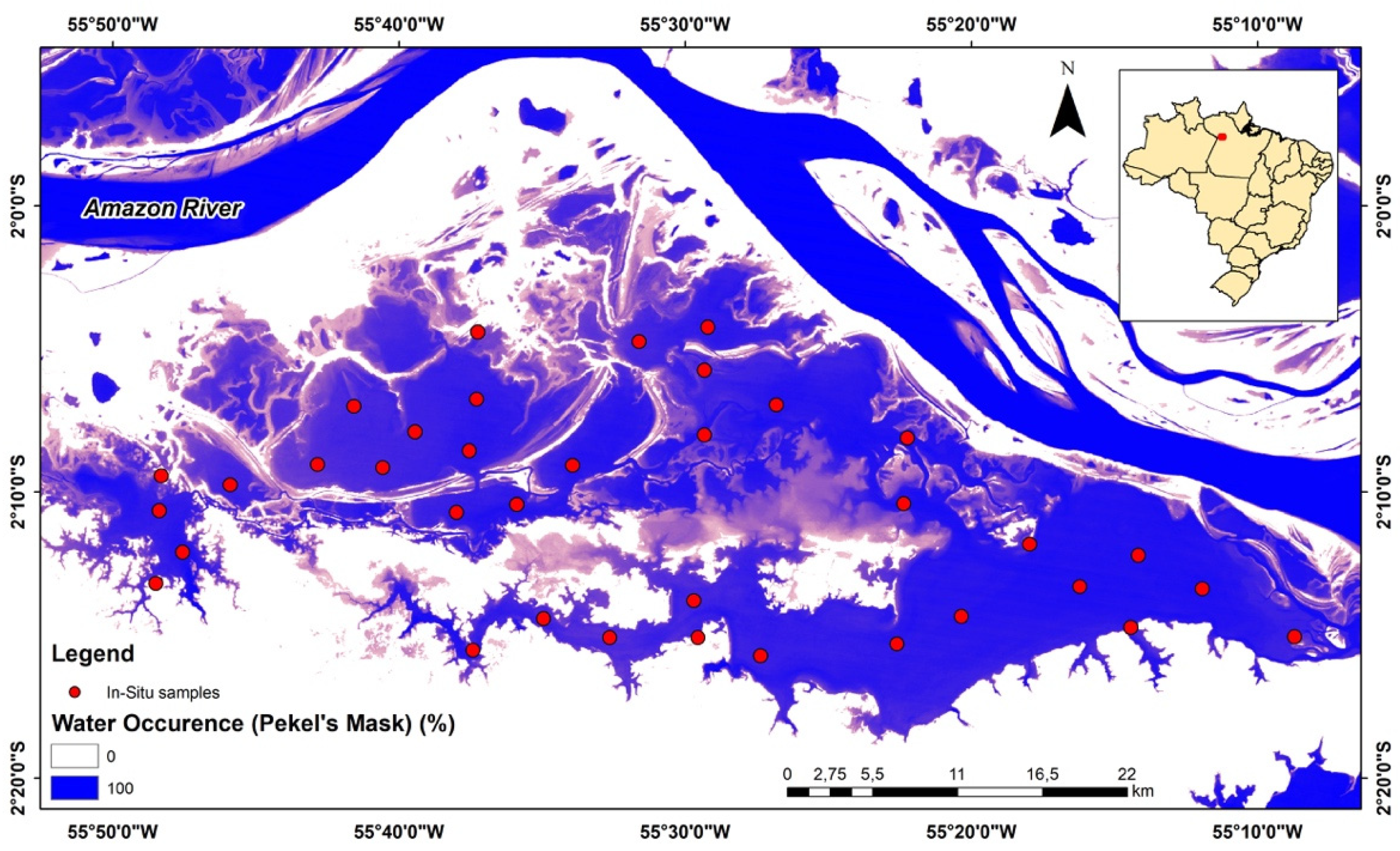
2.1. Study Area
2.2. Biological Data
2.3. Remote Sensing Reflectance Measurements
2.4. Kd Calculation Based Rrs Measurements
2.5. Statistical Analysis
3. Results
3.1. Phytoplankton and Multispectral Vertical Diffuse Attenuation Coefficient of Downward Irradiance
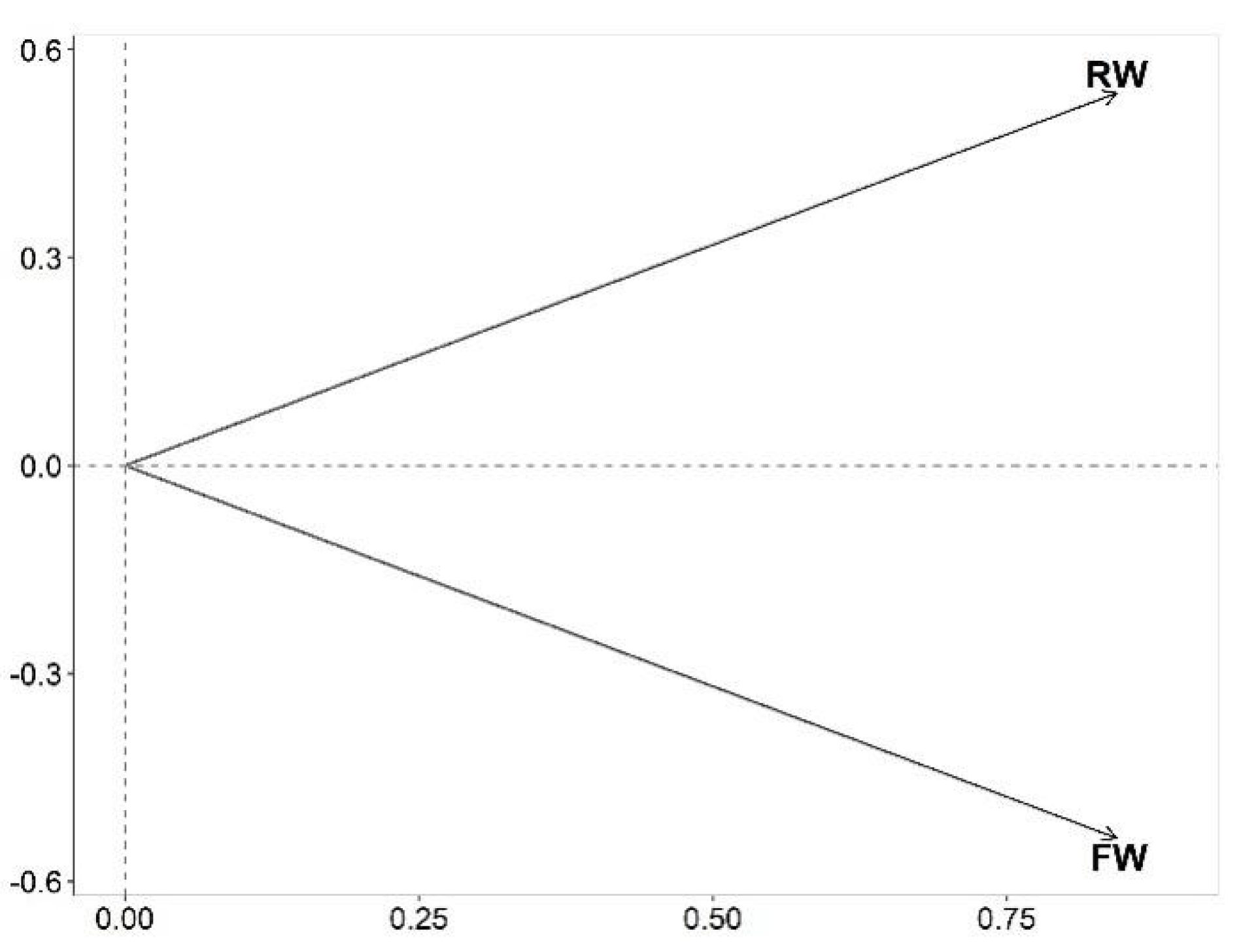
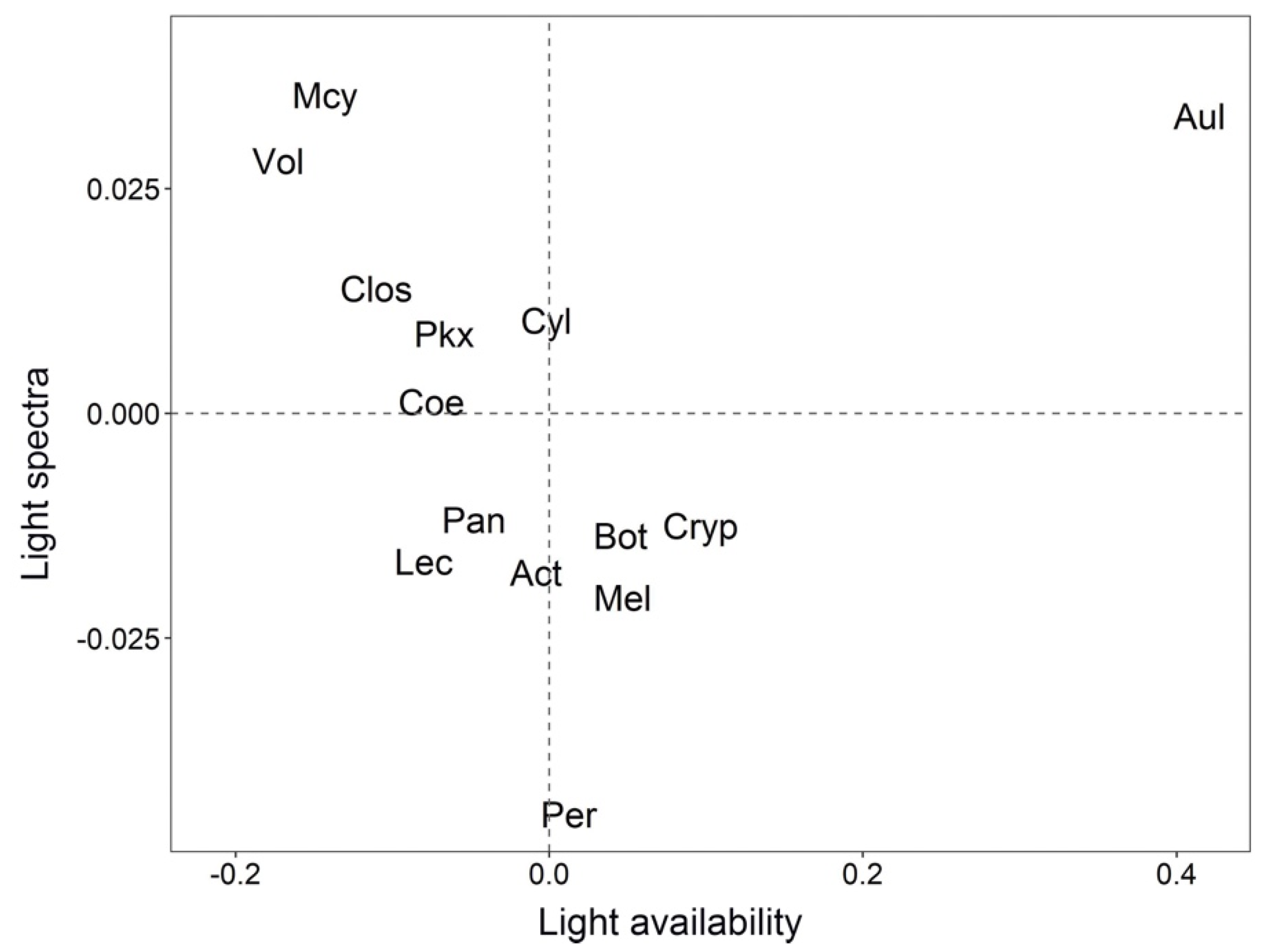
3.2. Biovolume of Phytoplankton Genera and Kd Relationships
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rastogi, R.P.; Madamwar, D.; Incharoensakdi, A. Bloom dynamics of cyanobacteria and their toxins: Environmental health impacts and mitigation strategies. Front. Microbiol. 2015, 6, 1254. [Google Scholar] [CrossRef] [PubMed]
- Vicentin, A.M.; Rodrigues, E.H.C.; Moschini-Carlos, V.; Pompêo, M.L.M. Is it possible to evaluate the ecological status of a reservoir using the phytoplankton community? Acta Limnol. Bras. 2018, 30, 1–15. [Google Scholar] [CrossRef][Green Version]
- Joa, S.J.C.; Lopes, P.M.; Bozelli, R.L.; Huszar, V.L.M.M.; Cardoso, S.J.; Nabout, J.C.; Farjalla, V.F.; Lopes, P.M.; Bozelli, R.L.; Huszar, V.L.M.M.; et al. Environmental factors driving phytoplankton taxonomic and functional diversity in Amazonian floodplain lakes. Hydrobiologia 2017, 802, 115–130. [Google Scholar] [CrossRef]
- Mellard, J.P.; Yoshiyama, K.; Litchman, E.; Klausmeier, C.A. The vertical distribution of phytoplankton in stratified water columns. J. Theor. Biol. 2011, 269, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Willis, A.; Burford, M.A. Differences in cyanobacterial strain responses to light and temperature reflect species plasticity. Harmful Algae 2017, 62, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Geider, R.J.; Maclntyre, H.L.; Kana, T.M. A dynamic regulatory model of phytoplanktonic acclimation to light, nutrients, and temperature. Limnol. Oceanogr. 1998, 43, 679–694. [Google Scholar] [CrossRef]
- Reynolds, C.S. What factors influence the species composition of phytoplankton in lakes of different trophic status? In Phytoplankton and Trophic Gradients; Springer: Dordrecht, The Netherlands, 1998; Volumes 369–370, pp. 11–26. [Google Scholar]
- Ma, J.; Song, K.; Wen, Z.; Zhao, Y.; Shang, Y.; Fang, C.; Du, J. Spatial distribution of diffuse attenuation of photosynthetic active radiation and its main regulating factors in Inland Waters of Northeast China. Remote Sens. 2016, 8, 964. [Google Scholar] [CrossRef]
- Vadakke-Chanat, S.; Shanmugam, P. Modeling the contributions of phytoplankton and non-algal particles to spectral scattering properties in near-shore and lagoon waters. Cont. Shelf Res. 2017, 135, 35–46. [Google Scholar] [CrossRef]
- Chapman, P.M.; Hayward, A.; Faithful, J. Total Suspended Solids Effects on Freshwater Lake Biota Other than Fish. Bull. Environ. Contam. Toxicol. 2017, 99, 423–427. [Google Scholar] [CrossRef]
- Kraus, C.N.; Bonnet, M.P.; Nogueira, I.d.S.; Lobo, M.T.M.P.S.; Marques, D.d.M.; Garnier, J.; Vieira, L.C.G. Unraveling flooding dynamics and nutrients’ controls upon phytoplankton functional dynamics in Amazonian floodplain lakes. Water 2019, 11, 154. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Falkowski, P.G. A consumer’s guide to phytoplankton primary productivity models. Limnol. Oceanogr. 1997, 42, 1479–1491. [Google Scholar] [CrossRef]
- Lisi, P.J.; Hein, C.L. Eutrophication drives divergent water clarity responses to decadal variation in lake level. Limnol. Oceanogr. 2019, 64, S49–S59. [Google Scholar] [CrossRef]
- Bernardo, N.; Alcântara, E.; Watanabe, F.; Rodrigues, T.; do Carmo, A.; Gomes, A.C.C.; Andrade, C. Light Absorption Budget in a Reservoir Cascade System with Widely Differing Optical Properties. Water 2019, 11, 229. [Google Scholar] [CrossRef]
- Le, C.; Li, Y.; Zha, Y.; Sun, D. Specific absorption coefficient and the phytoplankton package effect in Lake Taihu, China. Hydrobiologia 2009, 619, 27–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, Y.; Wang, M.; Liu, X. Effect of phytoplankton community composition and cell size on absorption properties in eutrophic shallow lakes: Field and experimental evidence. Opt. Express 2012, 20, 11882. [Google Scholar] [CrossRef]
- Paavel, B.; Kangro, K.; Arst, H.; Reinart, A.; Kutser, T.; Nõges, T. Parameterization of chlorophyll-specific phytoplankton absorption coefficients for productive lake waters. J. Limnol. 2016, 75, 423–438. [Google Scholar] [CrossRef]
- Chase, A.P.; Kramer, S.J.; Haëntjens, N.; Boss, E.S.; Karp-Boss, L.; Edmondson, M.; Graff, J.R. Evaluation of diagnostic pigments to estimate phytoplankton size classes. Limnol. Oceanogr. Methods 2020, 18, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, L.A.; Boss, E.; Behrenfeld, M.J.; Westberry, T.K.; Sarmiento, J.L. Seasonal modulation of phytoplankton biomass in the Southern Ocean. Nat. Commun. 2020, 11, 5364. [Google Scholar] [CrossRef] [PubMed]
- Matthews, M.W.; Bernard, S.; Evers-King, H.; Robertson Lain, L. Distinguishing cyanobacteria from algae in optically complex inland waters using a hyperspectral radiative transfer inversion algorithm. Remote Sens. Environ. 2020, 248, 111981. [Google Scholar] [CrossRef]
- Ryabov, A.B.; Rudolf, L.; Blasius, B. Vertical distribution and composition of phytoplankton under the influence of an upper mixed layer. J. Theor. Biol. 2010, 263, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Bricaud, A.; Babin, M.; Morel, A.; Claustre, H. Variability in the chlorophyll-specific absorption coefficients of natural phytoplankton: Analysis and parameterization. J. Geophys. Res. 1995, 100, 13321. [Google Scholar] [CrossRef]
- Serive, B.; Nicolau, E.; Bérard, J.B.; Kaas, R.; Pasquet, V.; Picot, L.; Cadoret, J.P. Community Analysis of Pigment Patterns from 37 Microalgae Strains Reveals New Carotenoids and Porphyrins Characteristic of Distinct Strains and Taxonomic Groups. PLoS ONE 2017, 12, e0171872. [Google Scholar] [CrossRef]
- Uitz, J.; Huot, Y.; Bruyant, F.; Babin, M.; Claustre, H. Relating phytoplankton photophysiological properties to community structure on large scales. Limnol. Oceanogr. 2008, 53, 614–630. [Google Scholar] [CrossRef]
- Lawrenz, E.; Smith, E.M.; Richardson, T.L. Spectral Irradiance, Phytoplankton Community Composition and Primary Productivity in a Salt Marsh Estuary, North Inlet, South Carolina, USA. Estuaries Coasts 2013, 36, 347–364. [Google Scholar] [CrossRef]
- Meler, J.; Ostrowska, M.; Ficek, D.; Zdun, A. Light absorption by phytoplankton in the southern Baltic and Pomeranian lakes: Mathematical expressions for remote sensing applications. Oceanologia 2017, 59, 195–212. [Google Scholar] [CrossRef]
- Wilhelm, C.; Becker, A.; Toepel, J.; Vieler, A.; Rautenberger, R. Photophysiology and primary production of phytoplankton in freshwater. Physiol. Plant. 2004, 120, 347–357. [Google Scholar] [CrossRef]
- Grimm, B.; Porra, R.J.; Scheer, H. Chlorophylls and Bacteriochlorophylls; Grimm, B., Porra, R.J., Rüdiger, W., Scheer, H., Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2006; Volume 25, ISBN 978-1-4020-4515-8. [Google Scholar]
- Zapata, M.; Garrido, J.L.; Jeffrey, S.W. Chlorophyll c Pigments: Current Status. In Chlorophylls and Bacteriochlorophylls; Springer: Dordrecht, The Netherlands, 2007; pp. 39–53. [Google Scholar] [CrossRef]
- Chase, A.P.; Boss, E.; Cetinić, I.; Slade, W. Estimation of Phytoplankton Accessory Pigments From Hyperspectral Reflectance Spectra: Toward a Global Algorithm. J. Geophys. Res. Ocean. 2017, 122, 9725–9743. [Google Scholar] [CrossRef]
- Bricaud, A. Natural variability of phytoplanktonic absorption in oceanic waters: Influence of the size structure of algal populations. J. Geophys. Res. 2004, 109, C11010. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Wright, S.W.; Zapata, M. Microalgal classes and their signature pigments. In Phytoplankton Pigments; Roy, S., Llewellyn, C., Egeland, E.S., Johnsen, G., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 3–77. ISBN 9780511732263. [Google Scholar]
- Mackey, M.; Mackey, D.; Higgins, H.; Wright, S. CHEMTAX—A program for estimating class abundances from chemical markers:application to HPLC measurements of phytoplankton. Mar. Ecol. Prog. Ser. 1996, 144, 265–283. [Google Scholar] [CrossRef]
- Hmimina, G.; Hulot, F.D.; Humbert, J.F.; Quiblier, C.; Tambosco, K.; Lemaire, B.J.; Vinçon-Leite, B.; Audebert, L.; Soudani, K. Linking phytoplankton pigment composition and optical properties: A framework for developing remote-sensing metrics for monitoring cyanobacteria. Water Res. 2019, 148, 504–514. [Google Scholar] [CrossRef]
- Heath, M.R.; Richardson, K.; Kirboe, T. Optical assessment of phytoplankton nutrient depletion. J. Plankton Res. 1990, 12, 381–396. [Google Scholar] [CrossRef]
- Soberón, J. Commentary on Ditch, Stitch and Pitch: The niche is here to stay. J. Biogeogr. 2014, 41, 414–417. [Google Scholar] [CrossRef]
- Grüner, N.; Gebühr, C.; Boersma, M.; Feudel, U.; Wiltshire, K.H.; Freund, J.A. Reconstructing the realized niche of phytoplankton species from environmental data: Fitness versus abundance approach. Limnol. Oceanogr. Methods 2011, 9, 432–442. [Google Scholar] [CrossRef]
- da Silva, M.N.; Granzotti, R.V.; de Carvalho, P.; Rodrigues, L.C.; Bini, L.M. Niche measures and growth rate do not predict interspecific variation in spatial synchrony of phytoplankton. Limnology 2020. [Google Scholar] [CrossRef]
- Karasiewicz, S.; Breton, E.; Lefebvre, A.; Hernández Fariñas, T.; Lefebvre, S. Realized niche analysis of phytoplankton communities involving HAB: Phaeocystis spp. as a case study. Harmful Algae 2018, 72, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Petchey, O.L.; Gaston, K.J. Functional diversity: Back to basics and looking forward. Ecol. Lett. 2006, 9, 741–758. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.S.; Huszar, V.; Kruk, C.; Naselli-Flores, L.; Melo, S. Towards a functional classification of the freshwater phytoplankton. J. Plankton Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Ptacnik, R.; Andersen, T.; Brettum, P.; Lepistö, L.; Willén, E. Regional species pools control community saturation in lake phytoplankton. Proc. R. Soc. B Biol. Sci. 2010, 277, 3755–3764. [Google Scholar] [CrossRef]
- Salmaso, N.; Naselli-Flores, L.; Padisák, J. Functional classifications and their application in phytoplankton ecology. Freshw. Biol. 2015, 60, 603–619. [Google Scholar] [CrossRef]
- Nair, A.; Sathyendranath, S.; Platt, T.; Morales, J.; Stuart, V.; Forget, M.-H.; Devred, E.; Bouman, H. Remote sensing of phytoplankton functional types. Remote Sens. Environ. 2008, 112, 3366–3375. [Google Scholar] [CrossRef]
- Uitz, J.; Stramski, D.; Reynolds, R.A.; Dubranna, J. Assessing phytoplankton community composition from hyperspectral measurements of phytoplankton absorption coefficient and remote-sensing reflectance in open-ocean environments. Remote Sens. Environ. 2015, 171, 58–74. [Google Scholar] [CrossRef]
- Muller-Karger, F.E.; Hestir, E.; Ade, C.; Turpie, K.; Roberts, D.A.; Siegel, D.; Miller, R.J.; Humm, D.; Izenberg, N.; Keller, M.; et al. Satellite sensor requirements for monitoring essential biodiversity variables of coastal ecosystems. Ecol. Appl. 2018, 28, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Pahlevan, N.; Smith, B.; Binding, C.; Gurlin, D.; Li, L.; Bresciani, M.; Giardino, C. Hyperspectral retrievals of phytoplankton absorption and chlorophyll-a in inland and nearshore coastal waters. Remote Sens. Environ. 2021, 253, 112200. [Google Scholar] [CrossRef]
- Wang, M.; Son, S.; Harding, L.W. Retrieval of diffuse attenuation coefficient in the Chesapeake Bay and turbid ocean regions for satellite ocean color applications. J. Geophys. Res. 2009, 114, C10011. [Google Scholar] [CrossRef]
- Mobley, C.D. Polarized reflectance and transmittance properties of windblown sea surfaces. Appl. Opt. 2015, 54, 4828. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.R.; Narumalani, S.; Rundquist, D.; Lawson, M. Characterizing the vertical diffuse attenuation coefficient for downwelling irradiance in coastal waters: Implications for water penetration by high resolution satellite data. ISPRS J. Photogramm. Remote Sens. 2005, 60, 48–64. [Google Scholar] [CrossRef]
- Smith, R.C.; Baker, K.S. Optical classification of natural waters 1. Limnol. Oceanogr. 1978, 23, 260–267. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Platt, T.; Caverhill, C.M.; Warnock, R.E.; Lewis, M.R. Remote sensing of oceanic primary production: Computations using a spectral model. Deep Sea Res. Part A. Oceanogr. Res. Pap. 1989, 36, 431–453. [Google Scholar] [CrossRef]
- Bergamino, N.; Horion, S.; Stenuite, S.; Cornet, Y.; Loiselle, S.; Plisnier, P.-D.; Descy, J.-P. Spatio-temporal dynamics of phytoplankton and primary production in Lake Tanganyika using a MODIS based bio-optical time series. Remote Sens. Environ. 2010, 114, 772–780. [Google Scholar] [CrossRef]
- Bonnet, M.P.; Barroux, G.; Martinez, J.M.; Seyler, F.; Moreira-Turcq, P.; Cochonneau, G.; Melack, J.M.; Boaventura, G.; Maurice-Bourgoin, L.; León, J.G.; et al. Floodplain hydrology in an Amazon floodplain lake (Lago Grande de Curuaí). J. Hydrol. 2008, 349, 18–30. [Google Scholar] [CrossRef]
- Moquet, J.-S.; Crave, A.; Viers, J.; Seyler, P.; Armijos, E.; Bourrel, L.; Chavarri, E.; Lagane, C.; Laraque, A.; Casimiro, W.S.L.; et al. Chemical weathering and atmospheric/soil CO2 uptake in the Andean and Foreland Amazon basins. Chem. Geol. 2011, 287, 1–26. [Google Scholar] [CrossRef]
- Lapo, K.E.; Hinkelman, L.M.; Raleigh, M.S.; Lundquist, J.D. Impact of errors in the downwelling irradiances on simulations of snow water equivalent, snow surface temperature, and the snow energy balance. Water Resour. Res. 2015, 51, 1649–1670. [Google Scholar] [CrossRef]
- Sioli, H. (Ed.) Monographiae Biologicae; Monographi; Springer: Dordrecht, The Netherlands, 1984; Volume 56, ISBN 978-94-009-6544-7. [Google Scholar]
- Bonnet, M.P.; Garnier, J.; Barroux, G.; Boaventura, G.R.; Seyler, P. Biogeochemical functioning of Amazonian floodplains: The case of Lago Grande de Curua. In Riparian Zones: Characteristics, Management Practices and Ecological Impacts; Pokrovsky, O.S., Viers, J., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2016; pp. 77–98. ISBN 9781634846363. [Google Scholar]
- Utermöhl, H. Zur vervollkommnung der quantitativen phytoplankton-methodik. Mitt. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Lund, J.W.G.; Kipling, C.; Le Cren, E.D. The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 1958, 11, 143–170. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication. 2018. Available online: http//www.algaebase.org (accessed on 1 October 2021).
- Hillebrand, H.; Dürselen, C.D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- da Silva, E.F.F.; Novo, E.; Lobo, F.; Barbosa, C.; Tressmann, C.; Noernberg, M.A.; Rotta, L.H.d.S. A machine learning approach for monitoring Brazilian optical water types using Sentinel-2 MSI. Remote Sens. Appl. Soc. Environ. 2021, 23, 100577. [Google Scholar]
- da Silva, E.F.F.; Novo, E.M.L.d.M.; Lobo, F.; Barbosa, C.; Noernberg, M.A.; Rotta, L.H.d.S.; Cairo, C.T.; Maciel, D.A.; Flores Júnior, R. Optical water types found in Brazilian waters. Limnology 2020. [Google Scholar] [CrossRef]
- Maciel, D.; Novo, E.; Sander de Carvalho, L.; Barbosa, C.; Flores Júnior, R.; de Lucia Lobo, F. Retrieving Total and Inorganic Suspended Sediments in Amazon Floodplain Lakes: A Multisensor Approach. Remote Sens. 2019, 11, 1744. [Google Scholar] [CrossRef]
- Maciel, D.A.; Barbosa, C.C.F.; Novo, E.M.L.d.M.; Cherukuru, N.; Martins, V.S.; Flores Júnior, R.; Jorge, D.S.; Sander de Carvalho, L.A.; Carlos, F.M. Mapping of diffuse attenuation coefficient in optically complex waters of amazon floodplain lakes. ISPRS J. Photogramm. Remote Sens. 2020, 170, 72–87. [Google Scholar] [CrossRef]
- Lee, Z.-P.; Hu, C.; Shang, S.; Du, K.; Lewis, M.; Arnone, R.; Brewin, R. Penetration of UV-visible solar radiation in the global oceans: Insights from ocean color remote sensing. J. Geophys. Res. Ocean. 2013, 118, 4241–4255. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, L. Estimating scattering of pure water from density fluctuation of the refractive index. Opt. Express 2009, 17, 3485–3489. [Google Scholar] [CrossRef]
- Legendre, P.; Borcard, D. Box–Cox-chord transformations for community composition data prior to beta diversity analysis. Ecography 2018, 41, 1820–1824. [Google Scholar] [CrossRef]
- Dray, S.; Chessel, D.; Thioulouse, J. Co-inertia analysis and the linking of ecological data tables. Ecology 2003, 84, 3078–3089. [Google Scholar] [CrossRef]
- Thioulouse, J. Simultaneous analysis of a sequence of paired ecological tables: A comparison of several methods. Ann. Appl. Stat. 2011, 5, 2300–2325. [Google Scholar] [CrossRef]
- Slimani, N.; Guilbert, E.; El Ayni, F.; Jrad, A.; Boumaiza, M.; Thioulouse, J. The use of STATICO and COSTATIS, two exploratory three-ways analysis methods: An application to the ecology of aquatic heteroptera in the Medjerda watershed (Tunisia). Environ. Ecol. Stat. 2017, 24, 269–295. [Google Scholar] [CrossRef]
- Kraus, C.N.; Bonnet, M.-P.; Miranda, C.A.; de Souza Nogueira, I.; Garnier, J.; Vieira, L.C.G. Interannual hydrological variations and ecological phytoplankton patterns in Amazonian floodplain lakes. Hydrobiologia 2019, 830, 135–149. [Google Scholar] [CrossRef]
- Lobo, M.T.M.P.S.; de Souza Nogueira, I.; Fabris Sgarbi, L.; Nunes Kraus, C.; de Oliveira Bomfim, E.; Garnier, J.; da Motta Marques, D.; Bonnet, M.-P. Morphology-based functional groups as the best tool to characterize shallow lake-dwelling phytoplankton on an Amazonian floodplain. Ecol. Indic. 2018, 95, 579–588. [Google Scholar] [CrossRef]
- Alcântara, E.; Novo, E.; Stech, J.; Lorenzzetti, J.; Barbosa, C.; Assireu, A.; Souza, A. A contribution to understanding the turbidity behaviour in an Amazon floodplain. Hydrol. Earth Syst. Sci. 2010, 14, 351–364. [Google Scholar] [CrossRef]
- Affonso, A.; Barbosa, C.; Novo, E. Water quality changes in floodplain lakes due to the Amazon River flood pulse: Lago Grande de Curuaí (Pará). Braz. J. Biol. 2011, 71, 601–610. [Google Scholar] [CrossRef]
- Kannaujiya, V.K.; Sundaram, S.; Sinha, R.P. Evolution of Phycobiliproteins. In Phycobiliproteins: Recent Developments and Future Applications; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- MacColl, R.; Guard-Friar, D. Phycobiliproteins; CRC Press: Boca Raton, FL, USA, 2018; ISBN 135107556X. [Google Scholar]
- Wang, G.; Lee, Z.; Mouw, C. Multi-spectral remote sensing of phytoplankton pigment absorption properties in cyanobacteria bloom waters: A regional example in the Western Basin of Lake Erie. Remote Sens. 2017, 9, 1309. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, Y.J.; He, W.; Liu, W.X.; Kong, X.Z.; Jørgensen, S.E.; Xu, F.L. The tempo-spatial variations of phytoplankton diversities and their correlation with trophic state levels in a large eutrophic Chinese lake. Ecol. Indic. 2016, 66, 153–162. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.S.; Walsby, A.E. Water blooms. Biol. Rev. Camb. Philos. Soc. 1975, 50, 437–481. [Google Scholar] [CrossRef]
- Hunter, P.D.; Tyler, A.N.; Willby, N.J.; Gilvear, D.J. The spatial dynamics of vertical migration by Microcystis aeruginosa in a eutrophic shallow lake: A case study using high spatial resolution time-series airborne remote sensing. Limnol. Oceanogr. 2008, 53, 2391–2406. [Google Scholar] [CrossRef]
- Visser, P.M.; Passarge, J.; Mur, L.R. Modelling vertical migration of the cyanobacterium Microcystis. Hydrobiologia 1997, 349, 99–109. [Google Scholar] [CrossRef]
- O’Farrell, I.; Tell, G.; Podlejski, A. Morphological variability of Aulacoseira granulata (Ehr.) Simonsen (Bacillariophyceae) in the Lower Paraná River (Argentina). Limnology 2001, 2, 65–71. [Google Scholar] [CrossRef]
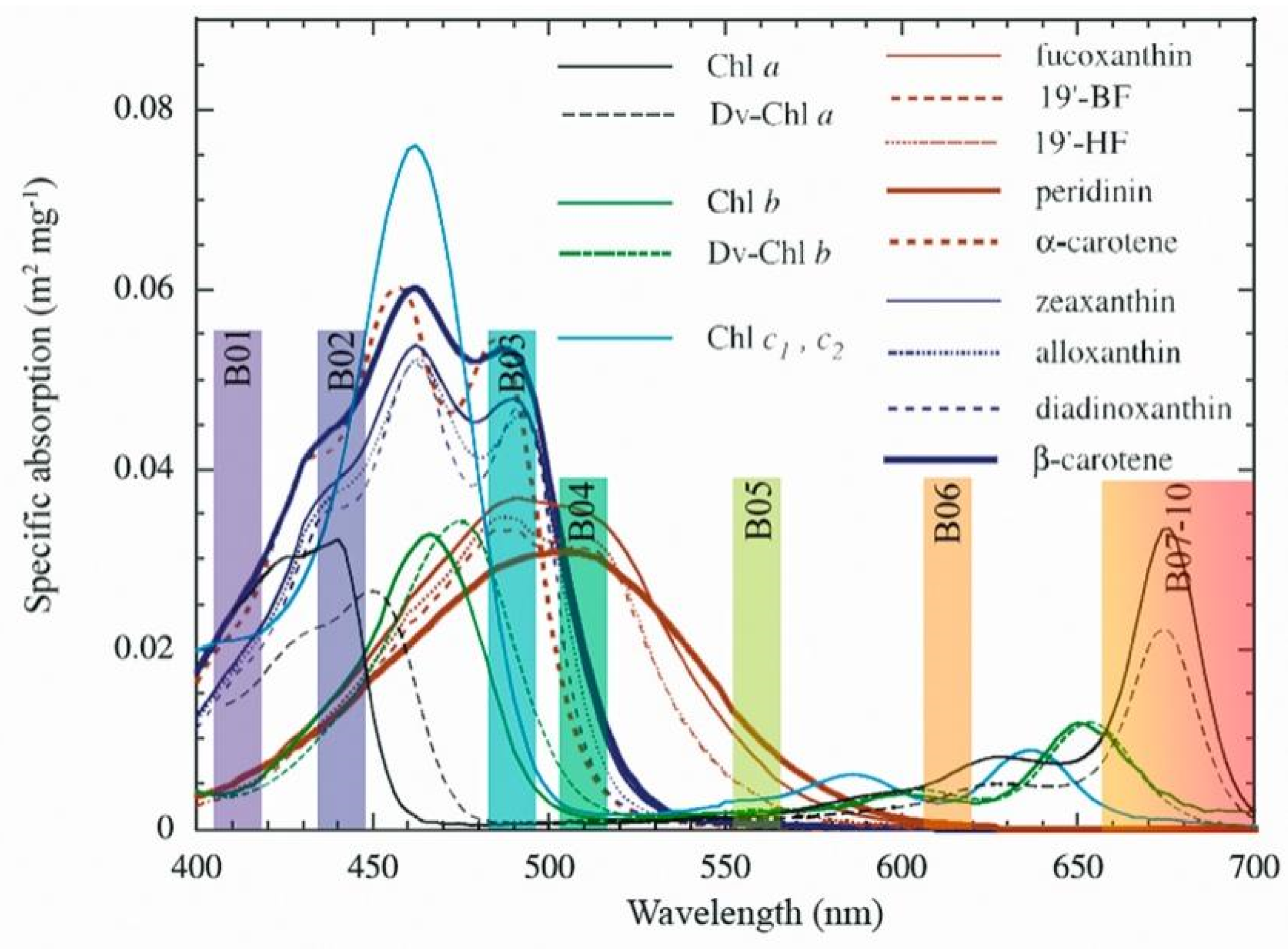

| Bands | CW (nm) | BW (nm) |
|---|---|---|
| B-01 | 412 | 405–419 |
| B-02 | 442 | 435–449 |
| B-03 | 490 | 483–498 |
| B-04 | 510 | 503–518 |
| B-05 | 560 | 552–567 |
| B-06 | 620 | 613–626 |
| B-07 | 665 | 658–672 |
| B-08 | 673 | 668–680 |
| B-09 | 681 | 675–687 |
| B-10 | 708 | 701–716 |
| Code | Genera | Class |
|---|---|---|
| Act | Actinastrum | Trebouxiophyceae |
| Aul | Aulacoseira | Bacilariophyceae |
| Bot | Botryococcus | Trebouxiophyceae |
| Clos | Closterium | Conjugatophyceae |
| Coe | Coelastrum | Chlorophyceae |
| Cryp | Cryptomonas | Cryptophyceae |
| Cyl | Cylindrospermopsis | Cyanophyceae |
| Lec | Lepocinclis | Euglenophyceae |
| Mel | Melosira | Coscinodiscophyceae |
| Mcy | Microcystis | Cyanophyceae |
| Pan | Pandorina | Chlorophyceae |
| Per | Peridinium | Dinophyceae |
| Pkx | Planktothrix | Cyanophyceae |
| Vol | Volvox | Chlorophyceae |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraus, C.N.; Maciel, D.A.; Bonnet, M.P.; Novo, E.M.L.d.M. Phytoplankton Genera Structure Revealed from the Multispectral Vertical Diffuse Attenuation Coefficient. Remote Sens. 2021, 13, 4114. https://doi.org/10.3390/rs13204114
Kraus CN, Maciel DA, Bonnet MP, Novo EMLdM. Phytoplankton Genera Structure Revealed from the Multispectral Vertical Diffuse Attenuation Coefficient. Remote Sensing. 2021; 13(20):4114. https://doi.org/10.3390/rs13204114
Chicago/Turabian StyleKraus, Cleber Nunes, Daniel Andrade Maciel, Marie Paule Bonnet, and Evlyn Márcia Leão de Moraes Novo. 2021. "Phytoplankton Genera Structure Revealed from the Multispectral Vertical Diffuse Attenuation Coefficient" Remote Sensing 13, no. 20: 4114. https://doi.org/10.3390/rs13204114
APA StyleKraus, C. N., Maciel, D. A., Bonnet, M. P., & Novo, E. M. L. d. M. (2021). Phytoplankton Genera Structure Revealed from the Multispectral Vertical Diffuse Attenuation Coefficient. Remote Sensing, 13(20), 4114. https://doi.org/10.3390/rs13204114







