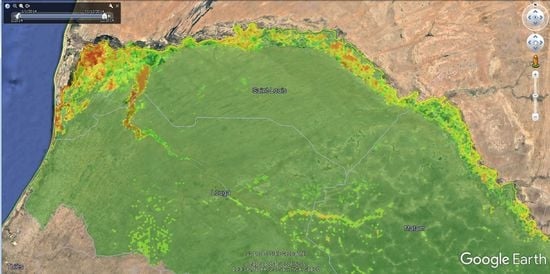
Spatial Modeling of Mosquito Vectors for Rift Valley Fever Virus in Northern Senegal: Integrating Satellite-Derived Meteorological Estimates in Population Dynamics Models
Abstract
1. Introduction
2. Materials and Methods
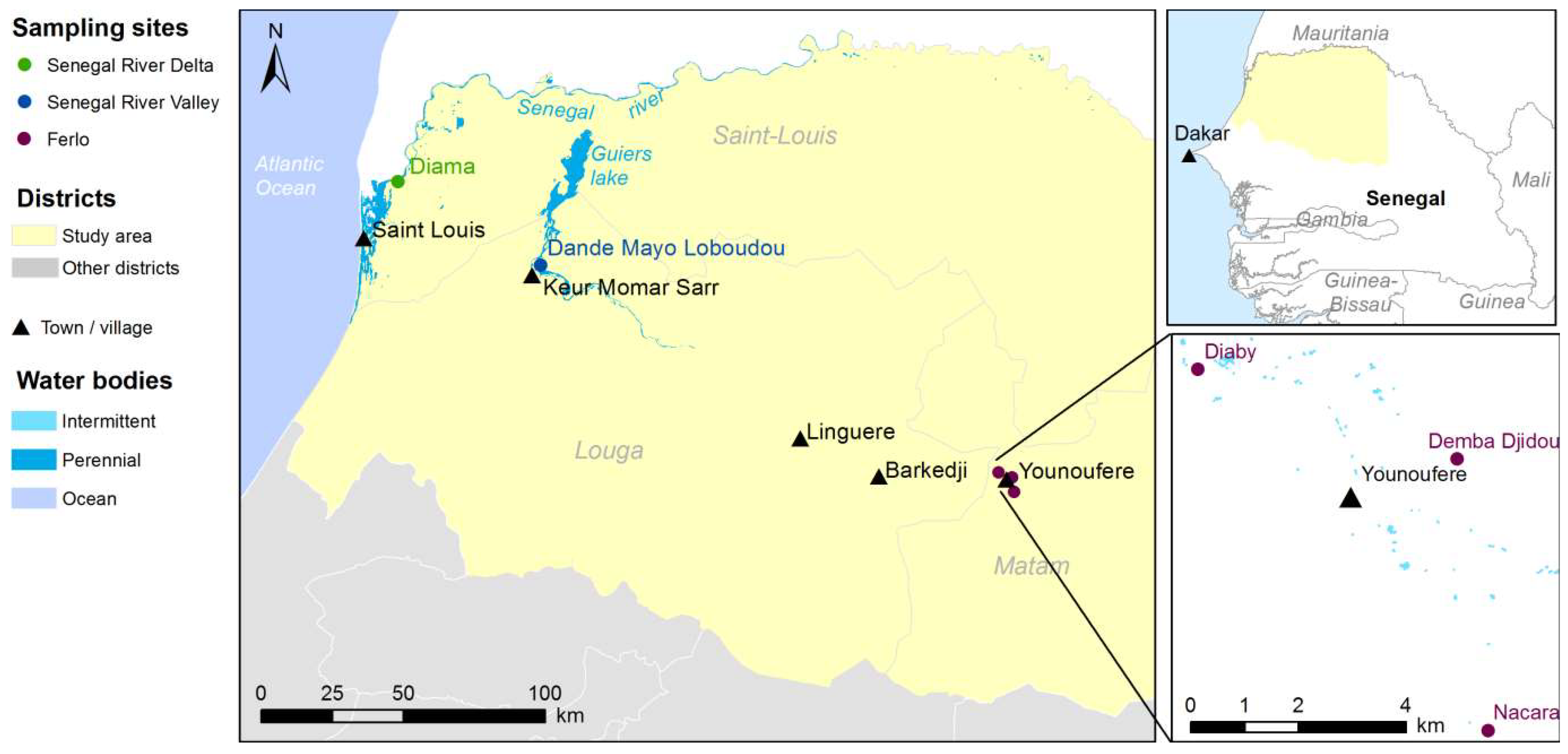
2.1. Study Sites
2.2. In-Situ Meteorological Data
2.3. Satellite-Derived Meteorological Data
2.4. Satellite Multispectral Images
2.5. Entomological Data
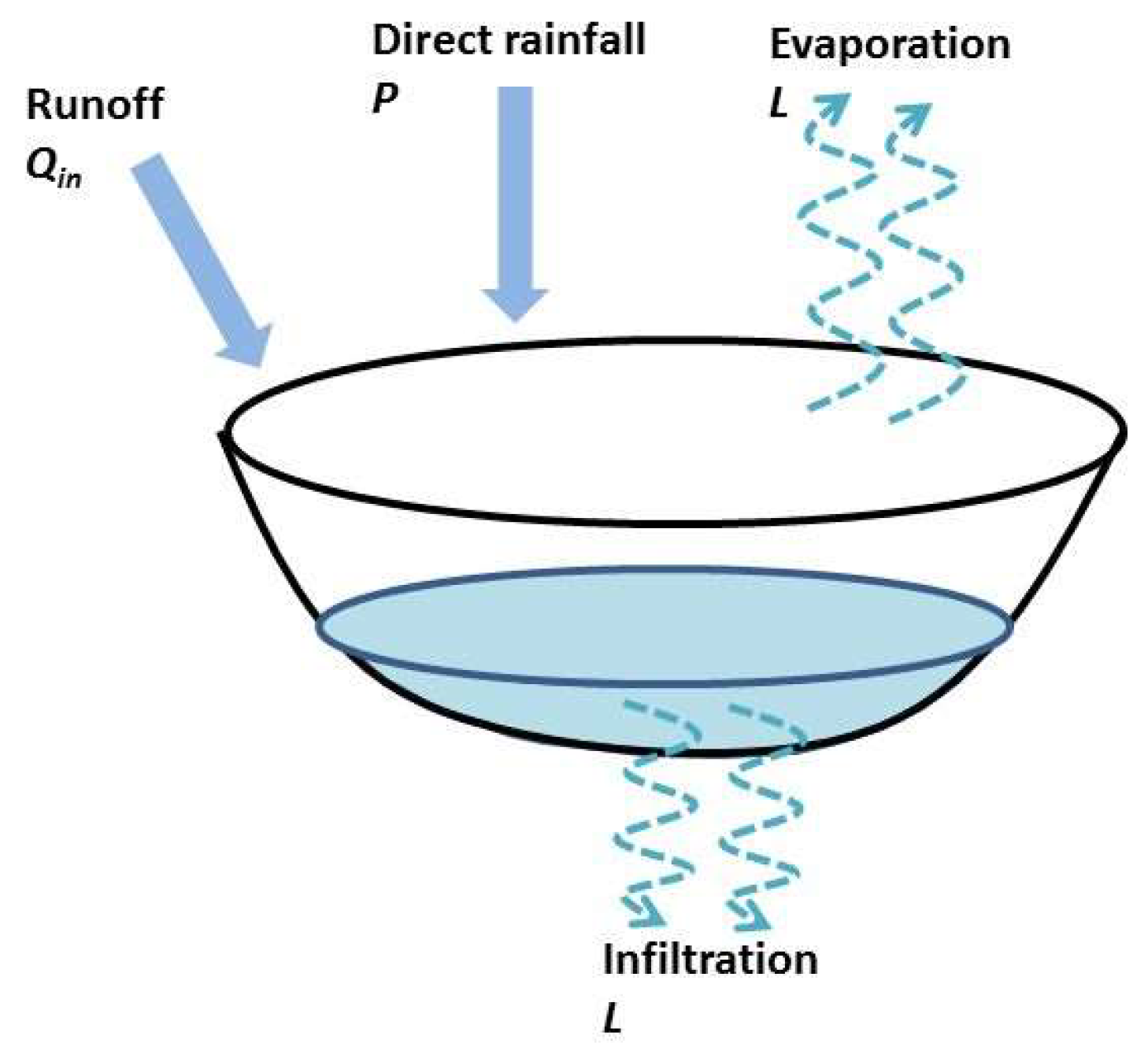
2.6. Overview of the Hydrologic Model Used in the Ferlo Area
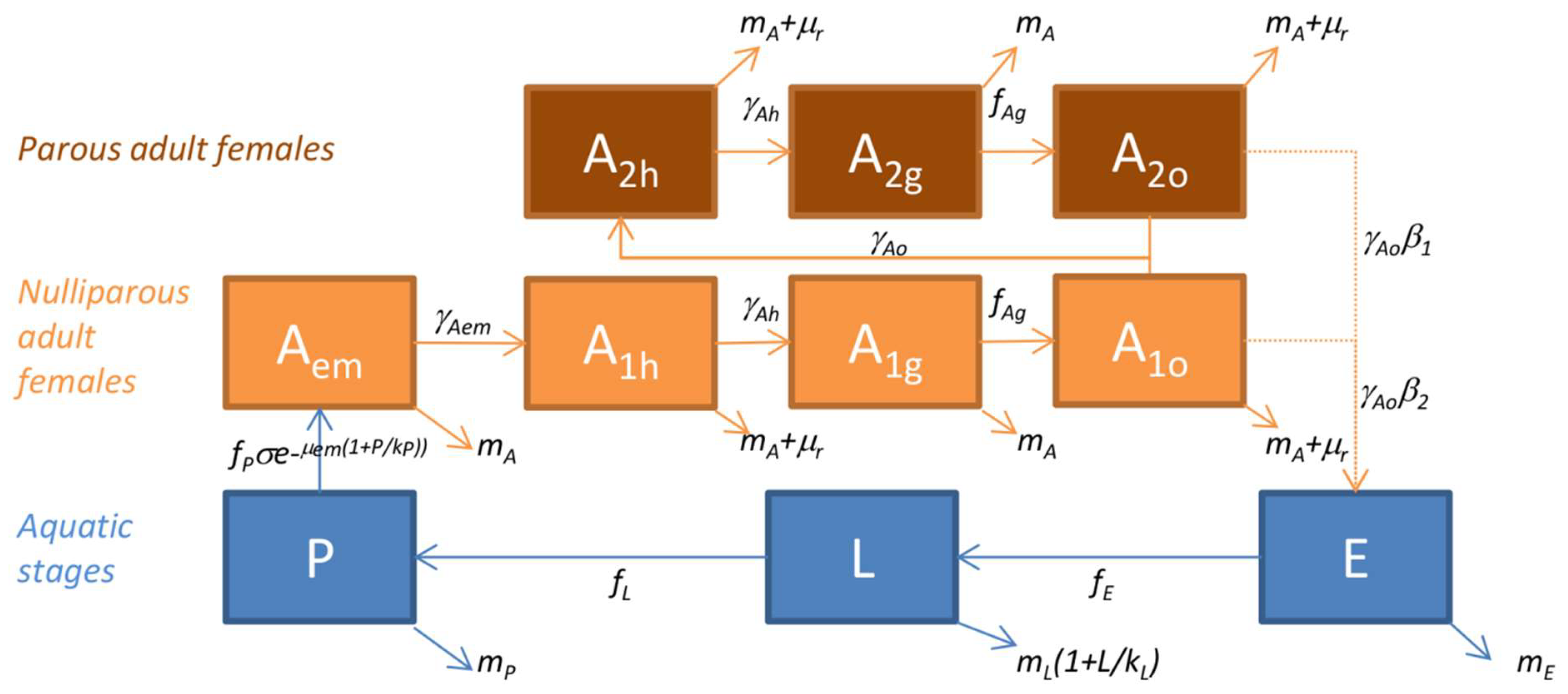
2.7. The Mosquito Population Dynamics Model
| Parameter | Definition | Ferlo | SRV and SRD | Reference | ||
|---|---|---|---|---|---|---|
| Ae. vexans | Cx. poic. | Cx. poic. | Cx. tritae. | |||
| Number of eggs laid/ovipositing nulliparous female | 80 | 100 | 100 | 100 | [13,51,52] | |
| Number of eggs laid/ovipositing parous female | 70 | 80 | 80 | 80 | [10,13,51] | |
| Sex-ratio at emergence | 0.5 | 0.5 | 0.5 | 0.5 | [13,51,53,54] | |
| Development rate of emerging adults (day−1) | 0.6 | 0.75 | 0.75 | 0.75 | [53,55,56] | |
| Transition rate from host-seeking to engorged adults (day−1) | 0.33 | 0.33 | 0.33 | 0.33 | [35,49,56] | |
| Transition rate from ovipositing to host-seeking adults (day−1) | 0.33 | 0.33 | 0.33 | 0.33 | [35,49,56] | |
| Mortality rate during emergence (day−1) | 0.1 | 0.1 | 0.1 | 0.1 | [51] | |
| Mortality rate related to seeking behavior (day−1) | 0.08 | 0.08 | 0.08 | 0.08 | * | |
| Minimum egg mortality rate (day−1) | 0.001 | 0.02 | 0.02 | 0.02 | * | |
| Minimum larvae mortality rate (day−1) | 0.01 | 0.01 | 0.01 | [51,53,55] | ||
| Minimum pupae mortality rate (day−1) | 0.02 | 0.01 | 0.01 | 0.01 | [51,53,55] | |
| Minimum environment carrying capacity for larvae (larvae ha−1) | 10,000 | 10,000 | 10,000 | 10,000 | * | |
| Minimum environment carrying capacity for pupae (pupae ha−1) | 10,000 | 10,000 | 10,000 | 10,000 | * | |
| Td | Minimal length of desiccation period for Aedes eggs (days) | 7 | _ | _ | _ | [11,35] |
| Start | First day of the favorable season | date of the first rain | date of the first rain | 1st of January | 1st of January | * |
| End | Last day of the favorable season | Max (date with S = 0; 15th Nov.) | Max (date with S = 0; 15th Nov.) | 31st Dec. | 31st Dec. | * |
| Fct 1 | Definition | Ae. vexans Ferlo | Cx. poic. Ferlo | Cx. poic. SRV/SRD | Cx. tritae. SRV/SRD | Ref. |
|---|---|---|---|---|---|---|
| Transition rate from egg to larvae (day−1) | *s | [11,57] | ||||
| Transition rate from larvae to pupae (day−1) | with q1 = −0.0007; q2 = 0.0392; q3 = −0.3911 | [51,57] | ||||
| Transition rate from pupae to emerging adults (day−1) | with q1 = −0.0008; q2 = −0.0051; q3 = −0.0319 | [51,57] | ||||
| Transition rate from engorged to ovipositing adults (day−1) | [51,53] | |||||
| Egg mortality rate (day−1) | + | * | ||||
| Larvae mortality rate (day−1) | * | |||||
| Pupae mortality rate (day−1) | * | |||||
| Adult mortality rate (day−1) | with q1 = 0.000148; q2 = −0.00667; q3 = 0.1 | , with q1 = 0.000148; q2 = −0.00667; q3 = 0.1 | [3] | |||
| Environment carrying capacity for larvae (larvae ha−1) | * | |||||
| Environment carrying capacity for pupae (pupae ha−1) | * | |||||
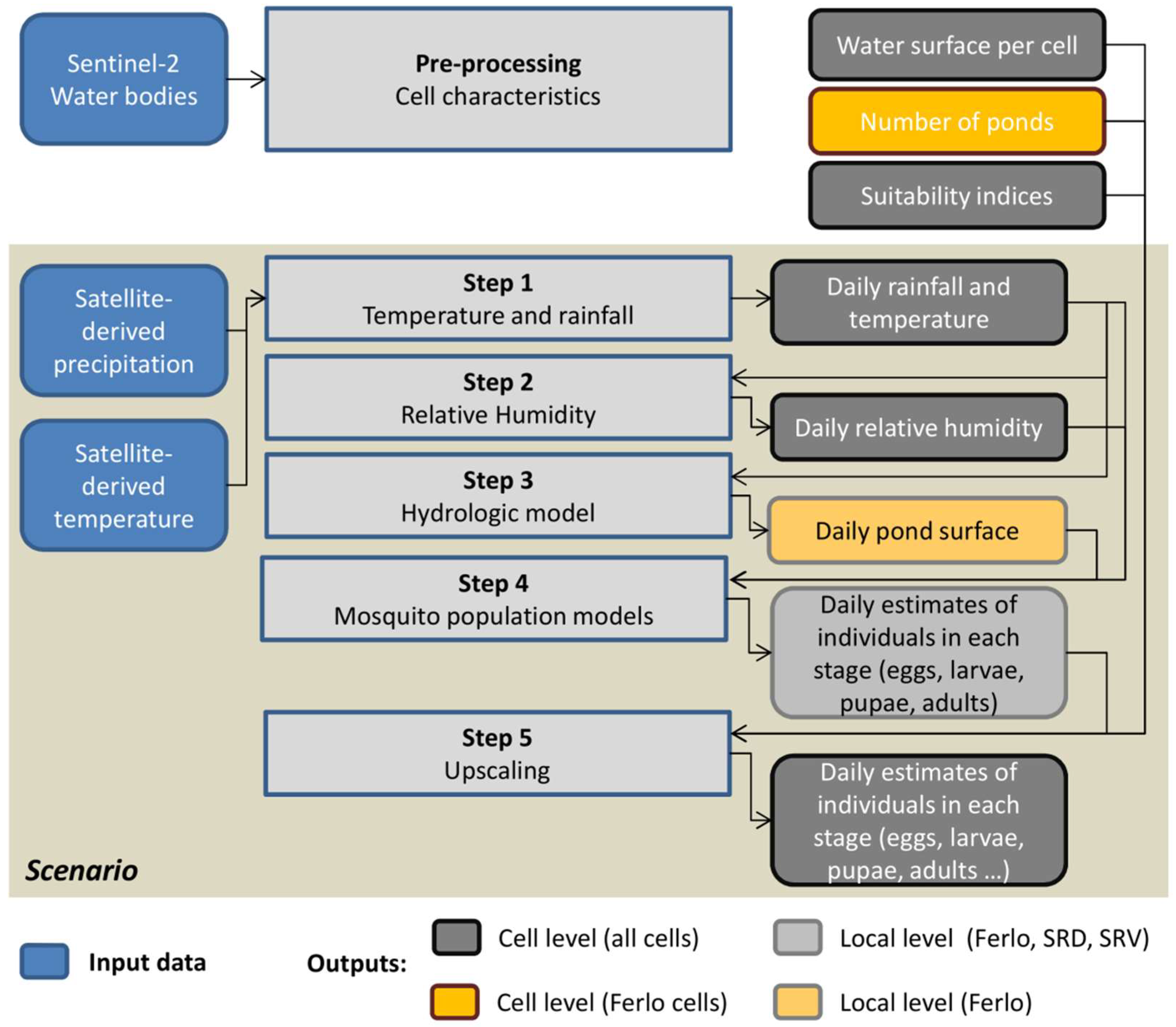
2.8. Application at Regional Scale
2.9. Validation
3. Results
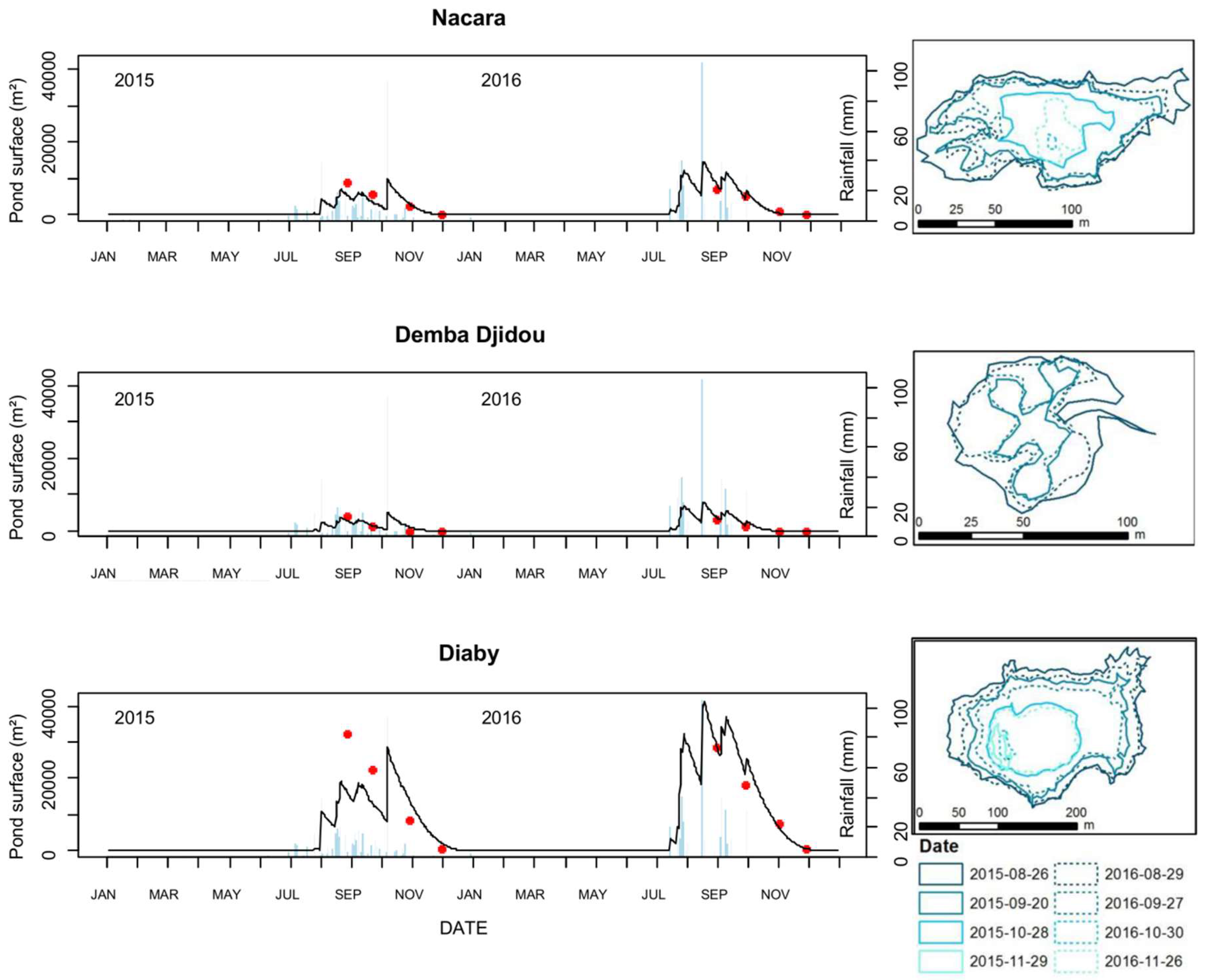
3.1. Assessment of the Hydrologic Model Used in the Ferlo Area
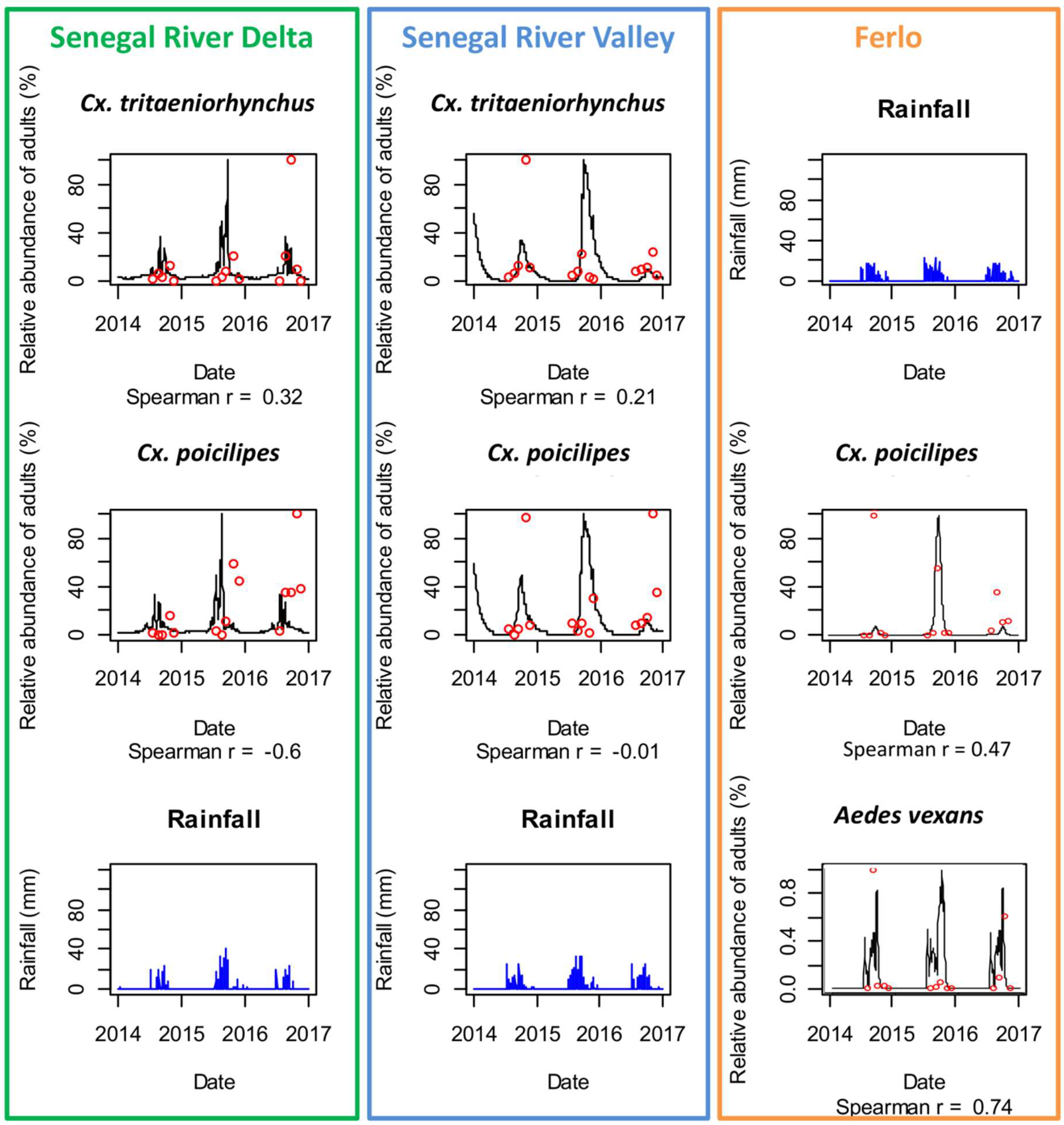
3.2. Mosquito Population Dynamics in the Three Study Sites
3.3. Model Predictions at Regional Scale
4. Discussion
4.1. Environmental Drivers of RVFV Vector Populations
4.2. Mosquito Population Modeling Using in-Situ Weather Data
4.3. Mosquito Population Modeling Using Satellite-Derived Weather Data
4.4. Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Hydrologic Model Description
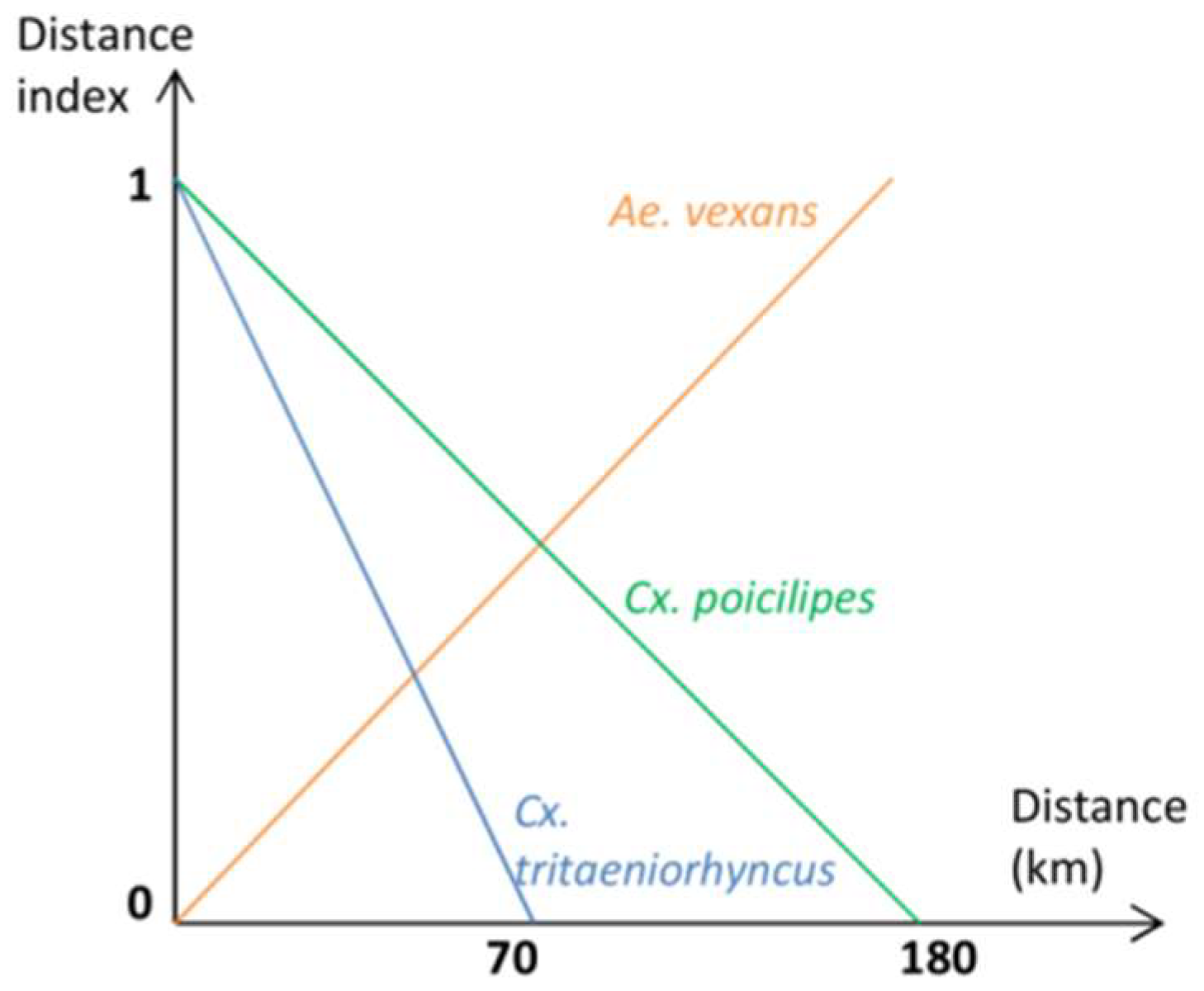
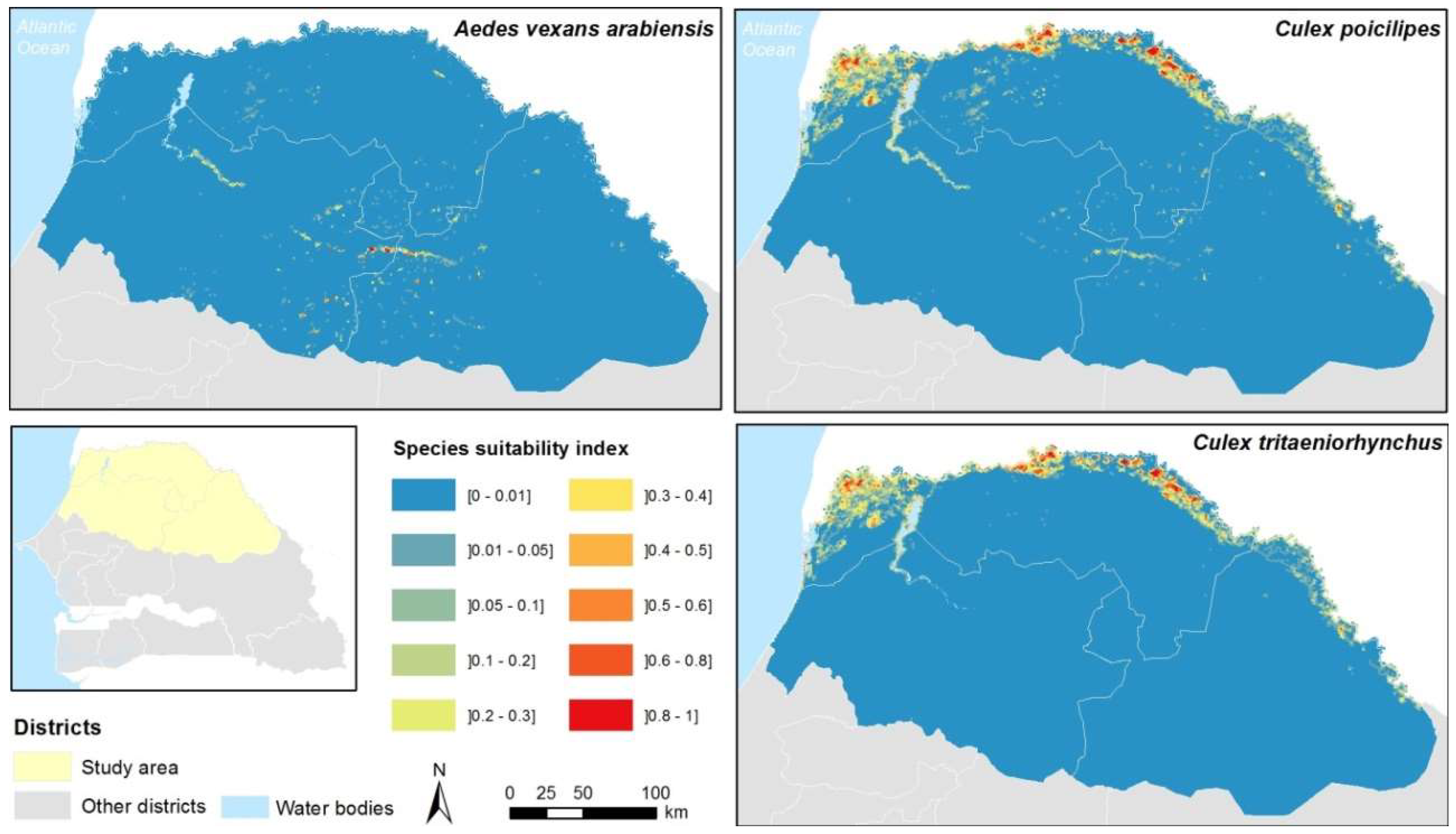
Appendix B. Cell Characterization-Estimations of the Water Surface in the Cell, the Number of Ponds, and a Suitability Index for the Presence of Cx. tritaeniorhynchus, Cx. poicilipes, and Ae. vexans
References
- Lord, C.C. Modeling and biological control of mosquitoes. J. Am. Mosq. Control Assoc. 2007, 23, 252–264. [Google Scholar] [CrossRef]
- Juliano, S.A. Population dynamics. J. Am. Mosq. Control Assoc. 2007, 23, 265–275. [Google Scholar] [CrossRef]
- Ezanno, P.; Aubry-Kientz, M.; Arnoux, S.; Cailly, P.; L’Ambert, G.; Toty, C.; Balenghien, T.; Tran, A. A generic weather-driven model to predict mosquito population dynamics applied to species of Anopheles, Culex and Aedes genera of southern France. Prev. Vet. Med. 2015, 120, 39–50. [Google Scholar] [CrossRef]
- Beck-Johnson, L.M.; Nelson, W.A.; Paaijmans, K.P.; Read, A.F.; Thomas, M.B.; Bjornstad, O.N. The effect of temperature on Anopheles mosquito population dynamics and the potential for malaria transmission. PLoS ONE 2013, 8, e79276. [Google Scholar] [CrossRef]
- Depinay, J.M.; Mbogo, C.M.; Killeen, G.; Knols, B.; Beier, J.; Carlson, J.; Dushoff, J.; Billingsley, P.; Mwambi, H.; Githure, J.; et al. A simulation model of African Anopheles ecology and population dynamics for the analysis of malaria transmission. Malar. J. 2004, 3, 29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Focks, D.A.; Haile, D.G.; Daniels, E.; Mount, G.A. Dynamic life table model for Aedes aegypti (diptera: Culicidae): Simulation results and validation. J. Med. Entomol. 1993, 30, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.; Solari, H.G.; Schweigmann, N. A stochastic population dynamics model for Aedes aegypti: Formulation and application to a city with temperate climate. Bull. Math. Biol. 2006, 68, 1945–1974. [Google Scholar] [CrossRef]
- Tran, A.; L’Ambert, G.; Lacour, G.; Benoît, R.; Demarchi, M.; Cros, M.; Cailly, P.; Aubry-Kientz, M.; Balenghien, T.; Ezanno, P. A rainfall- and temperature-driven abundance model for Aedes albopictus populations. Int. J. Environ. Res. Public Health 2013, 10, 1698–1719. [Google Scholar] [CrossRef] [PubMed]
- Erguler, K.; Smith-Unna, S.E.; Waldock, J.; Proestos, Y.; Christophides, G.K.; Lelieveld, J.; Parham, P.E. Large-Scale Modelling of the Environmentally-Driven Population Dynamics of Temperate Aedes albopictus (Skuse). PLoS ONE 2016, 11, e0149282. [Google Scholar] [CrossRef]
- Hammami, P.; Tran, A.; Kemp, A.; Tschikae, P.; Kgori, P.; Chevalier, V.; Paweska, J.; Jori, F. Rift Valley fever vector diversity and impact of meteorological and environmental factors on Culex pipiens dynamics in the Okavango Delta, Botswana. Parasit. Vectors 2016, 9, 434. [Google Scholar] [CrossRef]
- Soti, V.; Tran, A.; Degenne, P.; Chevalier, V.; Lo Seen, D.; Thiongane, Y.; Diallo, M.; Guegan, J.F.; Fontenille, D. Combining hydrology and mosquito population models to identify the drivers of rift valley Fever emergence in semi-arid regions of west Africa. PLoS Negl. Trop. Dis. 2012, 6, e1795. [Google Scholar] [CrossRef] [PubMed]
- Porphyre, T.; Bicout, D.J.; Sabatier, P. Modelling the abundance of mosquito vectors versus flooding dynamics. Ecol. Model. 2005, 183, 173–181. [Google Scholar] [CrossRef]
- Clements, A.N. The Biology of Mosquitoes: Development; Nutrition and Reproduction; CABI Publishing: Eastbourne, UK, 2000. [Google Scholar]
- Chuang, T.W.; Henebry, G.M.; Kimball, J.S.; Vanroekel-Patton, D.L.; Hildreth, M.B.; Wimberly, M.C. Satellite Microwave Remote Sensing for Environmental Modeling of Mosquito Population Dynamics. Remote Sens. Environ. 2012, 125, 147–156. [Google Scholar] [CrossRef]
- Tran, A.; Kassié, D.; Herbreteau, V. Applications of Remote Sensing to the Epidemiology of Infectious Diseases: Some Examples. In Land Surface Remote Sensing: Environment and Risks; Baghdadi, N., Zribi, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 295–316. [Google Scholar]
- Leedale, J.; Jones, A.E.; Caminade, C.; Morse, A.P. A dynamic, climate-driven model of Rift Valley fever. Geospat. Health 2016, 11, 394. [Google Scholar] [CrossRef] [PubMed]
- Anyamba, A.; Linthicum, K.J.; Small, J.; Britch, S.C.; Pak, E.; de La Rocque, S.; Formenty, P.; Hightower, A.W.; Breiman, R.F.; Chretien, J.P.; et al. Prediction, assessment of the Rift Valley fever activity in East and Southern Africa 2006-2008 and possible vector control strategies. Am. J. Trop. Med. Hyg. 2010, 83, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Linthicum, K.J.; Anyamba, A.; Tucker, C.J.; Kelley, P.W.; Myers, M.F.; Peters, C.J. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science 1999, 285, 397–400. [Google Scholar] [CrossRef]
- Metras, R.; Jewell, C.; Porphyre, T.; Thompson, P.N.; Pfeiffer, D.U.; Collins, L.M.; White, R.G. Risk factors associated with Rift Valley fever epidemics in South Africa in 2008-11. Sci. Rep. 2015, 5, 9492. [Google Scholar] [CrossRef] [PubMed]
- Adimi, F.; Soebiyanto, R.P.; Safi, N.; Kiang, R. Towards malaria risk prediction in Afghanistan using remote sensing. Malar. J. 2010, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Moiroux, N.; Djenontin, A.; Bio-Bangana, A.S.; Chandre, F.; Corbel, V.; Guis, H. Spatio-temporal analysis of abundances of three malaria vector species in southern Benin using zero-truncated models. Parasit. Vectors 2014, 7, 103. [Google Scholar] [CrossRef]
- Dambach, P.; Machault, V.; Lacaux, J.P.; Vignolles, C.; Sie, A.; Sauerborn, R. Utilization of combined remote sensing techniques to detect environmental variables influencing malaria vector densities in rural West Africa. Int. J. Health Geogr. 2012, 11, 8. [Google Scholar] [CrossRef]
- Peyre, M.; Chevalier, V.; Abdo-Salem, S.; Velthuis, A.; Antoine-Moussiaux, N.; Thiry, E.; Roger, F. A Systematic Scoping Study of the Socio-Economic Impact of Rift Valley Fever: Research Gaps and Needs. Zoonoses Public Health 2015, 62, 309–325. [Google Scholar] [CrossRef]
- Wilson, M.L. Rift Valley fever virus ecology and the epidemiology of disease emergence. Ann. N. Y. Acad. Sci. 1994, 740, 169–180. [Google Scholar] [CrossRef]
- Pepin, M.; Bouloy, M.; Bird, B.H.; Kemp, A.; Paweska, J. Rift Valley fever virus (Bunyaviridae: Phlebovirus): An update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet. Res. 2010, 41, 61. [Google Scholar] [CrossRef]
- Nicolas, G.; Durand, B.; Rakotoarimanana, T.T.; Lacote, S.; Chevalier, V.; Marianneau, P. A 3-year serological and virological cattle follow-up in Madagascar highlands suggests a non-classical transmission route of Rift Valley fever virus. Am. J. Trop. Med. Hyg. 2014, 90, 265–266. [Google Scholar] [CrossRef][Green Version]
- Jouan, A.; Le Guenno, B.; Digoutte, J.P.; Philippe, B.; Riou, O.; Adam, F. An RVF epidemic in southern Mauritania. Ann. Inst. Pasteur. Virol. 1988, 139, 307–308. [Google Scholar] [CrossRef]
- Zeller, H.G.; Fontenille, D.; Traore-Lamizana, M.; Thiongane, Y.; Digoutte, J.P. Enzootic activity of Rift Valley fever virus in Senegal. Am. J. Trop. Med. Hyg. 1997, 56, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, V.; Lancelot, R.; Thiongane, Y.; Sall, B.; Diaite, A.; Mondet, B. Rift Valley fever in small ruminants, Senegal, 2003. Emerg. Infect. Dis. 2005, 11, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Thonnon, J.; Picquet, M.; Thiongane, Y.; Lo, M.A.; Sylla, R.; Vercruysse, J. Rift valley fever surveillance in lower Senegal river basin: Update 10 years after the epidemic. Trop. Med. Int. Health 1999, 4, 580–585. [Google Scholar] [CrossRef]
- Sow, A.; Faye, O.; Ba, Y.; Diallo, D.; Fall, G.; Faye, O.; Bob, N.S.; Loucoubar, C.; Richard, V.; Dia, A.T.; et al. Widespread Rift Valley Fever Emergence in Senegal in 2013-2014. Open Forum Infect. Dis. 2016, 3, ofw149. [Google Scholar] [CrossRef] [PubMed]
- Diallo, M.; Lochouarn, L.; Ba, K.; Sall, A.A.; Mondo, M.; Girault, L.; Mathiot, C. First isolation of the Rift Valley fever virus from Culex poicilipes (Diptera: Culicidae) in nature. Am. J. Trop. Med. Hyg. 2000, 62, 702–704. [Google Scholar] [CrossRef]
- Fontenille, D.; Traore-Lamizana, M.; Diallo, M.; Thonnon, J.; Digoutte, J.P.; Zeller, H.G. New vectors of Rift Valley fever in west Africa. Emerg. Infect. Dis. 1998, 4, 289–293. [Google Scholar] [CrossRef]
- Diallo, M.; Nabeth, P.; Ba, K.; Sall, A.A.; Ba, Y.; Mondo, M.; Girault, L.; Abdalahi, M.O.; Mathiot, C. Mosquito vectors of the 1998–1999 outbreak of Rift Valley Fever and other arboviruses (Bagaza, Sanar, Wesselsbron and West Nile) in Mauritania and Senegal. Med. Vet. Entomol. 2005, 19, 119–126. [Google Scholar] [CrossRef]
- Mondet, B.; Diaite, A.; Ndione, J.A.; Fall, A.G.; Chevalier, V.; Lancelot, R.; Ndiaye, M.; Poncon, N. Rainfall patterns and population dynamics of Aedes (Aedimorphus) vexans arabiensis, Patton 1905 (Diptera: Culicidae), a potential vector of Rift Valley Fever virus in Senegal. J. Vector Ecol. 2005, 30, 102–106. [Google Scholar]
- Biteye, B.; Fall, A.G.; Ciss, M.; Seck, M.T.; Apolloni, A.; Fall, M.; Gimonneau, G. Ecological distribution and population dynamics of Rift Valley fever virus mosquito vectors (Diptera, Culicidae) in Senegal. Parasit. Vectors 2018, 11, 1–6. [Google Scholar] [CrossRef]
- Fall, A.G.; Diaite, A.; Lancelot, R.; Tran, A.; Soti, V.; Etter, E.; Konate, L.; Faye, O.; Bouyer, J. Feeding behaviour of potential vectors of West Nile virus in Senegal. Parasit. Vectors 2011, 4, 99. [Google Scholar] [CrossRef]
- Fall, A.G.; Diaite, A.; Seck, M.T.; Bouyer, J.; Lefrancois, T.; Vachiery, N.; Aprelon, R.; Faye, O.; Konate, L.; Lancelot, R. West Nile virus transmission in sentinel chickens and potential mosquito vectors, Senegal River Delta, 2008-2009. Int. J. Environ. Res. Public Health 2013, 10, 4718–4727. [Google Scholar] [CrossRef]
- Jupp, P.G.; Kemp, A.; Grobbelaar, A.; Leman, P.; Burt, F.J.; Alahmed, A.M.; Al Mujalli, D.; Al Khamees, M.; Swanepoel, R. The 2000 epidemic of Rift Valley fever in Saudi Arabia: Mosquito vector studies. Med. Vet. Entomol. 2002, 16, 245–252. [Google Scholar] [CrossRef]
- Bicout, D.J.; Sabatier, P. Mapping Rift Valley Fever vectors and prevalence using rainfall variations. Vector-Borne Zoonotic Dis. 2004, 4, 33–42. [Google Scholar] [CrossRef]
- Cailly, P.; Tran, A.; Balenghien, T.; L’Ambert, G.; Toty, C.; Ezanno, P. A climate-driven abundance model to assess mosquito control strategies. Ecol. Model. 2012, 227, 7–17. [Google Scholar] [CrossRef]
- OIE. Rift Valley Fever-Senegal: (DK) Bovine. Available online: http://www.promedmail.org (accessed on 18 December 2018).
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration (Guidelines for Computing Crop Water Requirements); FAO—Food and Agriculture Organization of the United Nations: Rome, Italy, 1998; Volume 56, Available online: http://www.fao.org/docrep/X0490E/X0490E00.htm#Contents (accessed on 18 December 2018).
- Xu, H.Q. Modification of normalised difference water index (NDWI) to enhance open water features in remotely sensed imagery. Int. J. Remote Sens. 2006, 27, 3025–3033. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, Y.; Ling, F.; Wang, Q.; Li, W.; Li, X. Water Bodies’ Mapping from Sentinel-2 Imagery with Modified Normalized Difference Water Index at 10-m Spatial Resolution Produced by Sharpening the SWIR Band. Remote Sens. 2016, 8, 354. [Google Scholar] [CrossRef]
- Soti, V.; Puech, C.; Lo Seen, D.; Bertran, A.; Vignolles, C.; Mondet, B.; Dessay, N.; Tran, A. The potential for remote sensing and hydrologic modelling to assess the spatio-temporal dynamics of ponds in the Ferlo Region (Senegal). Hydrol. Earth Syst. Sci. 2010, 14, 1449–1464. [Google Scholar] [CrossRef]
- Jetten, T.H.; Takken, W. Anophelism without Malaria in Europe: A Review of the Ecology and Distribution of the Genus Anopheles in Europe; Wageningen Agricultural University Press: Wageningen, The Netherlands, 1994. [Google Scholar]
- Diallo, D.; Talla, C.; Ba, Y.; Dia, I.; Sall, A.A.; Diallo, M. Temporal distribution and spatial pattern of abundance of the Rift Valley fever and West Nile fever vectors in Barkedji, Senegal. J. Vector Ecol. 2011, 36, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Ba, Y.; Diallo, D.; Kebe, C.M.F.; Dia, I.; Diallo, M. Aspects of bioecology of two Rift Valley fever virus vectors in Senegal (West Africa): Aedes vexans and Culex poicilipes (Diptera: Culicidae). J. Med Entomol. 2005, 42, 739–750. [Google Scholar] [CrossRef]
- Horsfall, W.R. Mosquitoes: Their Bionomics and Relation to Disease; The Royal Press Company: New York, NY, USA, 1955. [Google Scholar]
- Delatte, H.; Gimonneau, G.; Triboire, A.; Fontenille, D. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J. Med. Entomol. 2009, 46, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Briegel, H.; Timmermann, S.E. Aedes albopictus (Diptera: Culicidae): Physiological aspects of development and reproduction. J. Med Entomol. 2001, 38, 566–571. [Google Scholar] [CrossRef]
- Vinogradova, E. Culex Pipiens Pipiens Mosquitoes: Taxonomy, Distribution, Ecology, Physiology, Genetic, Applied Importance and Control; Pensoft Publishers Sofia-Moscow: Sofia, Bulgaria, 2000. [Google Scholar]
- Gjullin, C.M.; Yates, W.W.; Stage, H.H. Studies on Aedes vexans (Meigen) and Aedes sticticus (Meigen), flood-water mosquitoes, in the lower Columbia river valley. Ann. Entomol. Soc. Am. 1950, 43, 262–275. [Google Scholar] [CrossRef]
- Carron, A.; Bichaud, L.; Platz, N.; Bicout, D.J. Life history traits of Aedes caspius (Diptera: Culicidae): A laboratory study of larval stages. Bull. Entomol. Res. 2008, 98, 431–436. [Google Scholar] [CrossRef]
- Buei, K.; Ito, S.; Nakamura, H.; Yoshida, M. Field study on the gonotrophic cycle of Culex tritaeniorhynchus. Med. Entomol. Zool. 1980, 31, 57–62. [Google Scholar] [CrossRef]
- Rueda, L.M.; Patel, K.J.; Axtell, R.C.; Stinner, R.E. Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 1990, 27, 892–898. [Google Scholar] [CrossRef]
- Degenne, P.; Lo Seen, D. Ocelet: Simulating processes of landscape changes using interaction graphs. SoftwareX 2016, 5, 89–95. [Google Scholar] [CrossRef]
- Castets, M.; Degenne, P.; Poncelet, P.; Lo Seen, D. Integrating raster and vector spatial representations with interaction graphs for multi-scale environmental simulations. In Proceedings of the 7th International Congress on Environmental Modelling and Software, San Diego, CA, USA, 15–19 June 2014. [Google Scholar]
- Morris, M. Factorial sampling plans for preliminary computational experiments. Technometrics 1991, 33, 161–174. [Google Scholar] [CrossRef]
- Ba, Y.; Sall, A.A.; Diallo, D.; Mondo, M.; Girault, L.; Dia, I.; Diallo, M. Re-Emergence of Rift Valley Fever Virus in Barkedji (Senegal, West Africa) in 2002–2003: Identification of New Vectors and Epidemiological Implications. J. Am. Mosq. Control Assoc. 2012, 28, 170–178. [Google Scholar] [CrossRef]
- Talla, C.; Diallo, D.; Dia, I.; Ba, Y.; Ndione, J.A.; Sall, A.A.; Morse, A.; Diop, A.; Diallo, M. Statistical modeling of the abundance of vectors of West African Rift Valley fever in Barkedji, Senegal. PLoS ONE 2014, 9, e114047. [Google Scholar] [CrossRef]
- Talla, C.; Diallo, D.; Dia, I.; Ba, Y.; Ndione, J.A.; Morse, A.P.; Diop, A.; Diallo, M. Modelling hotspots of the two dominant Rift Valley fever vectors (Aedes vexans and Culex poicilipes) in Barkedji, Senegal. Parasit. Vectors 2016, 9, 111. [Google Scholar] [CrossRef]
- Soti, V.; Chevalier, V.; Maura, J.; Begue, A.; Lelong, C.; Lancelot, R.; Thiongane, Y.; Tran, A. Identifying landscape features associated with Rift Valley fever virus transmission, Ferlo region, Senegal, using very high spatial resolution satellite imagery. Int. J. Health Geogr. 2013, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Soti, V.; Tran, A.; Bailly, J.-S.; Puech, P.; Lo Seen, D.; Bégué, A. Assessing optical Earth observation systems for mapping and monitoring temporary ponds in arid areas. Int. J. Appl. Earth Obs. Geoinf. 2009, 11, 344–351. [Google Scholar] [CrossRef]
- Gouagna, L.C.; Dehecq, J.S.; Fontenille, D.; Dumont, Y.; Boyer, S. Seasonal variation in size estimates of Aedes albopictus population based on standard mark-release-recapture experiments in an urban area on Reunion Island. Acta Trop. 2015, 143, 89–96. [Google Scholar] [CrossRef]
- Fall, A.G.; Diaite, A.; Etter, E.; Bouyer, J.; Ndiaye, T.D.; Konate, L. The mosquito Aedes (Aedimorphus) vexans arabiensis as a probable vector bridging the West Nile virus between birds and horses in Barkedji (Ferlo, Senegal). Med. Vet. Entomol. 2012, 26, 106–111. [Google Scholar] [CrossRef]
- Anyamba, A.; Chretien, J.P.; Small, J.; Tucker, C.J.; Formenty, P.; Richardson, J.; Britch, S.; Linthicum, K.J. Forecasting the temporal and spatial distribution of a rift valley fever outbreak in east Africa: 2006–2007. Am. J. Trop. Med. Hyg. 2007, 77, 282–283. [Google Scholar]
- Hardy, A.; Ettritch, G.; Cross, D.E.; Bunting, P.; Liywalii, F.; Sakala, J.; Silumesii, A.; Singini, D.; Smith, M.; Willis, T.; et al. Automatic Detection of Open and Vegetated Water Bodies Using Sentinel 1 to Map African Malaria Vector Mosquito Breeding Habitats. Remote Sens. 2019, 11, 593. [Google Scholar] [CrossRef]
- Tran, A.; Trevennec, C.; Lutwama, J.; Sserugga, J.; Gely, M.; Pittiglio, C.; Pinto, J.; Chevalier, V. Development and Assessment of a Geographic Knowledge-Based Model for Mapping Suitable Areas for Rift Valley Fever Transmission in Eastern Africa. PLoS Negl. Trop. Dis. 2016, 10, e0004999. [Google Scholar] [CrossRef]
- Clements, A.C.; Pfeiffer, D.U.; Martin, V. Application of knowledge-driven spatial modelling approaches and uncertainty management to a study of Rift Valley fever in Africa. Int. J. Health Geogr. 2006, 5, 57. [Google Scholar] [CrossRef][Green Version]
- Clements, A.C.; Pfeiffer, D.U.; Martin, V.; Pittliglio, C.; Best, N.; Thiongane, Y. Spatial risk assessment of Rift Valley fever in Senegal. Vector Borne Zoonotic Dis. 2007, 7, 203–216. [Google Scholar] [CrossRef]
- Pedro, S.A.; Abelman, S.; Tonnang, H.E. Predicting Rift Valley Fever Inter-epidemic Activities and Outbreak Patterns: Insights from a Stochastic Host-Vector Model. PLoS Negl. Trop. Dis. 2016, 10, e0005167. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, G.; Chevalier, V.; Tantely, L.M.; Fontenille, D.; Durand, B. A spatially explicit metapopulation model and cattle trade analysis suggests key determinants for the recurrent circulation of rift valley Fever virus in a pilot area of madagascar highlands. PLoS Negl. Trop. Dis. 2014, 8, e3346. [Google Scholar] [CrossRef]
- Paul, P.N.T.; Bah, A.; Ndiaye, P.I.; Ndione, J.A. An Agent-Based Model for Studying the Impact of Herd Mobility on the Spread of Vector-Borne Diseases: The Case of Rift Valley Fever (Ferlo Senegal). Open J. Model. Simul. 2014, 2, 97–111. [Google Scholar] [CrossRef]
- Belkhiria, J.; Lo, M.; Sow, F.; Martínez-López, B.; Chevalier, V. Application of Exponential Random Graph Models to determine Nomadic herders’ movements in Senegal. Transbound Emerg. Dis. 2019. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, A.; Fall, A.G.; Biteye, B.; Ciss, M.; Gimonneau, G.; Castets, M.; Seck, M.T.; Chevalier, V. Spatial Modeling of Mosquito Vectors for Rift Valley Fever Virus in Northern Senegal: Integrating Satellite-Derived Meteorological Estimates in Population Dynamics Models. Remote Sens. 2019, 11, 1024. https://doi.org/10.3390/rs11091024
Tran A, Fall AG, Biteye B, Ciss M, Gimonneau G, Castets M, Seck MT, Chevalier V. Spatial Modeling of Mosquito Vectors for Rift Valley Fever Virus in Northern Senegal: Integrating Satellite-Derived Meteorological Estimates in Population Dynamics Models. Remote Sensing. 2019; 11(9):1024. https://doi.org/10.3390/rs11091024
Chicago/Turabian StyleTran, Annelise, Assane Gueye Fall, Biram Biteye, Mamadou Ciss, Geoffrey Gimonneau, Mathieu Castets, Momar Talla Seck, and Véronique Chevalier. 2019. "Spatial Modeling of Mosquito Vectors for Rift Valley Fever Virus in Northern Senegal: Integrating Satellite-Derived Meteorological Estimates in Population Dynamics Models" Remote Sensing 11, no. 9: 1024. https://doi.org/10.3390/rs11091024
APA StyleTran, A., Fall, A. G., Biteye, B., Ciss, M., Gimonneau, G., Castets, M., Seck, M. T., & Chevalier, V. (2019). Spatial Modeling of Mosquito Vectors for Rift Valley Fever Virus in Northern Senegal: Integrating Satellite-Derived Meteorological Estimates in Population Dynamics Models. Remote Sensing, 11(9), 1024. https://doi.org/10.3390/rs11091024