Hyperspectral Inversion of Phragmites Communis Carbon, Nitrogen, and Phosphorus Stoichiometry Using Three Models
Abstract
1. Introduction
2. Materials and Methods
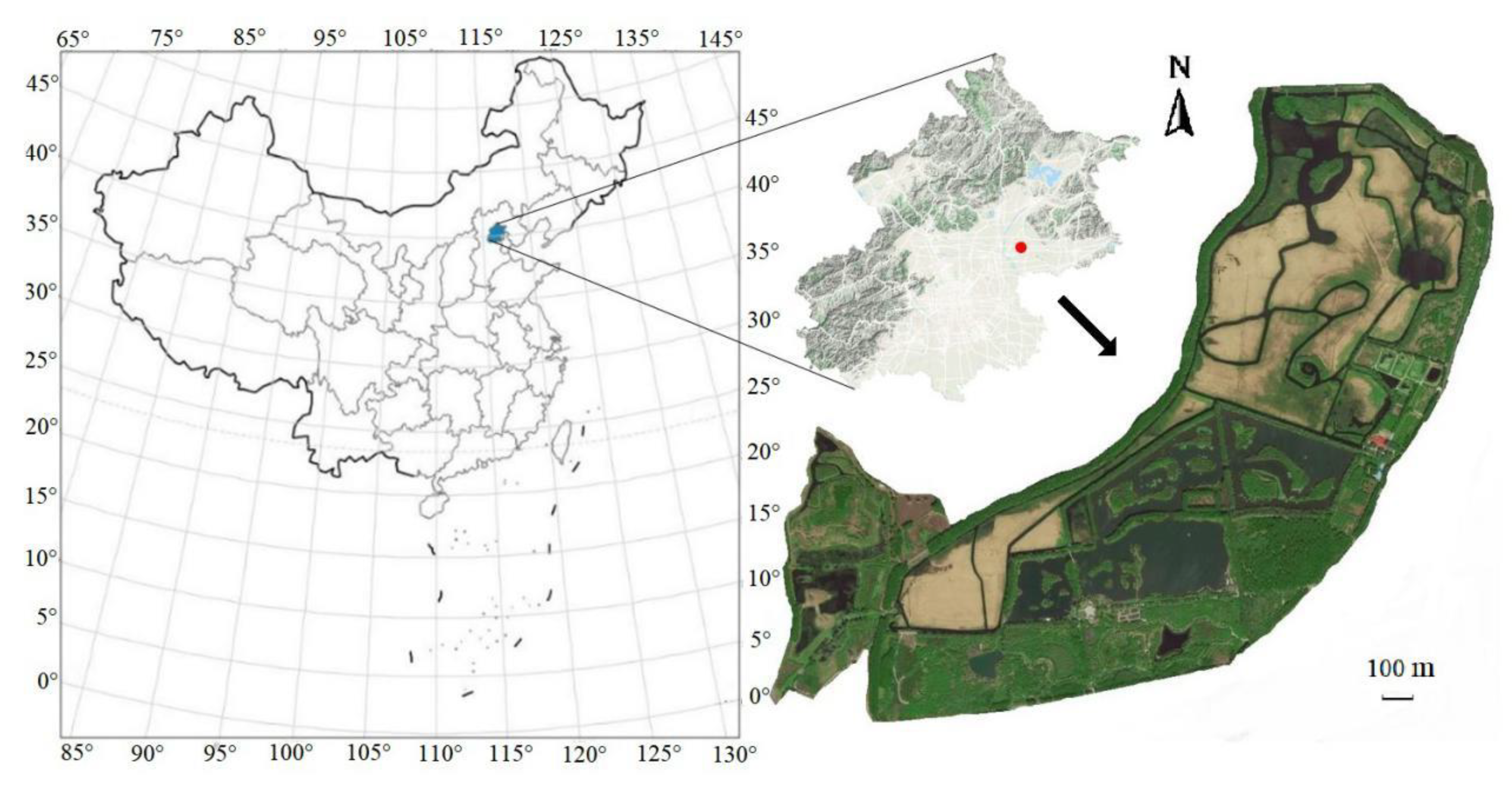
2.1. Study Area
2.2. Data Collection
2.3. Methods
3. Results
3.1. Stoichiometric Characteristics of Reed
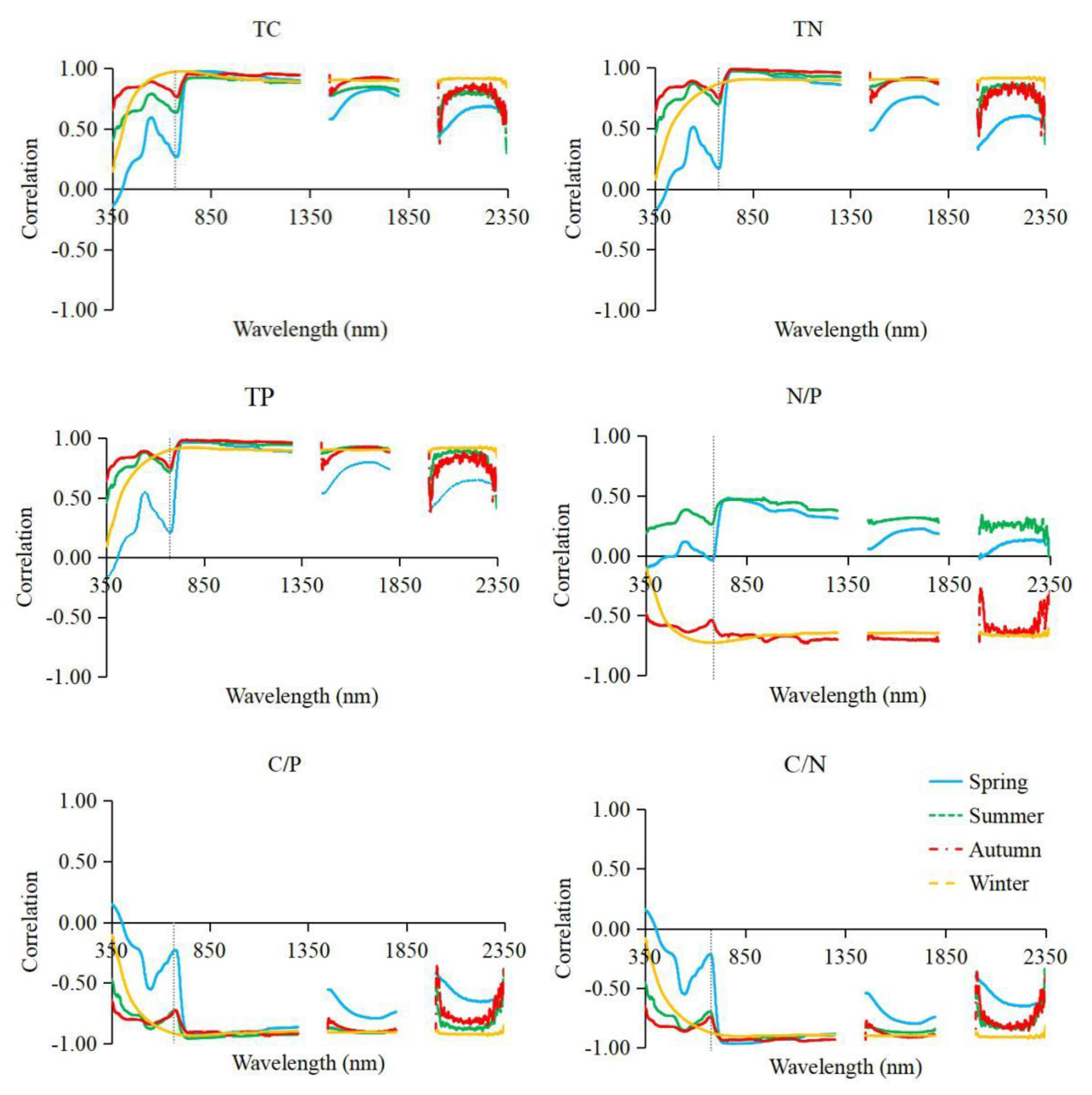
3.2. Correlation Analysis
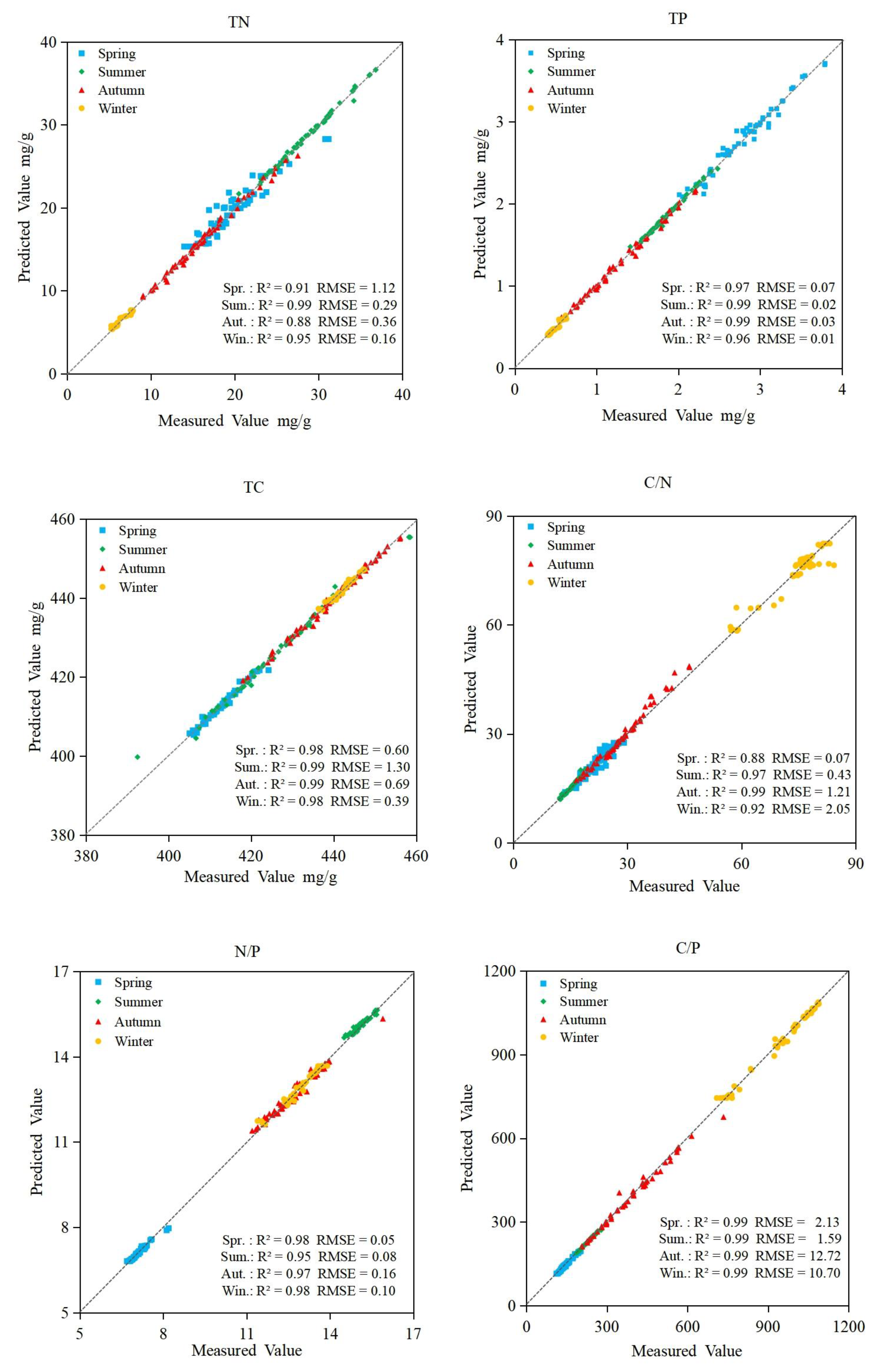
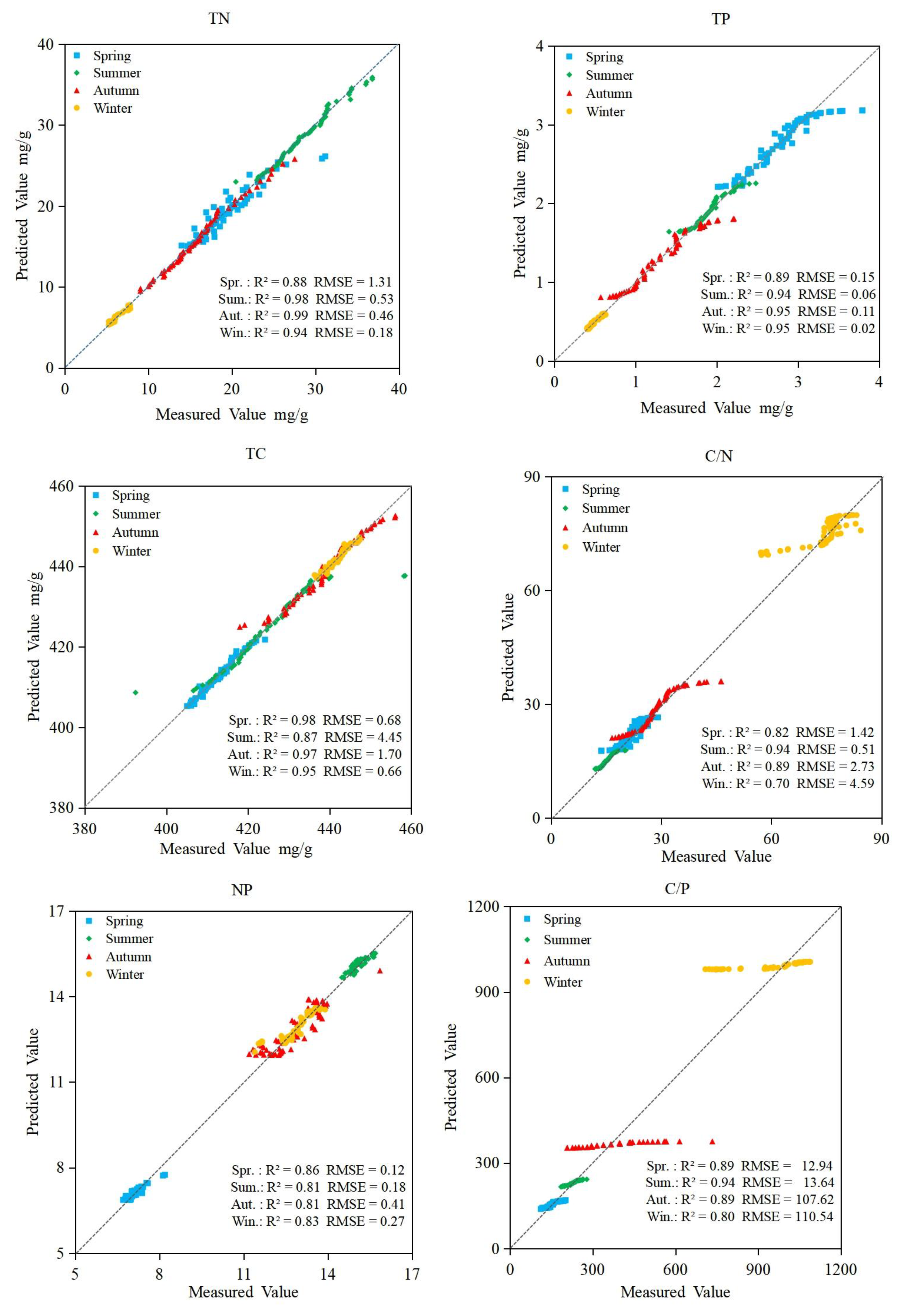
3.3. Random Forest Regression Models
3.4. Support Vector Machine Regression Models
3.5. BP Neural Network Regression Models
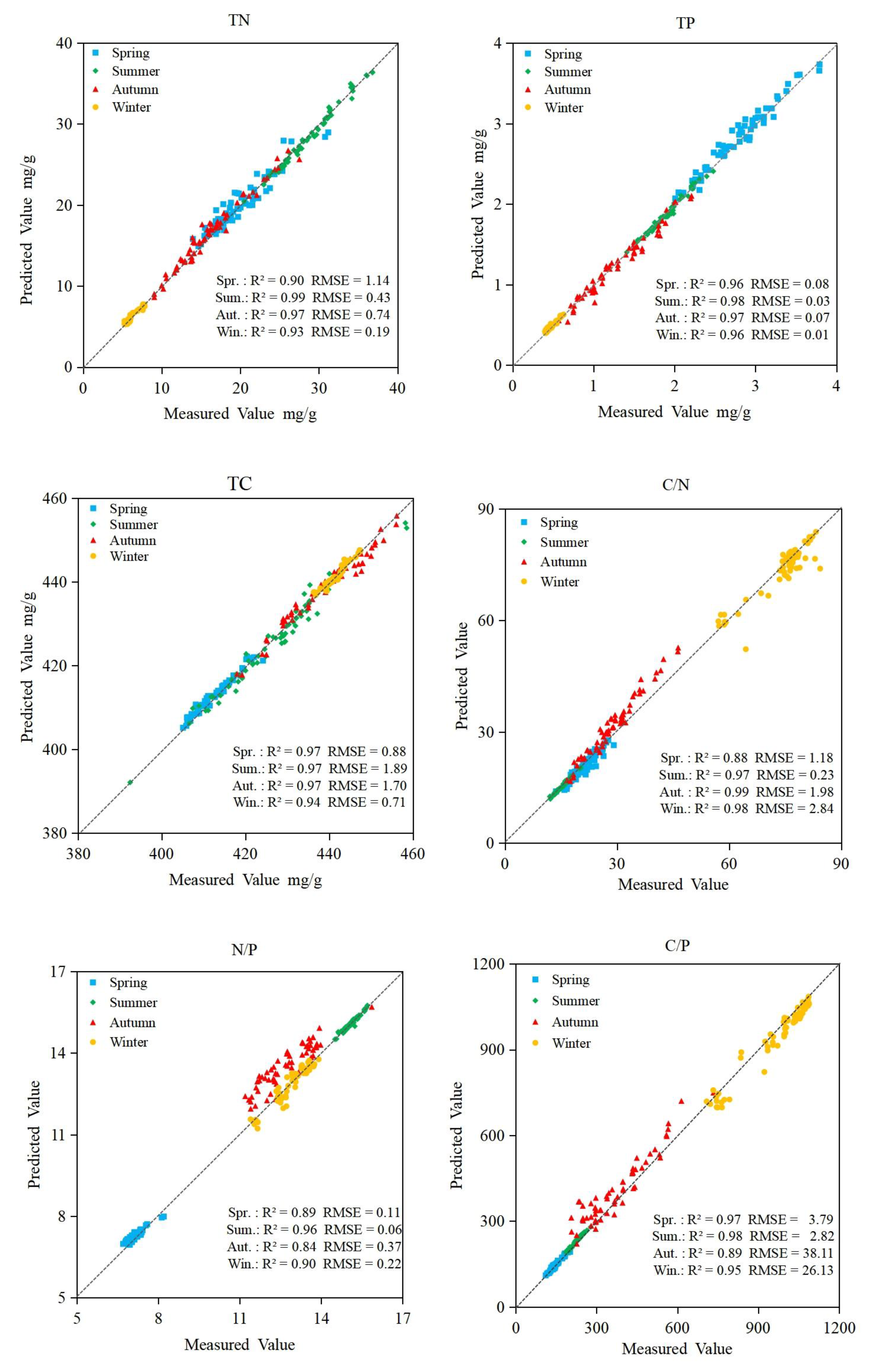
3.6. Accuracy of Prediction Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elser, J.J. Biological Stoichiometry: A Chemical Bridge between Ecosystem Ecology and Evolutionary Biology. Am. Nat. 2006, 168, S25–S35. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Reichert, P.; Schuwirth, N. A generic framework for deriving process stoichiometry in environmental models. Environ. Modell. Softw. 2010, 25, 1241–1251. [Google Scholar] [CrossRef]
- Michaels, A.F. The ratios of life. Science 2003, 300, 906–907. [Google Scholar] [CrossRef]
- Hall, E.K.; Maixner, F.; Franklin, O.; Daims, H.; Richter, A.; Battin, T. Linking microbial and ecosystem ecology using ecological stoichiometry: A synthesis of conceptual and empirical approaches. Ecosystems 2011, 14, 261–273. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C:N:P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Redfield, A.C. The biological control of chemical factors in the environment. Am. Sci. 1958, 46, 205–221. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.; Interlandi, S.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional constraints in terrestrial and freshwater food webs. Nature 2000, 408, 578–580. [Google Scholar] [CrossRef]
- Elser, J.J.; Hayakawa, K.; Urabe, J. Nutrient limitation reduces food quality for zooplankton Daphnia response to seston phosphorus enrichment. Ecology 2001, 82, 898–903. [Google Scholar] [CrossRef]
- Elser, J.J.; Nagy, J.D.; Kuang, Y. Biological stoichiometry: An ecological perspective on tumor dynamics. Bioscience 2003, 53, 1112–1120. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, W.; Yu, M.; Wang, G.; Wu, T. Latitudinal patterns of leaf N, P stoichiometry and nutrient resorption of Metasequoia glyptostroboides along the eastern coastline of china. Sci. Total Environ. 2018, 618, 1–6. [Google Scholar] [CrossRef]
- Niklas, K.J.; Owens, T.; Reich, P.B.; Cobb, E.D. Nitrogen/Phosphorus leaf stoichiometry and the scaling of plant growth. Ecol. Lett. 2005, 8, 636–642. [Google Scholar] [CrossRef]
- Reich, P.B.; Tjoelker, M.G.; Machado, J.L.; Oleksyn, J. Universal scaling of respiratory metabolism, size and nitrogen in plants. Nature 2006, 439, 457–461. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhu, L.; Li, W.; Zhang, Y.; Yan, F.; Dong, X.; Cui, L. Ecological stoichiometry characteristics of Carbon, Nitrogen and Phosphorus in various organs of aboveground part of Phragmites australis at the end of growing season and their fixation ability to 3 kinds of elements in Hanshiqiao wetlands. Wetl. Sci. 2019, 17, 311–317. (In Chinese) [Google Scholar] [CrossRef]
- Chapin, F.S.; Matson, P.A.; Mooney, H.A. Principles of Terrestrial Ecosystem Ecology; Springer: New York, NY, USA, 2002. [Google Scholar]
- Sterner, R.W.; Hessen, D.O. Algal nutrient limitation and the nutrition of aquatic herbivores. Annu. Rev. Ecol. Syst. 1994, 25, 1–29. [Google Scholar] [CrossRef]
- Peñuelas, J.; Sardans, J. Ecology: Elementary factors. Nature 2009, 460, 803–804. [Google Scholar] [CrossRef] [PubMed]
- Cleemput, E.V.; Vanierschot, L.; Fernández-Castilla, B.; Honnay, O.; Somers, B. The functional characterization of grass- and shrubland ecosystems using hyperspectral remote sensing: Trends, accuracy and moderating variables. Remote Sens. Environ. 2018, 209, 747–763. [Google Scholar] [CrossRef]
- Taddeo, S.; Dronova, I. Indicators of vegetation development in restored wetlands. Ecol. Indic. 2018, 94, 454–467. [Google Scholar] [CrossRef]
- Dou, Z.; Li, Y.; Cui, L.; Pan, X.; Ma, Q.; Huang, Y.; Lei, Y.; Li, J.; Zhao, X.; Li, W. Hyperspectral inversion of Suaeda salsa biomass under different types of human activities in Liaohe estuary wetland in northeast China. Mar. Freshw. Res. 2020, 71, 482–492. [Google Scholar] [CrossRef]
- Wang, J.J.; Wang, T.J.; Shi, T.Z.; Wu, G.F.; Skidmore, A.K. A Wavelet-Based Area Parameter for Indirectly Estimating Copper Concentration in Carex Leaves from Canopy Reflectance. Remote Sens. 2015, 7, 15340–15360. [Google Scholar] [CrossRef]
- Dou, Z.; Cui, L.; Li, J.; Zhu, Y.; Gao, C.; Pan, X.; Lei, Y.; Zhang, M.; Zhao, X.; Li, W. Hyperspectral Estimation of the Chlorophyll Content in Short-Term and Long-Term Restorations of Mangrove in Quanzhou Bay Estuary, China. Sustainability 2018, 10, 1127. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Zhang, M.; Wang, D.; Guo, Z.; Long, S.; Yang, S.; Wang, H.; Li, W.; Hu, Y.; et al. Estimating leaf mercury content in Phragmites australis based on leaf hyperspectral reflectance. Ecosyst. Health Sustain. 2020, 6, 1726211. [Google Scholar] [CrossRef]
- Zhang, M.; Li, M.; Liu, W.; Cui, L.; Li, W.; Wang, H.; Wei, Y.; Guo, Z.; Wang, D.; Hu, Y.; et al. Analyzing the performance of statistical models for estimating leaf nitrogen concentration of Phragmites australis based on leaf spectral reflectance. Spectrosc. Lett. 2019, 52, 483–491. [Google Scholar] [CrossRef]
- Osborne, S.L.; Schepers, J.S.; Francis, D.D.; Schlemmer, M.R. Detection of Phosphorus and Nitrogen Deficiencies in Corn Using Spectral Radiance Measurements. Agron. J. 2002, 94, 1215–1221. [Google Scholar] [CrossRef]
- Hansen, P.M.; Schjoerring, J.K. Reflectance measurement of canopy biomass and nitrogen status in wheat crops using normalized difference vegetation indices and partial least squares regression. Remote Sens. Environ. 2003, 86, 542–553. [Google Scholar] [CrossRef]
- Ata-Ul-Karim, S.T.; Cao, Q.; Qiang, C.; Yan, Z.; Liang, T.; Asif, R.M.I.; Weixing, C. Non-destructive assessment of plant nitrogen parameters using leaf chlorophyll measurements in rice. Front. Plant Sci. 2016, 7, 1829. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, F.; Kung, H.; Yu, H. Spectral response characteristics and identification of typical plant species in Ebinur lake wetland national nature reserve (ELWNNR) under a water and salinity gradient. Ecol. Indic. 2017, 81, 222–234. [Google Scholar] [CrossRef]
- Barbedo, J.G.A.B. Detection of nutrition deficiencies in plants using proximal images and machine learning: A review. Comput. Electron. Agric. 2019, 162, 482–492. [Google Scholar] [CrossRef]
- Gao, J.; Meng, B.; Liang, T.; Feng, Q.; Ge, J.; Yin, j.; Wu, C.; Cui, X.; Hou, M.; Liu, J.; et al. Modeling alpine grassland forage phosphorus based on hyperspectral remote sensing and a multi-factor machine learning algorithm in the east of Tibetan Plateau, China. ISPRS J. Photogramm. 2019, 147, 104–117. [Google Scholar] [CrossRef]
- Li, W.; Dou, Z.; Cui, L.; Wang, R.; Zhao, Z.; Cui, S.; Lei, Y.; Li, J.; Zhao, X.; Zhai, X. Suitability of hyperspectral data for monitoring nitrogen and phosphorus content in constructed wetlands. Remote Sens. Lett. 2020, 11, 495–504. [Google Scholar] [CrossRef]
- Li, W.; Dou, Z.; Wang, Y.; Wu, G.; Zhang, M.; Lei, Y.; Ping, Y.; Wang, J.; Cui, L.; Ma, W. Estimation of above-ground biomass of reed (Phragmites communis) based on in situ hyperspectral data in Beijing Hanshiqiao Wetland, China. Wetl. Ecol. Manag. 2019, 27, 87–102. [Google Scholar] [CrossRef]
- Datta, A.; Ghosh, S.; Ghosh, A. PCA, Kernel PCA and Dimensionality Reduction in Hyperspectral Images. In Advances in Principal Component Analysis; Naik, G., Ed.; Springer: Singapore, 2018. [Google Scholar]
- Mutanga, O.; Adam, E.; Cho, M.A. High density biomass estimation for wetland vegetation using WorldView-2 imagery and random forest regression algorithm. Int. J. Appl. Earth Obs. 2012, 18, 399–406. [Google Scholar] [CrossRef]
- Han, Z.; Zhu, X.; Fang, X.; Wang, Z.; Wang, L.; Jiang, Y. Hyperspectral estimation of apple tree canopy LAI based on SVM and RF regression. Spectrosc. Spect. Anal. 2016, 36, 800–805. [Google Scholar] [CrossRef]
- Yue, J.; Feng, H.; Yang, G.; Li, Z. A Comparison of Regression Techniques for Estimation of Above-Ground Winter Wheat Biomass Using Near-Surface Spectroscopy. Remote Sens. 2018, 10, 66. [Google Scholar] [CrossRef]
- Beltrami, M.; Silvab, A.C.L. A grid-quadtree model selection method for support vector machines. Expert Syst. Appl. 2020, 146, 113172. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Gu, G.; Bai, J. A Nonuniformity Correction Technique of IRFPA Based on BP Neural Network. Acta Photon. Sin. 2009, 38, 1504–1506. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Freshwater Phytoplankton; Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Prasad, S.T.; John, G.L. Hyperspectral Remote Sensing of Vegetation; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Li, X.; Liu, X.; Liu, M.; Wu, L. Random forest algorithm and regional applications of spectral inversion model for estimating canopy nitrogen concentration in rice. J. Remote Sens. 2014, 18, 923–945. [Google Scholar] [CrossRef]
- Calderón, R.; Navas-Cortés, J.A.; Zarco-Tejada, P.J. Early Detection and Quantification of Verticillium Wilt in Olive Using Hyperspectral and Thermal Imagery over Large Areas. Remote Sens. 2015, 7, 5584–5610. [Google Scholar] [CrossRef]
- Li, D.; Chen, S.; Chen, X. Research on method for extracting vegetation information based on hyperspectral remote sensing data. Trans. Chin. Soc. Agric. Eng. 2010, 26, 181–185. [Google Scholar]

| Classification | TN | TP | TC | C/N | N/P | C/P | |
|---|---|---|---|---|---|---|---|
| Spring | Max | 31.18 | 3.79 | 424.18 | 29.09 | 8.20 | 201.58 |
| Min | 13.96 | 2.01 | 405.07 | 13.60 | 6.70 | 111.37 | |
| Range | 17.22 | 1.78 | 19.11 | 15.49 | 1.50 | 90.21 | |
| Summer | Max | 36.81 | 2.48 | 458.49 | 19.17 | 15.70 | 278.14 |
| Min | 20.47 | 1.41 | 392.45 | 12.19 | 14.50 | 184.91 | |
| Range | 16.34 | 1.07 | 66.04 | 6.98 | 1.20 | 93.23 | |
| Autumn | Max | 27.51 | 2.21 | 456.11 | 46.32 | 15.90 | 733.59 |
| Min | 9.04 | 0.57 | 418.00 | 16.58 | 11.20 | 206.36 | |
| Range | 18.47 | 1.64 | 38.11 | 29.74 | 4.70 | 527.23 | |
| Winter | Max | 7.83 | 0.63 | 447.45 | 84.31 | 13.90 | 1088.28 |
| Min | 5.25 | 0.40 | 436.29 | 57.08 | 11.40 | 708.35 | |
| Range | 2.58 | 0.23 | 11.16 | 27.23 | 2.50 | 379.93 | |
| Test | RF | SVM | BPNN | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | ||
| TN | R2 | 0.95 | 0.98 | 0.99 | 0.95 | 0.94 | 0.97 | 0.93 | 0.96 | 0.95 | 0.97 | 0.96 | 0.91 |
| RMSE% | 5.67 | 1.01 | 2.22 | 2.66 | 6.64 | 1.84 | 2.83 | 2.99 | 5.77 | 1.49 | 4.55 | 3.16 | |
| TP | R2 | 0.98 | 0.97 | 0.98 | 0.94 | 0.91 | 0.91 | 0.99 | 0.93 | 0.96 | 0.96 | 0.94 | 0.89 |
| RMSE% | 2.52 | 1.05 | 3.32 | 2.11 | 5.40 | 3.16 | 8.49 | 4.25 | 2.88 | 1.58 | 5.41 | 2.13 | |
| TC | R2 | 0.98 | 0.91 | 0.98 | 0.97 | 0.99 | 0.83 | 0.95 | 0.96 | 0.96 | 0.95 | 0.86 | 0.92 |
| RMSE% | 0.15 | 0.31 | 0.16 | 0.09 | 0.16 | 1.04 | 0.39 | 0.15 | 0.21 | 0.44 | 0.39 | 0.16 | |
| C/N | R2 | 0.98 | 0.98 | 0.99 | 0.94 | 0.93 | 0.95 | 0.95 | 0.79 | 0.97 | 0.96 | 0.96 | 0.92 |
| RMSE% | 5.73 | 2.87 | 4.21 | 2.76 | 6.61 | 3.37 | 9.49 | 6.19 | 5.49 | 1.52 | 6.88 | 3.83 | |
| N/P | R2 | 0.98 | 0.95 | 0.97 | 0.98 | 0.86 | 0.64 | 0.81 | 0.83 | 0.87 | 0.96 | 0.84 | 0.90 |
| RMSE% | 0.75 | 0.50 | 1.29 | 0.74 | 1.72 | 1.18 | 3.20 | 2.07 | 1.54 | 0.37 | 2.91 | 1.69 | |
| C/P | R2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.89 | 0.94 | 0.89 | 0.81 | 0.97 | 0.98 | 0.90 | 0.95 |
| RMSE% | 1.40 | 0.70 | 3.41 | 1.12 | 8.49 | 6.01 | 28.80 | 11.52 | 2.48 | 1.24 | 10.22 | 2.72 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, L.; Dou, Z.; Liu, Z.; Zuo, X.; Lei, Y.; Li, J.; Zhao, X.; Zhai, X.; Pan, X.; Li, W. Hyperspectral Inversion of Phragmites Communis Carbon, Nitrogen, and Phosphorus Stoichiometry Using Three Models. Remote Sens. 2020, 12, 1998. https://doi.org/10.3390/rs12121998
Cui L, Dou Z, Liu Z, Zuo X, Lei Y, Li J, Zhao X, Zhai X, Pan X, Li W. Hyperspectral Inversion of Phragmites Communis Carbon, Nitrogen, and Phosphorus Stoichiometry Using Three Models. Remote Sensing. 2020; 12(12):1998. https://doi.org/10.3390/rs12121998
Chicago/Turabian StyleCui, Lijuan, Zhiguo Dou, Zhijun Liu, Xueyan Zuo, Yinru Lei, Jing Li, Xinsheng Zhao, Xiajie Zhai, Xu Pan, and Wei Li. 2020. "Hyperspectral Inversion of Phragmites Communis Carbon, Nitrogen, and Phosphorus Stoichiometry Using Three Models" Remote Sensing 12, no. 12: 1998. https://doi.org/10.3390/rs12121998
APA StyleCui, L., Dou, Z., Liu, Z., Zuo, X., Lei, Y., Li, J., Zhao, X., Zhai, X., Pan, X., & Li, W. (2020). Hyperspectral Inversion of Phragmites Communis Carbon, Nitrogen, and Phosphorus Stoichiometry Using Three Models. Remote Sensing, 12(12), 1998. https://doi.org/10.3390/rs12121998






