A Global Analysis of Anthropogenic Development of Marine Turtle Nesting Beaches
Abstract
1. Introduction
Coastal Squeeze and Marine Turtles
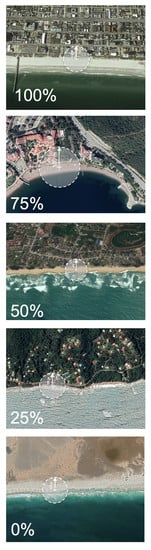
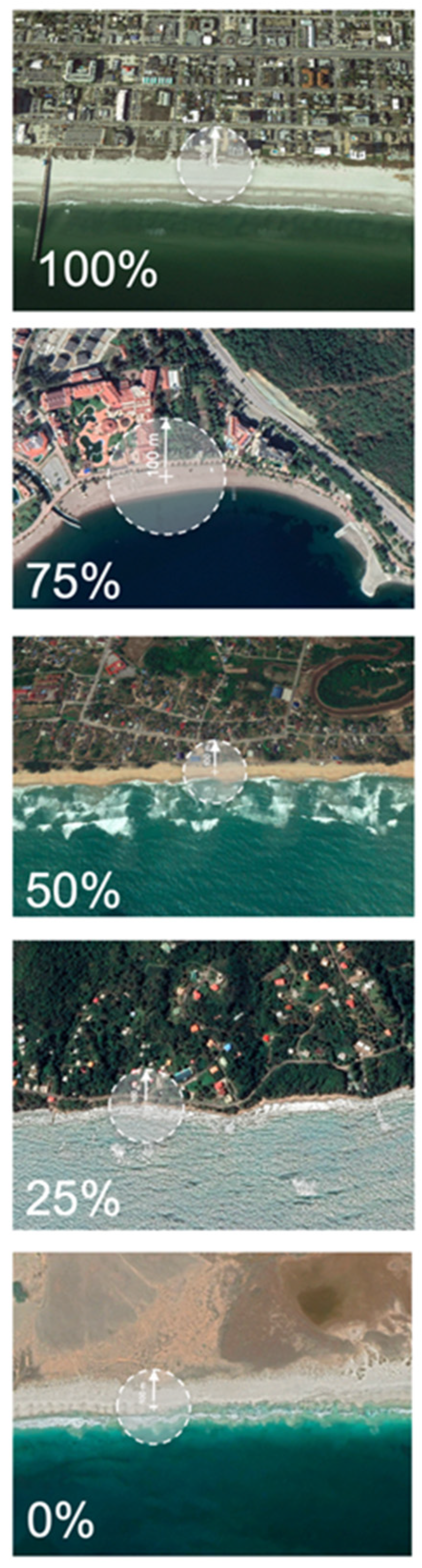
2. Materials and Methods
Statistical Analysis
3. Results
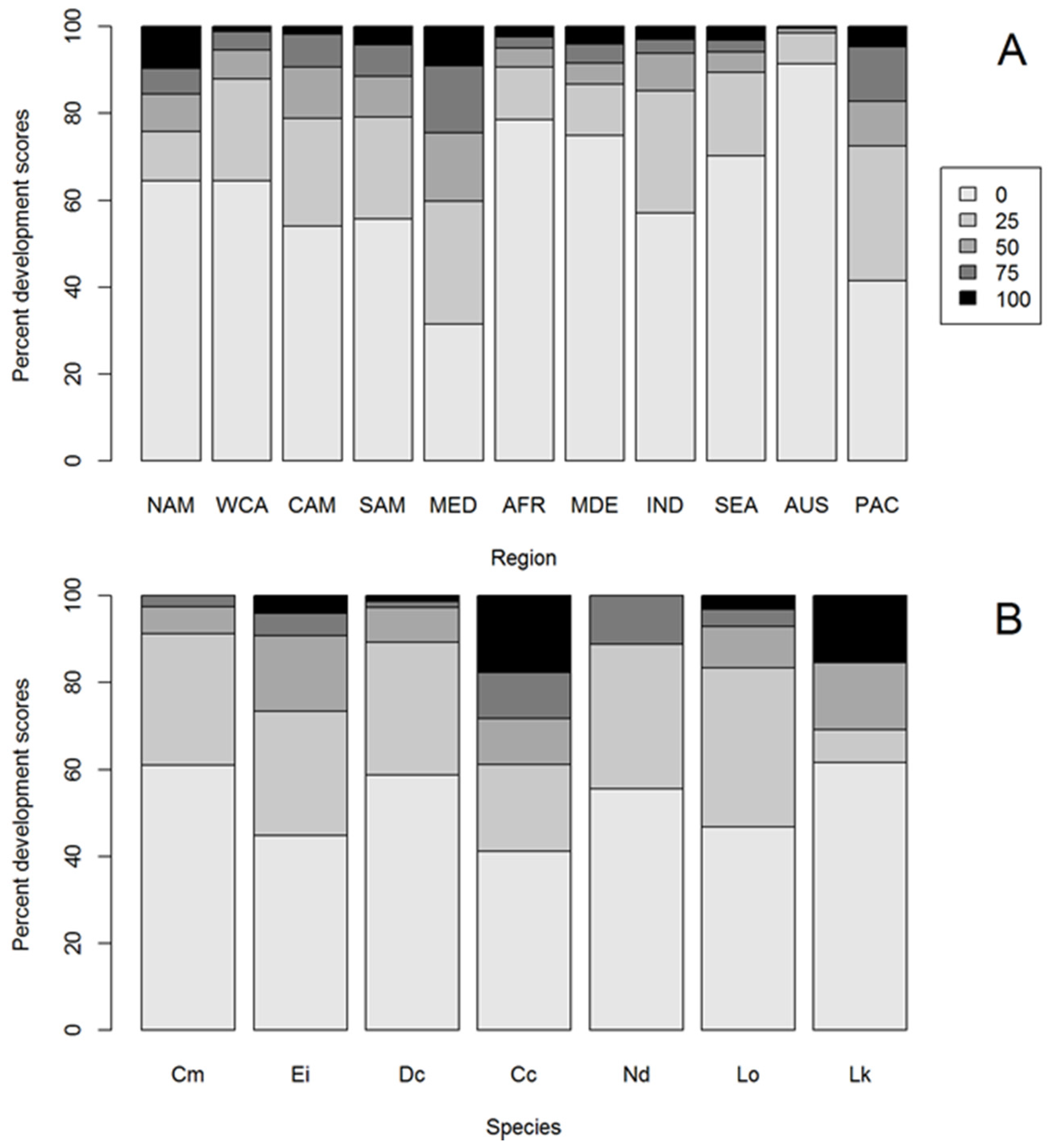
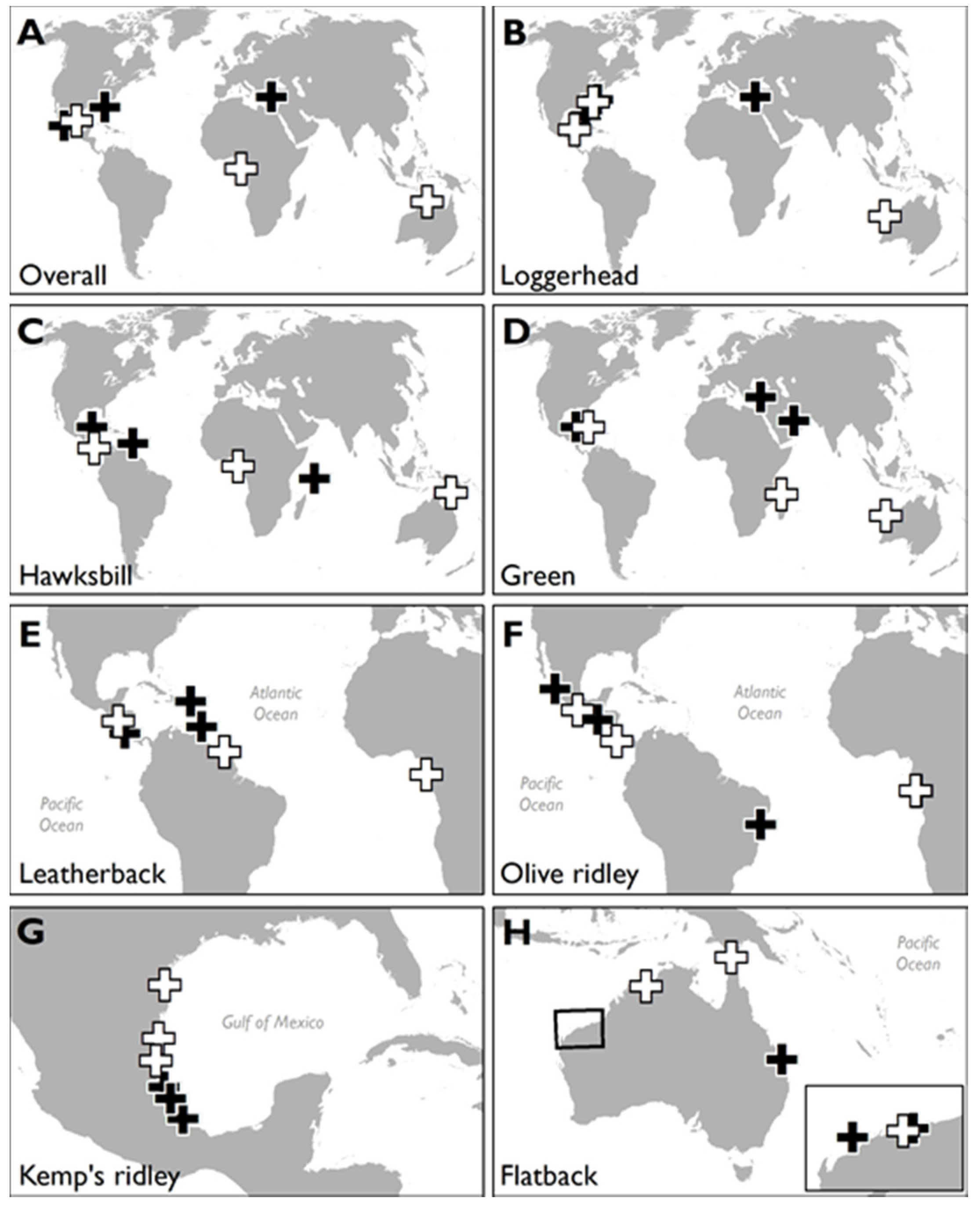
3.1. HACD by Regions
3.2. Most Developed Regions
3.3. Least Developed Regions
3.4. HACD by Species
3.5. Species with Most HACD
3.6. Species with Least HACD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tittensor, D.P.; Mora, C.; Jetz, W.; Lotze, H.K.; Ricard, D.; Berghe, E.V.; Worm, B. Global patterns and predictors of marine biodiversity across taxa. Nature 2010, 466, 1098–1101. [Google Scholar] [CrossRef]
- Small, C.; Nicholls, R. A global analysis of human settlement in coastal zones. J. Coast. Res. 2003, 3, 584–599. [Google Scholar]
- IPCC. Climate Change 2014 Synthesis report. In Summary for Policymakers. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Currin, C.A. Living Shorelines for Coastal Resilience. In Coastal Wetlands: An Integrated and Ecosystem Approach, 2nd ed.; Perillo, G.M.E., Wolanski, E., Cahoon, D.R., Hopkinson, C.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1023–1053. [Google Scholar]
- Firth, L.B.; Thompson, R.C.; Bohn, K.; Abbiati, M.; Airoldi, L.; Bouma, T.; Bozzeda, F.; Ceccherelli, V.; Colangelo, M.; Evans, A.; et al. Between a rock and a hard place: Environmental and engineering considerations when designing coastal defence structures. Coast. Eng. 2014, 87, 122–135. [Google Scholar] [CrossRef]
- Adger, W.N.; Hughes, T.P.; Folke, C.; Carpenter, S.R.; Rockström, J. Social-Ecological Resilience to Coastal Disasters. Science 2005, 309, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Gittman, R.K.; Fodrie, F.J.; Popowich, A.M.; Keller, D.; Bruno, J.F.; Currin, C.; Peterson, C.H.; Piehler, M.F. Engineering away our natural defenses: An analysis of shoreline hardening in the US. Front. Ecol. Environ. 2015, 13, 301–307. [Google Scholar] [CrossRef]
- Martínez, M.L.; Intralawan, A.; Vazquez, G.; Pérez-Maqueo, O.; Sutton, P.; Landgrave, R. The coasts of our world: Ecological, economic and social importance. Ecol. Econ. 2007, 63, 254–272. [Google Scholar] [CrossRef]
- Chu, M.L.; Muñoz-Carpena, R.; Kiker, G.; Emanuelsson, A.; Linkov, I. Exploring vulnerability of coastal habitats to sea level rise through global sensitivity and uncertainty analyses. Environ. Model. Softw. 2011, 26, 593–604. [Google Scholar]
- Von Holle, B.; Irish, J.L.; Spivy, A.; Weishampel, J.F.; Meylan, A.; Godfrey, M.H.; Dodd, M.; Schweitzer, S.H.; Keyes, T.; Sanders, F.; et al. Effects of future sea level rise on coastal habitat. J. Wildl. Manag. 2019, 83, 694–704. [Google Scholar] [CrossRef]
- Merino, G.; Barange, M.; Blanchard, J.L.; Harle, J.; Holmes, R.; Allen, I.; Allison, E.; Badjeck, M.-C.; Dulvy, N.K.; Holt, J.; et al. Can marine fisheries and aquaculture meet fish demand from a growing human population in a changing climate? Glob. Environ. Chang. 2012, 22, 795–806. [Google Scholar] [CrossRef]
- Arkema, K.K.; Guannel, G.; Verutes, G.; Wood, S.A.; Guerry, A.; Ruckelshaus, M.; Kareiva, P.; Lacayo, M.; Silver, J.M.; Lacayo-Emery, M. Coastal habitats shield people and property from sea-level rise and storms. Nat. Clim. Chang. 2013, 3, 913–918. [Google Scholar] [CrossRef]
- Narayan, S.; Beck, M.W.; Reguero, B.G.; Losada, I.J.; Van Wesenbeeck, B.; Pontee, N.; Sanchirico, J.N.; Ingram, J.C.; Lange, G.-M.; Burks-Copes, K.A. The Effectiveness, Costs and Coastal Protection Benefits of Natural and Nature-Based Defences. PLoS ONE 2016, 11, e0154735. [Google Scholar] [CrossRef] [PubMed]
- Temmerman, S.; Meire, P.; Bouma, T.J.; Herman, P.M.J.; Ysebaert, T.; De Vriend, H.J. Ecosystem-based coastal defence in the face of global change. Nature 2013, 504, 79–83. [Google Scholar] [CrossRef]
- Dutton, A.; Carlson, A.E.; Long, A.J.; Milne, G.A.; Clark, P.U.; DeConto, R.; Horton, B.P.; Rahmstorf, S.; Raymo, M.E. Sea-level rise due to polar ice-sheet mass loss during past warm periods. Science 2015, 349, aaa4019. [Google Scholar] [CrossRef] [PubMed]
- Mazaris, A.D.; Matsinos, G.; Pantis, J.D. Evaluating the impacts of coastal squeeze on sea turtle nesting. Ocean Coast. Manag. 2009, 52, 139–145. [Google Scholar] [CrossRef]
- Fish, M.R.; Côté, I.M.; Gill, J.A.; Jones, A.P.; Renshoff, S.; Watkinson, A.R. Predicting the Impact of Sea-Level Rise on Caribbean Sea Turtle Nesting Habitat. Conserv. Biol. 2005, 19, 482–491. [Google Scholar] [CrossRef]
- Katselidis, K.; Schofield, G.; Stamou, G.; Dimopoulos, P.; Pantis, J.D. Employing sea-level rise scenarios to strategically select sea turtle nesting habitat important for long-term management at a temperate breeding area. J. Exp. Mar. Biol. Ecol. 2014, 450, 47–54. [Google Scholar] [CrossRef]
- Fuentes, M.M.P.B.; Fish, M.R.; Maynard, J.A. Management strategies to mitigate the impacts of climate change on sea turtle’s terrestrial reproductive phase. Mitig. Adapt. Strat. Glob. Chang. 2011, 17, 51–63. [Google Scholar] [CrossRef]
- Fuentes, M.M.P.B.; Limpus, C.; Hamann, M. Vulnerability of sea turtle nesting grounds to climate change. Glob. Chang. Biol. 2010, 17, 140–153. [Google Scholar] [CrossRef]
- Fuentes, M.; Limpus, C.; Hamann, M.; Dawson, J.; Fuentes, M.M.P.B. Potential impacts of projected sea-level rise on sea turtle rookeries. Aquat. Conserv. Mar. Freshw. Ecosyst. 2010, 20, 132–139. [Google Scholar] [CrossRef]
- Varela, M.R.; Patrício, A.R.; Anderson, K.; Broderick, A.C.; Debell, L.; Hawkes, L.A.; Tilley, D.; Snape, R.T.E.; Westoby, M.; Godley, B.J. Assessing climate change associated sea-level rise impacts on sea turtle nesting beaches using drones, photogrammetry and a novel GPS system. Glob. Chang. Biol. 2018, 25, 753–762. [Google Scholar] [CrossRef]
- Butt, N.; Whiting, S.; Dethmers, K. Identifying future sea turtle conservation areas under climate change. Biol. Conserv. 2016, 204, 189–196. [Google Scholar] [CrossRef]
- Hinkel, J.; Nicholls, R.J.; Tol, R.S.; Wang, Z.-B.; Hamilton, J.M.; Boot, G.; Vafeidis, A.T.; McFadden, L.; Ganopolski, A.; Klein, R.J. A global analysis of erosion of sandy beaches and sea-level rise: An application of DIVA. Glob. Planet. Chang. 2013, 111, 150–158. [Google Scholar] [CrossRef]
- Yamamoto, K.H.; Powell, R.L.; Anderson, S.; Sutton, P. Using LiDAR to quantify topographic and bathymetric details for sea turtle nesting beaches in Florida. Remote. Sens. Environ. 2012, 125, 125–133. [Google Scholar] [CrossRef]
- The State of the World’s Sea Turtles Online Database: Data provided by the SWOT Team and hosted on OBIS-SEAMAP [Internet]. Oceanic Society, Conservation International, IUCN Marine Turtle Specialist Group (MTSG), and Marine Geospatial Ecology Lab, Duke University. 2015. Available online: http://seamap.env.duke.edu/swot (accessed on 1 January 2020).
- Fish, M.; Côte, I.; Horrocks, J.; Mulligan, B.; Watkinson, A.; Jones, A. Construction setback regulations and sea-level rise: Mitigating sea turtle nesting beach loss. Ocean Coast. Manag. 2008, 51, 330–341. [Google Scholar] [CrossRef]
- Ehrhart, L.M.; Bagley, D.A.; Redfoot, W.E. Loggerhead turtles in the Atlantic Ocean: Geographic distribution, abundance, and population status. In Loggerhead Sea Turtles; Bolten, A.B., Witherington, B.E., Eds.; Smithsonian: Washington, DC, USA, 2003; pp. 157–174. [Google Scholar]
- Plotkin, P.T. Biology and Conservation of Ridley Sea Turtles; John Hopkins University Press: Baltimore, MA, USA, 2007. [Google Scholar]
- Fitzgerald, D.M.; Fenster, M.S.; Argow, B.A.; Buynevich, I. Coastal Impacts Due to Sea-Level Rise. Annu. Rev. Earth Planet. Sci. 2008, 36, 601–647. [Google Scholar] [CrossRef]
- Plaziat, J.C.; Augustinius, P.G.E.F. Evolution of progradation/erosion along the French Guiana magrove coast: A comparison of mapped shorelines since the 18th century with Holocene data. Mar. Geol. 2004, 208, 127–143. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; ChurchiD, J.A.; Watson, C.S.; King, M.; Monselesan, D.; Legresy, B.; Harig, C. The increasing rate of global mean sea-level rise during 1993–2014. Nat. Clim. Chang. 2017, 7, 492–495. [Google Scholar] [CrossRef]
- Hill, M.K.; Monroe, M.C.; Ankersen, T.T.; Carthy, R.R.; Kay, T.A. Coastal Armoring and Sea Turtles: Beachfront Homeowners’ Opinions and Intent. Coast. Manag. 2019, 47, 594–610. [Google Scholar] [CrossRef]
- Zhang, K.; Douglas, B.C.; Leatherman, S.P. Global Warming and Coastal Erosion. Clim. Chang. 2004, 64, 41–58. [Google Scholar] [CrossRef]
- Kamel, S.J.; Mrosovsky, N. Repeatability of nesting preferences in the hawksbill sea turtle, Eretmochelys imbricata, and their fitness consequences. Anim. Behav. 2005, 70, 819–828. [Google Scholar] [CrossRef]
- Tucker, A. Nest site fidelity and clutch frequency of loggerhead turtles are better elucidated by satellite telemetry than by nocturnal tagging efforts: Implications for stock estimation. J. Exp. Mar. Biol. Ecol. 2010, 383, 48–55. [Google Scholar] [CrossRef]
- Walcott, J.; Eckert, K.; Horrocks, J.A. Tracking hawksbill sea turtles (Eretmochelys imbricata) during inter-nesting intervals around Barbados. Mar. Biol. 2012, 159, 927–938. [Google Scholar] [CrossRef]
- Wallace, B.P.; DiMatteo, A.D.; Bolten, A.; Chaloupka, M.Y.; Hutchinson, B.J.; Abreu-Grobois, F.A.; Mortimer, J.A.; Seminoff, J.A.; Amorocho, D.; Bjorndal, K.A.; et al. Global Conservation Priorities for Marine Turtles. PLoS ONE 2011, 6, e24510. [Google Scholar] [CrossRef]
- Boonzaier, L.; Pauly, D. Marine protection targets: An updated assessment of global progress. Oryx 2015, 50, 27–35. [Google Scholar] [CrossRef]
- Visbeck, M.; Kronfeld-Goharani, U.; Neumann, B.; Rickels, W.; Schmidt, J.O.; Van Doorn, E.; Matz-Lück, N.; Ott, K.; Quaas, M.F. Securing blue wealth: The need for a special sustainable development goal for the ocean and coasts. Mar. Policy 2014, 48, 184–191. [Google Scholar] [CrossRef]
- Pike, D.A. The benefits of nest relocation extend far beyond recruitment: A rejoinder to Mrosovsky. Environ. Manag. 2007, 41, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Studds, C.E.; Kendall, B.E.; Murray, N.J.; Wilson, H.B.; Rogers, D.I.; Clemens, R.S.; Gosbell, K.; Hassell, C.J.; Jessop, R.; Melville, D.S.; et al. Rapid population decline in migratory shorebirds relying on Yellow Sea tidal mudflats as stopover sites. Nat. Commun. 2017, 8, 14895. [Google Scholar] [CrossRef]
- Piersma, T.; Lindström, Å. Migrating shorebirds as integrative sentinels of global environmental change. Ibis 2004, 146, 61–69. [Google Scholar] [CrossRef]
- Temmerman, S.; De Vries, M.B.; Bouma, T.J. Coastal marsh die-off and reduced attenuation of coastal floods: A model analysis. Glob. Planet. Chang. 2012, 92, 267–274. [Google Scholar] [CrossRef]
- Wamsley, T.V.; Cialone, M.A.; Smith, J.M.; Atkinson, J.H.; Rosati, J.D. The potential of wetlands in reducing storm surge. Ocean Eng. 2010, 37, 59–68. [Google Scholar] [CrossRef]
- Shepard, C.C.; Crain, C.M.; Beck, M.W. The Protective Role of Coastal Marshes: A Systematic Review and Meta-analysis. PLoS ONE 2011, 6, e27374. [Google Scholar] [CrossRef] [PubMed]
- Gedan, K.B.; Kirwan, M.L.; Wolanski, E.; Barbier, E.; Silliman, B. The present and future role of coastal wetland vegetation in protecting shorelines: Answering recent challenges to the paradigm. Clim. Chang. 2010, 106, 7–29. [Google Scholar] [CrossRef]
- Barbier, E.; Koch, E.W.; Silliman, B.; Hacker, S.D.; Wolanski, E.; Primavera, J.; Granek, E.F.; Polasky, S.; Aswani, S.; Cramer, L.A.; et al. Coastal Ecosystem-Based Management with Nonlinear Ecological Functions and Values. Science 2008, 319, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, H.; Li, Y.; Xu, H.; Shen, J.; Rhome, J.; Smith, T.J. The role of mangroves in attenuating storm surges. Estuar. Coast. Shelf Sci. 2012, 102, 11–23. [Google Scholar] [CrossRef]
- Schlacher, T.A.; Dugan, J.; Schoeman, D.; Lastra, M.; Jones, A.; Scapini, F.; McLachlan, A.; Defeo, O. Sandy beaches at the brink. Divers. Distrib. 2007, 13, 556–560. [Google Scholar] [CrossRef]
- Nicholls, R.J.; Cazenave, A. Sea-Level Rise and Its Impact on Coastal Zones. Science 2010, 328, 1517–1520. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biddiscombe, S.J.; Smith, E.A.; Hawkes, L.A. A Global Analysis of Anthropogenic Development of Marine Turtle Nesting Beaches. Remote Sens. 2020, 12, 1492. https://doi.org/10.3390/rs12091492
Biddiscombe SJ, Smith EA, Hawkes LA. A Global Analysis of Anthropogenic Development of Marine Turtle Nesting Beaches. Remote Sensing. 2020; 12(9):1492. https://doi.org/10.3390/rs12091492
Chicago/Turabian StyleBiddiscombe, Sarah J., Elliott A. Smith, and Lucy A. Hawkes. 2020. "A Global Analysis of Anthropogenic Development of Marine Turtle Nesting Beaches" Remote Sensing 12, no. 9: 1492. https://doi.org/10.3390/rs12091492
APA StyleBiddiscombe, S. J., Smith, E. A., & Hawkes, L. A. (2020). A Global Analysis of Anthropogenic Development of Marine Turtle Nesting Beaches. Remote Sensing, 12(9), 1492. https://doi.org/10.3390/rs12091492