Examining the Utility of Visible Near-Infrared and Optical Remote Sensing for the Early Detection of Rapid ‘Ōhi‘a Death
Abstract
1. Introduction
- Characterize spectral progression changes for the two ROD fungal pathogens, C. lukuohia and C. huliohia, and extreme drought conditions, through frequent spectroradiometer measurements and RGB photography;
- Determine if the collected datasets, including derived simple vegetation indices (VIs), can be used to detect and discriminate between treated but visually asymptomatic ‘ōhi‘a trees; and,
- Examine how to effectively translate laboratory results into practical field-deployable diagnostic tools for the early detection of ROD across the Hawaiian Islands.
2. Materials and Methods
2.1. Laboratory Measurements
2.2. Data Processing
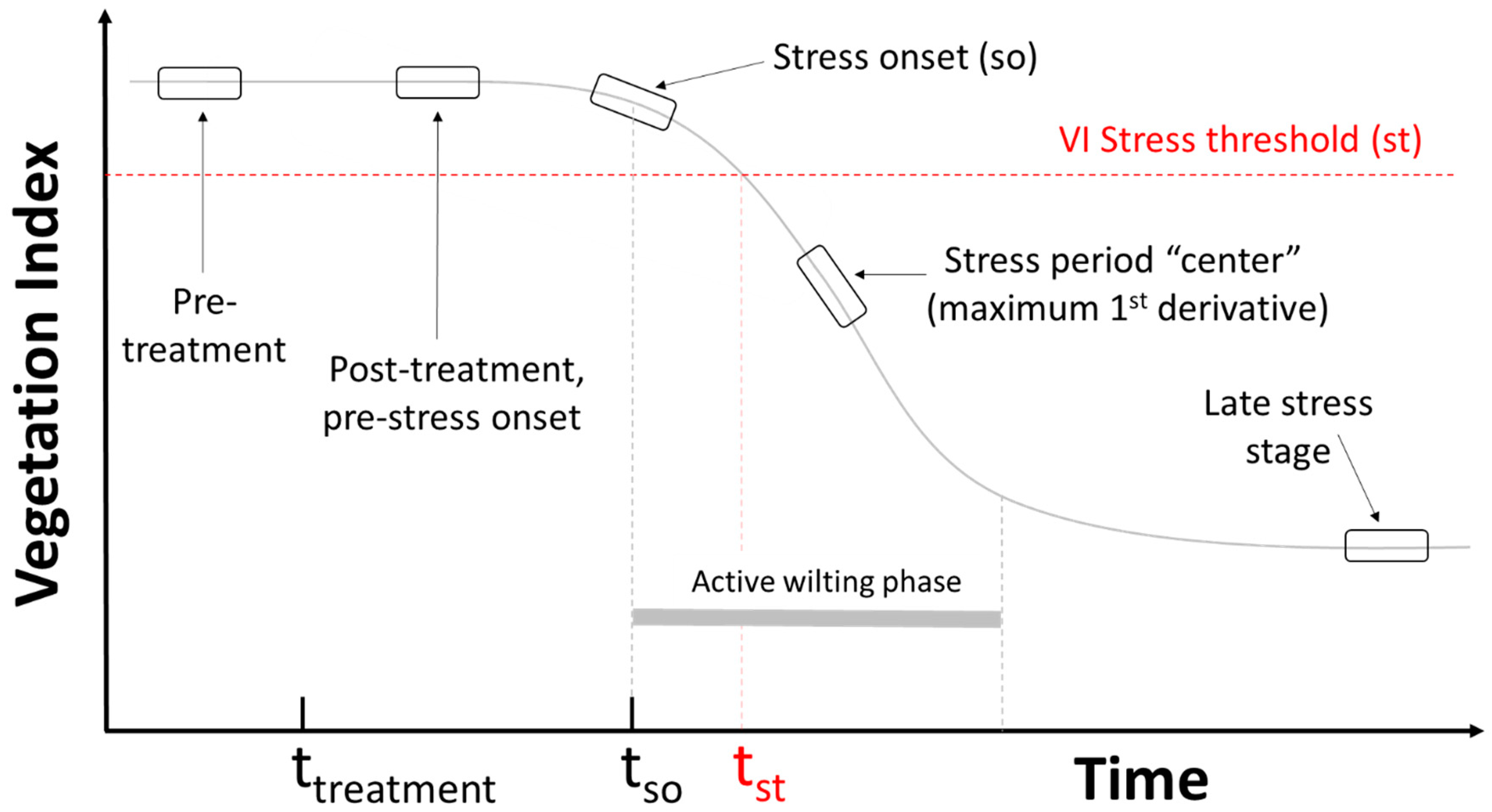
2.3. VI Stress Onset Detection
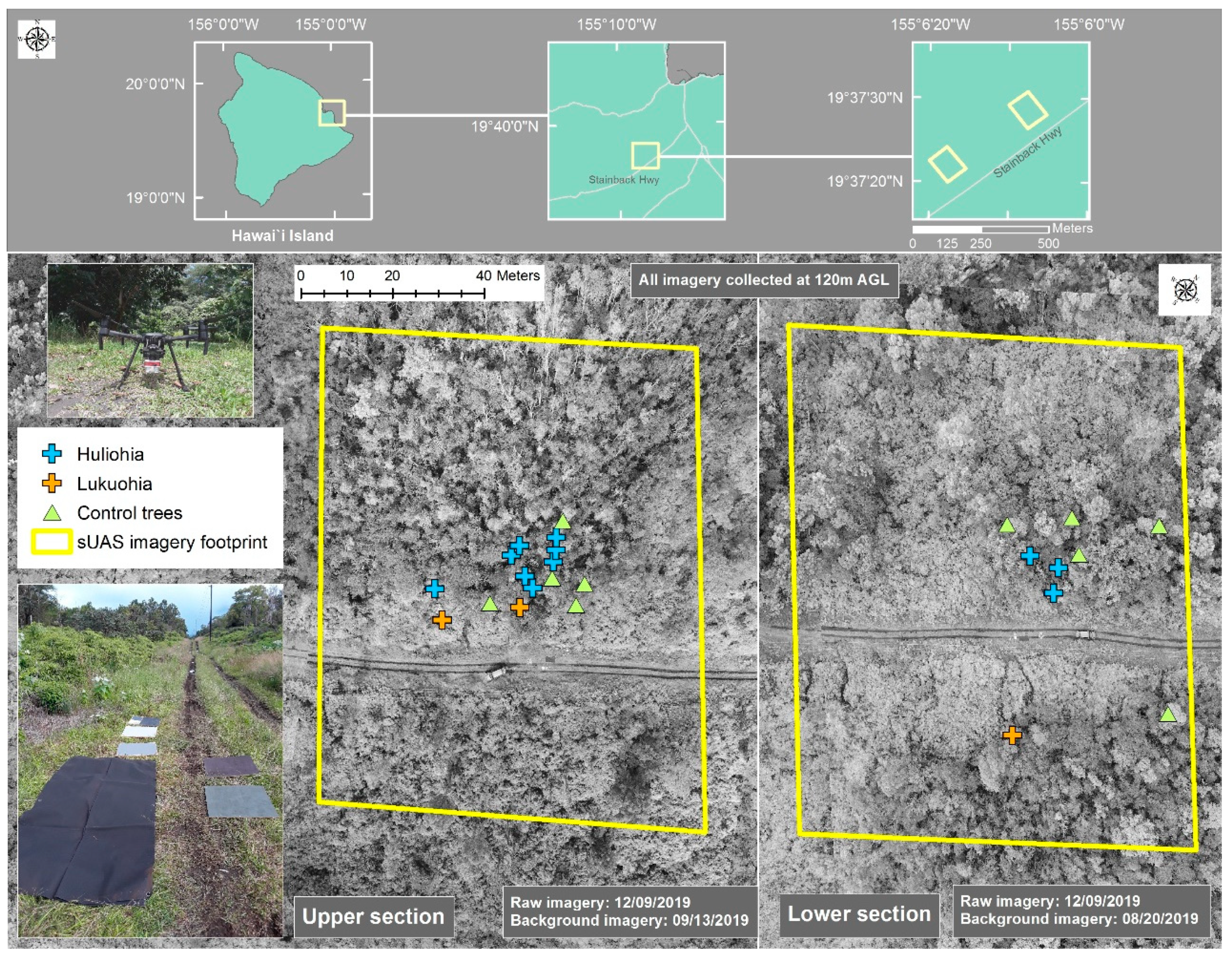
2.4. Field Measurements
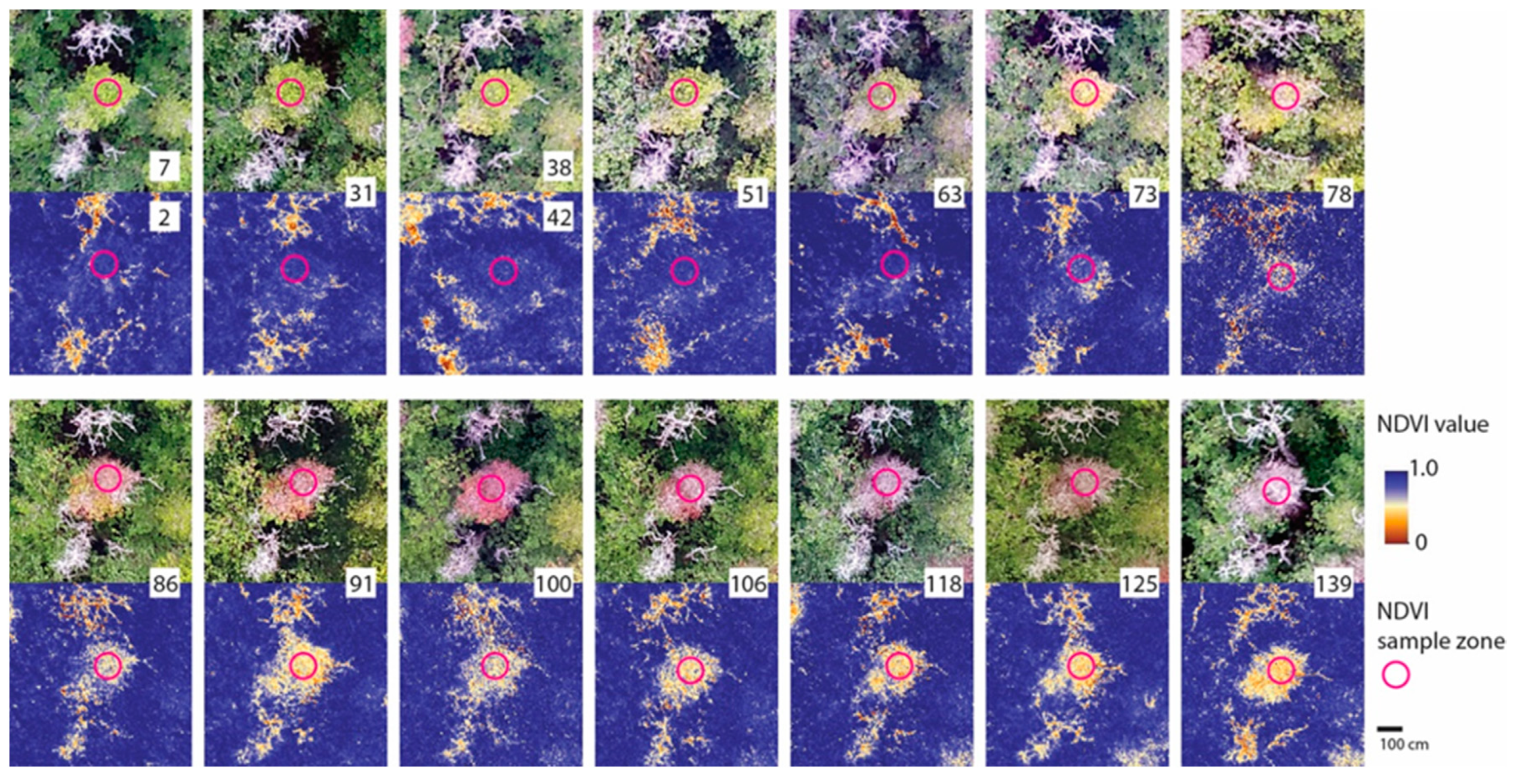
2.5. Aerial Image Processing and Analysis
2.6. Field Sample Collection
2.7. Treatment Discrimination via Classification
3. Results
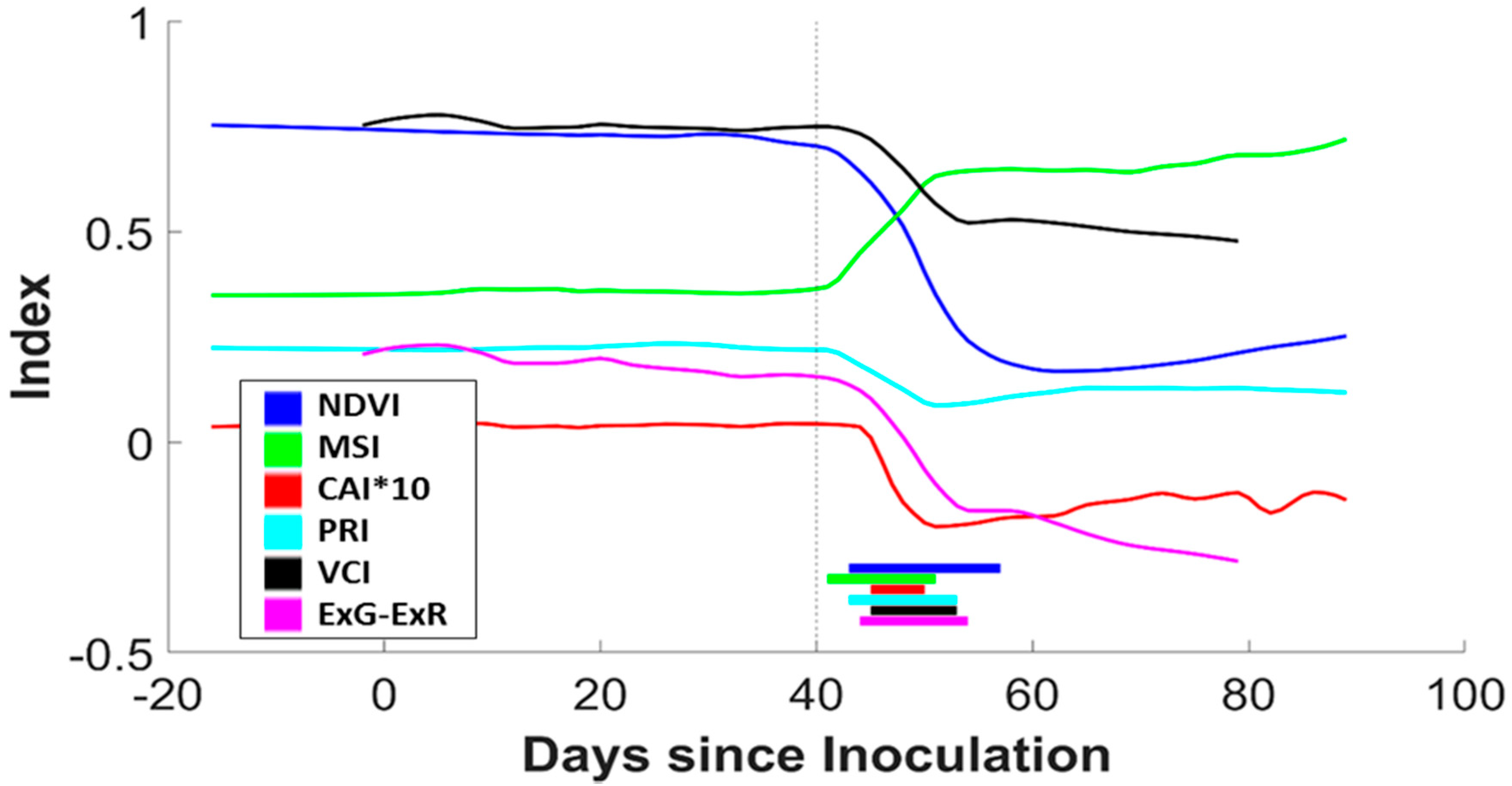
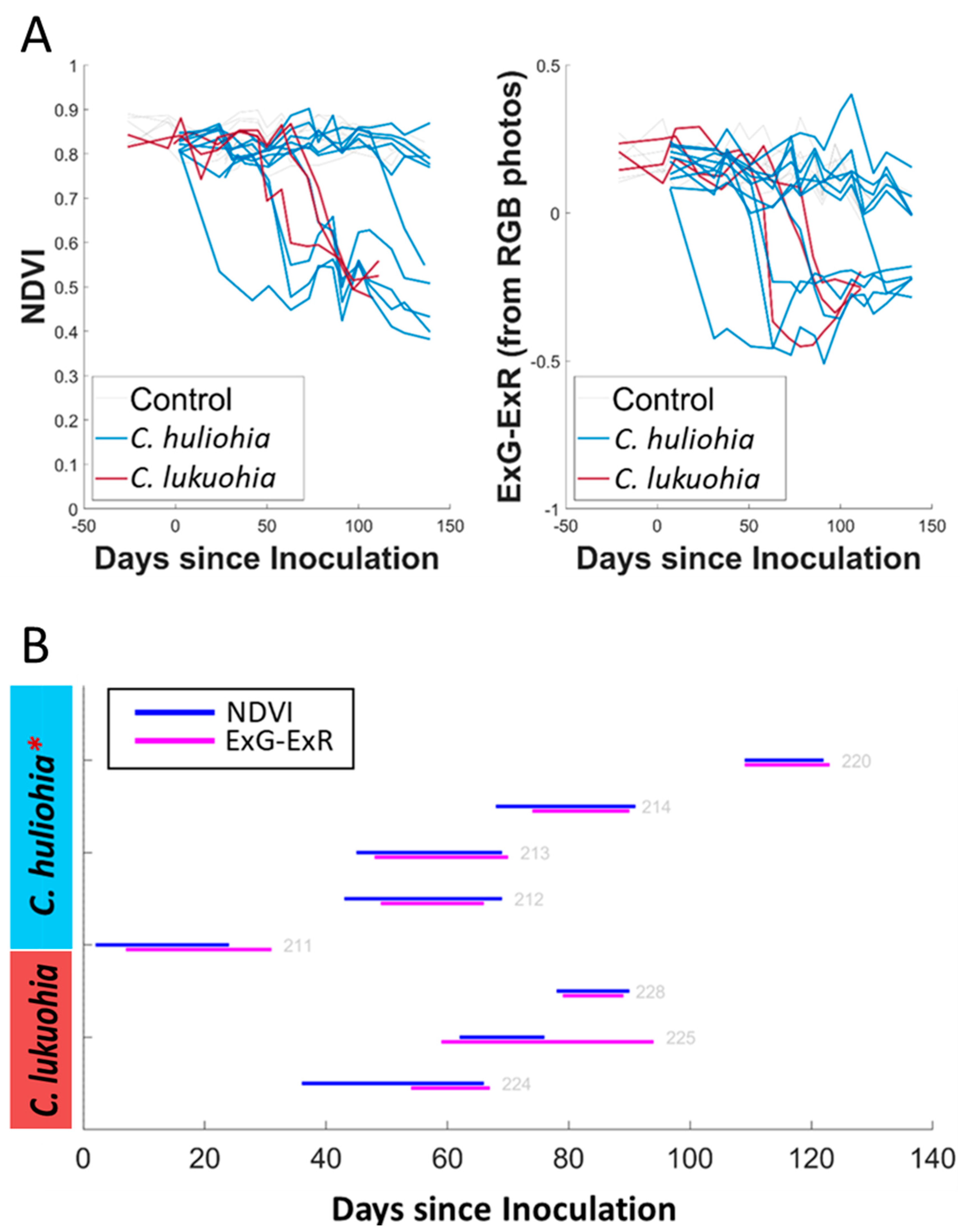
3.1. Laboratory Trials
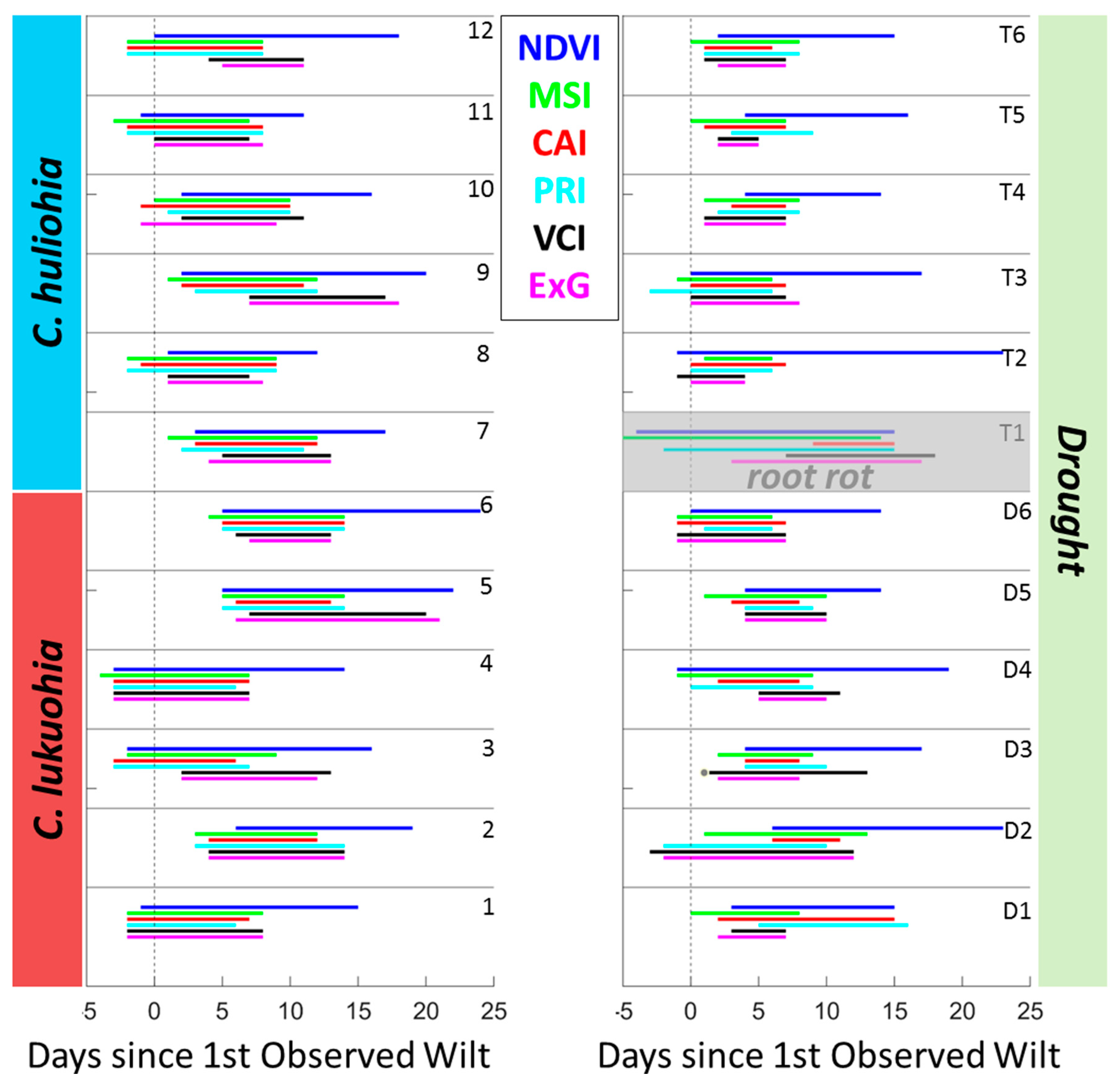
3.2. Field Inoculation Trials
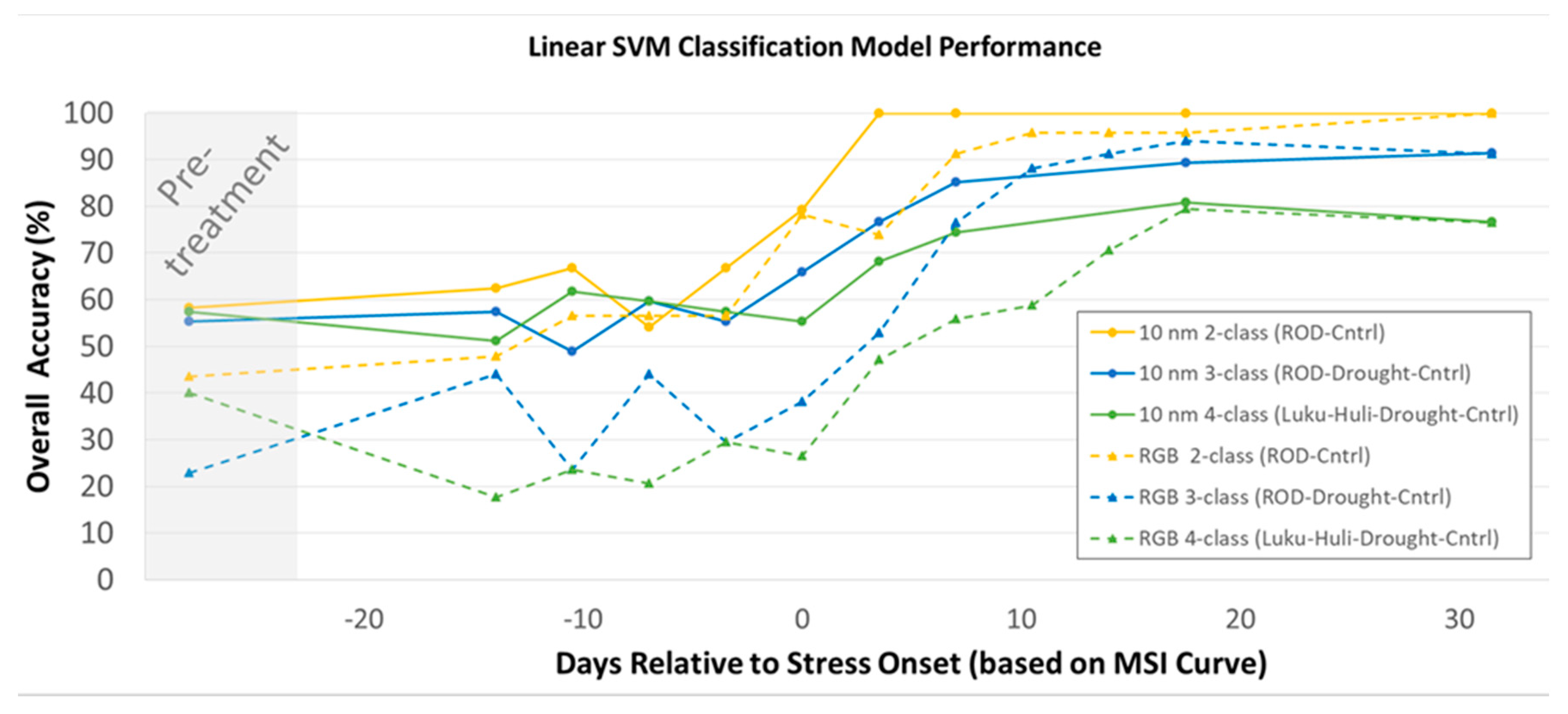
3.3. Classification Model results
4. Discussion
4.1. Laboratory Trials
4.2. Field Trials
4.3. Classification Modeling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Budde, K.B.; Nielsen, L.R.; Ravn, H.P.; Kjær, E.D. The Natural Evolutionary Potential of Tree Populations to Cope with Newly Introduced Pests and Pathogens—Lessons Learned From Forest Health Catastrophes in Recent Decades. Curr. For. Rep. 2016, 2, 18–29. [Google Scholar] [CrossRef]
- Ghelardini, L.; Luchi, N.; Pecori, F.; Pepori, A.L.; Danti, R.; Della Rocca, G.; Capretti, P.; Tsopelas, P.; Santini, A. Ecology of invasive forest pathogens. Boil. Invasions 2017, 19, 3183–3200. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Camp, R.J.; Lapointe, D.; Hart, P.J.; Sedgwick, D.E.; Canale, L.K. Large-scale tree mortality from Rapid Ohia Death negatively influences avifauna in lower Puna, Hawaii Island, USA. Condor 2019, 121, 007. [Google Scholar] [CrossRef]
- Fortini, L.B.; Kaiser, L.R.; Keith, L.; Price, J.; Hughes, R.F.; Jacobi, J.D.; Friday, J.B. The evolving threat of Rapid ‘Ōhi‘a Death (ROD) to Hawai‘i’s native ecosystems and rare plant species. For. Ecol. Manag. 2019, 448, 376–385. [Google Scholar] [CrossRef]
- Vaughn, N.; Asner, G.P.; Brodrick, P.; Martin, R.E.; Heckler, J.; Knapp, D.E.; Hughes, R.F. An Approach for High-Resolution Mapping of Hawaiian Metrosideros Forest Mortality Using Laser-Guided Imaging Spectroscopy. Remote. Sens. 2018, 10, 502. [Google Scholar] [CrossRef]
- Barnes, I.; Fourie, A.; Wingfield, M.; Harrington, T.; McNew, D.; Sugiyama, L.; Luiz, B.; Heller, W.; Keith, L. New Ceratocystis species associated with rapid death of Metrosideros polymorpha in Hawai’i. Persoonia-Mol. Phylogeny Evol. Fungi 2018, 40, 154–181. [Google Scholar] [CrossRef]
- Keith, L.M.; Hughes, R.F.; Sugiyama, L.S.; Heller, W.P.; Bushe, B.C.; Friday, J.B. First report of Ceratocystis wilt on ‘ohi‘a (Metrosideros polymorpha). Plant Dis. 2015, 99, 1276. [Google Scholar] [CrossRef]
- Hughes, M.A.; Juzwik, J.; Harrington, T.; Keith, L. Pathogenicity, symptom development and colonization of Metrosideros polymorpha by Ceratocystis lukuohia. Plant Dis. 2020. [Google Scholar] [CrossRef]
- Juzwik, J. Northern Research Station, USDA Forest Service, St. Paul, MN 55108, USA. Unpublished data. 2019. [Google Scholar]
- Heller, W.; Keith, L. Real-Time PCR Assays to Detect and Distinguish the Rapid ʻŌhiʻa Death Pathogens Ceratocystis lukuohia and C. huliohia. Phytopathology 2018, 108, 1395–1401. [Google Scholar] [CrossRef]
- Mortenson, L.A.; Hughes, R.F.; Friday, J.B.; Keith, L.M.; Barbosa, J.M.; Friday, N.J.; Liu, Z.; Sowards, T.G. Assessing spatial distribution, stand impacts and rate of Ceratocystis fimbriata induced ‘ōhi‘a (Metrosideros polymorpha) mortality in a tropical wet forest, Hawai‘i Island, USA. For. Ecol. Manag. 2016, 377, 83–92. [Google Scholar] [CrossRef]
- Clark, M.; Reeves, M.; Amidon, F.; Miller, S. Hawaiian Islands Wet Forest. In Reference Module in Earth Systems and Environmental Sciences; Elsevier BV: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Gregg, R.M. Hawaiian Islands Climate Vulnerability and Adaptation Synthesis; EcoAdapt: Bainbridge Island, WA, USA, 2018. [Google Scholar]
- Loope, L.; Hughes, F.; Keith, L.; Harrington, T.; Hauff, R.; Friday, J.B.; Martin, C. Guidance Document for Rapid ‘ōhi’a Death: Background for the 2017–2019 ROD Strategic Response Plan 2016; University of Hawaii: College of Tropical Agriculture and Human Resources: Honolulu, HI, USA, 2016. [Google Scholar]
- Asner, G.P.; Martin, R.E.; Keith, L.; Heller, W.; Hughes, M.A.; Vaughn, N.; Hughes, R.F.; Balzotti, C. A Spectral Mapping Signature for the Rapid Ohia Death (ROD) Pathogen in Hawaiian Forests. Remote. Sens. 2018, 10, 404. [Google Scholar] [CrossRef]
- Fallon, B.; Yang, A.; Lapadat, C.; Armour, I.; Juzwik, J.; Montgomery, R.A.; Cavender-Bares, J.; Nguyen, C. Spectral differentiation of oak wilt from foliar fungal disease and drought is correlated with physiological changes. Tree Physiol. 2020, 40, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Heim, R.; Wright, I.J.; Allen, A.; Geedicke, I.; Oldeland, J. Developing a spectral disease index for myrtle rust ( Austropuccinia psidii ). Plant Pathol. 2019, 68, 738–745. [Google Scholar] [CrossRef]
- Abdulridha, J.; Ehsani, R.; De Castro, A. Detection and Differentiation between Laurel Wilt Disease, Phytophthora Disease, and Salinity Damage Using a Hyperspectral Sensing Technique. Agriculture 2016, 6, 56. [Google Scholar] [CrossRef]
- Abdulridha, J.; Ampatzidis, Y.; Ehsani, R.; De Castro, A.I. Evaluating the performance of spectral features and multivariate analysis tools to detect laurel wilt disease and nutritional deficiency in avocado. Comput. Electron. Agric. 2018, 155, 203–211. [Google Scholar] [CrossRef]
- Hariharan, J.; Fuller, J.; Ampatzidis, Y.; Abdulridha, J.; Lerwill, A. Finite Difference Analysis and Bivariate Correlation of Hyperspectral Data for Detecting Laurel Wilt Disease and Nutritional Deficiency in Avocado. Remote. Sens. 2019, 11, 1748. [Google Scholar] [CrossRef]
- Calderón, R.; Navas-Cortés, J.A.; Zarco-Tejada, P.J. Early Detection and Quantification of Verticillium Wilt in Olive Using Hyperspectral and Thermal Imagery over Large Areas. Remote. Sens. 2015, 7, 5584–5610. [Google Scholar] [CrossRef]
- Smigaj, M.; Gaulton, R.; Suárez, J.C.; Barr, S.L. Canopy temperature from an Unmanned Aerial Vehicle as an indicator of tree stress associated with red band needle blight severity. For. Ecol. Manag. 2019, 433, 699–708. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Camino, C.; Beck, P.S.A.; Calderon, R.; Hornero, A.; Hernández-Clemente, R.; Kattenborn, T.; Montes-Borrego, M.; Susca, L.; Morelli, M.; et al. Previsual symptoms of Xylella fastidiosa infection revealed in spectral plant-trait alterations. Nat. Plants 2018, 4, 432–439. [Google Scholar] [CrossRef]
- Mendel, J.; Burns, C.; Kallifatidis, B.; Evans, E.; Crane, J.; Furton, K.G.; Mills, D. Agri-dogs: Using Canines for Earlier Detection of Laurel Wilt Disease Affecting Avocado Trees in South Florida. HortTechnology 2018, 28, 109–116. [Google Scholar] [CrossRef]
- Wilson, A.D.; Forse, L.B.; Babst, B.A.; Bataineh, M. Detection of Emerald Ash Borer Infestations in Living Green Ash by Noninvasive Electronic-Nose Analysis of Wood Volatiles. Biosensors 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Asaari, M.S.M.; Mishra, P.; Mertens, S.; Dhondt, S.; Inzé, D.; Wuyts, N.; Scheunders, P. Close-range hyperspectral image analysis for the early detection of stress responses in individual plants in a high-throughput phenotyping platform. ISPRS J. Photogramm. Remote. Sens. 2018, 138, 121–138. [Google Scholar] [CrossRef]
- Behmann, J.; Mahlein, A.-K.; Paulus, S.; Kuhlmann, H.; Oerke, E.-C.; Plümer, L. Calibration of hyperspectral close-range pushbroom cameras for plant phenotyping. ISPRS J. Photogramm. Remote. Sens. 2015, 106, 172–182. [Google Scholar] [CrossRef]
- Oliva, J.; Stenlid, J.; Martínez-Vilalta, J. The effect of fungal pathogens on the water and carbon economy of trees: Implications for drought-induced mortality. New Phytol. 2014, 203, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, W.K.; Bhaskar, R.; Sack, L.; Cordell, S.; Lunch, C.K. Adjustment of structure and function of Hawaiian Metrosideros polymorpha at high vs. low precipitation. Funct. Ecol. 2007, 21, 1063–1071. [Google Scholar] [CrossRef]
- Hueni, A.; Bialek, A. Cause, Effect, and Correction of Field Spectroradiometer Interchannel Radiometric Steps. IEEE J. Sel. Top. Appl. Earth Obs. Remote. Sens. 2017, 10, 1542–1551. [Google Scholar] [CrossRef]
- Wu, C. Normalized spectral mixture analysis for monitoring urban composition using ETM+ imagery. Remote. Sens. Environ. 2004, 93, 480–492. [Google Scholar] [CrossRef]
- Feilhauer, H.; Asner, G.P.; Martin, R.E.; Schmidtlein, S. Brightness-normalized Partial Least Squares Regression for hyperspectral data. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 1947–1957. [Google Scholar] [CrossRef]
- Rouse, J.; Haas, R.H.; Schell, J.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. In Third Earth Resources Technology Satellite Symposium; Freden, S., Ed.; NASA, Goddard Space Flight Center: Greenbelt, MD, USA, 1974; pp. 309–317. [Google Scholar]
- Haboudane, D. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote. Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Gamon, J.A.; Surfus, J.S. Assessing leaf pigment content and activity with a reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef]
- Huntjr, E.; Rock, B. Detection of changes in leaf water content using Near- and Middle-Infrared reflectances☆. Remote. Sens. Environ. 1989, 30, 43–54. [Google Scholar] [CrossRef]
- Daughtry, C.; Hunt, E.; McMurtrey, J. Assessing crop residue cover using shortwave infrared reflectance. Remote. Sens. Environ. 2004, 90, 126–134. [Google Scholar] [CrossRef]
- Meyer, G.E.; Neto, J.C. Verification of color vegetation indices for automated crop imaging applications. Comput. Electron. Agric. 2008, 63, 282–293. [Google Scholar] [CrossRef]
- Zhang, X.; Jayavelu, S.; Liu, L.; Friedl, M.A.; Henebry, G.M.; Liu, Y.; Schaaf, C.B.; Richardson, A.D.; Gray, J. Evaluation of land surface phenology from VIIRS data using time series of PhenoCam imagery. Agric. For. Meteorol. 2018, 256, 137–149. [Google Scholar] [CrossRef]
- Ramoelo, A.; Dzikiti, S.; Van Deventer, H.; Maherry, A.; Cho, M.A.; Gush, M.B. Potential to monitor plant stress using remote sensing tools. J. Arid. Environ. 2015, 113, 134–144. [Google Scholar] [CrossRef]
- White, J.C.; Coops, N.C.; Hilker, T.; Wulder, M.A.; Carroll, A.L. Detecting mountain pine beetle red attack damage with EO-1 Hyperion moisture indices. Int. J. Remote. Sens. 2007, 28, 2111–2121. [Google Scholar] [CrossRef]
- Gerhards, M.; Rock, G.; Schlerf, M.; Udelhoven, T. Water stress detection in potato plants using leaf temperature, emissivity, and reflectance. Int. J. Appl. Earth Obs. Geoinformation 2016, 53, 27–39. [Google Scholar] [CrossRef]
- Roberts, D.A.; Roth, K.L.; Perroy, R.L. Hyperspectral Vegetation Indices. In Hyperspectral Remote Sensing of Vegetation; CRC Press: Boca Raton, FL, USA, 2016; pp. 344–363. [Google Scholar]
- Fernández, E.; Gorchs, G.; Serrano, L. Use of consumer-grade cameras to assess wheat N status and grain yield. PLoS ONE 2019, 14, e0211889. [Google Scholar] [CrossRef]
- Woebbecke, D.M.; Meyer, G.E.; Von Bargen, K.; Mortensen, D.A. Color Indices for Weed Identification Under Various Soil, Residue, and Lighting Conditions. Trans. ASAE 1995, 38, 259–269. [Google Scholar] [CrossRef]
- Meyer, G.E.; Hindman, T.W.; Laksmi, K. Machine vision detection parameters for plant species identification. Photonics East (ISAM, VVDC, IEMB) 1999, 3543, 327–335. [Google Scholar] [CrossRef]
- Meneses, N.C.; Brunner, F.; Baier, S.; Geist, J.; Schneider, T. Quantification of Extent, Density, and Status of Aquatic Reed Beds Using Point Clouds Derived from UAV–RGB Imagery. Remote. Sens. 2018, 10, 1869. [Google Scholar] [CrossRef]
- Orfanidis, S.J. Introduction to Signal Processing; Prentice-Hall: Englewood Cliffs, NJ, USA, 1996. [Google Scholar]
- Aràndiga, F.; Donat, R.; Santágueda-Villanueva, M. The PCHIP subdivision scheme. Appl. Math. Comput. 2016, 272, 28–40. [Google Scholar] [CrossRef]
- Fritsch, F.N.; Carlson, R.E. Monotone Piecewise Cubic Interpolation. SIAM J. Numer. Anal. 1980, 17, 238–246. [Google Scholar] [CrossRef]
- Killick, R.; Fearnhead, P.; Eckley, I.A. Optimal Detection of Changepoints With a Linear Computational Cost. J. Am. Stat. Assoc. 2012, 107, 1590–1598. [Google Scholar] [CrossRef]
- Lavielle, M. Using penalized contrasts for the change-point problem. Signal Process. 2005, 85, 1501–1510. [Google Scholar] [CrossRef]
- Lowe, A.; Harrison, N.; French, A.P. Hyperspectral image analysis techniques for the detection and classification of the early onset of plant disease and stress. Plant Methods 2017, 13, 80. [Google Scholar] [CrossRef]
- Barbedo, J.G.A. A Review on the Use of Unmanned Aerial Vehicles and Imaging Sensors for Monitoring and Assessing Plant Stresses. Drones 2019, 3, 40. [Google Scholar] [CrossRef]
- Hernández-Clemente, R.; Hornero, A.; Mottus, M.; Penuelas, J.; González-Dugo, V.; Jiménez, J.C.; Suárez, L.; Alonso, L.; Zarco-Tejada, P.J. Early Diagnosis of Vegetation Health From High-Resolution Hyperspectral and Thermal Imagery: Lessons Learned From Empirical Relationships and Radiative Transfer Modelling. Curr. For. Rep. 2019, 5, 169–183. [Google Scholar] [CrossRef]
- Aasen, H.; Honkavaara, E.; Lucieer, A.; Zarco-Tejada, P.J. Quantitative Remote Sensing at Ultra-High Resolution with UAV Spectroscopy: A Review of Sensor Technology, Measurement Procedures, and Data Correction Workflows. Remote. Sens. 2018, 10, 1091. [Google Scholar] [CrossRef]
- Smith, G.M.; Milton, E.J. The use of the empirical line method to calibrate remotely sensed data to reflectance. Int. J. Remote. Sens. 1999, 20, 2653–2662. [Google Scholar] [CrossRef]
- Benjamin, A.; O’Brien, D.; Barnes, G.; Wilkinson, B.; Volkmann, W. Assessment of Structure from Motion (SfM) processing parameters on processing time, spatial accuracy, and geometric quality of unmanned aerial system derived mapping products. J. Unmanned Aerial Syst. 2017, 3, 27. [Google Scholar]
- Gross, J.W.; Heumann, B.W. A Statistical Examination of Image Stitching Software Packages for Use With Unmanned Aerial Systems. Photogramm. Eng. Remote. Sens. 2016, 82, 419–425. [Google Scholar] [CrossRef]
- Lin, Q.; Huang, H.; Wang, J.; Huang, K.; Liu, Y. Detection of Pine Shoot Beetle (PSB) Stress on Pine Forests at Individual Tree Level using UAV-Based Hyperspectral Imagery and Lidar. Remote. Sens. 2019, 11, 2540. [Google Scholar] [CrossRef]
- Rumpf, T.; Mahlein, A.-K.; Steiner, U.; Oerke, E.-C.; Dehne, H.-W.; Plümer, L. Early detection and classification of plant diseases with Support Vector Machines based on hyperspectral reflectance. Comput. Electron. Agric. 2010, 74, 91–99. [Google Scholar] [CrossRef]
- Bayat, B.; Van Der Tol, C.; Verhoef, W. Remote Sensing of Grass Response to Drought Stress Using Spectroscopic Techniques and Canopy Reflectance Model Inversion. Remote. Sens. 2016, 8, 557. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Samuels, L.; Kunst, L.; Jetter, R.; Samuels, L. Sealing plant surfaces: Cuticular wax formation by epidermal cells. Annu. Rev. Plant Boil. 2008, 59, 683–707. [Google Scholar] [CrossRef]
- Shigo, A.L. Compartmentalization: A conceptual framework for understanding how trees grow and defend themselves. Annu. Rev. Phytopathol. 1984, 22, 189–214. [Google Scholar] [CrossRef]
- Beier, G.L.; Held, B.W.; Giblin, C.P.; Blanchette, R.A.; Cavender-Bares, J. American elm cultivars: Variation in compartmentalization of infection by Ophiostoma novo-ulmi and its effects on hydraulic conductivity. For. Pathol. 2017, 47, e12369. [Google Scholar] [CrossRef]
- Rioux, D.; Blais, M.; Nadeau-Thibodeau, N.; Lagacé, M.; DesRochers, P.; Klimaszewska, K.; Bernier, L.; Nadeau-Thibodeau, N. First Extensive Microscopic Study of Butternut Defense Mechanisms Following Inoculation with the Canker PathogenOphiognomonia clavigignenti-juglandacearumReveals Compartmentalization of Tissue Damage. Phytopathology 2018, 108, 1237–1252. [Google Scholar] [CrossRef] [PubMed]
- Dimond, A.E. Biophysics and Biochemistry of the Vascular Wilt Syndrome. Annu. Rev. Phytopathol. 1970, 8, 301–322. [Google Scholar] [CrossRef]
- Inch, S.A.; Ploetz, R.C. Impact of laurel wilt, caused by Raffaelea lauricola, on xylem function in avocado, Persea americana. For. Pathol. 2011, 42, 239–245. [Google Scholar] [CrossRef]
- Yadeta, K.A.; Thomma, B.P.H.J. The xylem as battleground for plant hosts and vascular wilt pathogens. Front. Plant Sci. 2013, 4, 97. [Google Scholar] [CrossRef]
- Cordell, S.; Goldstein, G.; Meinzer, F.C.; Vitousek, P. Regulation of leaf life-span and nutrient-use efficiency of Metrosideros polymorpha trees at two extremes of a long chronosequence in Hawaii. Oecologia 2001, 127, 198–206. [Google Scholar] [CrossRef]
- Miranda, A.C.; De Moraes, M.L.T.; Tambarussi, E.V.; Furtado, E.; Mori, E.S.; Da Silva, P.H.M.; Sebbenn, A. Heritability for resistance to Puccinia psidii Winter rust in Eucalyptus grandis Hill ex Maiden in Southwestern Brazil. Tree Genet. Genomes 2012, 9, 321–329. [Google Scholar] [CrossRef]
- Loope, L. A Summary of Information on the Rust Puccinia Psidii Winter (guava rust) with Emphasis on Means to Prevent Introduction of Additional Strains to Hawaii; US Geological Survey: Reston, VA, USA, 2010; pp. 1–31.
- Sandino, J.; Pegg, G.S.; Gonzalez, F.; Smith, G.R. Aerial Mapping of Forests Affected by Pathogens Using UAVs, Hyperspectral Sensors, and Artificial Intelligence. Sensors 2018, 18, 944. [Google Scholar] [CrossRef]






| Data Source | Index | Formula | Reference |
|---|---|---|---|
| Spectroradiometer | NDVI | [34,35] | |
| PRI | [36] | ||
| MSI | [37] | ||
| CAI | [38] | ||
| RGB Camera | ExG-ExR | [39] | |
| VCI | [40] |
| Tree | Pre-Stress Onset Model Results | Stress Onset Model Results | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 Class | 3 Class | 4 Class | 2 Class | 3 Class | 4 Class | |||||||
| 10 nm | RGB | 10 nm | RGB | 10 nm | RGB | 10 nm | RGB | 10 nm | RGB | 10 nm | RGB | |
| 215 | ROD | Cntrl | Cntrl | D | Cntrl | D | ROD | Cntrl | ROD | Cntrl | D | Cntrl |
| 216 | ROD | Cntrl | D | Cntrl | D | D | ROD | Cntrl | ROD | Cntrl | D | Cntrl |
| 217 | ROD | Cntrl | Cntrl | ROD | Cntrl | luku | Cntrl | ROD | Cntrl | ROD | Cntrl | luku |
| 218 | ROD | Cntrl | ROD | Cntrl | Cntrl | D | ROD | Cntrl | ROD | Cntrl | D | Cntrl |
| 219 | ROD | ROD | D | ROD | Cntrl | luku | ROD | ROD | ROD | ROD | D | Cntrl |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perroy, R.L.; Hughes, M.; Keith, L.M.; Collier, E.; Sullivan, T.; Low, G. Examining the Utility of Visible Near-Infrared and Optical Remote Sensing for the Early Detection of Rapid ‘Ōhi‘a Death. Remote Sens. 2020, 12, 1846. https://doi.org/10.3390/rs12111846
Perroy RL, Hughes M, Keith LM, Collier E, Sullivan T, Low G. Examining the Utility of Visible Near-Infrared and Optical Remote Sensing for the Early Detection of Rapid ‘Ōhi‘a Death. Remote Sensing. 2020; 12(11):1846. https://doi.org/10.3390/rs12111846
Chicago/Turabian StylePerroy, Ryan L., Marc Hughes, Lisa M. Keith, Eszter Collier, Timo Sullivan, and Gabriel Low. 2020. "Examining the Utility of Visible Near-Infrared and Optical Remote Sensing for the Early Detection of Rapid ‘Ōhi‘a Death" Remote Sensing 12, no. 11: 1846. https://doi.org/10.3390/rs12111846
APA StylePerroy, R. L., Hughes, M., Keith, L. M., Collier, E., Sullivan, T., & Low, G. (2020). Examining the Utility of Visible Near-Infrared and Optical Remote Sensing for the Early Detection of Rapid ‘Ōhi‘a Death. Remote Sensing, 12(11), 1846. https://doi.org/10.3390/rs12111846






