Estimation of Size-Fractionated Primary Production from Satellite Ocean Colour in UK Shelf Seas
Abstract
:1. Introduction
2. Materials and Methods
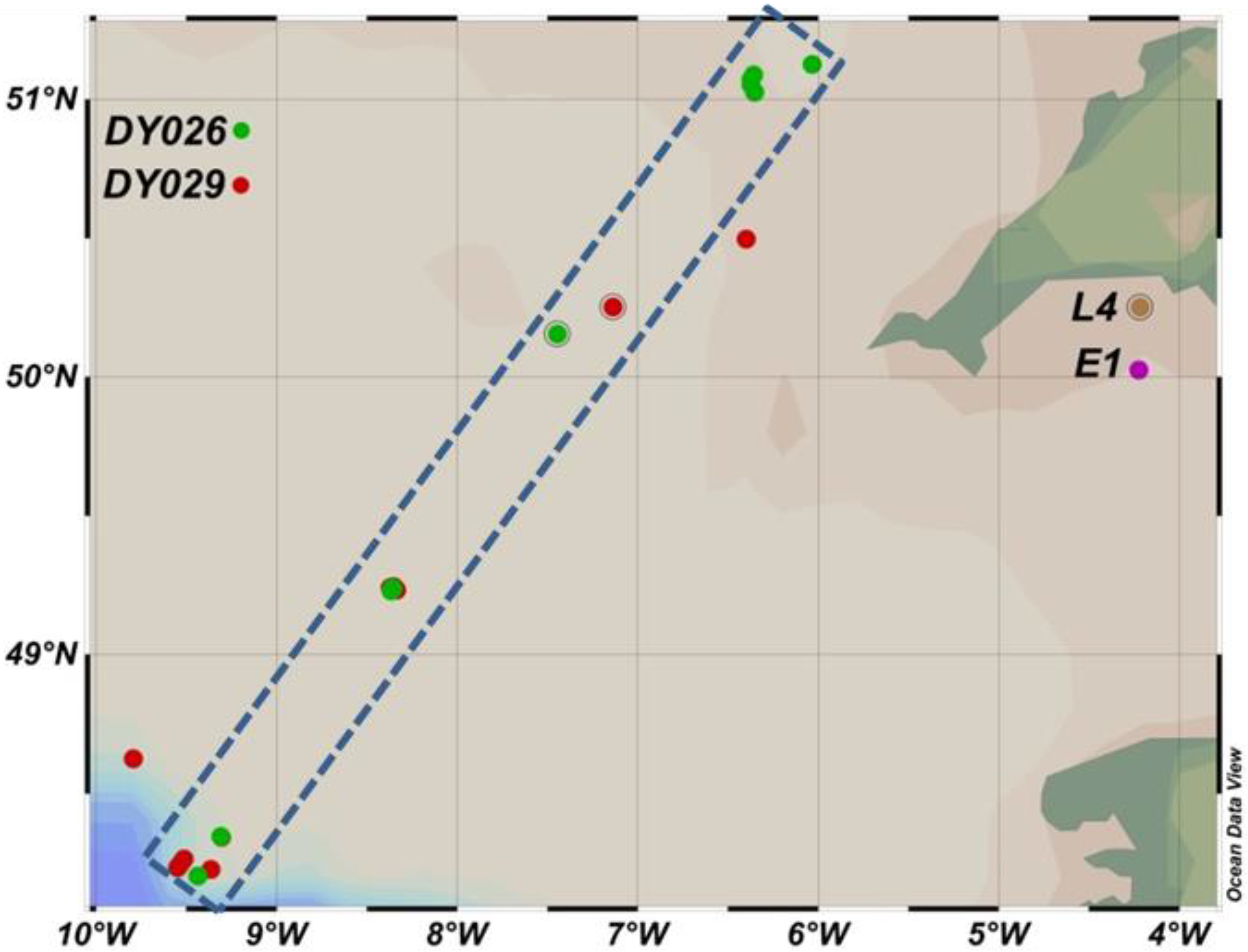
2.1. In Situ Measurements of Size Fractionated Primary Production
2.2. Satellite Model of Size Fractionated Primary Production
2.2.1. Estimation of Euphotic Depth
2.2.2. Estimation of Vertical Variability in Chlorophyll-a
2.2.3. Estimation of Photosynthetic Parameters
2.2.4. Estimation of Irradiance
2.2.5. Satellite Data
3. Results
3.1. Modelling Size-Fractionated Chlorophyll-a from Total Chlorophyll-a
3.2. Comparison of Measured and Modelled Photosynthetic Parameters
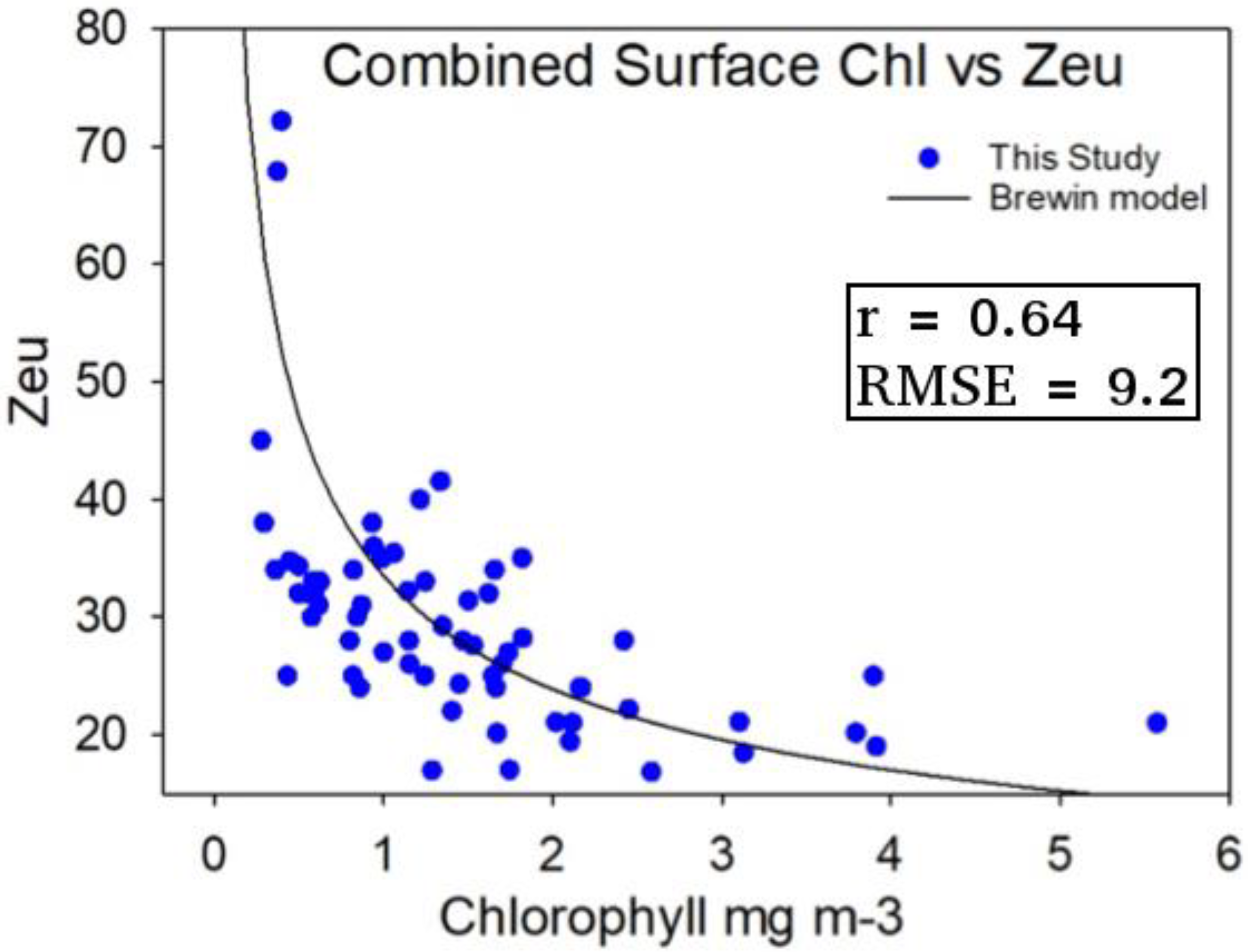
3.3. Estimation of Euphotic Depth from Surface Chlorophyll-a
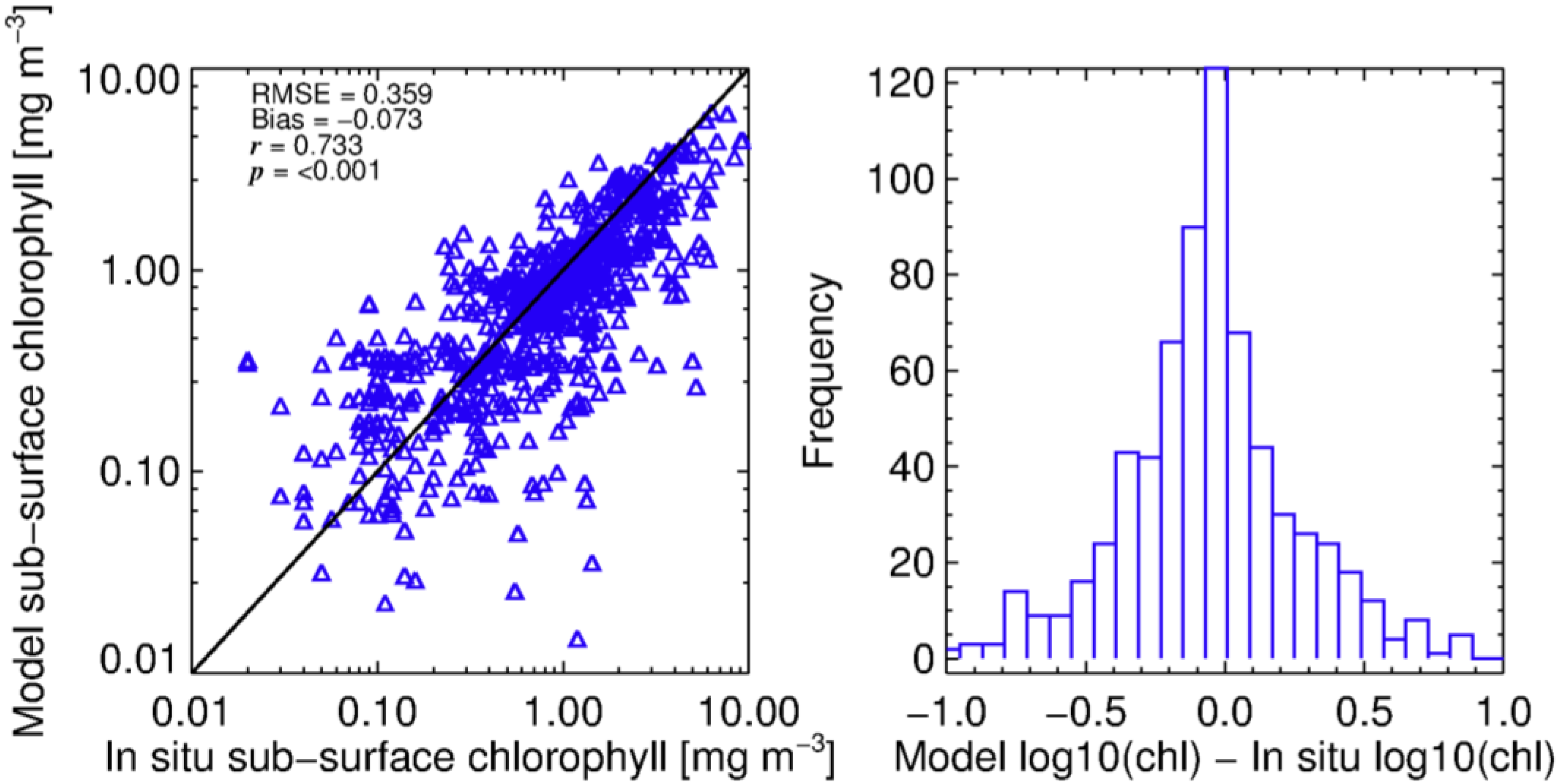
3.4. Estimation of the Vertical Distribution of Phytoplankton Biomass
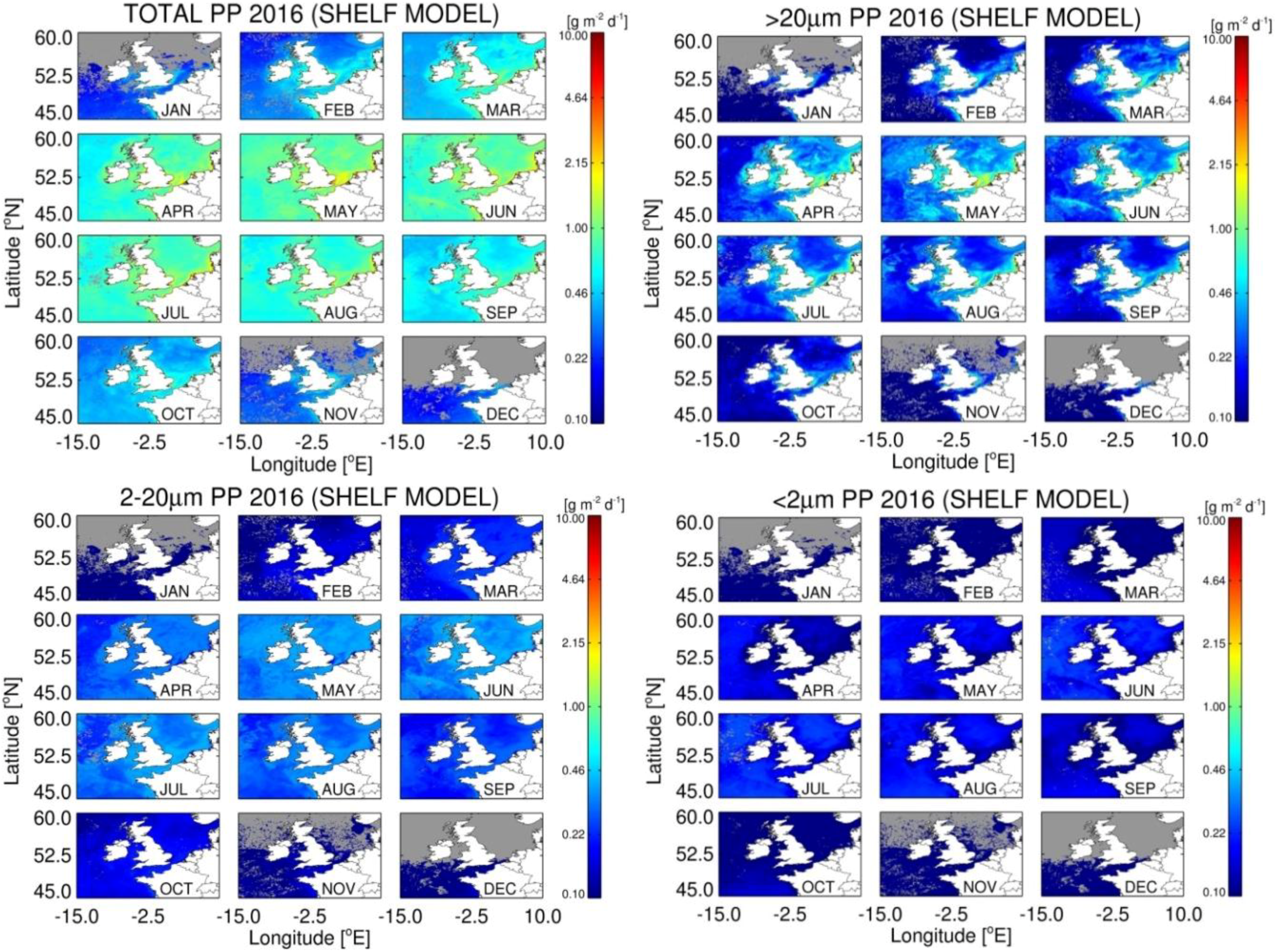
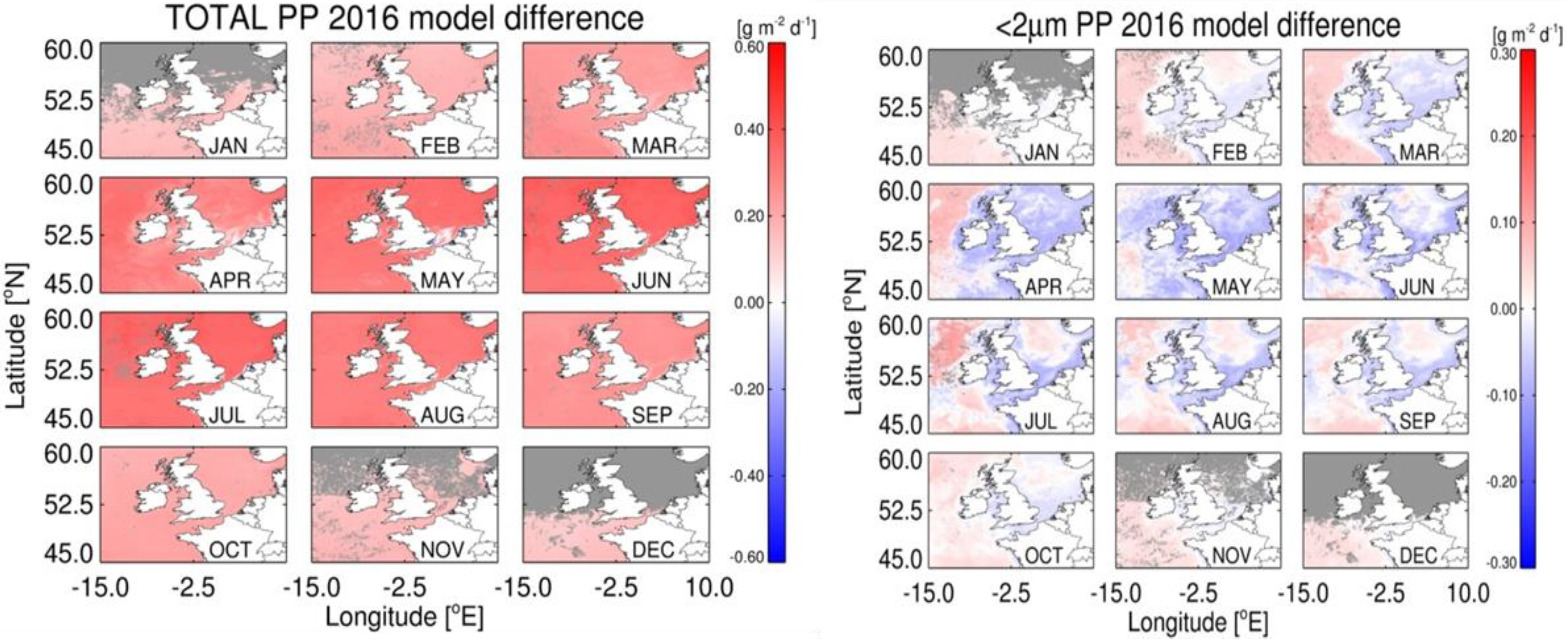
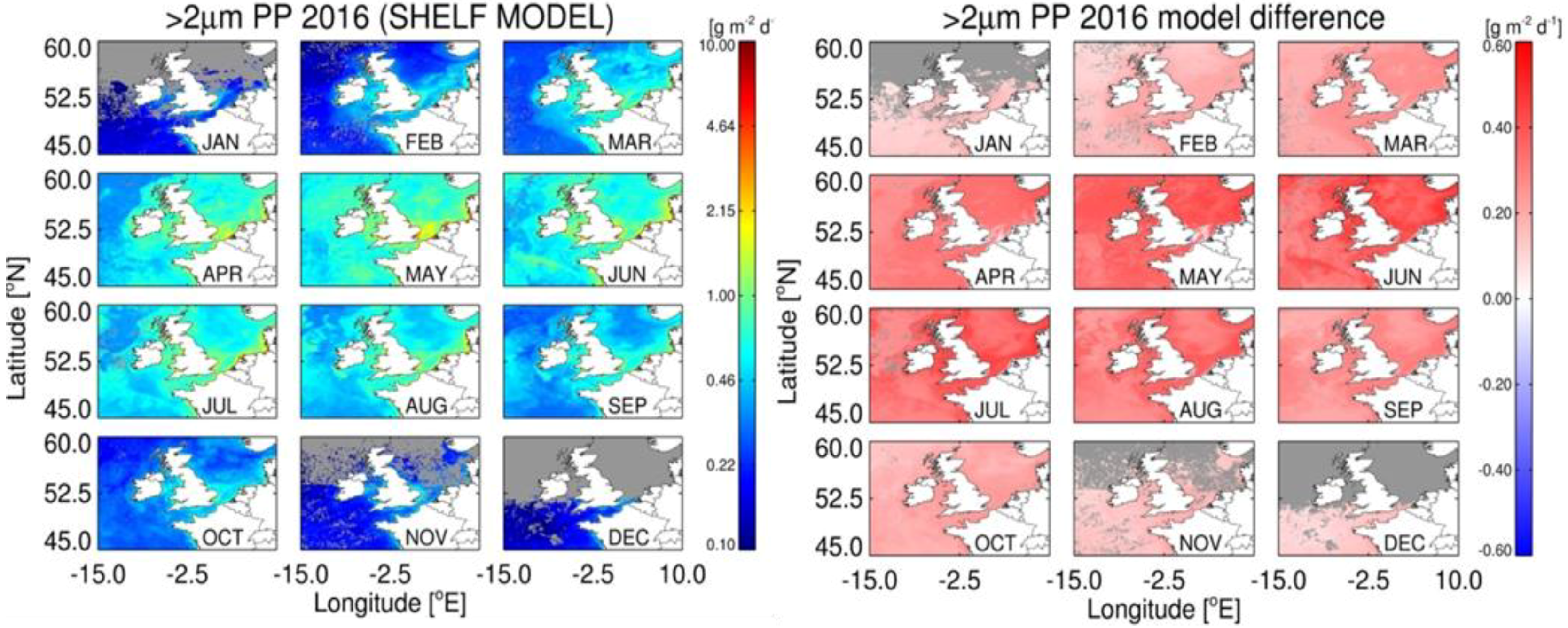
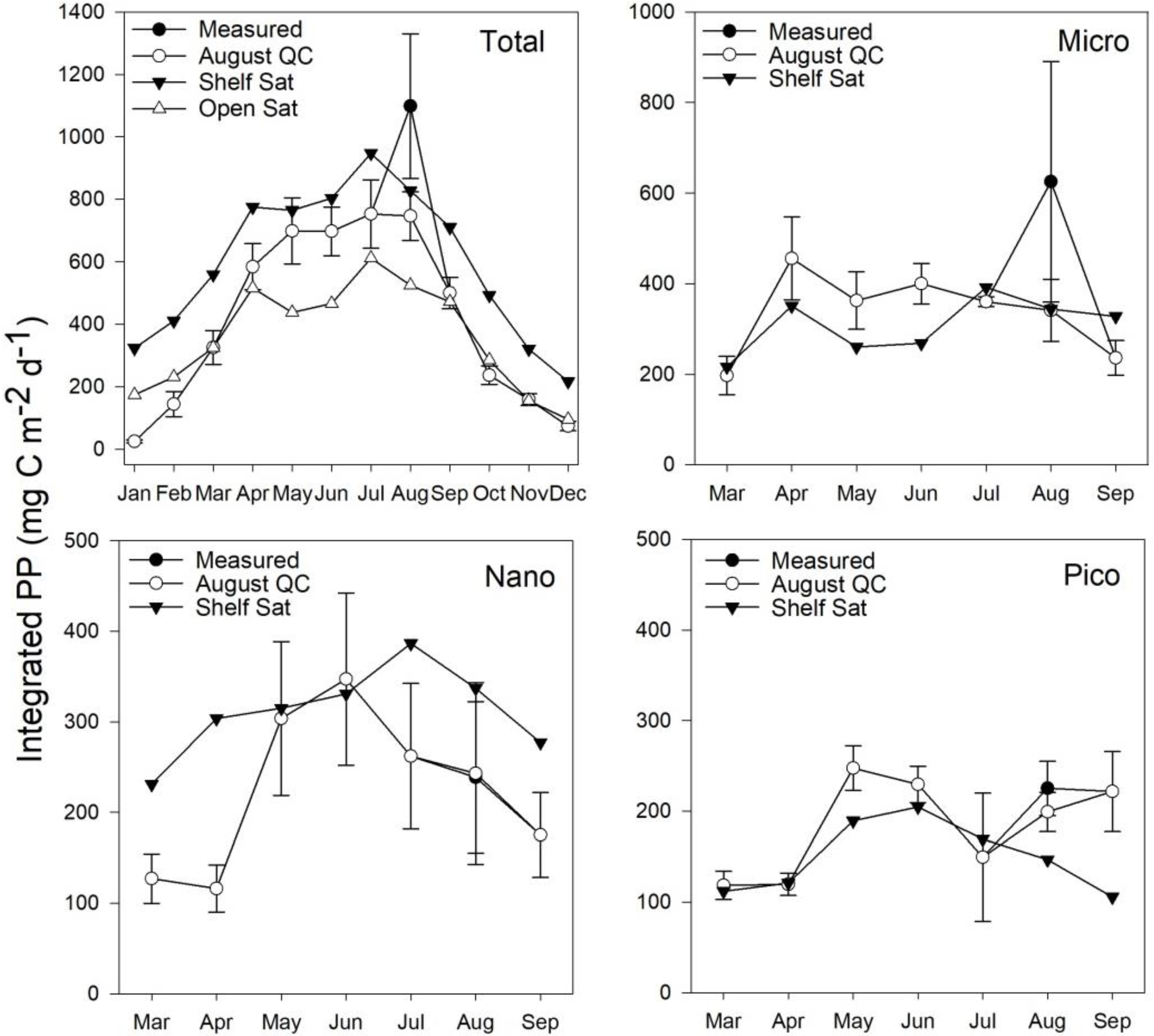
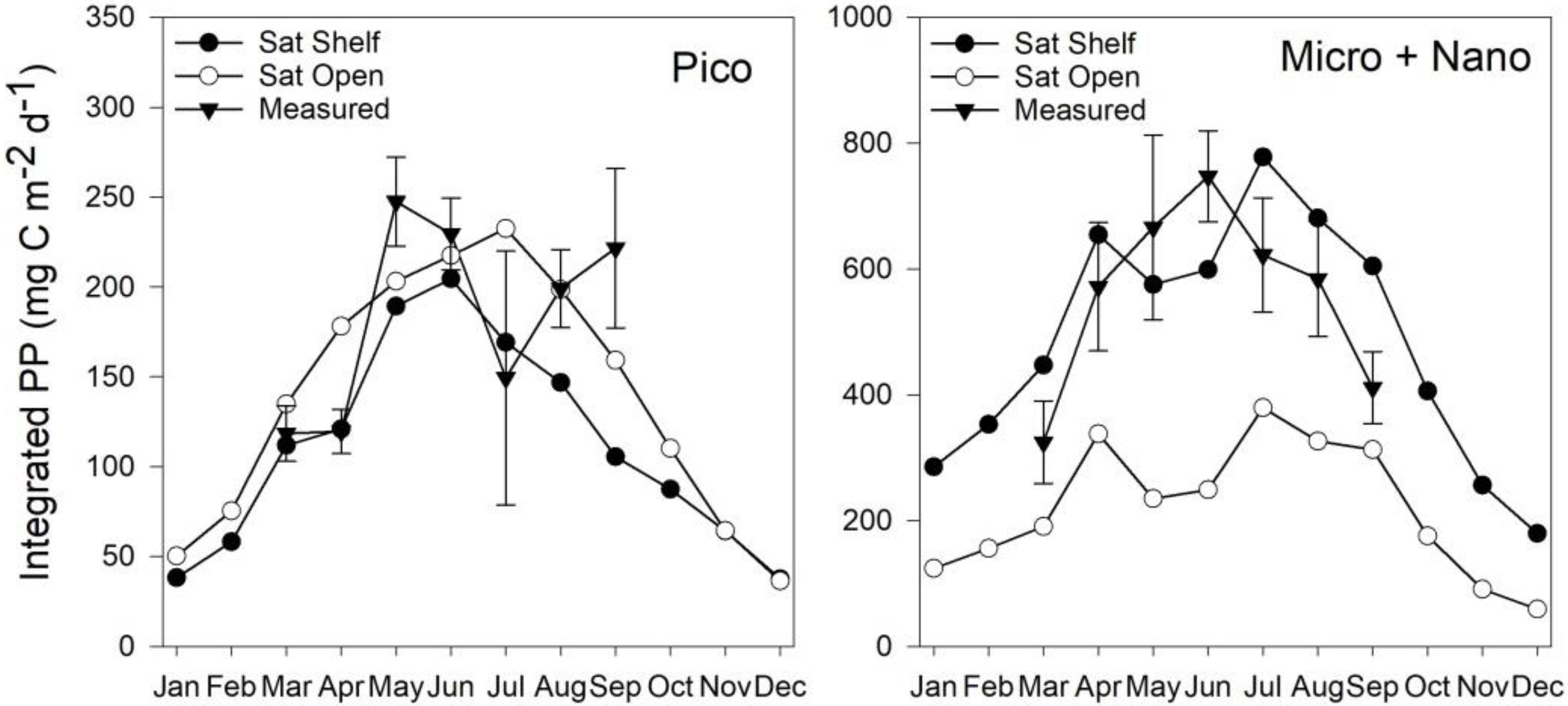
3.5. Estimates of Annual Size-Fractionated Primary Production
4. Discussion
4.1. Accuracy of Size-Fractionated Primary Production Satellite Estimates for the UK Shelf Seas
4.2. Accuracy of Size-Fractionated Photosynthetic Parameters and Biomass Distribution
4.3. Effect of Hydrographic and Optical Differences on Model Performance
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dacey, J.W.; Wakeham, S.G. Oceanic dimethylsulfide: Production during zooplankton grazing on phytoplankton. Science 1986, 233, 1314–1316. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Gruber, N.; Dunne, J.; Sarmiento, J.; Armstrong, R. Diagnosing the contribution of phytoplankton functional groups to the production and export of particulate organic carbon, CaCO3, and opal from global nutrient and alkalinity distributions. Glob. Biogeochem. Cycles 2006, 20. [Google Scholar] [CrossRef]
- Sinha, V.; Williams, J.; Meyerhöfer, M.; Riebesell, U.; Paulino, A.; Larsen, A. Air-sea fluxes of methanol, acetone, acetaldehyde, isoprene and dms from a Norwegian fjord following a phytoplankton bloom in a mesocosm experiment. Atmos. Chem. Phys. 2007, 7, 739–755. [Google Scholar] [CrossRef] [Green Version]
- Arnold, S.; Spracklen, D.; Williams, J.; Yassaa, N.; Sciare, J.; Bonsang, B.; Gros, V.; Peeken, I.; Lewis, A.; Alvain, S. Evaluation of the global oceanic isoprene source and its impacts on marine organic carbon aerosol. Atmos. Chem. Phys. 2009, 9, 1253–1262. [Google Scholar] [CrossRef] [Green Version]
- Pauly, D.; Christensen, V.; Guénette, S.; Pitcher, T.J.; Sumaila, U.R.; Walters, C.J.; Watson, R.; Zeller, D. Towards sustainability in world fisheries. Nature 2002, 418, 689–695. [Google Scholar] [CrossRef] [PubMed]
- De Haas, H.; van Weering, T.C.; de Stigter, H. Organic carbon in shelf seas: Sinks or sources, processes and products. Cont. Shelf Res. 2002, 22, 691–717. [Google Scholar] [CrossRef]
- Pace, M.; Glasser, J.; Pomeroy, L. A simulation analysis of continental shelf food webs. Mar. Biol. 1984, 82, 47–63. [Google Scholar] [CrossRef]
- Yunev, O.A.; Carstensen, J.; Moncheva, S.; Khaliulin, A.; Ærtebjerg, G.; Nixon, S. Nutrient and phytoplankton trends on the Western Black Sea shelf in response to cultural eutrophication and climate changes. Estuar. Coast. Shelf Sci. 2007, 74, 63–76. [Google Scholar] [CrossRef]
- McQuatters-Gollop, A.; Raitsos, D.E.; Edwards, M.; Pradhan, Y.; Mee, L.D.; Lavender, S.J.; Attrill, M.J. A long-term chlorophyll dataset reveals regime shift in North Sea phytoplankton biomass unconnected to nutrient levels. Limnol. Oceanogr. 2007, 52, 635–648. [Google Scholar] [CrossRef] [Green Version]
- Ridgway, J.; Shimmield, G. Estuaries as repositories of historical contamination and their impact on shelf seas. Estuar. Coast. Shelf Sci. 2002, 55, 903–928. [Google Scholar] [CrossRef]
- Gowen, R.J.; Hydes, D.; Mills, D.; Stewart, B.; Brown, J.; Gibson, C.; Shammon, T.; Allen, M.; Malcolm, S.J. Assessing trends in nutrient concentrations in coastal shelf seas: A case study in the Irish Sea. Estuar. Coast. Shelf Sci. 2002, 54, 927–939. [Google Scholar] [CrossRef]
- Arnold, H.E.; Kerrison, P.; Steinke, M. Interacting effects of ocean acidification and warming on growth and dms-production in the haptophyte coccolithophore Emiliania huxleyi. Glob. Chang. Biol. 2013, 19, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Platt, T.; Rao, D.S.; Irwin, B. Photosynthesis of picoplankton in the oligotrophic ocean. Nature 1983, 301, 702–704. [Google Scholar] [CrossRef]
- Viviani, D.A.; Björkman, K.M.; Karl, D.M.; Church, M.J. Plankton metabolism in surface waters of the tropical and subtropical Pacific Ocean. Aquat. Microb. Ecol. 2011, 62, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Montoya, J.P.; Holl, C.M.; Zehr, J.P.; Hansen, A.; Villareal, T.A.; Capone, D.G. High rates of N2 fixation by unicellular diazotrophs in the oligotrophic Pacific Ocean. Nature 2004, 430, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Zubkov, M.V.; Fuchs, B.M.; Tarran, G.A.; Burkill, P.H.; Amann, R. High rate of uptake of organic nitrogen compounds by prochlorococcus cyanobacteria as a key to their dominance in oligotrophic oceanic waters. Appl. Environ. Microbiol. 2003, 69, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Fehling, J.; Davidson, K.; Bolch, C.J.; Brand, T.D.; Narayanaswamy, B.E. The relationship between phytoplankton distribution and water column characteristics in North West European shelf sea waters. PLoS ONE 2012, 7, e34098. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.; Richardson, A.J. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 2004, 430, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.B.; Strom, S.L. Phytoplankton growth, microzooplankton herbivory and community structure in the southeast Bering Sea: Insight into the formation and temporal persistence of an Emiliania huxleyi bloom. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 5969–5990. [Google Scholar] [CrossRef]
- Siemering, B.; Bresnan, E.; Painter, S.C.; Daniels, C.J.; Inall, M.; Davidson, K. Phytoplankton distribution in relation to environmental drivers on the north west european shelf sea. PLoS ONE 2016, 11, e0164482. [Google Scholar] [CrossRef] [PubMed]
- Holligan, P. Biological implications of fronts on the northwest European continental shelf. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 1981, 302, 547–562. [Google Scholar] [CrossRef]
- Aumont, O.; Maier-Reimer, E.; Blain, S.; Monfray, P. An ecosystem model of the global ocean including Fe, Si, P colimitations. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.K.; Doney, S.C.; Kleypas, J.A.; Glover, D.M.; Fung, I.Y. An intermediate complexity marine ecosystem model for the global domain. Deep Sea Res. Part II Top. Stud. Oceanogr. 2001, 49, 403–462. [Google Scholar] [CrossRef] [Green Version]
- Moloney, C.L.; Field, J.G. The size-based dynamics of plankton food webs. I. A simulation model of carbon and nitrogen flows. J. Plankton Res. 1991, 13, 1003–1038. [Google Scholar] [CrossRef]
- Van den Berg, A.; Ridderinkhof, H.; Riegman, R.; Ruardij, P.; Lenhart, H. Influence of variability in water transport on phytoplankton biomass and composition in the southern North Sea: A modelling approach (fyfy). Cont. Shelf Res. 1996, 16, 907–931. [Google Scholar] [CrossRef]
- Baretta-Bekker, J.; Baretta, J.; Ebenhöh, W. Microbial dynamics in the marine ecosystem model ersem ii with decoupled carbon assimilation and nutrient uptake. J. Sea Res. 1997, 38, 195–211. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Beardall, J.; Flynn, K.J.; Quigg, A.; Rees, T.A.V.; Raven, J.A. Phytoplankton in a changing world: Cell size and elemental stoichiometry. J. Plankton Res. 2009, 32, 119–137. [Google Scholar] [CrossRef]
- Poulton, A.J.; Davis, C.E.; Daniels, C.J.; Mayers, K.M.J.; Harris, C.; Tarran, G.A.; Widdicombe, C.E.; Woodward, E.M.S. Seasonal phosphorus and carbon dynamics in a temperate shelf sea (Celtic Sea). Prog. Oceanogr. 2017. [Google Scholar] [CrossRef] [Green Version]
- Arrigo, K.R. Marine microorganisms and global nutrient cycles. Nature 2004, 437, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Hessen, D.O.; Færøvig, P.J.; Andersen, T. Light, nutrients, andP:C ratios in algae: Grazer performance related to food quality and quantity. Ecology 2002, 83, 1886–1898. [Google Scholar] [CrossRef]
- Key, T.; McCarthy, A.; Campbell, D.A.; Six, C.; Roy, S.; Finkel, Z.V. Cell size trade-offs govern light exploitation strategies in marine phytoplankton. Environ. Microbiol. 2010, 12, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Cermeno, P.; Estévez-Blanco, P.; Maranón, E.; Fernóndez, E. Maximum photosynthetic efficiency of size-fractionated phytoplankton assessed by 14C uptake and fast repetition rate fluorometry. Limnol. Oceanogr. 2005, 50, 1438–1446. [Google Scholar] [CrossRef]
- Cermeño, P.; Marañón, E.; Rodríguez, J.; Fernández, E. Large-sized phytoplankton sustain higher carbon-specific photosynthesis than smaller cells in a coastal eutrophic ecosystem. Mar. Ecol. Prog. Ser. 2005, 297, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Maraóón, E.; Cermeóo, P.; Rodríguez, J.; Zubkov, M.V.; Harris, R.P. Scaling of phytoplankton photosynthesis and cell size in the ocean. Limnol. Oceanogr. 2007, 52, 2190–2198. [Google Scholar] [CrossRef] [Green Version]
- Uitz, J.; Huot, Y.; Bruyant, F.; Babin, M.; Claustre, H. Relating phytoplankton photophysiological properties to community structure on large scales. Limnol. Oceanogr. 2008, 53, 614–630. [Google Scholar] [Green Version]
- Marañón, E.; Cermeño, P.; López-Sandoval, D.C.; Rodríguez-Ramos, T.; Sobrino, C.; Huete-Ortega, M.; Blanco, J.M.; Rodríguez, J. Unimodal size scaling of phytoplankton growth and the size dependence of nutrient uptake and use. Ecol. Lett. 2013, 16, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Joint, I.; Owens, N.; Pomroy, A.; Pomeroy, A. Seasonal production of photosynthetic picoplankton and nanoplankton in the Celtic Sea. Mar. Ecol. Prog. Ser. 1986, 251–258. [Google Scholar] [CrossRef]
- Stuart, V.; Sathyendranath, S.; Head, E.; Platt, T.; Irwin, B.; Maass, H. Bio-optical characteristics of diatom and prymnesiophyte populations in the Labrador Sea. Mar. Ecol. Prog. Ser. 2000, 201, 91–106. [Google Scholar] [CrossRef] [Green Version]
- Morán, X.A.G.; Scharek, R. Photosynthetic parameters and primary production, with focus on large phytoplankton, in a temperate mid-shelf ecosystem. Estuar. Coast. Shelf Sci. 2015, 154, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Joint, I.; Pomroy, A. Photosynthetic characteristics of nanoplankton and picoplankton from the surface mixed layer. Mar. Biol. 1986, 92, 465–474. [Google Scholar] [CrossRef]
- Morán, X.A.G. Annual cycle of picophytoplankton photosynthesis and growth rates in a temperate coastal ecosystem: A major contribution to carbon fluxes. Aquat. Microb. Ecol. 2007, 49, 267–279. [Google Scholar] [CrossRef]
- Chen, Y.-L.L. Comparisons of primary productivity and phytoplankton size structure in the marginal regions of southern East China Sea. Cont. Shelf Res. 2000, 20, 437–458. [Google Scholar] [CrossRef]
- Marañón, E.; Holligan, P.M.; Barciela, R.; González, N.; Mouriño, B.; Pazó, M.J.; Varela, M. Patterns of phytoplankton size structure and productivity in contrasting open-ocean environments. Mar. Ecol. Prog. Ser. 2001, 216, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Bricaud, A.; Claustre, H.; Ras, J.; Oubelkheir, K. Natural variability of phytoplanktonic absorption in oceanic waters: Influence of the size structure of algal populations. J. Geophys. Res. Ocean. 2004, 109. [Google Scholar] [CrossRef] [Green Version]
- Kulk, G.; de Vries, P.; van de Poll, W.H.; Visser, R.J.; Buma, A.G. Temperature-dependent growth and photophysiology of prokaryotic and eukaryotic oceanic picophytoplankton. Mar. Ecol. Prog. Ser. 2012, 466, 43–55. [Google Scholar] [CrossRef] [Green Version]
- Mino, Y.; Matsumura, S.; Lirdwitayaprasit, T.; Fujiki, T.; Yanagi, T.; Saino, T. Variations in phytoplankton photo-physiology and productivity in a dynamic eutrophic ecosystem: A fast repetition rate fluorometer-based study. J. Plankton Res. 2013, 36, 398–411. [Google Scholar] [CrossRef]
- Moore, C.M.; Suggett, D.J.; Hickman, A.E.; Kim, Y.-N.; Tweddle, J.F.; Sharples, J.; Geider, R.J.; Holligan, P.M. Phytoplankton photoacclimation and photoadaptation in response to environmental gradients in a shelf sea. Limnol. Oceanogr. 2006, 51, 936–949. [Google Scholar] [CrossRef] [Green Version]
- Banse, K. Rates of growth, respiration and photosynthesis of unicellular algae as related to cell size—A review. J. Phycol. 1976, 12, 135–140. [Google Scholar]
- Hirata, T.; Hardman-Mountford, N.J.; Barlow, R.; Lamont, T.; Brewin, R.; Smyth, T.; Aiken, J. An inherent optical property approach to the estimation of size-specific photosynthetic rates in eastern boundary upwelling zones from satellite ocean colour: An initial assessment. Prog. Oceanogr. 2009, 83, 393–397. [Google Scholar] [CrossRef]
- Uitz, J.; Claustre, H.; Gentili, B.; Stramski, D. Phytoplankton class-specific primary production in the world’s oceans: Seasonal and interannual variability from satellite observations. Glob. Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef]
- Blondeau-Patissier, D.; Gower, J.F.R.; Dekker, A.G.; Phinn, S.R.; Brando, V.E. A review of ocean color remote sensing methods and statistical techniques for the detection, mapping and analysis of phytoplankton blooms in coastal and open oceans. Prog. Oceanogr. 2014, 123, 123–144. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.; Sathyendranath, S.; Platt, T.; Morales, J.; Stuart, V.; Forget, M.-H.; Devred, E.; Bouman, H. Remote sensing of phytoplankton functional types. Remote. Sens. Environ. 2008, 112, 3366–3375. [Google Scholar] [CrossRef]
- Alvain, S.; Moulin, C.; Dandonneau, Y.; Bréon, F.-M. Remote sensing of phytoplankton groups in case 1 waters from global SeaWiFS imagery. Deep Sea Res. Part I Oceanogr. Res. Pap. 2005, 52, 1989–2004. [Google Scholar] [CrossRef] [Green Version]
- Astoreca, R.; Rousseau, V.; Ruddick, K.; Knechciak, C.; Van Mol, B.; Parent, J.-Y.; Lancelot, C. Development and application of an algorithm for detecting phaeocystis globosa blooms in the case 2 Southern North Sea waters. J. Plankton Res. 2008, 31, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Westberry, T.; Siegel, D.; Subramaniam, A. An improved bio-optical model for the remote sensing of trichodesmium spp. Blooms. J. Geophys. Res. Ocean. 2005, 110. [Google Scholar] [CrossRef]
- Subramaniam, A.; Brown, C.; Hood, R.R.; Carpenter, E.; Capone, D. Detecting Trichodesmium blooms in SeaWiFS imagery. Deep Sea Res. Part II Top. Stud. Oceanogr. 2001, 49, 107–121. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Watts, L.; Devred, E.; Platt, T.; Caverhill, C.; Maass, H. Discrimination of diatoms from other phytoplankton using ocean-colour data. Mar. Ecol. Prog. Ser. 2004, 272, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Iglesias-Rodríguez, M.D.; Brown, C.W.; Doney, S.C.; Kleypas, J.; Kolber, D.; Kolber, Z.; Hayes, P.K.; Falkowski, P.G. Representing key phytoplankton functional groups in ocean carbon cycle models: Coccolithophorids. Glob. Biogeochem. Cycles 2002, 16, 47-1–47-20. [Google Scholar] [CrossRef]
- Smyth, T.J.; Moore, G.F.; Groom, S.B.; Land, P.E.; Tyrrell, T. Optical modeling and measurements of a coccolithophore bloom. Appl. Opt. 2002, 41, 7679–7688. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.A.; Minnett, P.J.; Fleming, L.E.; Banzon, V.F.; Baringer, W. Satellite remote sensing of harmful algal blooms: A new multi-algorithm method for detecting the Florida Red Tide (Karenia brevis). Harmful Algae 2010, 9, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Siswanto, E.; Ishizaka, J.; Tripathy, S.C.; Miyamura, K. Detection of harmful algal blooms of Karenia mikimotoi using modis measurements: A case study of Seto-Inland Sea, Japan. Remote Sens. Environ. 2013, 129, 185–196. [Google Scholar] [CrossRef]
- Lou, X.; Hu, C. Diurnal changes of a harmful algal bloom in the East China Sea: Observations from goci. Remote Sens. Environ. 2014, 140, 562–572. [Google Scholar] [CrossRef]
- Saba, V.; Friedrichs, M.A.; Antoine, D.; Armstrong, R.; Asanuma, I.; Behrenfeld, M.; Ciotti, A.; Dowell, M.; Hoepffner, N.; Hyde, K.; et al. An evaluation of ocean color model estimates of marine primary productivity in coastal and pelagic regions across the globe. Biogeosciences 2011, 8, 489–503. [Google Scholar] [CrossRef] [Green Version]
- Kutser, T. Passive optical remote sensing of cyanobacteria and other intense phytoplankton blooms in coastal and inland waters. Int. J. Remote Sens. 2009, 30, 4401–4425. [Google Scholar] [CrossRef]
- Hunter, P.D.; Tyler, A.N.; Présing, M.; Kovács, A.W.; Preston, T. Spectral discrimination of phytoplankton colour groups: The effect of suspended particulate matter and sensor spectral resolution. Remote Sens. Environ. 2008, 112, 1527–1544. [Google Scholar] [CrossRef]
- Bouman, H.A.; Platt, T.; Sathyendranath, S.; Li, W.K.; Stuart, V.; Fuentes-Yaco, C.; Maass, H.; Horne, E.P.; Ulloa, O.; Lutz, V.; et al. Temperature as indicator of optical properties and community structure of marine phytoplankton: Implications for remote sensing. Mar. Ecol. Prog. Ser. 2003, 258, 19–30. [Google Scholar] [CrossRef]
- Bouman, H.; Platt, T.; Sathyendranath, S.; Stuart, V. Dependence of light-saturated photosynthesis on temperature and community structure. Deep Sea Res. Part I Oceanogr. Res. Pap. 2005, 52, 1284–1299. [Google Scholar] [CrossRef]
- Brewin, R.J.W.; Tilstone, G.H.; Jackson, T.; Cain, T.; Miller, P.I.; Lange, P.K.; Misra, A.; Airs, R.L. Modelling size-fractionated primary production in the Atlantic Ocean from remote sensing. Prog. Oceanogr. 2017, 158, 130–149. [Google Scholar] [CrossRef]
- Brewin, R.J.W.; Ciavatta, S.; Sathyendranath, S.; Jackson, T.; Tilstone, G.; Curran, K.; Airs, R.L.; Cummings, D.; Brotas, V.; Organelli, E.; et al. Uncertainty in ocean-color estimates of chlorophyll for phytoplankton groups. Front. Mar. Sci. 2017, 4, 104. [Google Scholar] [CrossRef]
- Tilstone, G.H.; Figueiras, F.; Lorenzo, L.M.; Arbones, B. Phytoplankton composition, photosynthesis and primary production during different hydrographic conditions at the Northwest Iberian upwelling system. Mar. Ecol. Prog. Ser. 2003, 252, 89–104. [Google Scholar] [CrossRef] [Green Version]
- Platt, T.; Gallegos, C.; Harrison, W. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Morel, A.; Huot, Y.; Gentili, B.; Werdell, P.J.; Hooker, S.B.; Franz, B.A. Examining the consistency of products derived from various ocean color sensors in open ocean (Case 1) waters in the perspective of a multi-sensor approach. Remote Sens. Environ. 2007, 111, 69–88. [Google Scholar] [CrossRef]
- Hickman, A.E.; Holligan, P.M.; Moore, C.M.; Sharples, J.; Krivtsov, V.; Palmer, M.R. Distribution and chromatic adaptation of phytoplankton within a shelf sea thermocline. Limnol. Oceanogr. 2009, 54, 525–536. [Google Scholar] [CrossRef] [Green Version]
- Hickman, A.E.; Moore, C.; Sharples, J.; Lucas, M.I.; Tilstone, G.H.; Krivtsov, V.; Holligan, P.M. Primary production and nitrate uptake within the seasonal thermocline of a stratified shelf sea. Mar. Ecol. Prog. Ser. 2012, 463, 39–57. [Google Scholar] [CrossRef] [Green Version]
- Pope, R.M.; Fry, E.S. Absorption spectrum (380–700 nm) of pure water. II. Integrating cavity measurements. Appl. Opt. 1997, 36, 8710–8723. [Google Scholar] [CrossRef] [PubMed]
- Sathyendranath, S.; Brewin, R.J.W.; Jackson, T.; Mélin, F.; Platt, T. Ocean-colour products for climate-change studies: What are their ideal characteristics? Remote Sens. Environ. 2017, 203, 125–138. [Google Scholar] [CrossRef]
- Jackson, T.; Sathyendranath, S.; Mélin, F. An improved optical classification scheme for the Ocean Colour Essential Climate Variable and its applications. Remote Sens. Environ. 2017, 203, 152–161. [Google Scholar] [CrossRef]
- De Boyer Montégut, C.; Madec, G.; Fischer, A.S.; Lazar, A.; Iudicone, D. Mixed layer depth over the global ocean: An examination of profile data and a profile-based climatology. J. Geophys. Res. Ocean. 2004, 109. [Google Scholar] [CrossRef] [Green Version]
- Sathyendranath, S.; Cota, G.; Stuart, V.; Maass, H.; Platt, T. Remote sensing of phytoplankton pigments: A comparison of empirical and theoretical approaches. Int. J. Remote. Sens. 2001, 22, 249–273. [Google Scholar] [CrossRef]
- Widdicombe, C.E.; Eloire, D.; Harbour, D.; Harris, R.P.; Somerfield, P.J. Long-term phytoplankton community dynamics in the western english channel. J. Plankton Res. 2010, 32, 643–655. [Google Scholar] [CrossRef]
- Wiltshire, K.H.; Malzahn, A.M.; Wirtz, K.; Greve, W.; Janisch, S.; Mangelsdorf, P.; Manly, B.F.; Boersma, M. Resilience of North Sea phytoplankton spring bloom dynamics: An analysis of long-term data at Helgoland roads. Limnol. Oceanogr. 2008, 53, 1294–1302. [Google Scholar] [CrossRef]
- De Madariaga, I.; Joint, I. Photosynthesis and carbon metabolism by size-fractionated phytoplankton in the southern North Sea in early summer. Cont. Shelf Res. 1994, 14, 295–311. [Google Scholar] [CrossRef]
- Barnes, M.K.; Tilstone, G.H.; Suggett, D.J.; Widdicombe, C.E.; Bruun, J.; Martinez-Vicente, V.; Smyth, T.J. Temporal variability in total, micro-and nano-phytoplankton primary production at a coastal site in the western English Channel. Prog. Oceanogr. 2015, 137, 470–483. [Google Scholar] [CrossRef]
- Segura, V.; Lutz, V.A.; Dogliotti, A.; Silva, R.I.; Negri, R.M.; Akselman, R.; Benavides, H. Phytoplankton types and primary production in the Argentine Sea. Mar. Ecol. Prog. Ser. 2013, 491, 15–31. [Google Scholar] [CrossRef]
- Pemberton, K.; Rees, A.P.; Miller, P.I.; Raine, R.; Joint, I. The influence of water body characteristics on phytoplankton diversity and production in the Celtic Sea. Cont. Shelf Res. 2004, 24, 2011–2028. [Google Scholar] [CrossRef]
- Birchill, A.J.; Milne, A.; Woodward, E.M.S.; Harris, C.; Annett, A.; Rusiecka, D.; Achterberg, E.P.; Gledhill, M.; Ussher, S.J.; Worsfold, P.J.; et al. Seasonal iron depletion in temperate shelf seas. Geophys. Res. Lett. 2017, 44, 8987–8996. [Google Scholar] [CrossRef] [Green Version]
- Holligan, P.; Groom, S. Phytoplankton distributions along the shelf break. Proc. R. Soc. Edinb. Sect. B Biol. Sci. 1986, 88, 239–263. [Google Scholar] [CrossRef]
- Martin, A.P.; Richards, K.J. Mechanisms for vertical nutrient transport within a North Atlantic mesoscale eddy. Deep Sea Res. Part II Top. Stud. Oceanogr. 2001, 48, 757–773. [Google Scholar] [CrossRef]
- Williams, R.G.; Follows, M.J. The Ekman transfer of nutrients and maintenance of new production over the North Atlantic. Deep Sea Res. Part I Oceanogr. Res. Pap. 1998, 45, 461–489. [Google Scholar] [CrossRef]
- McGillicuddy, D.; Anderson, L.; Doney, S.; Maltrud, M. Eddy-driven sources and sinks of nutrients in the upper ocean: Results from a 0.1 resolution model of the North Atlantic. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef]
- Palmer, M.R.; Stephenson, G.R.; Inall, M.E.; Balfour, C.; Düsterhus, A.; Green, J.A.M. Turbulence and mixing by internal waves in the Celtic Sea determined from ocean glider microstructure measurements. J. Mar. Syst. 2015, 144, 57–69. [Google Scholar] [CrossRef] [Green Version]
- Palmer, M.; Green, M.; Inall, M.; Hopkins, J. Internal tidal bores and turbulent mixing at the Celtic Sea shelf break. In Proceedings of the AGU Fall Meeting Abstracts, San Francisco, CA, USA, 3–7 December 2012. [Google Scholar]
- Sharples, J.; Tett, P. Modelling the effect of physical variability on the midwater chlorophyll maximum. J. Mar. Res. 1994, 52, 219–238. [Google Scholar] [CrossRef]
- Tweddle, J.F.; Sharples, J.; Palmer, M.R.; Davidson, K.; McNeill, S. Enhanced nutrient fluxes at the shelf sea seasonal thermocline caused by stratified flow over a bank. Prog. Oceanogr. 2013, 117, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Green, J.M.; Simpson, J.H.; Legg, S.; Palmer, M.R. Internal waves, baroclinic energy fluxes and mixing at the European shelf edge. Cont. Shelf Res. 2008, 28, 937–950. [Google Scholar] [CrossRef]
- Pingree, R.D.; Mardell, G.T.; Holligan, P.M.; Griffiths, D.K.; Smithers, J. Celtic Sea and Armorican current structure and the vertical distributions of temperature and chlorophyll. Cont. Shelf Res. 1982, 1, 99–116. [Google Scholar] [CrossRef]
- Pérez, V.; Fernández, E.; Marañón, E.; Morán, X.A.G.; Zubkov, M.V. Vertical distribution of phytoplankton biomass, production and growth in the Atlantic subtropical gyres. Deep Sea Res. I 2006, 53, 1616–1634. [Google Scholar]
- Reul, A.; Rodríguez, J.; Blanco, J.; Rees, A.; Burkill, P. Control of microplankton size structure in contrasting water columns of the Celtic Sea. J. Plankton Res. 2006, 28, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Schulien, J.A.; Behrenfeld, M.J.; Hair, J.W.; Hostetler, C.A.; Twardowski, M.S. Vertically-resolved phytoplankton carbon and net primary production from a high spectral resolution lidar. Opt. Express 2017, 25, 13577–13587. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Behrenfeld, M.; Hostetler, C.; Pelon, J.; Trepte, C.; Hair, J.; Slade, W.; Cetinic, I.; Vaughan, M.; Lu, X. Ocean lidar measurements of beam attenuation and a roadmap to accurate phytoplankton biomass estimates. In Proceedings of the EPJ Web of Conferences, Thessaloniki, Greece, 29 August–3 September 2016. [Google Scholar]
- Hostetler, C.A.; Behrenfeld, M.J.; Hu, Y.; Hair, J.W.; Schulien, J.A. Spaceborne lidar in the study of marine systems. Annu. Rev. Mar. Sci. 2017, 10, 121–147. [Google Scholar] [CrossRef] [PubMed]
- Tilstone, G.H.; Smyth, T.J.; Gowen, R.J.; Martinez-Vicente, V.; Groom, S.B. Inherent optical properties of the Irish Sea and their effect on satellite primary production algorithms. J. Plankton Res. 2005, 27, 1–22. [Google Scholar] [CrossRef]
- Siegel, D.; Maritorena, S.; Nelson, N.; Behrenfeld, M.; McClain, C. Colored dissolved organic matter and its influence on the satellite-based characterization of the ocean biosphere. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef] [Green Version]
- Nelson, N.B.; Siegel, D.A. The global distribution and dynamics of chromophoric dissolved organic matter. Annu. Rev. Mar. Sci. 2013, 5, 447–476. [Google Scholar] [CrossRef] [PubMed]
- Kratzer, S.; Bowers, D.; Tett, P. Seasonal changes in colour ratios and optically active constituents in the optical Case-2 waters of the Menai Strait, North Wales. Int. J. Remote Sens. 2000, 21, 2225–2246. [Google Scholar] [CrossRef]
- International Ocean-Colour Coordinating Group (IOCCG). Remote Sensing of Ocean Colour in Coastal and Other Optically-Complex Waters; IOCCG Report; IOCCG: Dartmouth, Germany, 2000; 137p. [Google Scholar]
- Groom, S.; Martinez-Vicente, V.; Fishwick, J.; Tilstone, G.; Moore, G.; Smyth, T.; Harbour, D. The western English Channel observatory: Optical characteristics of station L4. J. Mar. Syst. 2009, 77, 278–295. [Google Scholar] [CrossRef]
- Astoreca, R.; Rousseau, V.; Lancelot, C. Coloured dissolved organic matter (CDOM) in Southern North Sea waters: Optical characterization and possible origin. Estuar. Coast. Shelf Sci. 2009, 85, 633–640. [Google Scholar] [CrossRef]


| Parameter | Size Class | UK Shelf Model | Atlantic Model | Shelf Values % of Atlantic Values |
|---|---|---|---|---|
| Micro | 4.27 (±0.25) | 6.05 (±0.98) | 70.6 | |
| Nano | 4.43 (±0.28) | 5.13 (±0.94) | 86.4 | |
| Pico | 5.51 (±0.30) | 3.46 (±0.80) | 159.2 | |
| Micro | 0.050 (±0.004) | 0.016 (±0.004) | 312.5 | |
| Nano | 0.050 (±0.004) | 0.014 (±0.003) | 357.1 | |
| Pico | 0.062 (±0.004) | 0.011 (±0.001) | 563.6 | |
| Micro | 0.49 (±0.09) | 0.35 (±0.27) | 140.0 | |
| Nano | 0.38 (±0.09) | 0.59 (±0.29) | 64.4 | |
| Pico | 0.45 (±0.09) | 0.68 (±0.31) | 66.2 | |
| Micro | 0.51 (±0.12) | −0.07 (±0.30) | −728.6 | |
| Nano | 0.25 (±0.12) | −0.12 (±0.23) | −208.3 | |
| Pico | 0.30 (±0.10) | −0.32 (±0.17) | −93.75 |
| Modelled PP | r2 | Slope | Intercept | |||
|---|---|---|---|---|---|---|
| Shelf | Open | Shelf | Open | Shelf | Open | |
| Total shelf, n = 15 | 0.78 | 0.75 | 0.59 | 0.43 | 321 | 158 |
| Micro, Nano shelf, n = 10 | 0.56 | 0.39 | 0.54 | 0.33 | 309 | 101 |
| Pico shelf, n = 10 | 0.32 | 0.00 | 0.24 | -0.01 | 104 | 188 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curran, K.; Brewin, R.J.W.; Tilstone, G.H.; Bouman, H.A.; Hickman, A. Estimation of Size-Fractionated Primary Production from Satellite Ocean Colour in UK Shelf Seas. Remote Sens. 2018, 10, 1389. https://doi.org/10.3390/rs10091389
Curran K, Brewin RJW, Tilstone GH, Bouman HA, Hickman A. Estimation of Size-Fractionated Primary Production from Satellite Ocean Colour in UK Shelf Seas. Remote Sensing. 2018; 10(9):1389. https://doi.org/10.3390/rs10091389
Chicago/Turabian StyleCurran, Kieran, Robert J. W. Brewin, Gavin H. Tilstone, Heather A. Bouman, and Anna Hickman. 2018. "Estimation of Size-Fractionated Primary Production from Satellite Ocean Colour in UK Shelf Seas" Remote Sensing 10, no. 9: 1389. https://doi.org/10.3390/rs10091389
APA StyleCurran, K., Brewin, R. J. W., Tilstone, G. H., Bouman, H. A., & Hickman, A. (2018). Estimation of Size-Fractionated Primary Production from Satellite Ocean Colour in UK Shelf Seas. Remote Sensing, 10(9), 1389. https://doi.org/10.3390/rs10091389









