Characterizing the Variability of the Structure Parameter in the PROSPECT Leaf Optical Properties Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
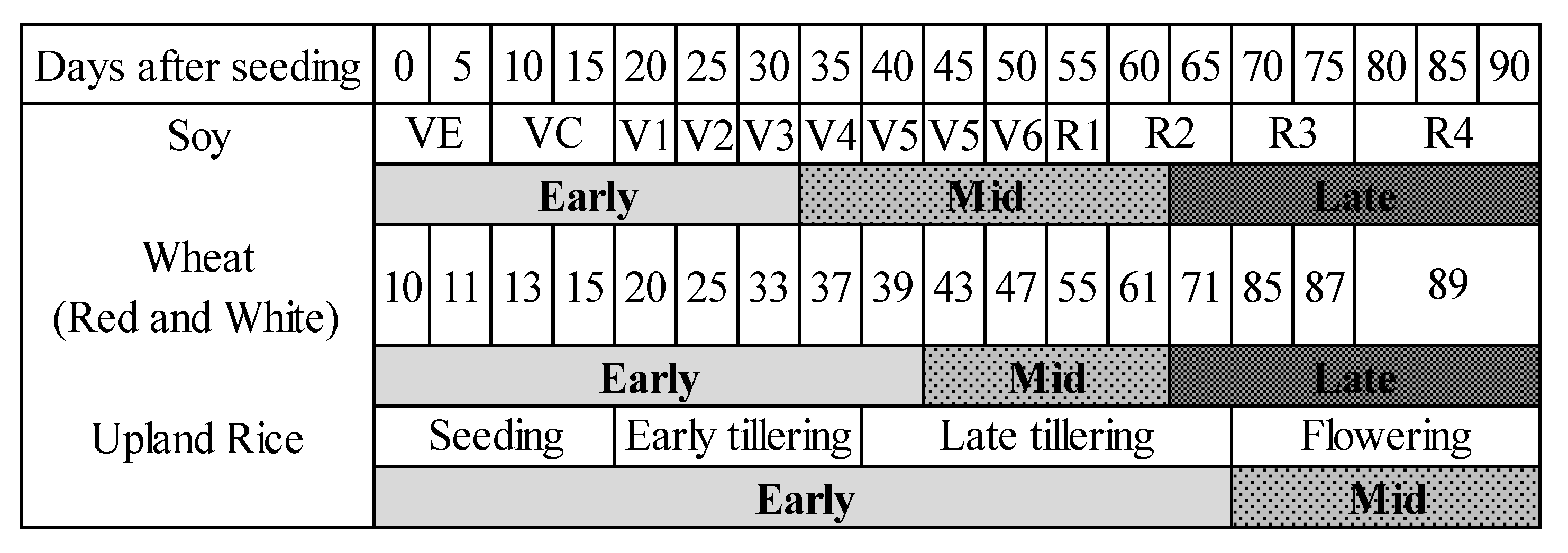
2.1.1. Experimental Design
2.1.2. Measurement Protocol
2.1.3. Spectral Data Processing
2.2. Data Analysis
- λ1: Wavelength of minimum absorbance (i.e., the maximum of the sum of measured transmittance and reflectance).
- λ2: Wavelength of maximum measured reflectance.
- λ3: Wavelength of maximum measured transmittance.
- The population of estimated Ns values was stratified into sub-populations according to the criteria defined in Section 2.1.
- A Welch’s two-sample t-test was performed to determine if the estimated means of monocotyledon and dicotyledon Ns differed significantly from one another.
- A Welch’s two-sample t-test was run to determine whether there was a significant difference between the estimated means of pre- and post-irrigation Ns for each plant type.
- Welch’s one-way ANOVA tests were run to test whether the variation of Ns in each plant type can be explained by phenological class (early/mid/late) and irrigation regime (irrigation at 85%, 75%, and 60% of the initial saturated weight)
- If the ANOVA test was significant at P ≤ 0.05, then Welch’s two-sample t-tests were carried out to compare the means of classes within the sub-population of the plant type. For example, if phenological class returned a significant result as an explanatory variable for Ns in the sampled monocotyledon leaves, then the means of ‘early’, ‘mid’, and ‘late’ monocotyledon Ns would be compared.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Running, S.W.; Coughlan, J.C. A general model of forest ecosystem processes for regional applications I. Hydrologic balance, canopy gas exchange and primary production processes. Ecol. Model. 1988, 42, 125–154. [Google Scholar] [CrossRef]
- Reichstein, M.; Bahn, M.; Mahecha, M.D.; Kattge, J.; Baldocchi, D.D. Linking plant and ecosystem functional biogeography. Proc. Natl. Acad. Sci. USA 2014, 111, 13697–13702. [Google Scholar] [CrossRef] [PubMed]
- Schimel, D.S.; Kittel, T.G.; Parton, W.J. Terrestrial biogeochemical cycles: Global interactions with the atmosphere and hydrology. Tellus B 1991, 43, 188–203. [Google Scholar] [CrossRef]
- Trumbore, S. Carbon respired by terrestrial ecosystems–recent progress and challenges. Glob. Chang. Biol. 2006, 12, 141–153. [Google Scholar] [CrossRef]
- Running, S.W.; Nemani, R.R.; Heinsch, F.A.; Zhao, M.; Reeves, M.; Hashimoto, H. A continuous satellite-derived measure of global terrestrial primary production. AIBS Bull. 2004, 54, 547–560. [Google Scholar] [CrossRef]
- Justice, C.O.; Vermote, E.; Townshend, J.R.; Defries, R.; Roy, D.P.; Hall, D.K.; Salomonson, V.V.; Privette, J.L.; Riggs, G.; Strahler, A. The Moderate Resolution Imaging Spectroradiometer (MODIS): Land remote sensing for global change research. IEEE Trans. Geosci. Remote Sens. 1998, 36, 1228–1249. [Google Scholar] [CrossRef]
- Ustin, S.L.; Roberts, D.A.; Gamon, J.A.; Asner, G.P.; Green, R.O. Using imaging spectroscopy to study ecosystem processes and properties. AIBS Bull. 2004, 54, 523–534. [Google Scholar] [CrossRef]
- Baret, F.; Guyot, G. Potentials and limits of vegetation indices for LAI and APAR assessment. Remote Sens. Environ. 1991, 35, 161–173. [Google Scholar] [CrossRef]
- Pinty, B.; Verstraete, M.M. Extracting information on surface properties from bidirectional reflectance measurements. J. Geophys. Res. Atmos. 1991, 96, 2865–2874. [Google Scholar] [CrossRef]
- Baret, F.; Buis, S. Estimating canopy characteristics from remote sensing observations. Review of methods and associated problems. In Advances in Land Remote Sensing: System, Modeling, Inversion and Application; Springer: Dordrecht, The Netherlands, 2008; pp. 173–201. [Google Scholar]
- Jacquemoud, S.; Bacour, C.; Poilve, H.; Frangi, J.-P. Comparison of four radiative transfer models to simulate plant canopies reflectance: Direct and inverse mode. Remote Sens. Environ. 2000, 74, 471–481. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, H.; Liang, Y.; Liu, S.; Qin, Q.; Ersoy, O.K. Advancing the PROSPECT-5 Model to Simulate the Spectral Reflectance of Copper-Stressed Leaves. Remote Sens. 2017, 9, 1191. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Atzberger, C.; Skidmore, A.; Schlerf, M. Mapping grassland leaf area index with airborne hyperspectral imagery: A comparison study of statistical approaches and inversion of radiative transfer models. ISPRS J. Photogramm. Remote Sens. 2011, 66, 894–906. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Baret, F. Estimating vegetation biophysical parameters by inversion of a reflectance model on high spectral resolution data. In Crop Structure and Light Microclimate: Characterization and Applications; Varlet-Grancher, C., Bonhomme, R., Sinoquet, H., Eds.; Institut National De La Recherche Agronomique: Versailles, France, 1993; pp. 339–350. [Google Scholar]
- Goel, N.S. Models of vegetation canopy reflectance and their use in estimation of biophysical parameters from reflectance data. Remote Sens. Rev. 1988, 4, 1–212. [Google Scholar] [CrossRef]
- Serbin, S.P.; Dillaway, D.N.; Kruger, E.L.; Townsend, P.A. Leaf optical properties reflect variation in photosynthetic metabolism and its sensitivity to temperature. J. Exp. Bot. 2011, 63, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.A.; Knapp, A.K. Leaf optical properties in higher plants: Linking spectral characteristics to stress and chlorophyll concentration. Am. J. Bot. 2001, 88, 677–684. [Google Scholar] [CrossRef]
- Ustin, S.L.; Gitelson, A.A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-Tejada, P. Retrieval of foliar information about plant pigment systems from high resolution spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar] [CrossRef]
- Govaerts, Y.; Verstraete, M.M. Evaluation of the capability of BRDF models to retrieve structural information on the observed target as described by a three-dimensional ray tracing code. In Proceedings of the European Symposium on Satellite Remote Sensing, Rome, Italy, 26–30 September 1994; pp. 9–20. [Google Scholar]
- Baret, F.; Vanderbilt, V.C.; Steven, M.D.; Jacquemoud, S. Use of spectral analogy to evaluate canopy reflectance sensitivity to leaf optical properties. Remote Sens. Environ. 1994, 48, 253–260. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Baret, F. PROSPECT: A model of leaf optical properties spectra. Remote Sens. Environ. 1990, 34, 75–91. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Verhoef, W.; Baret, F.; Bacour, C.; Zarco-Tejada, P.J.; Asner, G.P.; François, C.; Ustin, S.L. PROSPECT+ SAIL models: A review of use for vegetation characterization. Remote Sens. Environ. 2009, 113, S56–S66. [Google Scholar] [CrossRef]
- Sun, J.; Shi, S.; Yang, J.; Du, L.; Gong, W.; Chen, B.; Song, S. Analyzing the performance of PROSPECT model inversion based on different spectral information for leaf biochemical properties retrieval. ISPRS J. Photogramm. Remote Sens. 2018, 135, 74–83. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, Y.; Huang, Y.; Reddy, K.N.; Lee, M.A.; Fletcher, R.S.; Thomson, S.J. Early detection of crop injury from herbicide glyphosate by leaf biochemical parameter inversion. Int. J. Appl. Earth Obs. Geoinf. 2014, 31, 78–85. [Google Scholar] [CrossRef]
- Allen, W.A.; Gausman, H.W.; Richardson, A.J.; Thomas, J.R. Interaction of isotropic light with a compact plant leaf. JOSA 1969, 59, 1376–1379. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Ustin, S.; Verdebout, J.; Schmuck, G.; Andreoli, G.; Hosgood, B. Estimating leaf biochemistry using the PROSPECT leaf optical properties model. Remote Sens. Environ. 1996, 56, 194–202. [Google Scholar] [CrossRef]
- Le Maire, G.; Francois, C.; Dufrene, E. Towards universal broad leaf chlorophyll indices using PROSPECT simulated database and hyperspectral reflectance measurements. Remote Sens. Environ. 2004, 89, 1–28. [Google Scholar] [CrossRef]
- Féret, J.-B.; François, C.; Asner, G.P.; Gitelson, A.A.; Martin, R.E.; Bidel, L.P.; Ustin, S.L.; Le Maire, G.; Jacquemoud, S. PROSPECT-4 and 5: Advances in the leaf optical properties model separating photosynthetic pigments. Remote Sens. Environ. 2008, 112, 3030–3043. [Google Scholar] [CrossRef]
- Féret, J.-B.; Gitelson, A.; Noble, S.; Jacquemoud, S. PROSPECT-D: Towards modeling leaf optical properties through a complete lifecycle. Remote Sens. Environ. 2017, 193, 204–215. [Google Scholar] [CrossRef]
- Combal, B.; Baret, F.; Weiss, M.; Trubuil, A.; Mace, D.; Pragnere, A.; Myneni, R.; Knyazikhin, Y.; Wang, L. Retrieval of canopy biophysical variables from bidirectional reflectance: Using prior information to solve the ill-posed inverse problem. Remote Sens. Environ. 2003, 84, 1–15. [Google Scholar] [CrossRef]
- Koetz, B.; Baret, F.; Poilvé, H.; Hill, J. Use of coupled canopy structure dynamic and radiative transfer models to estimate biophysical canopy characteristics. Remote Sens. Environ. 2005, 95, 115–124. [Google Scholar] [CrossRef]
- Lavergne, T.; Kaminski, T.; Pinty, B.; Taberner, M.; Gobron, N.; Verstraete, M.M.; Vossbeck, M.; Widlowski, J.-L.; Giering, R. Application to MISR land products of an RPV model inversion package using adjoint and Hessian codes. Remote Sens. Environ. 2007, 107, 362–375. [Google Scholar] [CrossRef]
- Danson, F.; Bowyer, P. Estimating live fuel moisture content from remotely sensed reflectance. Remote Sens. Environ. 2004, 92, 309–321. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, J.; Wan, H.; Li, X.; Zhou, G. A Bayesian network algorithm for retrieving the characterization of land surface vegetation. Remote Sens. Environ. 2008, 112, 613–622. [Google Scholar] [CrossRef]
- Laurent, V.C.; Schaepman, M.E.; Verhoef, W.; Weyermann, J.; Chávez, R.O. Bayesian object-based estimation of LAI and chlorophyll from a simulated Sentinel-2 top-of-atmosphere radiance image. Remote Sens. Environ. 2014, 140, 318–329. [Google Scholar] [CrossRef]
- Govaerts, Y.M.; Jacquemoud, S.; Verstraete, M.M.; Ustin, S.L. Three-dimensional radiation transfer modeling in a dicotyledon leaf. Appl. Opt. 1996, 35, 6585–6598. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.K.; Vogelmann, T.C.; DeLucia, E.H.; Bell, D.T.; Shepherd, K.A. Leaf form and photosynthesis. Bioscience 1997, 47, 785–793. [Google Scholar] [CrossRef]
- Oguchi, R.; Onoda, Y.; Terashima, I.; Tholen, D. Leaf Anatomy and Function. In The Leaf: A Platform for Performing Photosynthesis; Springer: Cham, Switzerland, 2018; pp. 97–139. [Google Scholar]
- Ollinger, S. Sources of variability in canopy reflectance and the convergent properties of plants. New Phytol. 2011, 189, 375–394. [Google Scholar] [CrossRef] [PubMed]
- Ceccato, P.; Flasse, S.; Tarantola, S.; Jacquemoud, S.; Grégoire, J.-M. Detecting vegetation leaf water content using reflectance in the optical domain. Remote Sens. Environ. 2001, 77, 22–33. [Google Scholar] [CrossRef]
- Demarez, V. Seasonal variation of leaf chlorophyll content of a temperate forest. Inversion of the PROSPECT model. Int. J. Remote Sens. 1999, 20, 879–894. [Google Scholar] [CrossRef]
- Gausman, H.; Allen, W.; Myers, V.; Cardenas, R. Reflectance and Internal Structure of Cotton Leaves, Gossypium hirsutum L. 1. Agron. J. 1969, 61, 374–376. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Rueda, C.; Ustin, S. Water content estimation in vegetation with MODIS reflectance data and model inversion methods. Remote Sens. Environ. 2003, 85, 109–124. [Google Scholar] [CrossRef]
- Gerber, F.; Marion, R.; Olioso, A.; Jacquemoud, S.; Da Luz, B.R.; Fabre, S. Modeling directional–hemispherical reflectance and transmittance of fresh and dry leaves from 0.4 μm to 5.7 μm with the PROSPECT-VISIR model. Remote Sens. Environ. 2011, 115, 404–414. [Google Scholar] [CrossRef]
- Jurdao, S.; Yebra, M.; Guerschman, J.P.; Chuvieco, E. Regional estimation of woodland moisture content by inverting radiative transfer models. Remote Sens. Environ. 2013, 132, 59–70. [Google Scholar] [CrossRef]
- Baret, F.; Fourty, T. Estimation of leaf water content and specific leaf weight from reflectance and transmittance measurements. Agronomie 1997, 17, 455–464. [Google Scholar] [CrossRef]
- Arraudeau, M.; Vergara, B.S. A Farmer’s Primer on Growing Upland Rice; International Rice Research Institute: Los Baños, Philippines, 1988. [Google Scholar]
- Pedersen, P.; Kumudini, S.; Board, J.; Conley, S. Soybean Growth and Development; Iowa State University, University Extension: Ames, IA, USA, 2004. [Google Scholar]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Doorenbos, J.; Kassam, A. Yield response to water. Irrig. Drain. Pap. 1979, 33, 257. [Google Scholar]
- Lam, H.; Rotman, S. Performance Verification of NIR Spectrophotometers. In Practical Approaches to Method Validation and Essential Instrument Qualification; Wiley: Hoboken, NJ, USA, 2010; pp. 177–199. [Google Scholar]
- Arellano, P.; Tansey, K.; Balzter, H.; Boyd, D.S. Field spectroscopy and radiative transfer modelling to assess impacts of petroleum pollution on biophysical and biochemical parameters of the Amazon rainforest. Environ. Earth Sci. 2017, 76, 217. [Google Scholar] [CrossRef]
- Jay, S.; Bendoula, R.; Hadoux, X.; Féret, J.-B.; Gorretta, N. A physically-based model for retrieving foliar biochemistry and leaf orientation using close-range imaging spectroscopy. Remote Sens. Environ. 2016, 177, 220–236. [Google Scholar] [CrossRef]
- Bousquet, L.; Lachérade, S.; Jacquemoud, S.; Moya, I. Leaf BRDF measurements and model for specular and diffuse components differentiation. Remote Sens. Environ. 2005, 98, 201–211. [Google Scholar] [CrossRef]
- Li, D.; Cheng, T.; Jia, M.; Zhou, K.; Lu, N.; Yao, X.; Tian, Y.; Zhu, Y.; Cao, W. PROCWT: Coupling PROSPECT with continuous wavelet transform to improve the retrieval of foliar chemistry from leaf bidirectional reflectance spectra. Remote Sens. Environ. 2018, 206, 1–14. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Hosgood, B.; Jacquemoud, S.; Andreoli, G.; Verdebout, J.; Pedrini, G.; Schmuck, G. Leaf Optical Properties Experiment 93 (LOPEX93); European Commission, Joint Research Centre Institute of Remote Sensing Applications: Luxembourg, 1995. [Google Scholar]
- Nelder, J.A.; Mead, R. A simplex method for function minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- Suplick-Ploense, M.; Alshammary, S.; Qian, Y. Spectral reflectance response of three turfgrasses to leaf dehydration. Asian J. Plant Sci. 2011, 10, 67. [Google Scholar]
- Gitelson, A.; Merzlyak, M.N. Spectral reflectance changes associated with autumn senescence of Aesculus hippocastanum L. and Acer platanoides L. leaves. Spectral features and relation to chlorophyll estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]





| Symbol | Biochemical Parameter | Units |
|---|---|---|
| Ccab | Leaf chlorophyll content | µg cm−2 |
| Ccar | Leaf carotenoid content | µg cm−2 |
| Cw | Equivalent water thickness | g cm−2 |
| Cm | Dry matter content | g cm−2 |
| Plant Type | Total | Early | Mid | Late |
|---|---|---|---|---|
| Monocotyledon (n = 118) | ||||
| µ | 1.46 | 1.35 | 1.5 | 1.66 |
| σ | 0.29 | 0.28 | 0.23 | 0.25 |
| Max/Min | 2.23/1.00 | 1.92/1.00 | 1.84/1.00 | 2.23/1.15 |
| Upper/Lower IQ | 1.68/1.27 | 1.57/1.03 | 1.70/1.39 | 1.73/1.52 |
| Dicotyledon (n = 112) | ||||
| µ | 2.07 | 2.17 | 1.96 | 2.14 |
| σ | 0.27 | 0.19 | 0.25 | 0.29 |
| Max/Min | 2.96/1.61 | 2.54/1.91 | 2.89/1.61 | 2.96/1.64 |
| Upper/Lower IQ | 2.21/1.86 | 2.26/2.05 | 2.04/1.81 | 2.36/1.96 |
| Data | Independent Variable | F-Value | T-Statistic |
|---|---|---|---|
| All | Species | 10.93 (< 0.001) | |
| Monocotyledon | Season period | 10.227 (< 0.001) | |
| 2015 Irrigation regime | 0.896 (0.413) | ||
| 2016 Irrigation regime | −1.122 (0.271) | ||
| Dicotyledon | Season period | 4.46 (0.017) | |
| 2015 Irrigation regime | 2.422 (0.101) | ||
| 2016 Irrigation regime | −1.251 (0.22) |
| Population | Independent Variable | T-Value | df | Probability of > T |
|---|---|---|---|---|
| Monocotyledon | Early vs Mid | −2.486 | 88 | 0.015 |
| Mid vs Late | −2.626 | 58 | 0.011 | |
| Early vs Late | −4.912 | 84 | <0.001 | |
| Dicotyledon | Early vs Mid | 3.002 | 59 | 0.004 |
| Mid vs Late | −3.3 | 95 | 0.001 | |
| Early vs Late | 0.373 | 64 | 0.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boren, E.J.; Boschetti, L.; Johnson, D.M. Characterizing the Variability of the Structure Parameter in the PROSPECT Leaf Optical Properties Model. Remote Sens. 2019, 11, 1236. https://doi.org/10.3390/rs11101236
Boren EJ, Boschetti L, Johnson DM. Characterizing the Variability of the Structure Parameter in the PROSPECT Leaf Optical Properties Model. Remote Sensing. 2019; 11(10):1236. https://doi.org/10.3390/rs11101236
Chicago/Turabian StyleBoren, Erik J., Luigi Boschetti, and Dan M. Johnson. 2019. "Characterizing the Variability of the Structure Parameter in the PROSPECT Leaf Optical Properties Model" Remote Sensing 11, no. 10: 1236. https://doi.org/10.3390/rs11101236
APA StyleBoren, E. J., Boschetti, L., & Johnson, D. M. (2019). Characterizing the Variability of the Structure Parameter in the PROSPECT Leaf Optical Properties Model. Remote Sensing, 11(10), 1236. https://doi.org/10.3390/rs11101236





