Water Column Optical Properties of Pacific Coral Reefs Across Geomorphic Zones and in Comparison to Offshore Waters
Abstract
1. Introduction
2. Materials and Methods
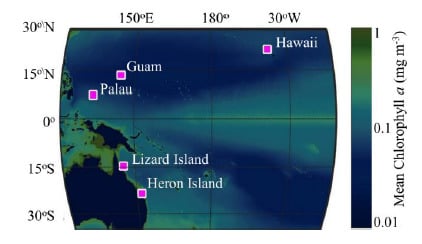
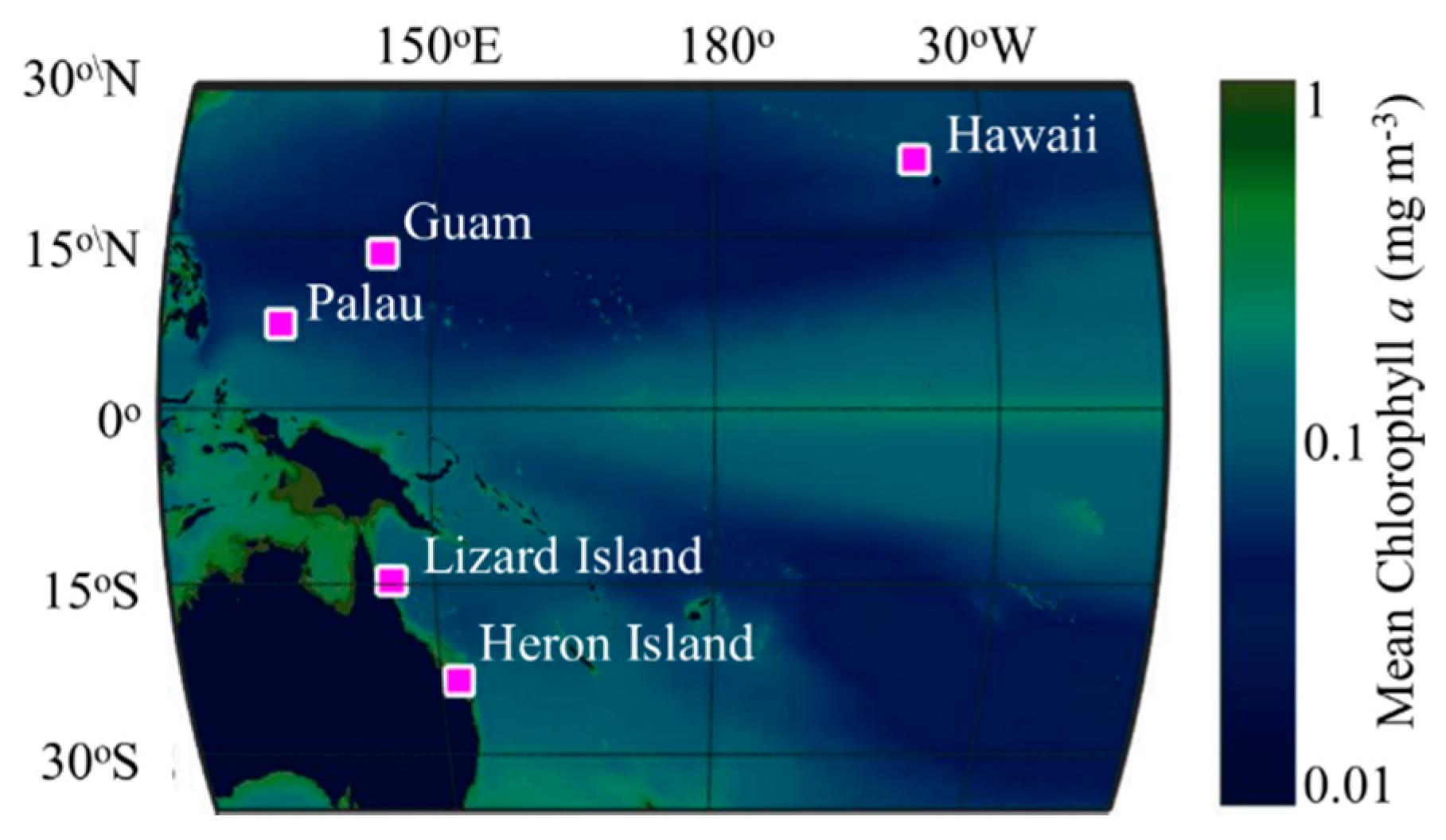
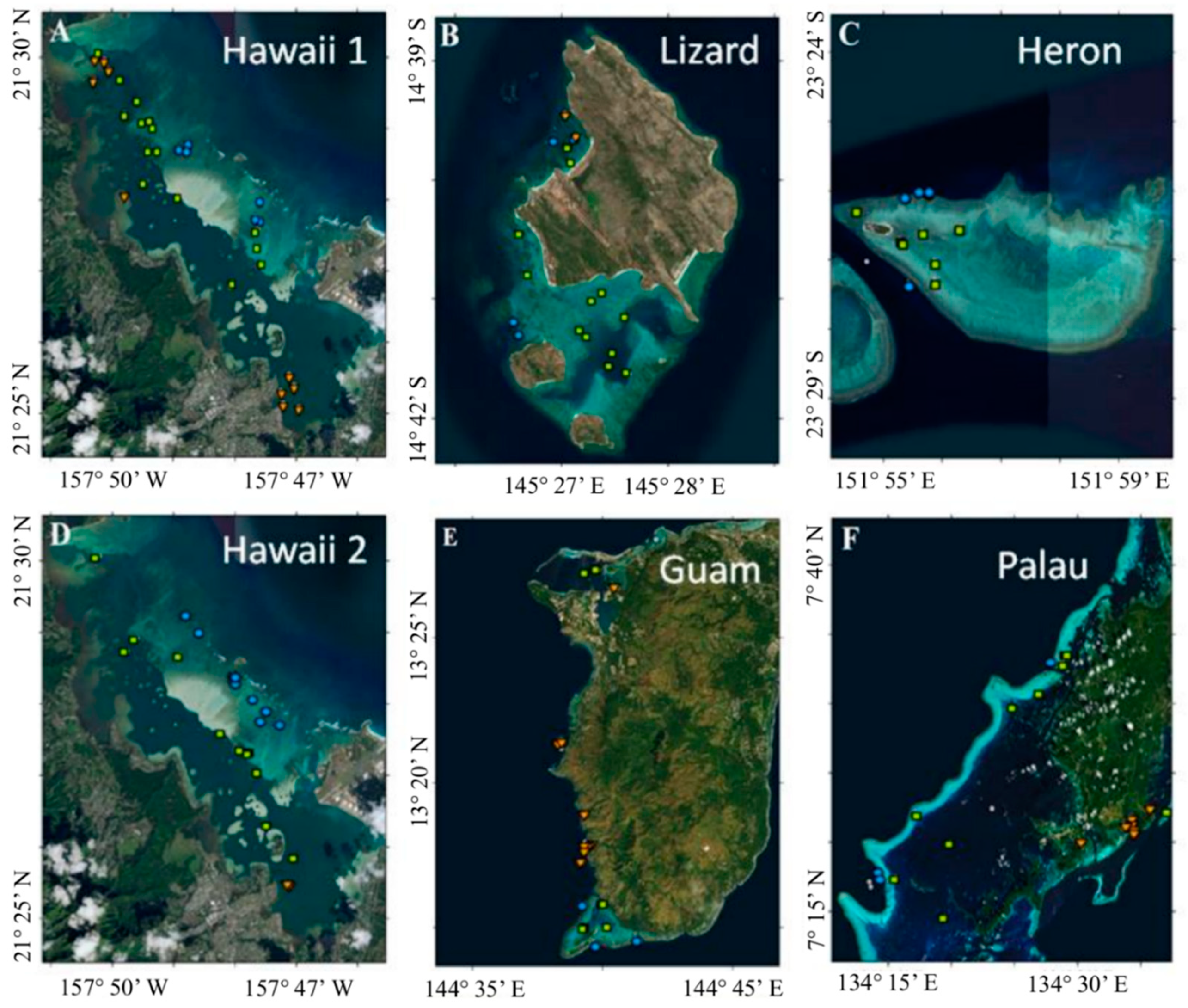
2.1. Data Collection
2.2. Data Processing
2.3. Data Screening
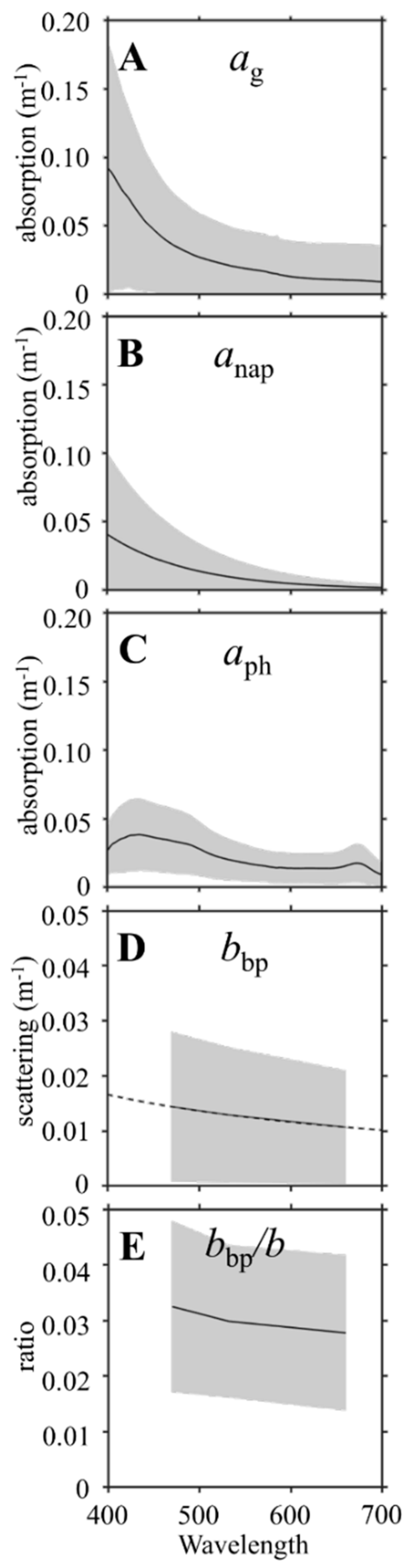
2.4. Inherent Optical Properties and Derived Parameters
2.5. Offshore Data Retrieval
2.6. Statistical Analysis
3. Results
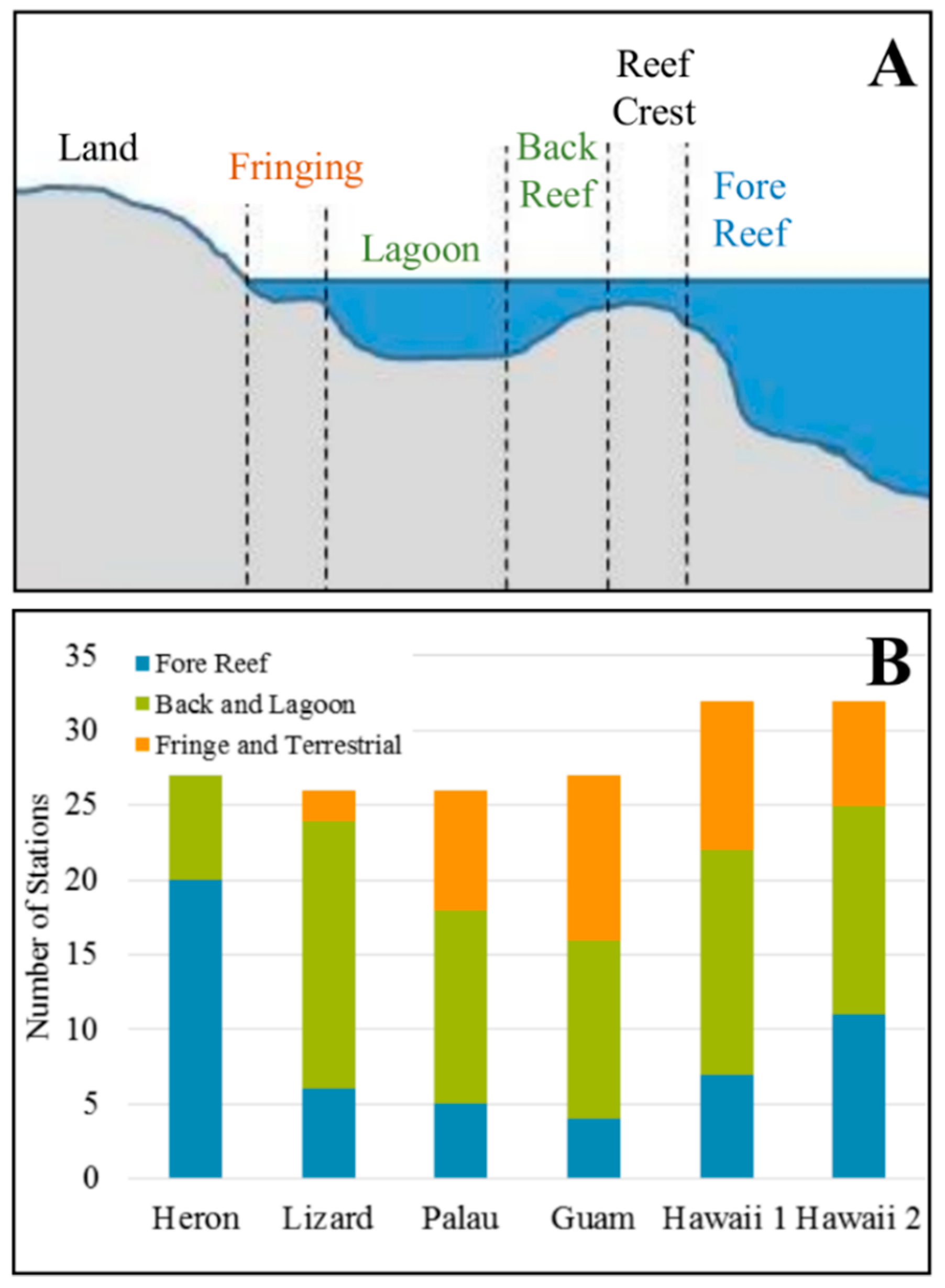
- Fore reef, which is most exposed to open ocean waters (n = 54)
- Back reef, shallow lagoons and deep lagoons (n = 80)
- Fringing reefs and terrestrial reefs which are impacted by land and river runoff (n = 38).
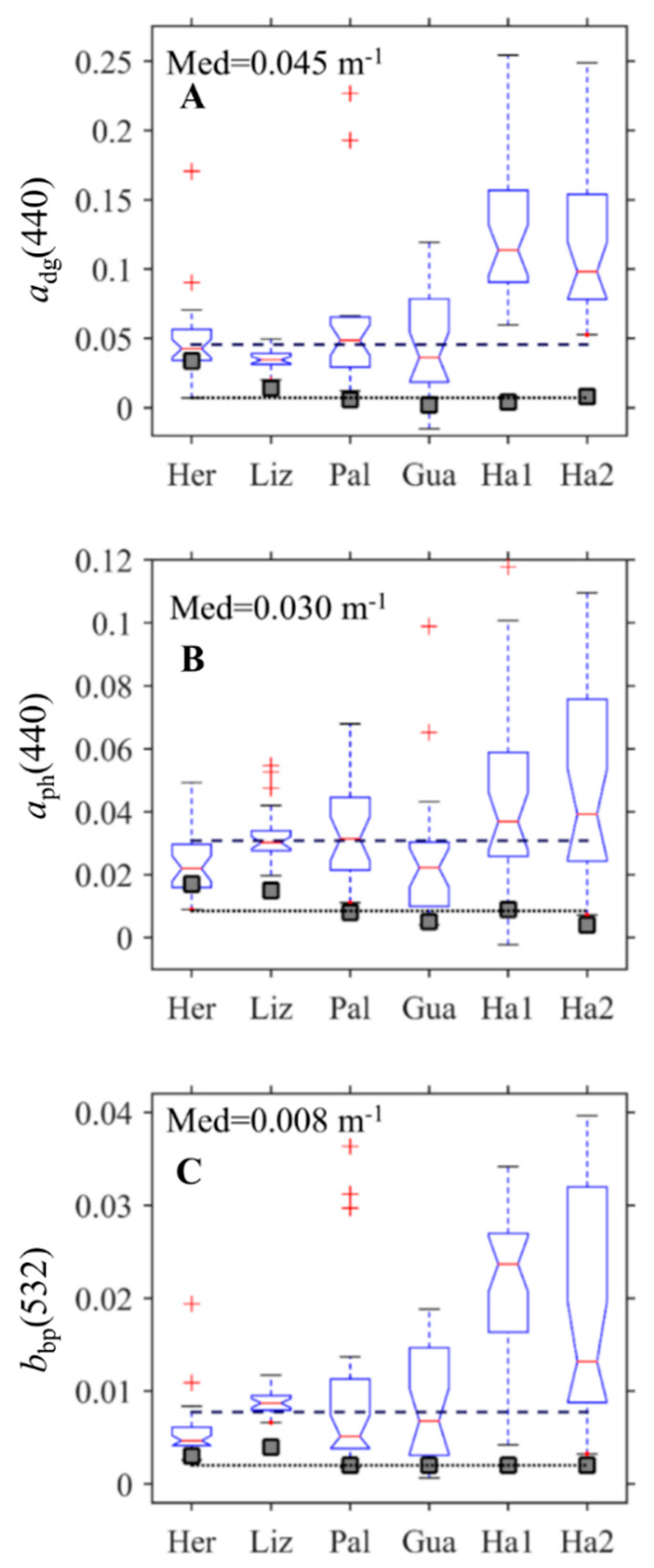
3.1. Interregional Comparison
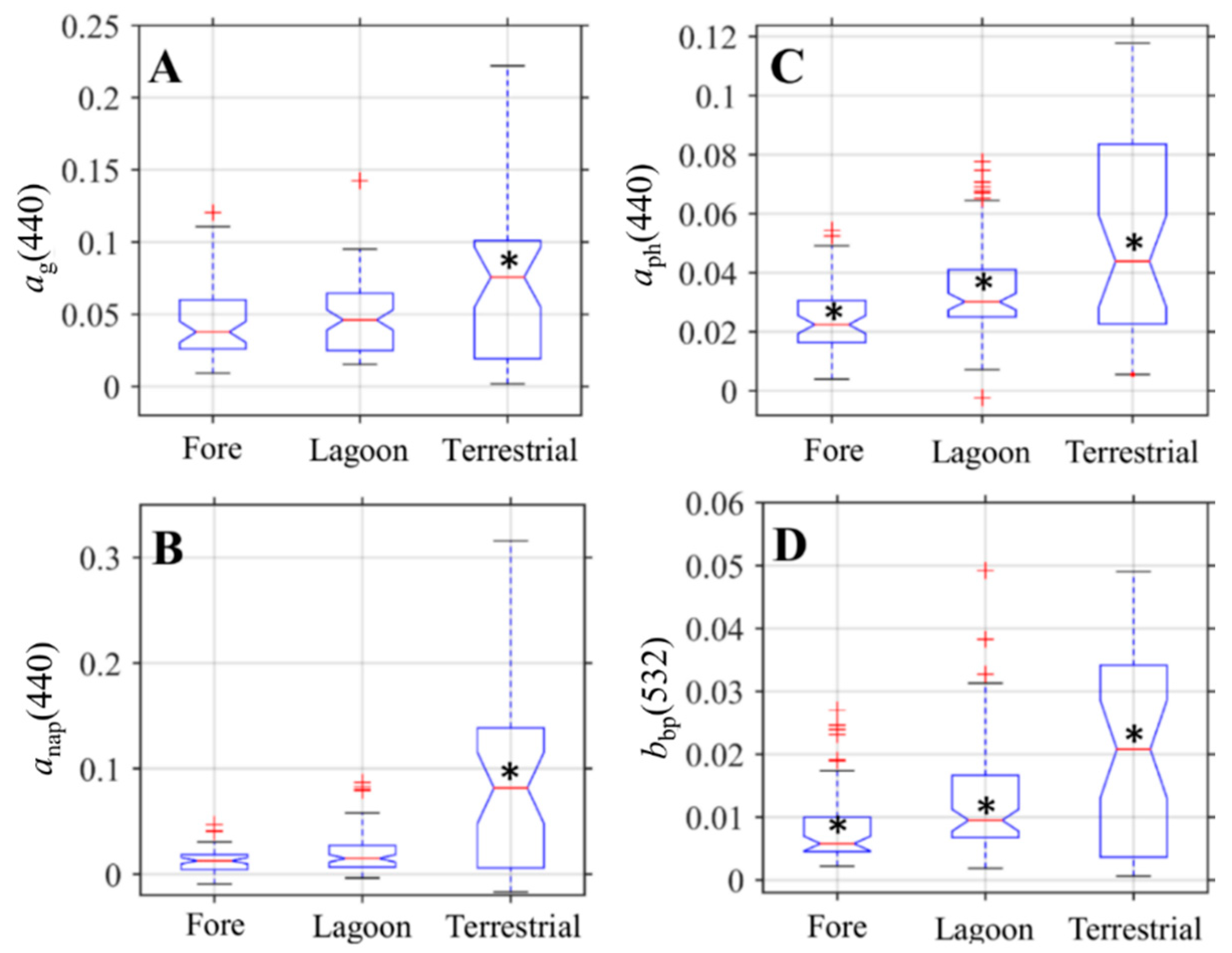
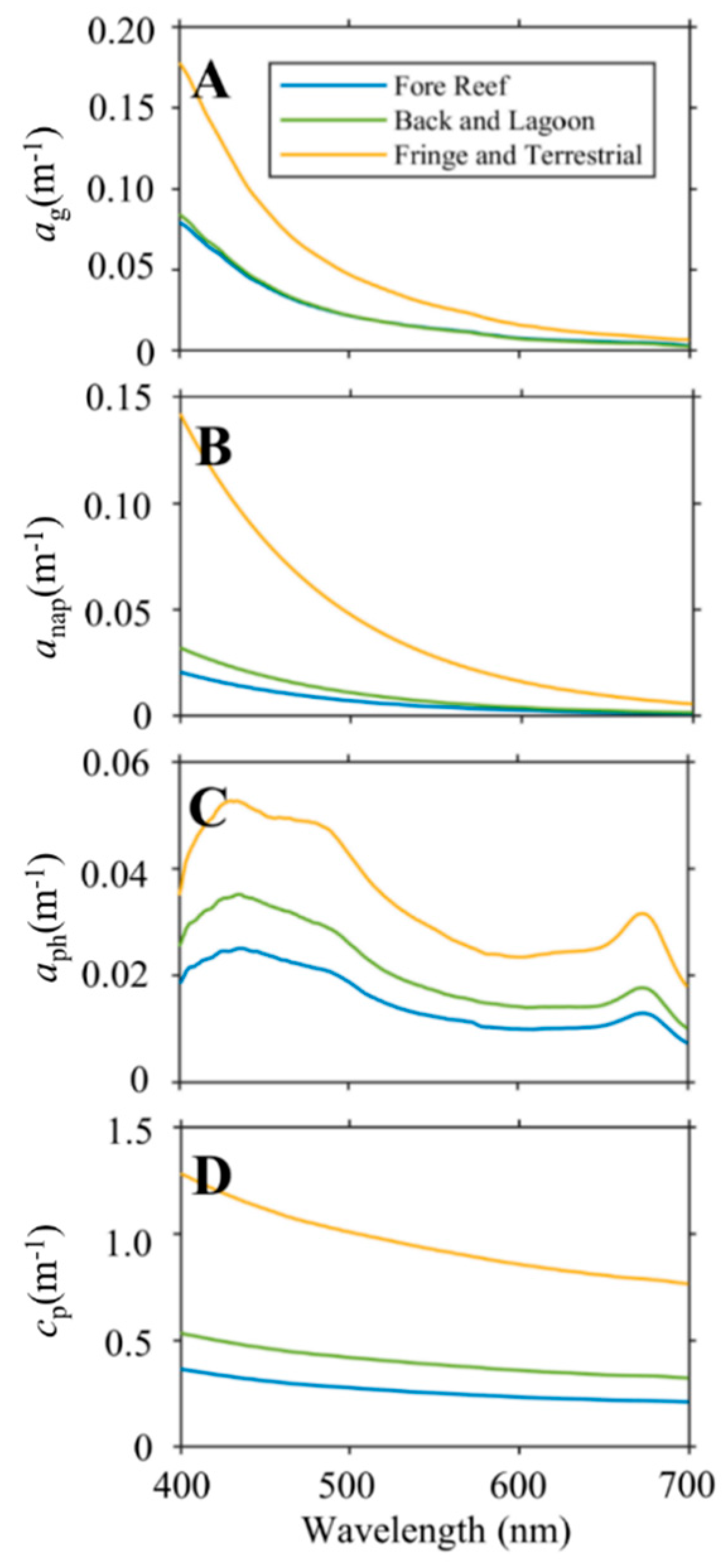
3.2. Comparison of IOPs across Geomorphic Zone
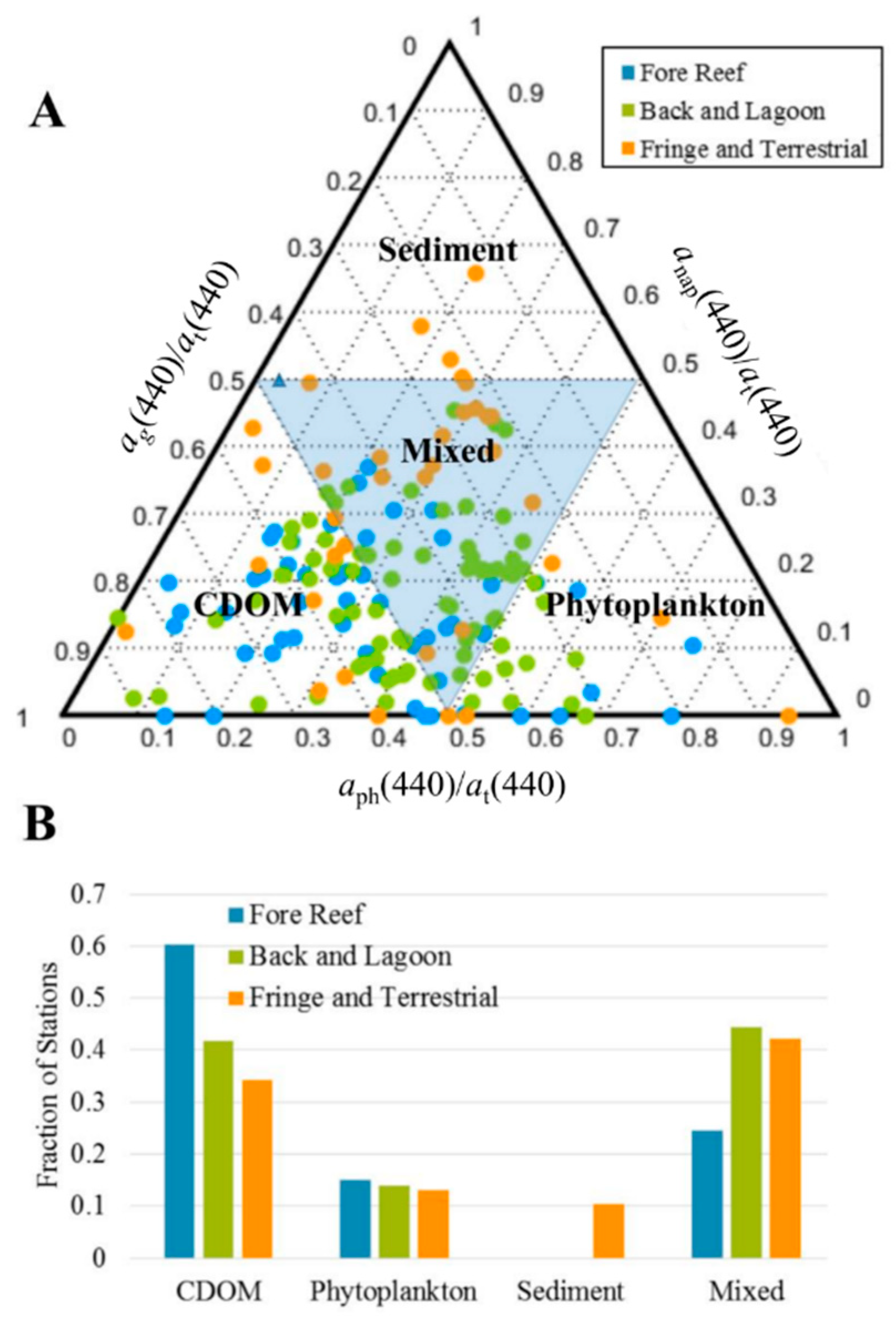
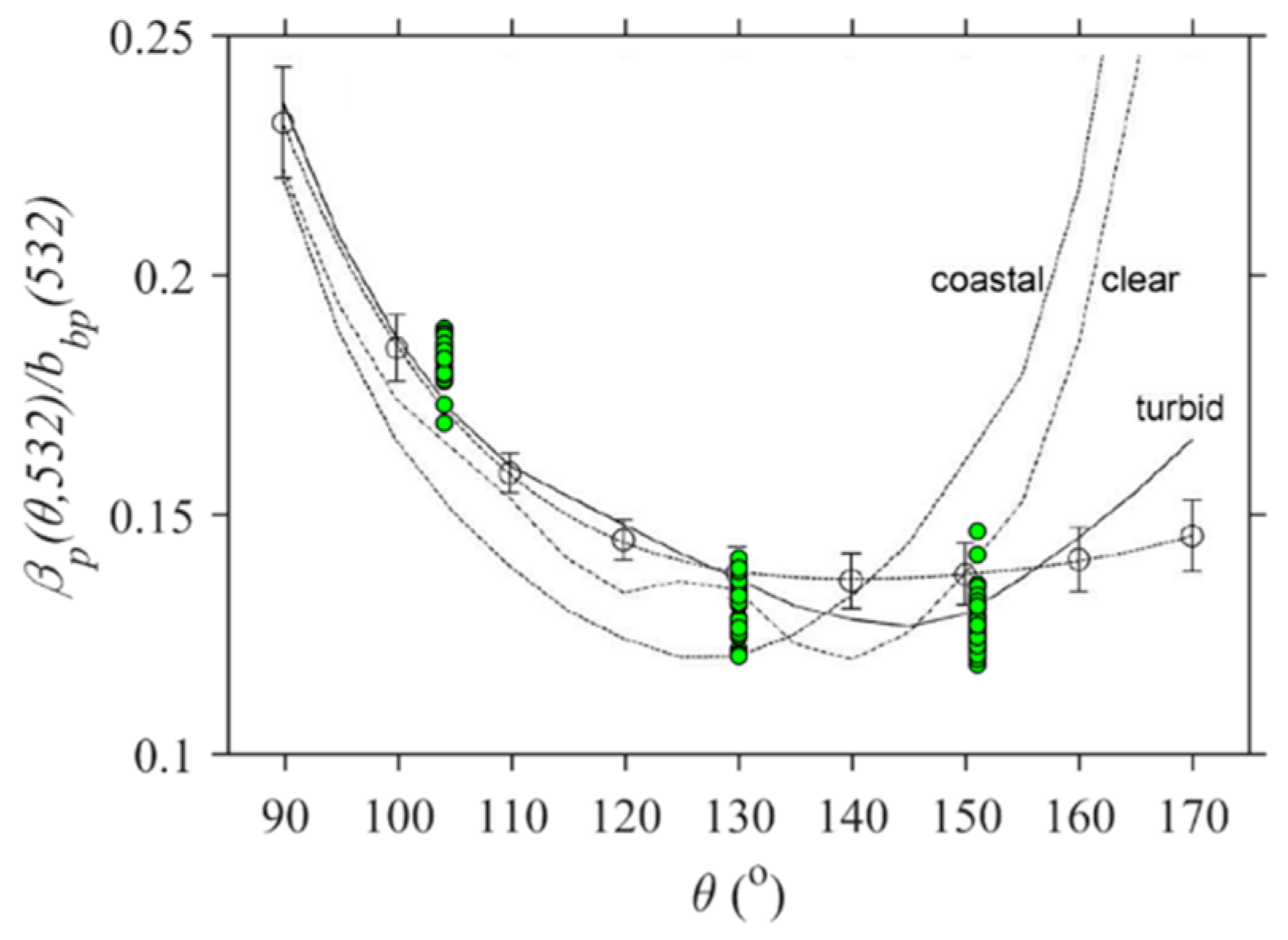
3.3. Optical Shape Parameters Across Geomorphic Zones
4. Discussion
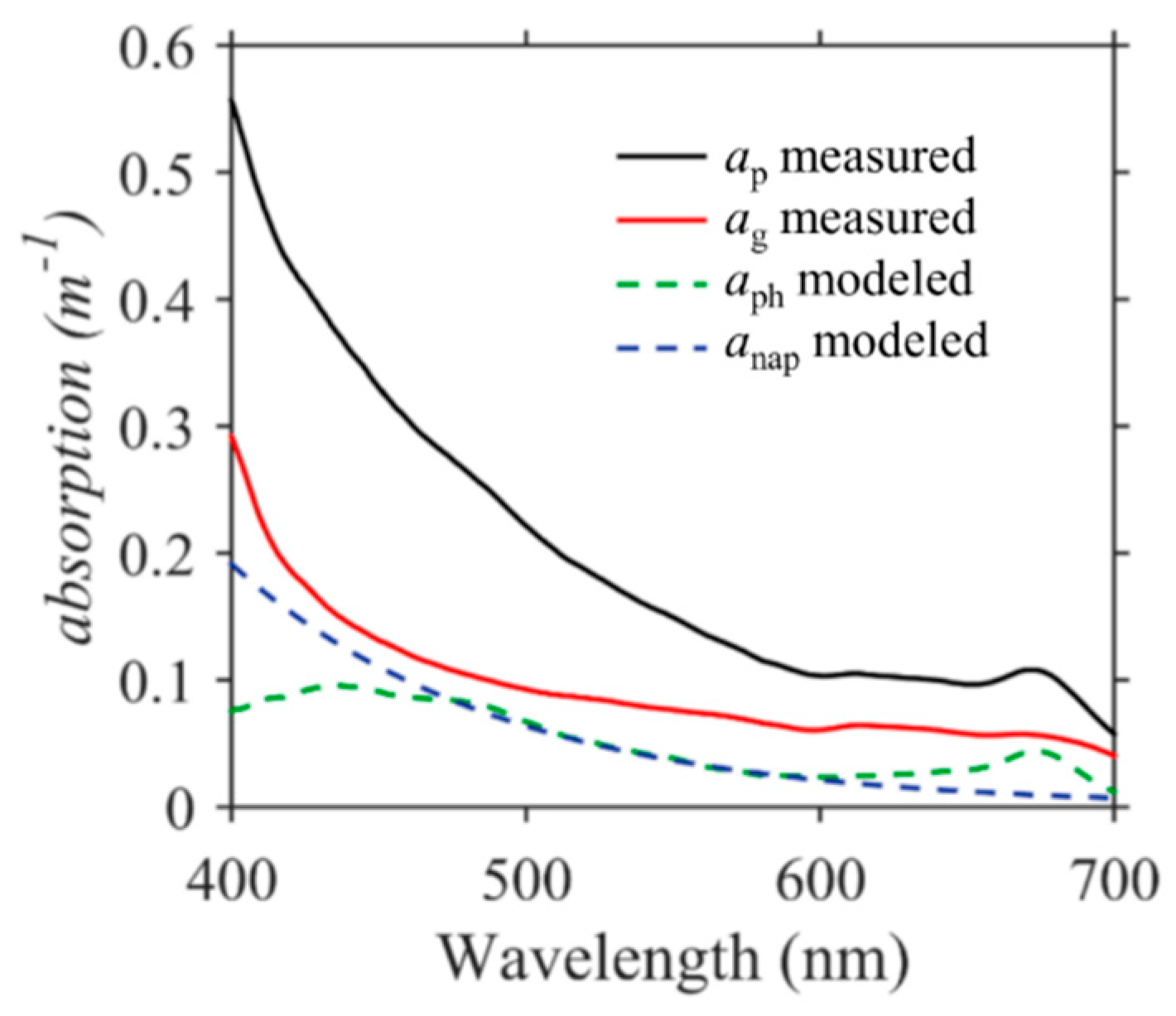
4.1. Colored Dissolved Organic Matter
4.2. Particulates
4.3. Impact on Light Availability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Golbuu, Y.; Victor, S.; Penland, L.; Idip, D.; Emaurois, C.; Okaji, K.; Yukihira, H.; Iwase, A.; van Woesik, R. Palau’s coral reefs show differential habitat recovery following the 1998-bleaching event. Coral Reefs 2007, 26, 319–332. [Google Scholar] [CrossRef]
- Banaszak, A.T.; Lesser, M.P. Effects of solar ultraviolet radiation on coral reef organisms. Photochem. Photobiol. Sci. 2009, 8, 1276. [Google Scholar] [CrossRef] [PubMed]
- Naumann, M.S.; Richter, C.; Mott, C.; el-Zibdah, M.; Manasrah, R.; Wild, C. Budget of coral-derived organic carbon in a fringing coral reef of the Gulf of Aqaba, Red Sea. J. Mar. Syst. 2012, 105–108, 20–29. [Google Scholar] [CrossRef]
- Messer, L.F.; Brown, M.V.; Furnas, M.J.; Carney, R.L.; McKinnon, A.D.; Seymour, J.R. Diversity and Activity of Diazotrophs in Great Barrier Reef Surface Waters. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Purkis, S.J. Remote Sensing Tropical Coral Reefs: The View from Above. Annu. Rev. Mar. Sci. 2018, 10, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Malthus, T.J.; Mumby, P.J. Remote sensing of the coastal zone: An overview and priorities for future research. Int. J. Remote Sens. 2003, 24, 2805–2815. [Google Scholar] [CrossRef]
- Phinn, S.R.; Dekker, A.G.; Brando, V.E.; Roelfsema, C.M. Mapping water quality and substrate cover in optically complex coastal and reef waters: an integrated approach. Mar. Pollut. Bull. 2005, 51, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Hedley, J.D.; Roelfsema, C.M.; Phinn, S.R.; Mumby, P.J. Environmental and Sensor Limitations in Optical Remote Sensing of Coral Reefs: Implications for Monitoring and Sensor Design. Remote Sens. 2012, 4, 271–302. [Google Scholar] [CrossRef]
- Maritorena, S.; Guillocheau, N. Optical properties of water and spectral light absorption by living and non-living particles and by yellow substances in coral reef waters of French Polynesia. Mar. Ecol. Prog. Ser. 1996, 245–255. [Google Scholar] [CrossRef]
- Zepp, R.G. UV exposure of coral assemblages in the Florida Keys. Environ. Prot. Agency Rep. No 2003, 600. [Google Scholar]
- Zepp, R.G.; Shank, G.C.; Stabenau, E.; Patterson, K.W.; Cyterski, M.; Fisher, W.; Bartels, E.; Anderson, S.L. Spatial and temporal variability of solar ultraviolet exposure of coral assemblages in the Florida Keys: Importance of colored dissolved organic matter. Limnol. Oceanogr. 2008, 53, 1909–1922. [Google Scholar] [CrossRef]
- Ayoub, L.M. Can Colored Dissolved Organic Material Protect Coral Reefs by Reducing Exposure to Ultraviolet Radiation? University of South Florida: Tampa, FL, USA, 2009. [Google Scholar]
- Kuwahara, V.S.; Nakajima, R.; Othman, B.H.R.; Kushairi, M.R.M.; Toda, T. Spatial variability of UVR attenuation and bio-optical factors in shallow coral-reef waters of Malaysia. Coral Reefs 2010, 29, 693–704. [Google Scholar] [CrossRef]
- Toro-Farmer, G.; Muller-Karger, F.; Vega-Rodríguez, M.; Melo, N.; Yates, K.; Cerdeira-Estrada, S.; Herwitz, S. Characterization of Available Light for Seagrass and Patch Reef Productivity in Sugarloaf Key, Lower Florida Keys. Remote Sens. 2016, 8, 86. [Google Scholar] [CrossRef]
- Karpouzli, E.; Malthus, T.; Place, C.; Chui, A.M.; Garcia, M.I.; Mair, J. Underwater light characterisation for correction of remotely sensed images. Int. J. Remote Sens. 2003, 24, 2683–2702. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems, 3rd ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2011; ISBN 978-0-521-15175-7. [Google Scholar]
- Morel, A.Y.; Bricaud, A. Theoretical results concerning light absorption in a discrete medium, and application to specific absorption of phytoplankton. Deep-Sea Res. 1981, 28A, 1375–1393. [Google Scholar] [CrossRef]
- Roesler, C.S.; Perry, M.J.; Carder, K.L. Modeling in situ phytoplankton absorption from total absorption spectra in productive inland marine waters. Limnol. Oceanogr. 1989, 34, 1510–1523. [Google Scholar] [CrossRef]
- Mobley, C.D. Light and Water: Radiative Transfer in Natural Waters; Academic Press: San Diego, CA, USA, 1994. [Google Scholar]
- Ulloa, O.; Sathyendranath, S.; Platt, T. Effect of the particle-size distribution on the backscattering ratio in seawater. Appl. Opt. 1994, 33, 7070–7077. [Google Scholar] [CrossRef]
- Sullivan, J.M.; Twardowski, M.S.; Donaghay, P.L.; Freeman, S.A. Use of optical scattering to discriminate particle types in coastal waters. Appl. Opt. 2005, 44, 1667–1680. [Google Scholar] [CrossRef]
- Aurin, D.A.; Dierssen, H.M.; Twardowski, M.S.; Roesler, C.S. Optical complexity in Long Island Sound and implications for coastal ocean color remote sensing. J. Geophys. Res. 2010, 115. [Google Scholar] [CrossRef]
- Odum, H.T.; Odum, E.P. Trophic Structure and Productivity of a Windward Coral Reef Community on Eniwetok Atoll. Ecol. Monogr. 1955, 25, 291–320. [Google Scholar] [CrossRef]
- Ferrier-Pagès, C.; Gattuso, J.-P.; Cauwet, G.; Jaubert, J.; Allemand, D. Release of dissolved organic carbon and nitrogen by the zooxanthellate coral Galaxea fascicularis. Mar. Ecol. Prog. Ser. 1998, 172, 265–274. [Google Scholar] [CrossRef]
- Boss, E.; Zaneveld, J.R.V. The effect of bottom substrate on inherent optical properties: evidence of biogeochemical processes. Limnol. Oceanogr. 2003, 48, 346–354. [Google Scholar] [CrossRef]
- Tribollet, A.; Langdon, C.; Golubic, S.; Atkinson, M. Endolithic microflora are major primary producers in dead carbonate substrates of Hawaiian coral reefs. J. Phycol. 2006, 42, 292–303. [Google Scholar] [CrossRef]
- Wild, C.; Niggl, W.; Naumann, M.; Haas, A. Organic matter release by Red Sea coral reef organisms—potential effects on microbial activity and in situ O2 availability. Mar. Ecol. Prog. Ser. 2010, 411, 61–71. [Google Scholar] [CrossRef]
- Perry, R.A.; Vaudrey, J.M.P.; Dierssen, H.M. Long range transport and carbon and nitrogen dynamics of floating seagrass wracks in Greater Florida Bay. Estuar. Coast. Shelf Sci. 2018, 209, 7–17. [Google Scholar] [CrossRef]
- Ortega-Retuerta, E.; Frazer, T.K.; Duarte, C.M.; Ruiz-Halpern, S.; Tovar-Sánchez, A.; Arrieta, J.M.; Rechea, I. Biogeneration of chromophoric dissolved organic matter by bacteria and krill in the Southern Ocean. Limnol. Oceanogr. 2009, 54, 1941–1950. [Google Scholar] [CrossRef]
- Jørgensen, L.; Stedmon, C.A.; Kragh, T.; Markager, S.; Middelboe, M.; Søndergaard, M. Global trends in the fluorescence characteristics and distribution of marine dissolved organic matter. Mar. Chem. 2011, 126, 139–148. [Google Scholar] [CrossRef]
- Wiebe, W.J.; Johannes, R.E.; Webb, K. Nitrogen fixation in a coral reef community. Science 1975, 188, 257–259. [Google Scholar] [CrossRef]
- Larkum, A.W.; Kennedy, I.; Muller, W. Nitrogen fixation on a coral reef. Mar. Biol. 1988, 98, 143–155. [Google Scholar] [CrossRef]
- Tanaka, Y.; Miyajima, T.; Umezawa, Y.; Hayashibara, T.; Ogawa, H.; Koike, I. Net release of dissolved organic matter by the scleractinian coral Acropora pulchra. J. Exp. Mar. Biol. Ecol. 2009, 377, 101–106. [Google Scholar] [CrossRef]
- Haas, A.F.; Nelson, C.E.; Wegley Kelly, L.; Carlson, C.A.; Rohwer, F.; Leichter, J.J.; Wyatt, A.; Smith, J.E. Effects of Coral Reef Benthic Primary Producers on Dissolved Organic Carbon and Microbial Activity. PLoS ONE 2011, 6, e27973. [Google Scholar] [CrossRef] [PubMed]
- Rix, L.; de Goeij, J.M.; Mueller, C.E.; Struck, U.; Middelburg, J.J.; van Duyl, F.C.; Al-Horani, F.A.; Wild, C.; Naumann, M.S.; van Oevelen, D. Coral mucus fuels the sponge loop in warm- and cold-water coral reef ecosystems. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Rix, L.; de Goeij, J.M.; van Oevelen, D.; Struck, U.; Al-Horani, F.A.; Wild, C.; Naumann, M.S. Differential recycling of coral and algal dissolved organic matter via the sponge loop. Funct. Ecol. 2017, 31, 778–789. [Google Scholar] [CrossRef]
- Fabricius, K.E. Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar. Pollut. Bull. 2005, 50, 125–146. [Google Scholar] [CrossRef] [PubMed]
- Martias, C.; Tedetti, M.; Lantoine, F.; Jamet, L.; Dupouy, C. Characterization and sources of colored dissolved organic matter in a coral reef ecosystem subject to ultramafic erosion pressure (New Caledonia, Southwest Pacific). Sci. Total Environ. 2018, 616–617, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.L.; Evans, C.W. Coral reefs in Kaneohe Bay, Hawaii: two centuries of western influence and two decades of data. Bull. Mar. Sci. 1995, 57, 501–515. [Google Scholar]
- Abal, E.G.; Dennison, W.C. Seagrass depth range and water quality in southern Moreton Bay, Queensland, Australia. Mar. Freshw. Res. 1996, 47, 763–771. [Google Scholar] [CrossRef]
- D’Angelo, C.; Wiedenmann, J. Impacts of nutrient enrichment on coral reefs: new perspectives and implications for coastal management and reef survival. Curr. Opin. Environ. Sustain. 2014, 7, 82–93. [Google Scholar] [CrossRef]
- Yeakel, K.L.; Andersson, A.J.; Bates, N.R.; Noyes, T.J.; Collins, A.; Garley, R. Shifts in coral reef biogeochemistry and resulting acidification linked to offshore productivity. Proc. Natl. Acad. Sci. 2015, 112, 14512–14517. [Google Scholar] [CrossRef]
- Australian Institute of Marine Science. The Great Barrier Reef Long-term Chlorophyll Monitoring. Available online: https://apps.aims.gov.au/metadata/view/eb16c150-c7b4-11dc-b99b-00008a07204e (accessed on 8 June 2018).
- Perry, C.T.; Taylor, K.G.; Machent, P.G. Temporal shifts in reef lagoon sediment composition, Discovery Bay, Jamaica. Estuar. Coast. Shelf Sci. 2006, 67, 133–144. [Google Scholar] [CrossRef]
- Perry, C.T.; Morgan, K.M.; Salter, M.A. Sediment generation by Halimeda on atoll interior coral reefs of the southern Maldives: A census-based approach for estimating carbonate production by calcareous green algae. Sediment. Geol. 2016, 346, 17–24. [Google Scholar] [CrossRef]
- Tebbett, S.B.; Goatley, C.H.; Bellwood, D.R. Algal turf sediments and sediment production by parrotfishes across the continental shelf of the northern Great Barrier Reef. PLoS ONE 2017, 12, e0170854. [Google Scholar] [CrossRef] [PubMed]
- Dierssen, H.M.; Zimmerman, R.C.; Burdige, D.J. Optics and remote sensing of Bahamian carbonate sediment whitings and potential relationship to wind-driven Langmuir circulation. Biogeosciences 2009, 6, 487–500. [Google Scholar] [CrossRef]
- Bessell-Browne, P.; Negri, A.P.; Fisher, R.; Clode, P.L.; Duckworth, A.; Jones, R. Impacts of turbidity on corals: The relative importance of light limitation and suspended sediments. Mar. Pollut. Bull. 2017, 117, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Erftemeijer, P.L.A.; Riegl, B.; Hoeksema, B.W.; Todd, P.A. Environmental impacts of dredging and other sediment disturbances on corals: A review. Mar. Pollut. Bull. 2012, 64, 1737–1765. [Google Scholar] [CrossRef] [PubMed]
- Junjie, R.K.; Browne, N.K.; Erftemeijer, P.L.A.; Todd, P.A. Impacts of Sediments on Coral Energetics: Partitioning the Effects of Turbidity and Settling Particles. PLoS ONE 2014, 9, e107195. [Google Scholar] [CrossRef] [PubMed]
- Wolanski, E.; Richmond, R.; McCook, L.; Sweatman, H. Mud, Marine Snow and Coral Reefs. Am. Sci. 2003, 91, 44. [Google Scholar] [CrossRef]
- Lee, Z.; Carder, K.L.; Mobley, C.D.; Steward, R.G.; Patch, J.S. Hyperspectral remote sensing for shallow waters. I. A semianalytical model. Appl. Opt. 1998, 37, 6329–6338. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.; Hedley, J.; Tin, H.; Fearns, P. A Method to Analyze the Potential of Optical Remote Sensing for Benthic Habitat Mapping. Remote Sens. 2015, 7, 13157–13189. [Google Scholar] [CrossRef]
- Thompson, D.R.; Hochberg, E.J.; Asner, G.P.; Green, R.O.; Knapp, D.E.; Gao, B.-C.; Garcia, R.; Gierach, M.; Lee, Z.; Maritorena, S.; et al. Airborne mapping of benthic reflectance spectra with Bayesian linear mixtures. Remote Sens. Environ. 2017, 200, 18–30. [Google Scholar] [CrossRef]
- Ackleson, S.G.; Smith, J.P.; Rodriguez, L.M.; Moses, W.J.; Russell, B.J. Autonomous Coral Reef Survey in Support of Remote Sensing. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Garcia, R.; Lee, Z.; Hochberg, E. Hyperspectral Shallow-Water Remote Sensing with an Enhanced Benthic Classifier. Remote Sens. 2018, 10, 147. [Google Scholar] [CrossRef]
- Hedley, J.; Roelfsema, C.; Chollett, I.; Harborne, A.; Heron, S.; Weeks, S.; Skirving, W.; Strong, A.; Eakin, C.; Christensen, T.; et al. Remote Sensing of Coral Reefs for Monitoring and Management: A Review. Remote Sens. 2016, 8, 118. [Google Scholar] [CrossRef]
- Lyzenga, D.R. Passive remote sensing techniques for mapping water depth and bottom features. Appl. Opt. 1978, 17, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, R.P.; Holderied, K. Determination of water depth with high-resolution satellite imagery over variable bottom types. Limnol. Oceanogr. 2003, 48, 547–556. [Google Scholar] [CrossRef]
- Dierssen, H.M.; Russell, B.J. COral Reef Airborne Laboratory. SeaWIFS Bio-optical Archive and Storage System (SeaBASS); NASA: Washington, DC, USA, 2017.
- Twardowski, M.S.; Sullivan, J.M.; Donaghay, P.L.; Zaneveld, J.R.V. Microscale quantification of the absorption by dissolved and particulate material in coastal waters with an ac-9. J. Atmospheric Ocean. Technol. 1999, 16, 691–707. [Google Scholar] [CrossRef]
- Sullivan, J.M.; Twardowski, M.S.; Ronald, J.; Zaneveld, V.; Moore, C.C. Measuring optical backscattering in water. In Light Scattering Reviews 7; Springer: Berlin/Heidelberg, Germany, 2013; pp. 189–224. ISBN 978-3-642-21906-1. [Google Scholar]
- Röttgers, R.; McKee, D.; Woźniak, S.B. Evaluation of scatter corrections for ac-9 absorption measurements in coastal waters. Methods Oceanogr. 2013, 7, 21–39. [Google Scholar] [CrossRef]
- Stockley, N.D.; Röttgers, R.; McKee, D.; Lefering, I.; Sullivan, J.M.; Twardowski, M.S. Assessing uncertainties in scattering correction algorithms for reflective tube absorption measurements made with a WET Labs ac-9. Opt. Express 2017, 25, A1139. [Google Scholar] [CrossRef]
- Schofield, O.; Bergmann, T.; Oliver, M.J.; Irwin, A.; Kirkpatrick, G.; Bissett, W.P.; Moline, M.A.; Orrico, C. Inversion of spectral absorption in the optically complex coastal waters of the Mid-Atlantic Bight. J. Geophys. Res. 2004, 109. [Google Scholar] [CrossRef]
- Twardowski, M.S.; Boss, E.; Sullivan, J.M.; Donaghay, P.L. Modeling the spectral shape of absorption by chromophoric dissolved organic matter. Mar. Chem. 2004, 89, 69–88. [Google Scholar] [CrossRef]
- Lee, Z.; Carder, K.L.; Mobley, C.D.; Steward, R.G.; Patch, J.S. Hyperspectral remote sensing for shallow waters. II. Deriving bottom depths and water properties by optimization. Appl. Opt. 1999, 38, 3831–3843. [Google Scholar] [CrossRef] [PubMed]
- Boss, E.; Pegau, W.S.; Gardner, W.D.; Zaneveld, J.R.V.; Barnard, A.H.; Twardowski, M.S.; Chang, G.C.; Dickey, T.D. Spectral particulate attenuation and particle size distribution in the bottom boundary layer of a continental shelf. J. Geophys. Res. Oceans 2001, 106, 9509–9516. [Google Scholar] [CrossRef]
- Kostadinov, T.S.; Siegel, D.A.; Maritorena, S. Retrieval of the particle size distribution from satellite ocean color observations. J. Geophys. Res. 2009, 114. [Google Scholar] [CrossRef]
- Slade, W.H.; Boss, E. Spectral attenuation and backscattering as indicators of average particle size. Appl. Opt. 2015, 54, 7264. [Google Scholar] [CrossRef] [PubMed]
- Diehl, P.; Haardt, H. Measurement of the spectral attenuation to support biological-research in a plankton tube experiment. Oceanol. Acta 1980, 3, 89–96. [Google Scholar]
- Nardelli, S.C.; Twardowski, M.S. Assessing the link between chlorophyll concentration and absorption line height at 676 nm over a broad range of water types. Opt. Express 2016, 24, A1374. [Google Scholar] [CrossRef] [PubMed]
- Roesler, C.S.; Barnard, A.H. Optical proxy for phytoplankton biomass in the absence of photophysiology: Rethinking the absorption line height. Methods Oceanogr. 2013, 7, 79–94. [Google Scholar] [CrossRef]
- Babin, M.; Morel, A.; Fournier-Sicre, V.; Fell, F.; Stramski, D. Light scattering properties of marine particles in coastal and open ocean waters as related to the particle mass concentration. Limnol. Oceanogr. 2003, 48, 843–859. [Google Scholar] [CrossRef]
- Snyder, W.A.; Arnone, R.A.; Davis, C.O.; Goode, W.; Gould, R.W.; Ladner, S.; Lamela, G.; Rhea, W.J.; Stavn, R.; Sydor, M. Optical scattering and backscattering by organic and inorganic particulates in US coastal waters. Appl. Opt. 2008, 47, 666–677. [Google Scholar] [CrossRef]
- Han, B.; Loisel, H.; Vantrepotte, V.; Mériaux, X.; Bryère, P.; Ouillon, S.; Dessailly, D.; Xing, Q.; Zhu, J. Development of a Semi-Analytical Algorithm for the Retrieval of Suspended Particulate Matter from Remote Sensing over Clear to Very Turbid Waters. Remote Sens. 2016, 8, 211. [Google Scholar] [CrossRef]
- Werdell, P.J.; Franz, B.A.; Bailey, S.W.; Feldman, G.C.; Boss, E.; Brando, V.E.; Dowell, M.; Hirata, T.; Lavender, S.J.; Lee, Z.; et al. Generalized ocean color inversion model for retrieving marine inherent optical properties. Appl. Opt. 2013, 52, 2019. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.; Arnone, R.; Hu, C.; Werdell, P.J.; Lubac, B. Uncertainties of optical parameters and their propagations in an analytical ocean color inversion algorithm. Appl. Opt. 2010, 49, 369. [Google Scholar] [CrossRef] [PubMed]
- Werdell, P.J.; McKinna, L.I.W.; Boss, E.; Ackleson, S.G.; Craig, S.E.; Gregg, W.W.; Lee, Z.; Maritorena, S.; Roesler, C.S.; Rousseaux, C.S.; et al. An overview of approaches and challenges for retrieving marine inherent optical properties from ocean color remote sensing. Prog. Oceanogr. 2018, 160, 186–212. [Google Scholar] [CrossRef] [PubMed]
- Goreau, T.F. The ecology of Jamaican coral reefs I. Species composition and zonation. Ecology 1959, 40, 67–90. [Google Scholar] [CrossRef]
- Veron, J.E.N. Corals of the World; Australian Institute of Marine Science: Townsville, Australia, 2000; ISBN 0-642-32236-8. [Google Scholar]
- Kennedy, D.M.; Woodroffe, C.D. Fringing reef growth and morphology: a review. Earth-Sci. Rev. 2002, 57, 255–277. [Google Scholar] [CrossRef]
- Stoddart, D. Ecology and morphology of recent coral reefs. Biol. Rev. 1969, 44, 433–498. [Google Scholar] [CrossRef]
- Boss, E.; Stramski, D.; Bergmann, T.; Pegau, S.; Lewis, M. Why Should We Measure the Optical Backscattering Coefficient? Oceanography 2004, 17, 44–49. [Google Scholar] [CrossRef][Green Version]
- Laws, E.A.; Allen, C.B. Water quality in a subtropical embayment more than a decade after diversion of sewage discharges. Pac. Sci. 1996, 50, 194–210. [Google Scholar]
- Falter, J.L.; Atkinson, M.J.; Merrifield, M.A. Mass-transfer limitation of nutrient uptake by a wave-dominated reef flat community. Limnol. Oceanogr. 2004, 49, 1820–1831. [Google Scholar] [CrossRef]
- Shamberger, K.E.F.; Feely, R.A.; Sabine, C.L.; Atkinson, M.J.; DeCarlo, E.H.; Mackenzie, F.T.; Drupp, P.S.; Butterfield, D.A. Calcification and organic production on a Hawaiian coral reef. Mar. Chem. 2011, 127, 64–75. [Google Scholar] [CrossRef]
- Dierssen, H.M.; Zimmerman, R.C.; Drake, L.A.; Burdige, D. Benthic ecology from space: optics and net primary production in seagrass and benthic algae across the Great Bahama Bank. Mar. Ecol. Prog. Ser. 2010, 411, 1–15. [Google Scholar] [CrossRef]
- Fournier, G.; Forand, J.L. Analytic phase function for ocean water. SPIE 1994, 2258, 194–201. [Google Scholar]
- Mobley, C.D.; Sundman, L.K.; Boss, E. Phase function effects on oceanic light fields. Appl. Opt. 2002, 41, 1035–1050. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.M.; Twardowski, M.S. Angular shape of the oceanic particulate volume scattering function in the backward direction. Appl. Opt. 2009, 48, 6811. [Google Scholar] [CrossRef] [PubMed]
- Petzold, T.J. Volume Scattering Functions for Selected Ocean Waters; Scripps Institution of Oceanography: San Diego, CA, USA, 1972; pp. 72–78. [Google Scholar]
- Blondeau-Patissier, D.; Brando, V.E.; Oubelkheir, K.; Dekker, A.G.; Clementson, L.A.; Daniel, P. Bio-optical variability of the absorption and scattering properties of the Queensland inshore and reef waters, Australia. J. Geophys. Res. 2009, 114. [Google Scholar] [CrossRef]
- Branco, A.B.; Kremer, J.N. The relative importance of chlorophyll and colored dissolved organic matter (CDOM) to the prediction of the diffuse attenuation coefficient in shallow estuaries. Estuaries 2005, 28, 643–652. [Google Scholar] [CrossRef]
- Ferrier-Pagès, C.; Richard, C.; Forcioli, D.; Allemand, D.; Pichon, M.; Shick, J.M. Effects of temperature and UV radiation increases on the photosynthetic efficiency in four scleractinian coral species. Biol. Bull. 2007, 213, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, L.M.; Hallock, P.; Coble, P.G.; Bell, S.S. MAA-like absorbing substances in Florida Keys phytoplankton vary with distance from shore and CDOM: Implications for coral reefs. J. Exp. Mar. Biol. Ecol. 2012, 420–421, 91–98. [Google Scholar] [CrossRef]
- Barnes, B.B.; Hallock, P.; Hu, C.; Muller-Karger, F.; Palandro, D.; Walter, C.; Zepp, R. Prediction of coral bleaching in the Florida Keys using remotely sensed data. Coral Reefs 2015, 34, 491–503. [Google Scholar] [CrossRef]
- Dunne, R.P.; Brown, B.E. Penetration of solar UVB radiation in shallow tropical waters and its potential biological effects on coral reefs; results from the central Indian Ocean and Andaman Sea. Mar. Ecol. Prog. Ser. 1996, 144, 109–118. [Google Scholar] [CrossRef]
- Lyons, M.M.; Aas, P.; Pakulski, J.D.; Van Waasbergen, L.; Miller, R.V.; Mitchell, D.L.; Jeffrey, W.H. DNA damage induced by ultraviolet radiation in coral-reef microbial communities. Mar. Biol. 1998, 130, 537–543. [Google Scholar] [CrossRef]
- Anderson, S.; Zepp, R.; Machula, J.; Santavy, D.; Hansen, L.; Mueller, E. Indicators of UV Exposure in Corals and Their Relevance to Global Climate Change and Coral Bleaching. Hum. Ecol. Risk Assess. Int. J. 2001, 7, 1271–1282. [Google Scholar] [CrossRef]
- Carder, K.L.; Steward, R.G.; Harvey, G.R.; Ortner, P.B. Marine humic and fulvic acids: Their effects on remote sensing of ocean chlorophyll. Limnol. Oceanogr. 1989, 34, 68–81. [Google Scholar] [CrossRef]
- Coble, P.G. Marine optical biogeochemistry: the chemistry of ocean color. Chem. Rev. 2007, 107, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Blough, N.; Green, S. Spectroscopic characterization and remote sensing of non-living organic matter. In The Role of Non-Living Organic Matter in the Earth’s Carbon Cycle; Zepp, R.G., Sonntag, C., Eds.; Wiley: Chichester, UK, 1995; pp. 23–45. [Google Scholar]
- Vodacek, A.; Blough, N.V.; DeGrandpre, M.D.; Nelson, R.K. Seasonal variation of CDOM and DOC in the Middle Atlantic Bight: Terrestrial inputs and photooxidation. Limnol. Oceanogr. 1997, 42, 674–686. [Google Scholar] [CrossRef]
- Boss, E.; Pegau, W.S.; Zaneveld, J.R.V.; Barnard, A.H. Spatial and temporal variability of absorption by dissolved material at a continental shelf. J. Geophys. Res. 2001, 106, 9499–9507. [Google Scholar] [CrossRef]
- Twardowski, M.S. Photobleaching of aquatic dissolved materials: Absorption removal, spectral alteration, and their interrelationship. J. Geophys. Res. 2002, 107. [Google Scholar] [CrossRef]
- Matsuoka, A.; Ortega-Retuerta, E.; Bricaud, A.; Arrigo, K.R.; Babin, M. Characteristics of colored dissolved organic matter (CDOM) in the Western Arctic Ocean: Relationships with microbial activities. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015, 118, 44–52. [Google Scholar] [CrossRef]
- Zanardi-Lamardo, E.; Moore, C.A.; Zika, R.G. Seasonal variation in molecular mass and optical properties of chromophoric dissolved organic material in coastal waters of southwest Florida. Mar. Chem. 2004, 89, 37–54. [Google Scholar] [CrossRef]
- Oubelkheir, K.; Clementson, L.A.; Webster, I.T.; Ford, P.W.; Dekker, A.G.; Radke, L.C.; Daniel, P. Using inherent optical properties to investigate biogeochemical dynamics in a tropical macrotidal coastal system. J. Geophys. Res. 2006, 111. [Google Scholar] [CrossRef]
- Schaffelke, B.; Carleton, J.; Skuza, M.; Zagorskis, I.; Furnas, M.J. Water quality in the inshore Great Barrier Reef lagoon: Implications for long-term monitoring and management. Mar. Pollut. Bull. 2012, 65, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; de Beer, D.; Lott, C.; Polerecky, L.; Kohls, K.; Abed, R.M.M.; Ferdelman, T.G.; Fabricius, K.E. Mechanisms of damage to corals exposed to sedimentation. Proc. Natl. Acad. Sci. 2012, 109, E1558–E1567. [Google Scholar] [CrossRef] [PubMed]
- Bartley, R.; Bainbridge, Z.T.; Lewis, S.E.; Kroon, F.J.; Wilkinson, S.N.; Brodie, J.E.; Silburn, D.M. Relating sediment impacts on coral reefs to watershed sources, processes and management: A review. Sci. Total Environ. 2014, 468–469, 1138–1153. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Ricardo, G.F.; Negri, A.P. Effects of sediments on the reproductive cycle of corals. Mar. Pollut. Bull. 2015, 100, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Bessell-Browne, P.; Fisher, R.; Klonowski, W.; Slivkoff, M. Assessing the impacts of sediments from dredging on corals. Mar. Pollut. Bull. 2016, 102, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Yahel, G.; Post, A.F.; Fabricius, K.; Marie, D.; Vaulot, D.; Genin, A. Phytoplankton distribution and grazing near coral reefs. Limnol. Oceanogr. 1998, 43, 551–563. [Google Scholar] [CrossRef]
- Leipe, T.; Knoppers, B.; Marone, E.; Camargo, R. Suspended matter transport in coral reef waters of the Abrolhos Bank, Brazil. Geo-Mar. Lett. 1999, 19, 186–195. [Google Scholar] [CrossRef]
- Abdel-Hamid, A.M.A.; Hamed, M.A. Impact of water quality deterioration on coral reef community structure in the Northern Red Sea, Egypt. Egypt J Aquat Biol Fish 2006, 10, 117–146. [Google Scholar]
- Thompson, A.; Schroeder, T.; Brando, V.E.; Schaffelke, B. Coral community responses to declining water quality: Whitsunday Islands, Great Barrier Reef, Australia. Coral Reefs 2014, 33, 923–938. [Google Scholar] [CrossRef]
- Cortés, J.N.; Risk, M.J. A reef under siltation stress: Cahuita, Costa Rica. Bull. Mar. Sci. 1985, 36, 339–356. [Google Scholar]
- Van Katwijk, M.M.; Meier, N.F.; Van Loon, R.; Van Hove, E.M.; Giesen, W.; Van der Velde, G.; Den Hartog, C. Sabaki River sediment load and coral stress: correlation between sediments and condition of the Malindi-Watamu reefs in Kenya (Indian Ocean). Mar. Biol. 1993, 117, 675–683. [Google Scholar] [CrossRef]
- Stramski, D.; Bricaud, A.; Morel, A. Modeling the inherent optical properties of the ocean based on the detailed composition of the planktonic community. Appl. Opt. 2001, 40, 2929–2945. [Google Scholar] [CrossRef]
- Twardowski, M.S.; Boss, E.; Macdonald, J.B.; Pegau, W.S.; Barnard, A.H.; Zaneveld, J.R.V. A model for estimating bulk refractive index from the optical backscattering ratio and the implications for understanding particle composition in case I and case II waters. J. Geophys. Res. 2001, 106, 14129–14142. [Google Scholar] [CrossRef]
- Zaneveld, J.R.V.; Kitchen, J.C. The variation in the inherent optical properties of phytoplankton near an absorption peak as determined by various models of cell structure. J. Geophys. Res. Oceans 1995, 100, 13309–13320. [Google Scholar] [CrossRef]
- Morel, A.Y.; Ahn, Y.-H. Optical efficiency factors of free-living marine bacteria: Influence of bacterioplankton upon the optical properties and particulate organic carbon in oceanic waters. J. Mar. Res. 1990, 48, 145–175. [Google Scholar] [CrossRef]
- Torres-Pérez, J.; Guild, L.; Armstrong, R. Hyperspectral Distinction of Two Caribbean Shallow-Water Corals Based on Their Pigments and Corresponding Reflectance. Remote Sens. 2012, 4, 3813–3832. [Google Scholar] [CrossRef]
- Torres-Pérez, J.L.; Guild, L.S.; Armstrong, R.A.; Corredor, J.; Zuluaga-Montero, A.; Polanco, R. Relative Pigment Composition and Remote Sensing Reflectance of Caribbean Shallow-Water Corals. PLOS ONE 2015, 10, e0143709. [Google Scholar] [CrossRef]
- Fitt, W.K.; McFarland, F.K.; Warner, M.E.; Chilcoat, G.C. Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol. Oceanogr. 2000, 45, 677–685. [Google Scholar] [CrossRef]
- Lesser, M.P.; Farrell, J.H. Exposure to solar radiation increases damage to both host tissues and algal symbionts of corals during thermal stress. Coral Reefs 2004, 23, 367–377. [Google Scholar] [CrossRef]
- Kinsey, D. Metabolism, calcification and carbon production I: Systems level studies. In Proceedings of the Fifth International Coral Reef Congress, Tahiti, France, 27 May–1 June 1985; Volume 4, pp. 505–526. [Google Scholar]
- Klumpp, D.; McKinnon, A. Temporal and spatial patterns in primary production of a coral-reef epilithic algal community. J. Exp. Mar. Biol. Ecol. 1989, 131, 1–22. [Google Scholar] [CrossRef]
- Berner, T. Coral-reef algae. In Coral Reefs; Dubinsky, Z., Ed.; Ecosystems of the World; Elsevier: Amsterdam, The Netherlands, 1990; Volume 25, pp. 253–264. [Google Scholar]
- Glynn, P.W. Feeding ecology of coral-reef macroconsumers. In Coral Reefs; Dubinsky, Z., Ed.; Ecosystems of the World; Elsevier: Amsterdam, The Netherlands, 1990; Volume 25. [Google Scholar]
- Uthicke, S. Photosynthetic efficiency and rapid light curves of sediment-biofilms along a water quality gradient in the Great Barrier Reef, Australia. Mar. Ecol. Prog. Ser. 2006, 322, 61–73. [Google Scholar] [CrossRef][Green Version]
- Enríquez, S.; Iglesias-Prieto, R.; Merino, M. Variations in the photosynthetic performance along the leaves of the tropical seagrass Thalassia testudinum. Mar. Biol. 2002, 140, 891–900. [Google Scholar]
- Andréfouët, S.; Kramer, P.; Torres-Pulliza, D.; Joyce, K.E.; Hochberg, E.J.; Garza-Pérez, R.; Mumby, P.J.; Riegl, B.; Yamano, H.; White, W.H.; et al. Multi-site evaluation of IKONOS data for classification of tropical coral reef environments. Remote Sens. Environ. 2003, 88, 128–143. [Google Scholar] [CrossRef]
- Hedley, J.; Russell, B.; Randolph, K.; Dierssen, H. A physics-based method for the remote sensing of seagrasses. Remote Sens. Environ. 2016, 174, 134–147. [Google Scholar] [CrossRef]
- Hedley, J.D.; Russell, B.J.; Randolph, K.; Pérez-Castro, M.Á.; Vásquez-Elizondo, R.M.; Enríquez, S.; Dierssen, H.M. Remote Sensing of Seagrass Leaf Area Index and Species: The Capability of a Model Inversion Method Assessed by Sensitivity Analysis and Hyperspectral Data of Florida Bay. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Joyce, K.E.; Phinn, S.R.; Roelfsema, C.M.; Neil, D.T.; Dennison, W.C. Combining Landsat ETM+ and Reef Check classifications for mapping coral reefs: a critical assessment from the southern Great Barrier Reef, Australia. Coral Reefs 2004, 23, 21–25. [Google Scholar] [CrossRef]
- Aronson, R.B.; Precht, W.F. Conservation, precaution, and Caribbean reefs. Coral Reefs 2006, 25, 441–450. [Google Scholar] [CrossRef]
- Walsh, S.M. Ecosystem-Scale Effects of Nutrients and Fishing on Coral Reefs. J. Mar. Biol. 2011, 2011, 1–13. [Google Scholar] [CrossRef]
- Bruno, J.F.; Valdivia, A. Coral reef degradation is not correlated with local human population density. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Crane, N.L.; Nelson, P.; Abelson, A.; Precoda, K.; Rulmal Jr, J.; Bernardi, G.; Paddack, M. Atoll-scale patterns in coral reef community structure: Human signatures on Ulithi Atoll, Micronesia. PLoS ONE 2017, 12, e0177083. [Google Scholar] [CrossRef]
- Mazel, C.H. Diver-operated instrument for in situ measurement of spectral fluorescence and reflectance of benthic marine organisms and substrates. Opt. Eng. 1997, 36, 2612–2617. [Google Scholar] [CrossRef]
- Chennu, A.; Färber, P.; De’ath, G.; de Beer, D.; Fabricius, K.E. A diver-operated hyperspectral imaging and topographic surveying system for automated mapping of benthic habitats. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Johnsen, G.; Volent, Z.; Dierssen, H.; Pettersen, R.; Ardelan, M.V.; Søreide, F.; Fearns, P.; Ludvigsen, M.; Moline, M. Underwater hyperspectral imagery to create biogeochemical maps of seafloor properties. In Subsea Optics and Imaging; Elsevier: Sawston, UK, 2013; pp. 508–540e. ISBN 978-0-85709-341-7. [Google Scholar]
- Chirayath, V.; Earle, S.A. Drones that see through waves - preliminary results from airborne fluid lensing for centimetre-scale aquatic conservation: Airborne Fluid Lensing for Centimetre-Scale Aquatic Conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 237–250. [Google Scholar] [CrossRef]
- Long, M.H.; Berg, P.; de Beer, D.; Zieman, J.C. In situ coral reef oxygen metabolism: An eddy correlation study. PLoS ONE 2013, 8, e58581. [Google Scholar] [CrossRef]
- Hedley, J.; Mirhakak, M.; Wentworth, A.; Dierssen, H. Influence of Three-Dimensional Coral Structures on Hyperspectral Benthic Reflectance and Water-Leaving Reflectance. Appl. Sci. 2018, 8, 2688. [Google Scholar] [CrossRef]
| Parameter | Unit | Definition | Method |
|---|---|---|---|
| a(λ) | m−1 | Total absorption coefficient (=400–750 nm) 1 | unfiltered ac-s |
| apg(λ) | m−1 | Non-water absorption coefficient | unfiltered ac-s, corrected |
| ag(λ) | m−1 | Colored Dissolved Organic Matter (CDOM) absorption coefficient | filtered ac-s |
| ap(λ) | m−1 | Particulate absorption coefficient | filtered and unfiltered ac-s |
| aph(λ) | m−1 | Phytoplankton absorption coefficient | derived |
| anap(λ) | m−1 | Non-algal particulate absorption coefficient | derived |
| c(λ) | m−1 | Total attenuation coefficient | unfiltered ac-s |
| cpg(λ) | m−1 | Non-water attenuation coefficient | unfiltered ac-s, corrected |
| cg(λ) | m−1 | Dissolved attenuation coefficient | filtered ac-s |
| cp(λ) | m−1 | Particulate attenuation coefficient | filtered and unfiltered ac-s |
| bp(λ) | m−1 | Particulate scattering coefficient | unfiltered ac-s |
| bbp(λ) | m−1 | Particulate backscattering coefficient (=470, 532, 660 nm) | ECO VSF-3 |
| bbp/bp(λ) | Particulate backscattering ratio (=470, 532, 660 nm) | ECO VSF-3 and ac-s | |
| β(θ,λ) | m−1 | Volume scattering function (θ = 104°, 130°, 151°; =470, 532, 660 nm) | ECOVSF-3 |
| Sg | nm−1 | Slope of dissolved absorption | filtered ac-s |
| Y | Slope of particulate backscattering | ECO VSF-3 | |
| γ | Slope of particulate attenuation | unfiltered ac-s | |
| [Chl a] | mg m−3 | Estimated chlorophyll a concentration | derived aph(λ) |
| np | Particulate index of refraction | Derived |
| Campaign | SeaBASS ID | Dates (Local) | Location | Stations |
|---|---|---|---|---|
| Hawaii Summer 2016 | CHI1606 | 11–19 June 2016 | Kaneohe Bay, Oahu, Hawaii, USA | 32 |
| Lizard Island | CAL1609 | 4–10 September 2016 | Great Barrier Reef, Queensland, Australia | 26 |
| Heron Island | CAH1609 | 17–22 September 2016 | Great Barrier Reef, Queensland, Australia | 27 |
| Hawaii Winter 2017 | CHI1702 | 15–24 February 2017 | Kaneohe Bay, Oahu, Hawaii, USA | 34 |
| Guam | CGU1704 | 9–16 April 2017 | Guam, USA | 27 |
| Palau | CPA1705 | 2–13 May 2017 | Koror, Palau | 26 |
| Geomorphic Zone | Description | Stations |
|---|---|---|
| Fore Reef | Seaward of main wave breaking zone | 54 |
| Back Reef | Behind reef crest | 26 |
| Shallow Lagoon | Inside reef crests, <10 m depth | 41 |
| Operationally Deep Lagoon | Inside reef crests, >10 m depth | 13 |
| Fringing Reef | Coral directly adjacent to land mass | 17 |
| Terrestrial | Areas where water column or benthic properties are likely to be dominated by terrestrial inputs, such as near river mouths | 21 |
| Reef Type | ag(440) (m−1) | anap(440) (m−1) | aph(440) (m−1) | bbp(532) (m−1) |
|---|---|---|---|---|
| Offshore (MODIS) | 0.004 ± < 0.001 a | 0.009 ± 0.001 | 0.002 ± < 0.001 | |
| Fore Reef | 0.038 ± 0.015 | 0.013 ± 0.007 | 0.022 ± 0.007 | 0.006 ± 0.002 |
| Back Reef & Lagoon | 0.046 ± 0.021 | 0.015 ± 0.009 | 0.030 ± 0.007 | 0.001 ± 0.003 |
| Fringing Reef & Terrestrial | 0.076 ± 0.028 | 0.082 ± 0.065 | 0.044 ± 0.029 | 0.021 ± 0.014 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russell, B.J.; Dierssen, H.M.; Hochberg, E.J. Water Column Optical Properties of Pacific Coral Reefs Across Geomorphic Zones and in Comparison to Offshore Waters. Remote Sens. 2019, 11, 1757. https://doi.org/10.3390/rs11151757
Russell BJ, Dierssen HM, Hochberg EJ. Water Column Optical Properties of Pacific Coral Reefs Across Geomorphic Zones and in Comparison to Offshore Waters. Remote Sensing. 2019; 11(15):1757. https://doi.org/10.3390/rs11151757
Chicago/Turabian StyleRussell, Brandon J., Heidi M. Dierssen, and Eric J. Hochberg. 2019. "Water Column Optical Properties of Pacific Coral Reefs Across Geomorphic Zones and in Comparison to Offshore Waters" Remote Sensing 11, no. 15: 1757. https://doi.org/10.3390/rs11151757
APA StyleRussell, B. J., Dierssen, H. M., & Hochberg, E. J. (2019). Water Column Optical Properties of Pacific Coral Reefs Across Geomorphic Zones and in Comparison to Offshore Waters. Remote Sensing, 11(15), 1757. https://doi.org/10.3390/rs11151757