Evaluation of Nearshore and Offshore Water Quality Assessment Using UAV Multispectral Imagery
Abstract
1. Introduction
2. Materials and Methods
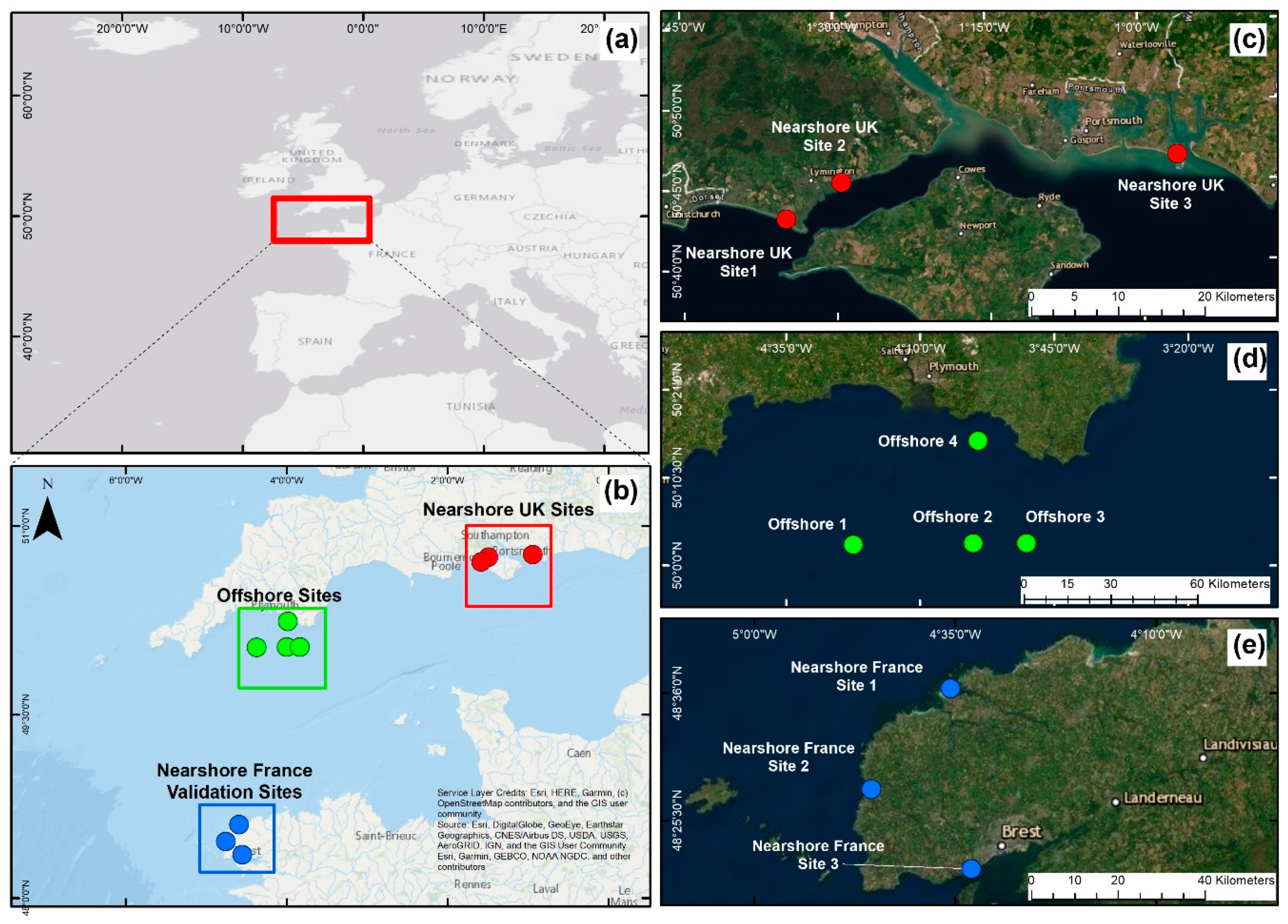
2.1. Study Area
2.2. Sampling Strategy
2.3. Methods
2.3.1. In-Situ Measurements
2.3.2. UAV Multispectral Surveys
2.4. Data Processing
2.4.1. Image Reflectance Correction
2.4.2. Image Mosaics
2.4.3. Reflectance Value Extraction
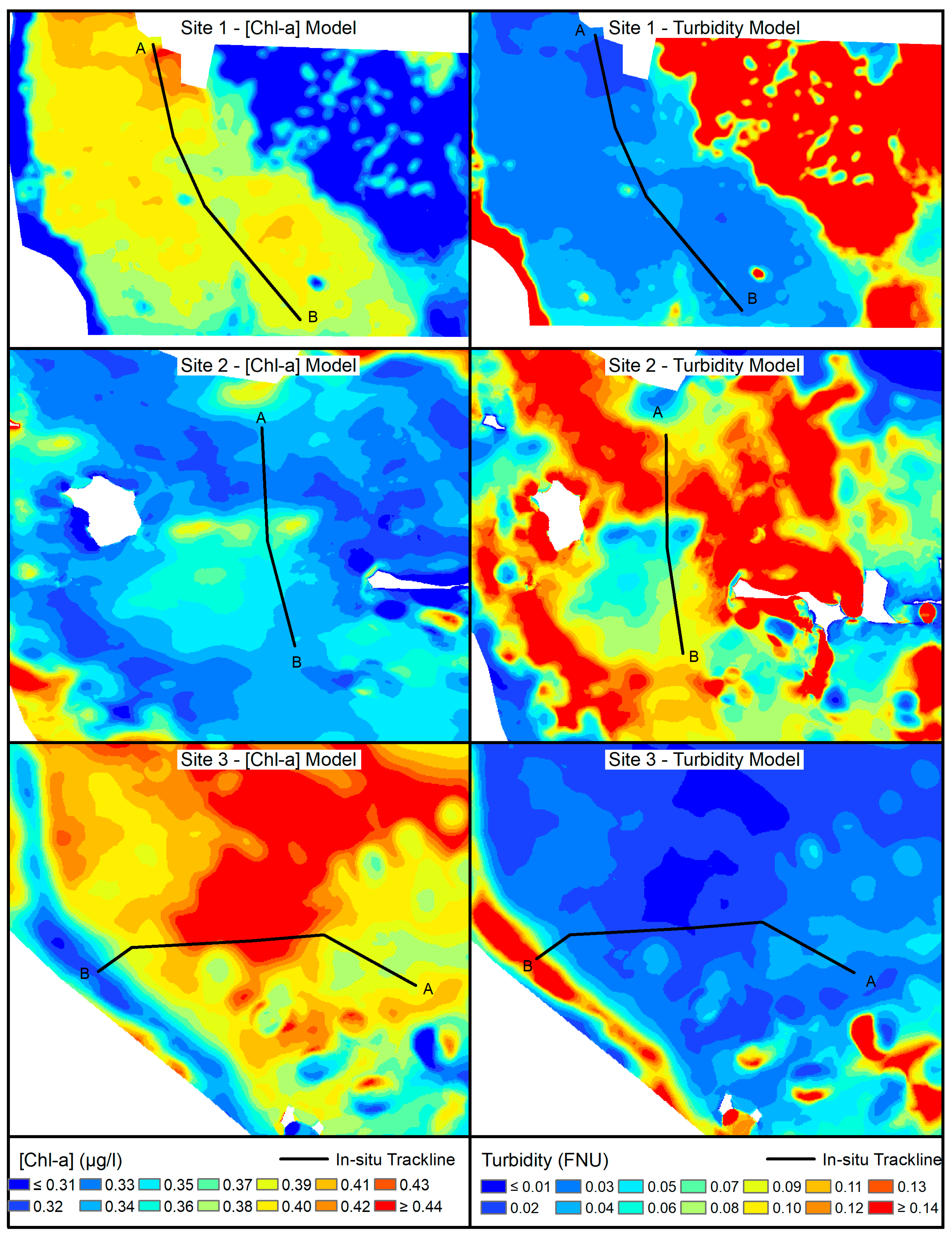
3. Results
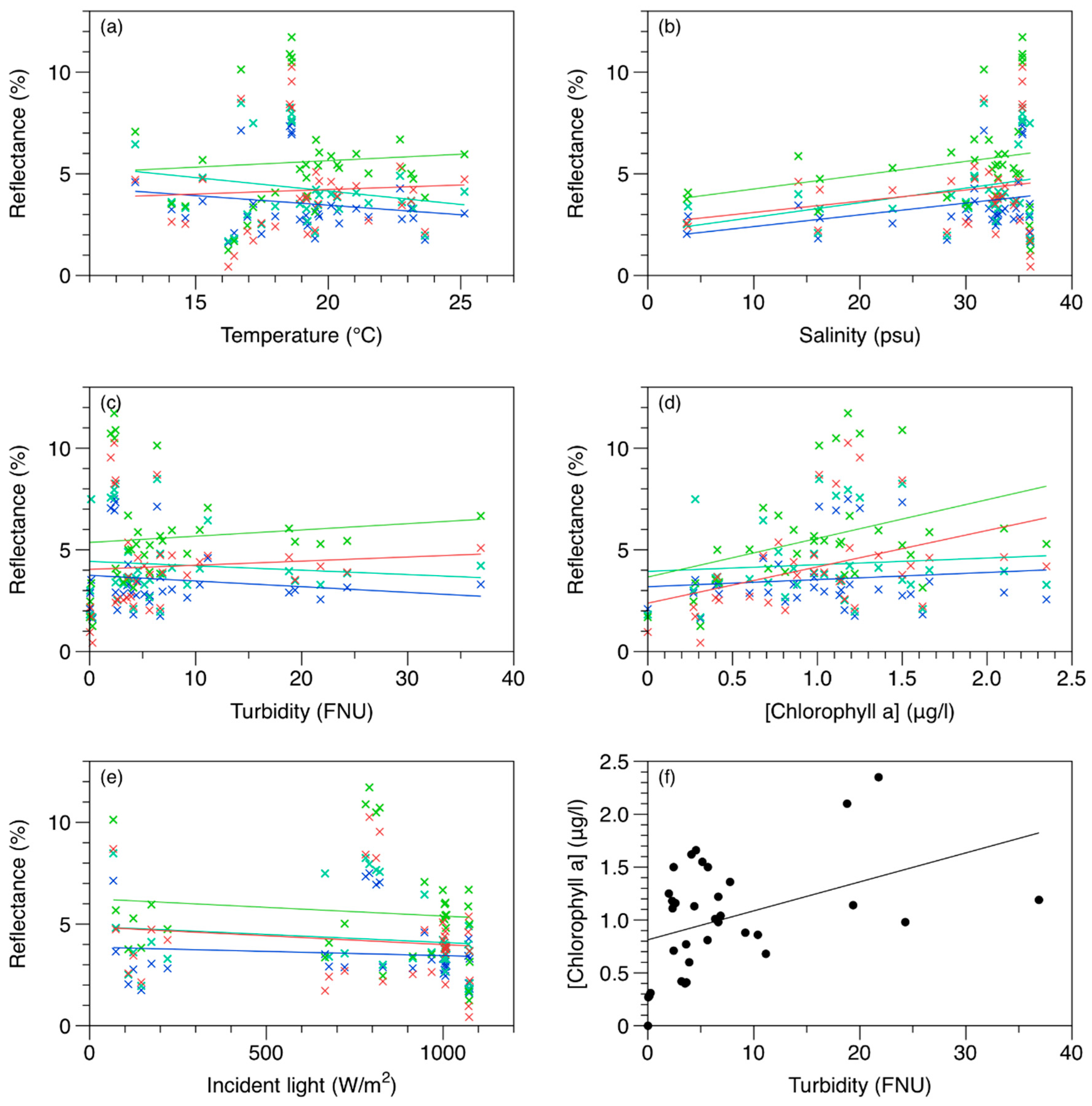
3.1. In-Situ Data Analysis
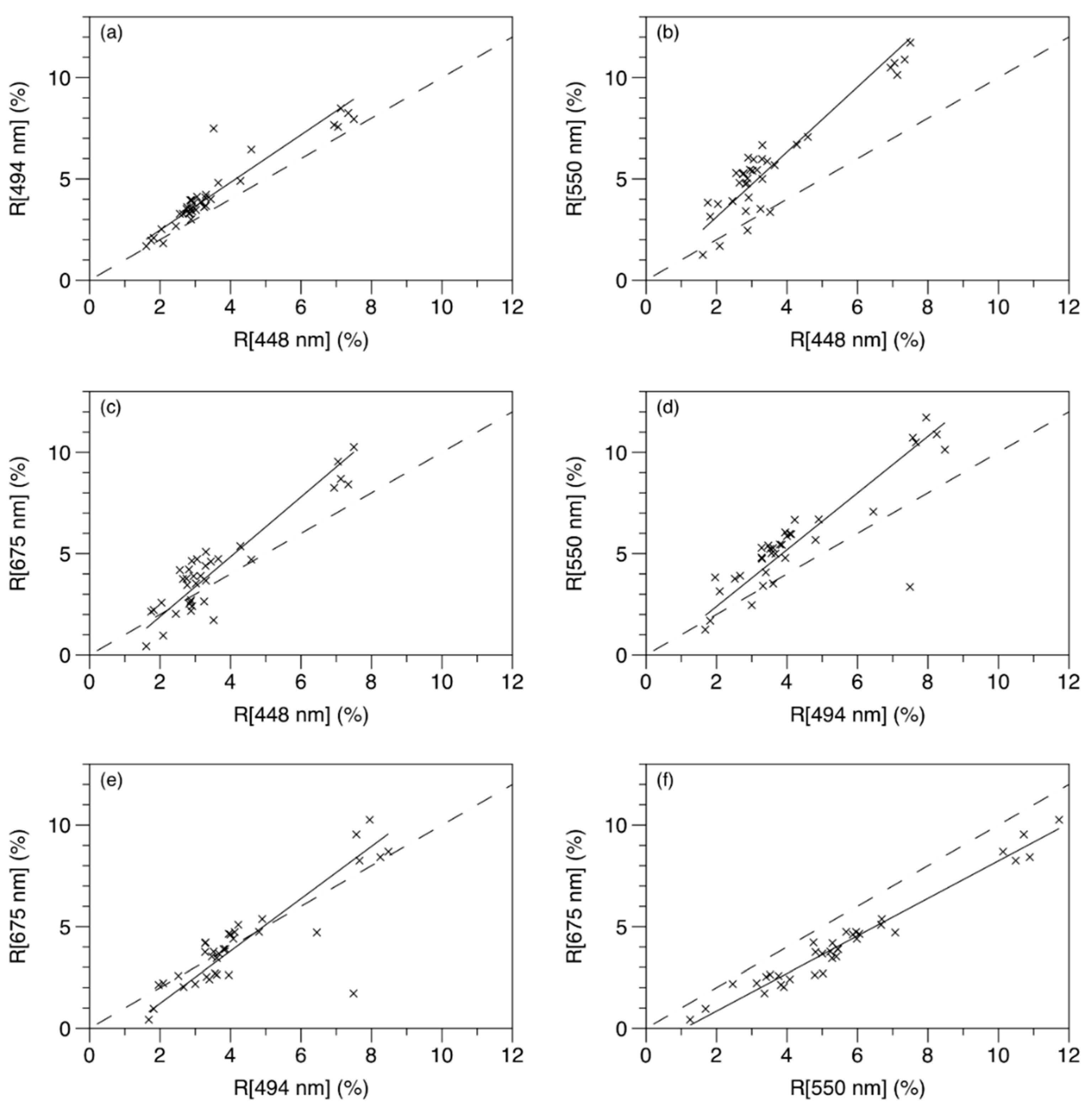
3.2. Multispectral Data Analysis
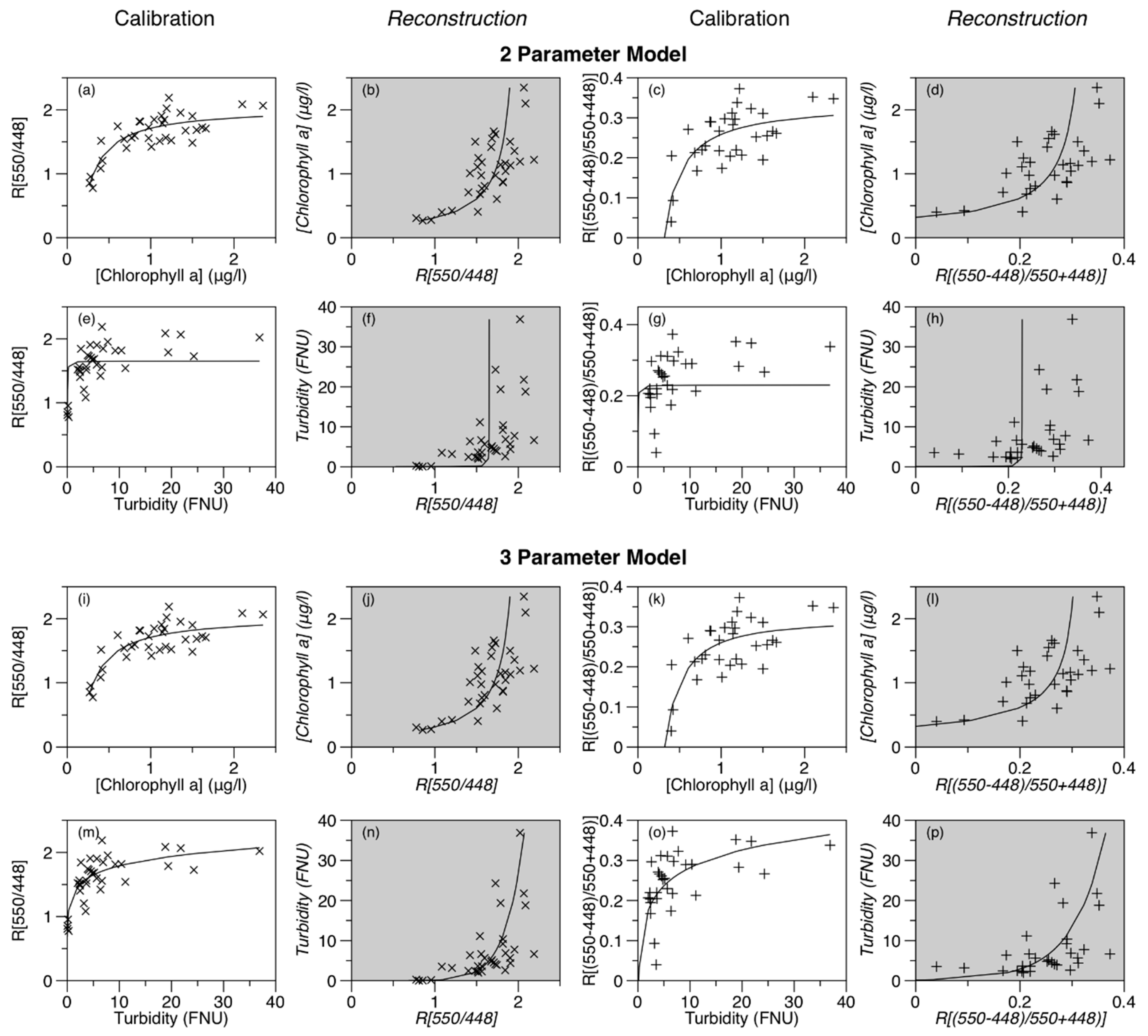
3.3. Algorithm Development
3.4. Calibration Results
3.5. Model Validation
4. Discussion
Limitations and Future Applications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, V.H. Eutrophication of Freshwater and Coastal Marine Ecosystems a Global Problem. Environ. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Chelsea Nagy, R.; Graeme Lockaby, B.; Kalin, L.; Anderson, C. Effects of Urbanization on Stream Hydrology and Water Quality: The Florida Gulf Coast. Hydrol. Process. 2012, 26, 2019–2030. [Google Scholar] [CrossRef]
- Schoonover, J.E. Channel Morphology and Sediment Origin in Streams Draining the Georgia Piedmont. J. Hydrol. 2007, 342, 110–123. [Google Scholar] [CrossRef]
- European Union. Directive 2008/56 edited by of the European Parliament and of the Council of 17 June 2008. In Official Journal of the European Union 164/136; European Union: Brussels, Belgium, 2008. [Google Scholar]
- Doxaran, D.; Froidefond, J.M.; Lavender, S.; Castaing, P. Spectral Signature of Highly Turbid Waters: Application with Spot Data to Quantify Suspended Particulate Matter Concentrations. Remote Sens. Environ. 2002, 81, 149–161. [Google Scholar] [CrossRef]
- Novoa, S.; Chust, G.; Valencia, V.; Froidefond, J.M.; Morichon, D. Estimation of Chlorophyll-a Concentration in Waters over the Continental Shelf of the Bay of Biscay: A Comparison of Remote Sensing Algorithms. Int. J. Remote Sens. 2011, 32, 8349–8371. [Google Scholar] [CrossRef]
- Odermatt, D.; Gitelson, A.; Brando, V.E.; Schaepman, M. Review of Constituent Retrieval in Optically Deep and Complex Waters from Satellite Imagery. Remote Sens. Environ. 2012, 118, 116–126. [Google Scholar] [CrossRef]
- Ouillon, S.; Petrenko, A.A. Above-Water Measurements of Reflectance and Chlorophyll-a Algorithms in the Gulf of Lions, Nw Mediterranean Sea. Opt. Express 2005, 13, 2531–2548. [Google Scholar] [CrossRef]
- O’Reilly, J.E.; Maritorena, S.; Mitchell, B.G.; Siegel, D.A.; Carder, K.L.; Garver, S.A.; Kahru, M.; McClain, C. Ocean Color Chlorophyll Algorithms for Seawifs. J. Geophys. Res. Ocean. 1998, 103, 24937–24953. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Moses, W.J.; Gitelson, A.A.; Perk, R.L.; Gurlin, D.; Rundquist, D.C.; Leavitt, B.C.; Barrow, T.M.; Brakhage, P. Estimation of Chlorophyll-a Concentration in Turbid Productive Waters Using Airborne Hyperspectral Data. Water Res. 2012, 46, 993–1004. [Google Scholar] [CrossRef]
- Palmer, S.C.; Kutser, T.; Hunter, P.D. Remote Sensing of Inland Waters: Challenges, Progress and Future Directions. Remote Sens. Environ. 2015, 157, 1–8. [Google Scholar] [CrossRef]
- Flynn, K.F.; Chapra, S.C. Remote Sensing of Submerged Aquatic Vegetation in a Shallow Non-Turbid River Using an Unmanned Aerial Vehicle. Remote Sens. 2014, 6, 12815–12836. [Google Scholar] [CrossRef]
- Hardin, P.; Jensen, R. Small-Scale Unmanned Aerial Vehicles in Environmental Remote Sensing: Challenges and Opportunities. GIScience Remote Sens. 2011, 48, 99–111. [Google Scholar] [CrossRef]
- Anderson, K.; Gaston, K.J. Lightweight Unmanned Aerial Vehicles Will Revolutionize Spatial Ecology. Front. Ecol. Environ. 2013, 11, 138–146. [Google Scholar] [CrossRef]
- Kageyama, Y.; Takahashi, J.; Nishida, M.; Kobori, B.; Nagamoto, D. Analysis of Water Quality in Miharu Dam Reservoir, Japan, Using Uav Data. IEEJ Trans. Electr. Electron. Eng. 2016, 11, S183–S185. [Google Scholar] [CrossRef]
- Su, T.C.; Chou, H.T. Application of Multispectral Sensors Carried on Unmanned Aerial Vehicle (Uav) to Trophic State Mapping of Small Reservoirs: A Case Study of Tain-Pu Reservoir in Kinmen, Taiwan. Remote Sens. 2015, 7, 10078–10097. [Google Scholar] [CrossRef]
- Su, T.C. A Study of a Matching Pixel by Pixel (Mpp) Algorithm to Establish an Empirical Model of Water Quality Mapping, as Based on Unmanned Aerial Vehicle (Uav) Images. Int. J. Appl. Earth Obs. Geoinf. 2017, 58, 213–224. [Google Scholar] [CrossRef]
- Cillero Castro, C.; Domínguez Gómez, J.A.; Delgado Martín, J.; Hinojo Sánchez, B.A.; Cereijo Arango, J.L.; Cheda Tuya, F.A.; Díaz-Varela, R. An Uav and Satellite Multispectral Data Approach to Monitor Water Quality in Small Reservoirs. Remote Sens. 2020, 12, 1514. [Google Scholar] [CrossRef]
- Klemas, V.V. Coastal and Environmental Remote Sensing from Unmanned Aerial Vehicles: An Overview. J. Coast. Res. 2015, 31, 1260–1267. [Google Scholar] [CrossRef]
- Zang, W.; Lin, J.; Wang, Y.; Tao, H. Investigating Small-Scale Water Pollution with Uav Remote Sensing Technology. In World Automation Congress; Puerto Vallarta, Mexico, 2012; pp. 1–4. Available online: https://www.semanticscholar.org/paper/Investigating-small-scale-water-pollution-with-UAV-Zang-Lin/767fa8b350ee6fbb79cc121da625722b012bcf59 (accessed on 12 June 2020).
- Yang, C. A High-Resolution Airborne Four-Camera Imaging System for Agricultural Remote Sensing. Comput. Electron. Agric. 2012, 88, 13–24. [Google Scholar] [CrossRef]
- Welschmeyer, N.A. Fluorometric Analysis of Chlorophyll a in the Presence of Chlorophyll B and Pheopigments. Limnol. Oceanogr. 1994, 39, 1985–1992. [Google Scholar] [CrossRef]
- Brown, M.; Lowe, D.G. Automatic Panoramic Image Stitching Using Invariant Features. Int. J. Comput. Vis. 2007, 74, 59–73. [Google Scholar] [CrossRef]
- Song, H.; Yang, C.; Zhang, J.; Hoffmann, W.C.; He, D.; Thomasson, J.A. Comparison of Mosaicking Techniques for Airborne Images from Consumer-Grade Cameras. J. Appl. Remote Sens. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Frouin, R.; Lingner, D.W.; Gautier, C.; Baker, K.S.; Smith, R.C. A Simple Analytical Formula to Compute Clear Sky Total and Photosynthetically Available Solar Irradiance at the Ocean Surface. J. Geophys. Res. Ocean. 1989, 94, 9731–9742. [Google Scholar] [CrossRef]
- Nelder, J.A.; Mead, R. A Simplex Method for Function Minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- Efron, B. Bootstrap Methods: Another Look at the Jackknife. Ann. Statist. 1979, 7, 1–26. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and Photographic Infrared Linear Combinations for Monitoring Vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- ITU. Drones for Agriculture: E-Agriculture in Action; ITU: Geneva, Switzerland, 2018; Available online: http://handle.itu.int/11.1002/pub/8111728e-en (accessed on 14 May 2020).
- McKinnon, T.; Hoff, P. Comparing Rgb-Based Vegetation Indices with Ndvi for Drone Based Agricultural Sensing. Agribotix. Com. 2017, 21, 1–8. Available online: https://agribotix.com/wp-content/uploads/2017/05/Agribotix-VARI-TGI-Study.pdf (accessed on 12 June 2020).
- Metsamaa, L.; Kutser, T.; Strömbeck, N. Recognising Cyanobacterial Blooms Based on Their Optical Signature: A Modelling Study. Boreal Environ. Res. 2006, 11, 493–506. [Google Scholar]
- Cannizzaro, J.P.; Carder, K.L. Estimating Chlorophyll a Concentrations from Remote-Sensing Reflectance in Optically Shallow Waters. Remote Sens. Environ. 2006, 101, 13–24. [Google Scholar] [CrossRef]
- Minghelli-Roman, A.; Dupouy, C. Influence of Water Column Chlorophyll Concentration on Bathymetric Estimations in the Lagoon of New Caledonia, Using Several Meris Images. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2013, 6, 739–745. [Google Scholar] [CrossRef]
- Wattelez, G.; Dupouy, C.; Mangeas, M.; Lefèvre, J.; Frouin, R. A Statistical Algorithm for Estimating Chlorophyll Concentration in the New Caledonian Lagoon. Remote Sens. 2016, 8, 45. [Google Scholar] [CrossRef]
- Minghelli-Roman, A.; Dupouy, C. Correction of the Water Column Attenuation: Application to the Seabed Mapping of the Lagoon of New Caledonia Using Meris Images. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 2619–2629. [Google Scholar] [CrossRef]
- Schaeffer, B.A.; Hagy, J.D.; Conmy, R.N.; Lehrter, J.C.; Stumpf, R.P. An Approach to Developing Numeric Water Quality Criteria for Coastal Waters Using the Seawifs Satellite Data Record. Environ. Sci. Technol. 2012, 46, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.B.; Hu, C.; Schaeffer, B.A.; Lee, Z.; Palandro, D.A.; Lehrter, J.C. Modis-Derived Spatiotemporal Water Clarity Patterns in Optically Shallow Florida Keys Waters: A New Approach to Remove Bottom Contamination. Remote Sens. Environ. 2013, 134, 377–391. [Google Scholar] [CrossRef]
- Dekker, A.G.; Phinn, S.R.; Anstee, J.; Bissett, P.; Brando, V.E.; Casey, B.; Fearns, P.; Hedley, J.; Klonowski, W.; Lee, Z.P.; et al. Intercomparison of Shallow Water Bathymetry, Hydro-Optics, and Benthos Mapping Techniques in Australian and Caribbean Coastal Environments. Limnol. Oceanogr. Methods 2011, 9, 396–425. [Google Scholar] [CrossRef]
- Nechad, B.; Ruddick, K.G.; Neukermans, G. Calibration and Validation of a Generic Multisensor Algorithm for Mapping of Turbidity in Coastal Waters. In Proceedings of the SPIE—The International Society for Optical Engineering, Berlin, Germany, 9 September 2009; p. 7473. [Google Scholar]
- Binding, C.E.; Jerome, J.H.; Bukata, R.P.; Booty, W.G. Suspended Particulate Matter in Lake Erie Derived from Modis Aquatic Colour Imagery. Int. J. Remote Sens. 2010, 31, 5239–5255. [Google Scholar] [CrossRef]
| Color | Center Wavelength (nm) | Wavelength Range (nm) |
|---|---|---|
| Blue | 448 | 438.0–458.0 |
| Blue | 494 | 484.0–504.0 |
| Green | 550 | 537.5–652.5 |
| Red | 675 | 662.5–687.5 |
| Plot in Figure 2 | λ1 (nm) | λ2 (nm) | Slope | Intercept | R2 |
|---|---|---|---|---|---|
| a | 448 | 494 | 1.17 | 0.13 | 0.86 |
| b | 448 | 550 | 1.60 | −0.08 | 0.86 |
| c | 448 | 675 | 1.33 | −0.54 | 0.86 |
| d | 494 | 550 | 1.40 | −0.40 | 0.73 |
| e | 494 | 675 | 1.29 | −1.35 | 0.69 |
| f | 550 | 675 | 0.92 | −1.00 | 0.96 |
| [Chl-a] (2 parameters) | [550/448] | [(550 − 448)/(550 + 448)] | [675/448] | [(675 − 448)/675 + 448)] | [550/494] | [(550 − 494)/(550 + 494)] | [675/494] | [(675 − 494)/675 + 494)] |
| Best(x1) | 2.03 | 2.02 | 1.53 | 1.50 | 1.67 | 1.69 | 1.26 | 1.25 |
| Best(x2) | −0.32 | −0.32 | −0.28 | −0.27 | −0.26 | −0.28 | −0.23 | −0.23 |
| R² | 0.70 | 0.78 | 0.65 | 0.65 | 0.68 | 0.74 | 0.60 | 0.61 |
| Mean(x1) | 2.03 | 2.02 | 1.53 | 1.51 | 1.68 | 1.69 | 1.25 | 1.24 |
| Mean(x2) | −0.32 | −0.32 | -0.29 | −0.28 | −0.27 | −0.28 | −0.23 | −0.23 |
| Var(x1) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Var(x2) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Corr(x1,x2) | −0.84 | −0.87 | −0.83 | −0.84 | −0.77 | −0.82 | −0.85 | −0.87 |
| [Chl-a] (3 parameters) | [550/448] | [(550 − 448)/(550 + 448)] | [675/448] | [(675 − 448)/675 + 448)] | [550/494] | [(550 − 494)/(550 + 494)] | [675/494] | [(675 − 494)/675 + 494)] |
| Best(x1) | 2.05 | 1.93 | 2.26 | 1.70 | 1.55 | 1.50 | 1.45 | 1.25 |
| Best(x2) | −0.34 | −0.17 | −1.04 | −0.44 | −0.13 | −0.07 | −0.43 | −0.24 |
| Best(x3) | 0.97 | 1.49 | 0.38 | 0.98 | 1.48 | 2.09 | 0.65 | 1.25 |
| R² | 0.70 | 0.78 | 0.67 | 0.66 | 0.69 | 0.75 | 0.60 | 0.61 |
| Mean(x1) | 2.29 | 1.96 | 2.53 | 2.44 | 1.63 | 1.53 | 1.75 | 1.51 |
| Mean(x2) | −0.59 | −0.19 | −1.29 | −1.11 | −0.21 | −0.09 | −0.73 | −0.50 |
| Mean(x3) | 1.04 | 1.56 | 0.59 | 1.04 | 1.56 | 2.11 | 0.79 | 1.35 |
| Var(x1) | 0.84 | 0.03 | 2.94 | 10.06 | 0.19 | 0.01 | 1.23 | 1.49 |
| Var(x2) | 0.86 | 0.02 | 2.96 | 8.78 | 0.20 | 0.01 | 1.27 | 1.57 |
| Var(x3) | 0.26 | 0.20 | 0.15 | 0.25 | 0.40 | 0.36 | 0.21 | 0.32 |
| Corr(x1,x2) | −1.00 | −0.97 | −1.00 | −1.00 | −1.00 | −0.91 | −1.00 | −1.00 |
| Corr(x1,x3) | −0.61 | −0.83 | −0.60 | −0.39 | −0.46 | −0.75 | −0.60 | −0.45 |
| Corr(x2,x3) | 0.62 | 0.87 | 0.61 | 0.39 | 0.48 | 0.84 | 0.61 | 0.45 |
| Turbidity (2 parameters) | [550/448] | [(550 − 448)/(550 + 448)] | [675/448] | [(675 − 448)/675 + 448)] | [550/494] | [(550 − 494)/(550+494)] | [675/494] | [(675 − 494)/675 + 494)] |
| Best(x1) | 1.65 | 1.60 | 1.19 | 1.12 | 1.36 | 1.32 | 0.98 | 0.94 |
| Best(x2) | −0.02 | −0.02 | −0.02 | −0.01 | −0.01 | −0.01 | −0.01 | −0.01 |
| R² | 0.31 | 0.34 | 0.25 | 0.25 | 0.19 | 0.15 | 0.17 | 0.14 |
| Mean(x1) | 1.66 | 1.59 | 1.19 | 1.11 | 1.36 | 1.32 | 0.99 | 0.93 |
| Mean(x2) | −0.02 | −0.02 | −0.02 | −0.01 | −0.01 | −0.01 | −0.01 | −0.01 |
| Var(x1) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Var(x2) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Corr(x1,x2) | −0.17 | −0.46 | −0.18 | −0.42 | −0.31 | −0.41 | −0.29 | −0.38 |
| Turbidity (3 parameters) | [550/448] | [(550 − 448)/(550+448)] | [675/448] | [(675 − 448)/675+448)] | [550/494] | [(550 − 494)/(550+494)] | [675/494] | [(675 − 494)/675+494)] |
| Best(x1) | −2.25 | −2.26 | −1.56 | −1.08 | −8.82 | −1.20 | −13.83 | −1.00 |
| Best(x2) | 3.59 | 3.10 | 2.50 | 2.08 | 9.98 | 2.19 | 14.65 | 2.00 |
| Best(x3) | −0.05 | 0.03 | −0.06 | 0.00 | −0.01 | 0.01 | −0.01 | 0.00 |
| R² | 0.68 | 0.74 | 0.56 | 0.58 | 0.51 | 0.53 | 0.41 | 0.44 |
| Mean(x1) | −1.41 | −4.19 | −0.86 | −1.20 | −1.17 | -1.59 | −2.53 | −1.00 |
| Mean(x2) | 2.73 | 4.76 | 1.78 | 2.21 | 2.30 | 2.56 | 3.31 | 2.00 |
| Mean(x3) | −0.11 | 0.02 | −0.14 | 0.00 | −0.13 | 0.00 | −0.17 | 0.00 |
| Var(x1) | 6.49 | 77.14 | 2.92 | 1.08 | 8.39 | 5.78 | 27.02 | 0.00 |
| Var(x2) | 6.51 | 57.83 | 2.93 | 1.21 | 8.45 | 5.21 | 27.23 | 0.00 |
| Var(x3) | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.02 | 0.00 |
| Corr(x1,x2) | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 |
| Corr(x1,x3) | −0.70 | −0.64 | -0.68 | −0.58 | −0.61 | −0.53 | −0.65 | −0.74 |
| Corr(x2,x3) | 0.70 | 0.64 | 0.68 | 0.59 | 0.61 | 0.53 | 0.64 | 0.73 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McEliece, R.; Hinz, S.; Guarini, J.-M.; Coston-Guarini, J. Evaluation of Nearshore and Offshore Water Quality Assessment Using UAV Multispectral Imagery. Remote Sens. 2020, 12, 2258. https://doi.org/10.3390/rs12142258
McEliece R, Hinz S, Guarini J-M, Coston-Guarini J. Evaluation of Nearshore and Offshore Water Quality Assessment Using UAV Multispectral Imagery. Remote Sensing. 2020; 12(14):2258. https://doi.org/10.3390/rs12142258
Chicago/Turabian StyleMcEliece, Ryan, Shawn Hinz, Jean-Marc Guarini, and Jennifer Coston-Guarini. 2020. "Evaluation of Nearshore and Offshore Water Quality Assessment Using UAV Multispectral Imagery" Remote Sensing 12, no. 14: 2258. https://doi.org/10.3390/rs12142258
APA StyleMcEliece, R., Hinz, S., Guarini, J.-M., & Coston-Guarini, J. (2020). Evaluation of Nearshore and Offshore Water Quality Assessment Using UAV Multispectral Imagery. Remote Sensing, 12(14), 2258. https://doi.org/10.3390/rs12142258






