The Impact of Tourist Traffic on the Condition and Cell Structures of Alpine Swards
Abstract
:1. Introduction
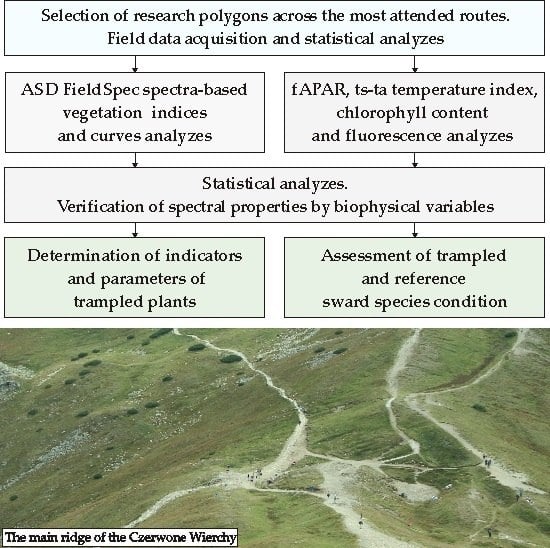
2. Study Area and Research Objects
3. Materials and Methods
- Hyperspectral properties of plants using an ASD FieldSpec 3/4 spectrometer fitted with a contact probe (ASD PlantProbe), operating in the range 350–2500 nm (ASD Inc., Longmont, CO, USA). The ASD PlantProbe uses an artificial light and closed chamber for data acquisition at the leaf-level, offering comparable results for all measurements within test areas during the field campaigns. The instrument was configured by taking 25 measurements of the Dark Current (DC), then 25 of a White Reference spectralon reflectance target (SG 33151 Zenith Lite) in the cap of the ASD PlantProbe, which was repeated before every plant patch (Table 1). The plant spectral properties were acquired from 25 independent plant measurements, which were averaged into one spectral characteristic (a spectral record) with 10 records acquired for one plant patch, i.e., totaling 250 measurements that affording a higher data objectivity (Table 1). The measurements were made directly on the leaves using a fiber cable (25° field of view (FOV)) placed in the ASD PlantProbe, which takes measurements from a one cm of FOV circle area of leaves in the ASD PlantProbe (Figure 3). All leaves were measured in the upper (5 measurements) and middle (5) parts of plants. In the case of narrow leaves, a set of touching leaves were used [32].
- The fraction of accumulated radiation in the range of photosynthesis (fAPAR) [34] that is the total radiation used by the vegetation for photosynthesis, measured using an AccuPAR linear ceptometer at the canopy level. In each field course, two sets of the following Absorbed Photosynthetically Active Radiation (APAR) components were made: PAR0, amount of radiation reaching the surface of the plant; PARc, amount of radiation reflected by plants; PARt, amount of radiation that has penetrated the plants; and PARs, amount of radiation that has been reflected from the ground; this biophysical variable is directly related to the primary productivity of photosynthesis.
- The temperature index, ts-ta, is the difference between ts (the plant surface temperature), and ta (the air temperature), which defines evapotranspiration and water stress (pyrometer IRtec MiniRay) at the canopy level. Ten independent measurements of ts and ta were made.
- Chlorophyll content in leaves was measured as Chlorophyll Content Meter (CCM-200) at the leaf level. Twenty independent measurements were made.
- Chlorophyll fluorescence—a Plant Stress Meter (PSM Mark II) fluorometer for the analysis of the actual state of the photosynthetic apparatus (the peak wavelength for the Far Red source is ~690 nm); fluorescence measurements were made for dark adapted leaves (20 min; Fv/Fm; Figure 3) as 10 independent measurements; the level of half rise-time (t½) using leaf clips. Measurements of chlorophyll fluorescence provide a useful probe of photosynthetic performance in vivo, and the extent to which performance is limited by photochemical and non-photochemical processes [38]. The parameters that determine the photosynthetic efficiency and state of photosynthetic apparatus are the maximum quantum efficiency of photosystem II (PSII) photochemistry (Fv/Fm, where Fm is maximal fluorescence; Fv is variable fluorescence equal to Fm-Fo; and Fo is minimal fluorescence) and t½ (half rise time from Fo to Fm, which indicates the size of antenna systems). Fv/Fm describes the photochemical efficiency of the PSII, i.e., the parameter most sensitive to stress factors. Fv/Fm represents the maximum quantum efficiency of PSII, if all capable reaction centers were opened, and so a value from 0.79 to 0.84 is the optimal value for many plant species, with lower values indicating plant stress [39,40]. In dark adapted plants (measured part of the leaf has been in the dark for an extended period of time before measurement), the electron transport chain is not active, whereas, in the light, all chlorophyll molecules are fully activated and transfers light energy to a reaction center.
- Modified Normalized Difference Vegetation Index 705 (mNDVI705) [46], Transformed Chlorophyll Absorption Reflectance Index (TCARI) [47], Modified Chlorophyll Absorption Ratio Index (MCARI) [48], Normalized Pigment Chlorophyll Index (NPCI) [49], Simple Ratio Pigment Index (SRPI) [50], and Normalized Phaeophytinization Index (NPQI) [51];
- Normalized Difference Nitrogen Index (NDNI) [54];
- Normalized Difference Lignin Index (NDLI) [54];
4. Results
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gamon, J.A.; Field, C.B.; Roberts, D.A.; Ustin, S.L.; Valentini, R. Functional patterns in an annual grassland during an AVIRIS overflight. Remote Sens. Environ. 1993, 44, 239–253. [Google Scholar] [CrossRef]
- Roberts, D.A.; Batista, G.T.; Pereira, J.; Waller, E.K.; Nelson, B.W. Change identification using multitemporal spectral mixture analysis: Applications in Eastern Amazonia. In Remote Sensing Change Detection: Environmental Monitoring Applications and Methods; Elvidge, C., Lunetta, R., Eds.; Ann Arbor Press: Chelsea, MI, USA, 1998; pp. 137–161. [Google Scholar]
- Kupková, L.; Červená, L.; Suchá, R.; Jakešová, L.; Zagajewski, B.; Březina, S.; Albrechtová, J. Classification of Tundra Vegetation in the Krkonoše Mts. National Park Using APEX, AISA Dual and Sentinel-2A Data. Eur. J. Remote Sens. 2017, 50, 29–46. [Google Scholar] [CrossRef]
- Raczko, E.; Zagajewski, B.; Ochtyra, A.; Jarocińska, A.; Marcinkowska-Ochtyra, A.; Dobrowolski, M. Forest species identification of Mount Chojnik (Karkonoski National Park) using airborne hyperspectal APEX data. Sylwan 2015, 159, 593–599. [Google Scholar]
- Raczko, E.; Zagajewski, B. Comparison of Support Vector Machine, Random Forest and Neural Network Classifiers for Tree Species Classification on Airborne Hyperspectral APEX images. Eur. J. Remote Sens. 2017, 50, 144–154. [Google Scholar] [CrossRef]
- Tucker, C.J.; Sellers, P.J. Satellite remote sensing of primary production. Int. J. Remote Sens. 1986, 7, 1395–1416. [Google Scholar] [CrossRef]
- Knipling, E.B. Physical and physiological basis for the reflectance of visible and near-infrared radiation from vegetation. Remote Sens. Environ. 1970, 1, 155–159. [Google Scholar] [CrossRef]
- Whinam, J.; Chilcott, N.M. Impact after four years of experimental trampling on alpine/sub-alpine environments in western Tasmania. J. Environ. Manag. 2003, 67, 339–351. [Google Scholar] [CrossRef]
- Cierniewski, J.; Kazmierowski, C.; Krolewicz, S.; Piekarczyk, J.; Wrobel, M.; Zagajewski, B. Effects of Different Illumination and Observation Techniques of Cultivated Soils on Their Hyperspectral Bidirectional Measurements under Field and Laboratory Conditions. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 2525–2530. [Google Scholar] [CrossRef]
- Cierniewski, J.; Ceglarek, J.; Karnieli, A.; Królewicz, S.; Kaźmierowski, C.; Zagajewski, B. Predicting the diurnal blue-sky albedo of soils using their laboratory reflectance spectra and roughness indices. J. Quant. Spectrosc. Radiat. Transf. 2017, 200, 25–31. [Google Scholar] [CrossRef]
- Cole, D.N. Experimental trampling of vegetation. I. Relationship between trampling intensity and vegetation response. J. Appl. Ecol. 1995, 32, 203–214. [Google Scholar] [CrossRef]
- Cole, D.N. Experimental trampling of vegetation. II. Prediction of resistance and resilience. J. App. Ecol. 1995, 32, 215–224. [Google Scholar] [CrossRef]
- Cole, D.N.; Bayfield, N.G. Recreational trampling of vegetation: Standard experimental procedures. Biol. Conserv. 1993, 63, 209–215. [Google Scholar] [CrossRef]
- Gremmen, N.J.M.; Smith, V.R.; van Tongeren, O.F.R. Impact of trampling on the vegetation of subantarctic Marion Island. Arct. Antarct. Alp. Res. 2003, 35, 442–446. [Google Scholar] [CrossRef]
- Jägerbrand, A.K.; Alatalo, J.M. Effects of human trampling on abundance and diversity of vascular plants, bryophytes and lichens in alpine heath vegetation, Northern Sweden. SpringerPlus 2015, 4, 95. [Google Scholar] [CrossRef] [PubMed]
- McDougall, K.L.; Wright, G.T. The impact of trampling on feldmark vegetation in Kosciuszko National Park, Australia. Aust. J. Bot. 2004, 52, 315–320. [Google Scholar] [CrossRef]
- Barros, A.; Gonnet, J.; Pickering, C. Impacts of informal trails on vegetation and soils in the highest protected area in the Southern Hemisphere. J. Environ. Manag. 2013, 127, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, M.; Pickering, C.M.; McDougall, K.L.; Wright, G.T. Sustained impacts of a hiking trail on changing windswept feldmark vegetation in the Australian Alps. Aust. J. Bot. 2014, 62, 263–275. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kaya, H.; Goto, K.; Iwabuchi, M.; Araki, T. A pair of related genes with antagonistic roles in mediating flowering signals. Science 1999, 286, 1960–1962. [Google Scholar] [CrossRef] [PubMed]
- Cole, D.N.; Monz, C.A. Trampling Disturbance of High-Elevation Vegetation, Wind River Mountains, Wyoming, U.S.A. Arct. Antarct. Alp. Res. 2002, 34, 365–376. [Google Scholar] [CrossRef]
- Sunohara, Y.; Ikeda, H.; Tsukgashi, S.; Murata, Y.; Sakurai, N.; Noma, Y. Effects of trampling on morphology and ethylene production in asiatic plantain. Weed Sci. 2002, 50, 479–484. [Google Scholar] [CrossRef]
- Sunohara, Y.; Ikeda, H. Effects of trampling and ethephon on leaf morphology in trampling-tolerant Plantago asiatica and Eleusine indica. Weed Res. 2003, 43, 155–162. [Google Scholar] [CrossRef]
- Striker, G.G.; Mollard, F.P.O.; Grimoldi, A.A.; Leon, R.J.C.; Insausti, P. Trampling enhances the dominance of graminoids over forbs in flooded grassland mesocosms. Appl. Veg. Sci. 2010, 14, 95–106. [Google Scholar] [CrossRef]
- Klug, B.; Scharfetter-Lehrl, G.; Scharfetter, E. Effects of trampling on vegetation above the timberline in the eastern Alps, Austria. Arct. Antarct. Alp. Res. 2002, 34, 377–388. [Google Scholar] [CrossRef]
- Scott, J.J.; Kirkpatrick, J.B. Effects of human trampling on the sub-Antarctic vegetation of Macquarie Island. Polar Rec. 1994, 30, 207–220. [Google Scholar] [CrossRef]
- Hortensteiner, S. Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 2006, 57, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Tsimilli-Michael, M.; Strasser, R.J. In vivo assessment of plants’ vitality: Applications in detecting and evaluating the impact of mycorrhization on host plants. In Mycorrhiza: State of the Art, Genetics and Molecular Biology, Eco-Function, Biotechnology, Eco-Physiology, Structure and Systematics, 3rd ed.; Varma, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 679–703. [Google Scholar] [CrossRef]
- Kalaji, M.H.; Bosa, K.; Kościelniak, J.; Hossain, Z. Chlorophyll a fluorescence—A useful tool for the early detection of temperature stress in spring barley (Hordeum vulgare L.). OMICS 2011, 15, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, M.H.; Guo, P. Chlorophyll fluorescence: A useful tool in barley plant breeding programs. In Photochemistry Research Progress; Sanchez, A., Gutierrez, S.J., Eds.; Nova Publishers: New York, NY, USA, 2008; pp. 439–463. [Google Scholar]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Kycko, M.; Zagajewski, B.; Zwijacz-Kozica, M.; Cierniewski, J.; Romanowska, E.; Orłowska, K.; Ochtyra, A.; Jarocińska, A. Assessment of Hyperspectral Remote Sensing for Analyzing the Impact of Human Trampling on Alpine Swards. Mt. Res. Dev. 2017, 37, 66–74. [Google Scholar] [CrossRef]
- Zagajewski, B.; Jarocinska, A. Analysis of plant condition of the Bystrzanka catchment. In Remote Sensing for a Changing Europe, Proceedings of the 28th EARSeL Symposium, Istanbul, Turkey, 2–5 June 2008; Maktav, D., Ed.; IOS Press: Amsterdam, The Netherlands, 2009; pp. 498–504. [Google Scholar] [CrossRef]
- Bareth, G.; Aasen, H.; Bendig, J.; Gnyp, M.L.; Bolten, A.; Jung, A.; Michels, R.; Soukkamäki, J. Low-weight and UAV-based Hyperspectral Full-frame Cameras for Monitoring Crops: Spectral Comparison with Portable Spectroradiometer Measurements. Photogramm. Fernerkund. Geoinf. 2015, 1, 69–79. [Google Scholar] [CrossRef]
- Kycko, M.; Zagajewski, B.; Kozłowska, A. Variability in spectral characteristics of trampled high-mountain grasslands. Misc. Geogr. 2014, 18, 10–14. [Google Scholar] [CrossRef]
- Kozłowska, A.; Rączkowska, Z.; Zagajewski, B. Links between vegetation and morphodynamics of high-mountain slopes in the Tatra Mountain. Geogr. Pol. 2006, 79, 27–39. [Google Scholar]
- Pitman, J.I. Absorption of Photosynthetically Active Radiation, Radiation Use Efficiency and Spectral Reflectance of Bracken [Pteridium aquilinum (L.) Kuhnl] Canopies. Ann. Bot. 2000, 85 (Suppl. B), 101–111. [Google Scholar] [CrossRef]
- Kozłowska, A. Detailed mapping of high vegetation in the Tatra Mts. Pol. Bot. Stud. 2006, 22, 333–341. [Google Scholar]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.N.; Young, A.J.; Scholes, J.D.; Horton, P. The dissipation of excess excitation energy in British plant species. Plant Cell Environ. 1993, 16, 673–679. [Google Scholar] [CrossRef]
- Jarocińska, A.M.; Kacprzyk, M.; Marcinkowska-Ochtyra, A.; Ochtyra, A.; Zagajewski, B.; Meuleman, K. The application of APEX images in the assessment of the state of non-forest vegetation in the Karkonosze Mountains. Misc. Geogr. 2016, 20, 21–27. [Google Scholar] [CrossRef]
- Gitelson, A.A. Wide Dynamic Range Vegetation Index for Remote Quantification of Biophysical Characteristics of Vegetation. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Huete, A. A Soil-Adjusted Vegetation Index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Bejron, A.; Miller, J.R. Stress Detection in Crops with Hyperspectral Remote Sensing and Physical Simulation Models. In Proceedings of the Airborne Imaging Spectroscopy Workshop, Bruges, Belgium, 8 October 2004. [Google Scholar]
- Dawson, T.P.; Curran, P.J. Technical note: A new technique for interpolating the reflectance red edge position. Int. J. Remote Sens. 1998, 11, 2133–2139. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships Between Leaf Pigment Content and Spectral Reflectance Across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral Vegetation Indices and Novel Algorithms for Predicting Green LAI of Crop Canopies: Modeling and Validation in the Context of Precision Agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Daughtry, C.; Walthall, C.L.; Kim, M.S.; Brown de Colstoun, E.; McMurtreym, J.E. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Peñuelas, J.; Gamon, J.A.; Fredeen, A.L.; Merino, J.; Field, C.B. Reflectance Indices Associated with Physiological Changes in Nitrogen and Water Limited Sunflower Leaves. Remote Sens. Environ. 1994, 48, 135–146. [Google Scholar] [CrossRef]
- Peñuelas, J.; Baret, F.; Filella, I. Semi-Empirical Indices to Assess Carotenoids/Chlorophyll—A Ratio from Leaf Spectral Reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Gamon, J.A.; Penuelas, J.; Field, C.B. A Narrow-Waveband Spectral Index That Tracks Diurnal Changes in Photosynthetic Efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Gamon, J.A.; Field, C.B.; Bilger, W.; Bjorkman, O.; Fredeen, A.L.; Peñuelas, J. Remote sensing of xanthophyll cycle and chlorophyll fluorescence in sunflower leaves and canopies. Oecologia 1990, 85, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fourty, T.; Baret, F.; Jacquemoud, S.; Schmuck, G.; Verdebout, J. Leaf Optical Properties with Explicit Description of Its Biochemical Composition. Direct and Inverse Problems. Remote Sens. Environ. 1996, 56, 104–117. [Google Scholar] [CrossRef]
- Merzlyak, J.R.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive Optical Detection of Pigment Changes During Leaf Senescence and Fruit Ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Nagler, P.L.; Inoue, Y.; Glenn, E.P.; Russ, A.L.; Daughtry, C.S.T. Cellulose absorption index (CAI) to quantify mixed soil–plant litter scenes. Remote Sens. Environ. 2003, 87, 310–325. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing carotenoid content in plant leaves with reflectance spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzylak, M.N.; Chivkunowam, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Rock, B.N.; Williams, D.L.; Vogehnann, J.E. Field and airborne spectral characterization of suspected acid deposition damage in red spruce (Picea rubens) form Vermont. In Proceedings of the 11th International Symposium Machine Processing of Remotely Sensed Data, Lafayette, IN, USA, 25–27 June 1985; pp. 71–81. [Google Scholar]
- Hardisky, M.A.; Klemas, V.; Smart, R.M. The Influences of Soil Salinity, Growth Form, and Leaf Moisture on the Spectral Reflectance of Spartina Alterniflora Canopies. Photogramm. Eng. Remote Sens. 1983, 49, 77–83. [Google Scholar]
- Gao, B.C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Ruban, A.V.; Young, A.J.; Horton, P. Induction of Nonphotochemical Energy Dissipation and Absorbance Changes in Leaves (Evidence for Changes in the State of the Light-Harvesting System of Photosystem II In Vivo). Plant Physiol. 1993, 102, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Agapiou, A.; Hadjimitsis, D.G.; Alexakis, D.D. Evaluation of Broadband and Narrowband Vegetation Indices for the Identification of Archaeological Crop Marks. Remote Sens. 2012, 4, 3892–3919. [Google Scholar] [CrossRef]
- Rodriguez-Perez, J.R.; Riano, D.; Carlisle, E.; Ustin, S.; Smart, D.R. Evaluation of hyperspectral reflectance indices to detect grapevine water status in vineyard. Am. J. Enol. Vitic. 2007, 58, 302–317. [Google Scholar]
- Sobczak, M.; Folbrier, A.; Kozłowska, A.; Krówczyńska, M.; Pabjanek, P.; Wrzesień, M.; Zagajewski, B. Assessment of the potential of hyperspectral data and techniques for mountain vegetation analysis. In Imaging Spectroscopy. New Quality in Environmental Studies; Zagajewski, B., Sobczak, M., Eds.; EARSeL & Warsaw University, Faculty of Geography and Regional Studies: Warsaw, Poland, 2005; pp. 761–780. [Google Scholar]
- Zagajewski, B. Assessment of neural networks and Imaging Spectroscopy for vegetation classification of the High Tatras. Teledetekcja Środowiska 2010, 43, 113. [Google Scholar]
- Zagajewski, B.; Tømmervik, H.; Bjerke, J.W.; Raczko, E.; Bochenek, Z.; Kłos, A.; Jarocińska, A.; Lavender, S.; Ziółkowski, D. Intraspecific Differences in Spectral Reflectance Curves as Indicators of Reduced Vitality in High-Arctic Plants. Remote Sens. 2017, 9, 1289. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Berjón, A.; López-Lozano, R.; Miller, J.R.; Martín, P.; Cachorro, V.; González, M.R.; de Frutos, A. Assessing vineyard condition with hyperspectral indices: Leaf and canopy reflectance simulation in a row-structured discontinuous canopy. Remote Sens. Environ. 2005, 99, 271–287. [Google Scholar] [CrossRef]
- Marcinkowska-Ochtyra, A.; Zagajewski, B.; Ochtyra, A.; Jarocińska, A.; Wojtuń, B.; Rogass, C.; Mielke, C.; Lavender, S. Subalpine and alpine vegetation classification based on hyperspectral APEX and simulated EnMAP images. Int. J. Remote Sens. 2017, 38, 1839–1864. [Google Scholar] [CrossRef]
- Liddle, M.J. A theoretical relationship between the primary productivity of vegetation and its ability to tolerate trampling. Biol. Conserv. 1975, 8, 251–255. [Google Scholar] [CrossRef]
- Liddle, M.J. Recreation Ecology: The Ecological Impact of Outdoor Recreation and Ecotourism; Chapman & Hall: London, UK, 1997; p. 639. [Google Scholar]
- Cole, D.N.; Spildie, D.R. Hiker, horse and llama trampling effects on native vegetation in Montana, USA. J. Environ. Manag. 1998, 53, 61–71. [Google Scholar] [CrossRef]
- Littlemore, J.; Barker, S. The ecological response of forest ground flora and soils to experimental trampling in British urban woodlands. Urban Ecosyst. 2001, 5, 257–276. [Google Scholar] [CrossRef]
- Sun, D.; Liddle, M.J. Plant morphological characteristics and resistance to simulated trampling. Environ. Manag. 1993, 17, 511–521. [Google Scholar] [CrossRef]
- Price, M.F. Impacts of recreational activities on alpine vegetation in western North America. Mt. Res. Dev. 1985, 5, 263–277. [Google Scholar] [CrossRef]
- Monz, C.A. The response of two arctic tundra plant communities to human trampling disturbance. J. Environ. Manag. 2002, 64, 207–217. [Google Scholar] [CrossRef]
- Bell, K.L.; Bliss, L.C. Alpine disturbance studies: Olympic National Park USA. Biol. Conserv. 1973, 5, 25–32. [Google Scholar] [CrossRef]
- Calais, S.S.; Kirkpatrick, J.B. Impact of Trampling on Natural Ecosystems in the Cradle Mountain-Lake St Clair National Park. Aust. Geogr. 1986, 17, 6–15. [Google Scholar] [CrossRef]
- Bjerke, J.W.; Karlsen, S.R.; Høgda, K.A.; Malnes, E.; Jepsen, J.U.; Lovibond, S.; Vikhamar Schuler, D.; Tømmervik, H. Record-low primary productivity and high plant damage in the Nordic Arctic Region in 2012 caused by multiple weather events and pest outbreaks. Environ. Res. Lett. 2014, 9, 084006. [Google Scholar] [CrossRef]
- Dumitrascu, M.; Marin, A.; Preda, E.; Tibirnac, M.; Vadineanu, A. Trampling effects on plant species morphology. Rom. J. Biol. Plant Biol. 2010, 55, 89–96. [Google Scholar]
- Carter, G.A.; Knapp, A.K. Leaf optical properties in higher plants: Linking spectral characteristics to stress and chlorophyll concentration. Am. J. Bot. 2001, 88, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Broge, N.; Leblanc, E. Comparing Prediction Power and Stability of Broadband and Hyperspectral Vegetation Indices for Estimation of Green Leaf Area and Canopy Chlorophyll Density. Remote Sens. Environ. 2000, 76, 156–172. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Bolhàr-Nordenkampf, H.R.; Öquist, G.O. Chlorophyll fluorescence as a tool in photosynthesis research. In Photosynthesis and Production in a Changing Environment. A Field and Laboratory Manual; Hall, D.O., Scurlock, J.M.O., Bolhàr-Nordenkampf, H.R., Leegoood, R.C., Long, S.P., Eds.; Chapman & Hall: London, UK, 1993; pp. 193–206. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor and Francis: London, UK, 2000; pp. 443–480. [Google Scholar]
- Gola Golan, T.; Li, X.P.; Müller-Moulé, P.; Niyogi, K.K. Using mutants to understand light stress acclimationin plants. In Chlorophyll Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Ed.; Springer: Dordrecht, The Netherlands, 2004; pp. 525–554. [Google Scholar]
- Kaiser, W.M. Effect of water deficit on photosynthetic capacity. Physiol. Plant. 1987, 71, 142–149. [Google Scholar] [CrossRef]
- Ohashi, Y.; Nakayama, N.; Saneoka, H.; Fujita, K. Effects of drought stress on photosynthetic gas exchange, chlorophyll fluorescence and stem diameter of soybean plants. Biol. Plant. 2006, 50, 138–141. [Google Scholar] [CrossRef]
- Guóth, A.; Tari, I.; Gallé, A.; Csiszár, J.; Horváth, F.; Pécsváradi, A.; Cseuz, L.; Erdei, L. Chlorophyll a fluorescence induction parameters of flag leaves characterize genotypes and not the drought tolerance of wheat during grain filling under water deficit. Acta Biol. Szeged. 2009, 53, 1–7. [Google Scholar]
- Tan, C.W.; Huang, W.J.; Jin, X.L.; Wang, J.C.; Tong, L.; Wang, J.H.; Guo, W.S. Monitoring the chlorophyll fluorescence parameter Fv/Fm in compact corn based on different hyperspectral vegetation indices. Spectrosc. Spectr. Anal. 2012, 32, 1287–1291. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N. Spectral Reflectance Changes Associated with Autumn Senescence of Aesculus hippocastanum L. and Acer platanoides L. Leaves. Spectral Features and Relation to Chlorophyll Estimation. J. Plant. Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Pickering, C.M.; Growcock, A.J. Impacts of experimental trampling on tall alpine herbfields and subalpine grasslands in the Australian Alps. J. Environ. Manag. 2009, 91, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Ketchledge, E.H.; Leonard, R.E.; Richards, N.A.; Craul, P.F.; Eschner, A.R. Rehabilitation of Alpine Vegetation in the Adirondack Mountains of New York State; Research Paper NE-553; US Department of Agriculture Forest Service, Northeast Research Station: Newtown Square, PA, USA, 1985; pp. 1–10. [Google Scholar]
- Jędrych, M.; Zagajewski, B.; Marcinkowska-Ochtyra, A. Application of Sentinel-2 and EnMAP new satellite data to the mapping of alpine vegetation of the Karkonosze Mountains. Polish Carto. Rev. 2017, 49, 107–119. [Google Scholar] [CrossRef]
- Ochtyra, A.; Zagajewski, B.; Kozłowska, A.; Marcinkowska-Ochtyra, A.; Jarocińska, A. Assessment of the Tatra National Park forests condition using decision tree method and multispectral Landsat TM satellite images. Sylwan 2016, 160, 256–264. [Google Scholar]
- Mason, S.; Newsome, D.; Moore, S.; Admiraal, R. Recreational trampling negatively impacts vegetation structure of an Australian biodiversity hotspot. Biodivers. Conserv. 2015, 24, 2685–2707. [Google Scholar] [CrossRef]



| Number of Patches | Number of Measurements | Number of Measurements for Trampled Plants | Number of Measurements for Reference Plants | |
|---|---|---|---|---|
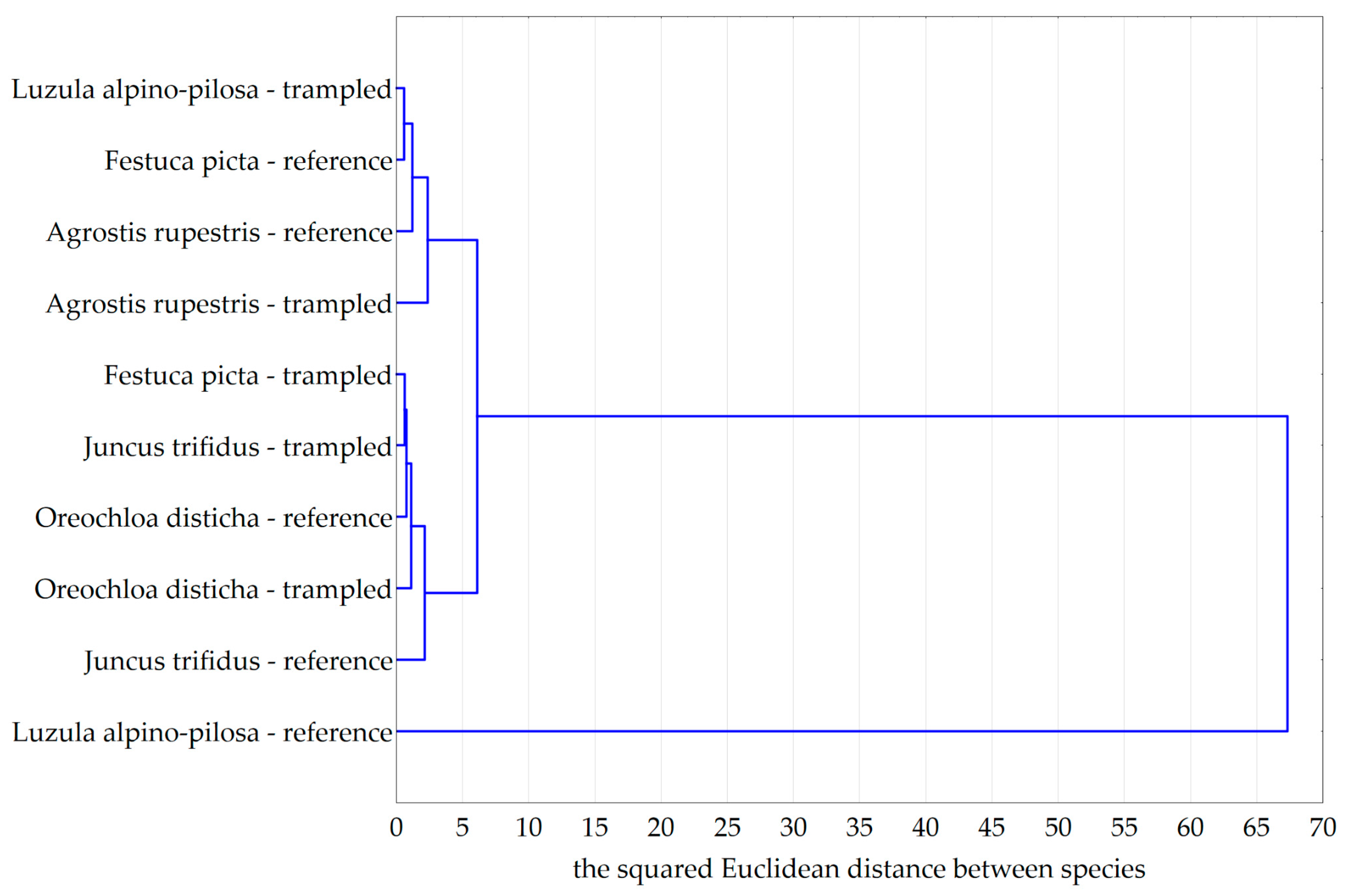
| Juncus trifidus | 62 | 1300 | 610 | 690 |
| Oreochloa disticha | 32 | 630 | 290 | 340 |
| Agrostis rupestris | 98 | 1970 | 940 | 1030 |
| Festuca picta | 28 | 530 | 250 | 280 |
| Luzula alpino-pilosa | 76 | 1610 | 770 | 840 |
| VIS (nm) | NIR (nm) | SWIR (nm) | |
|---|---|---|---|
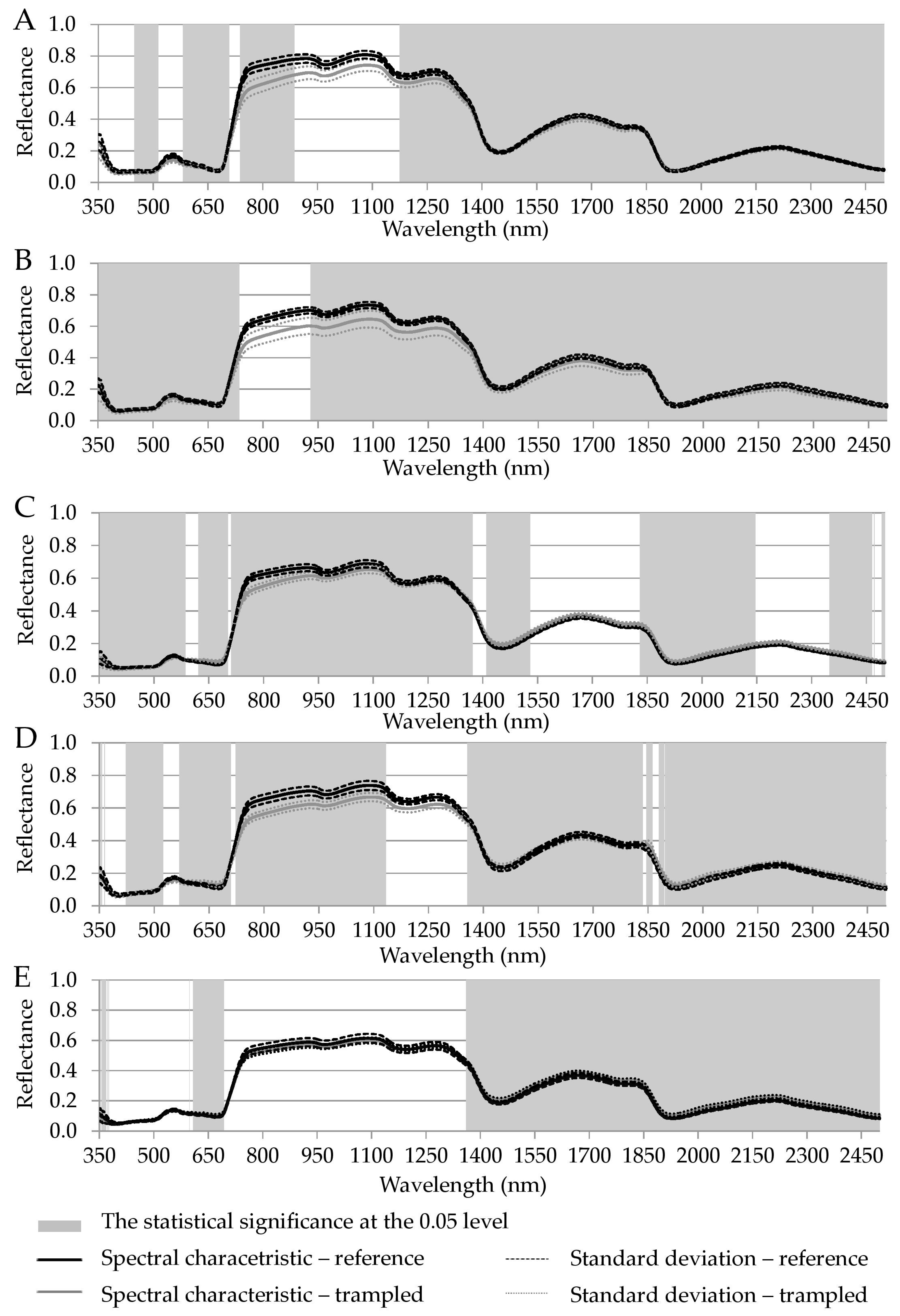
| Luzula alpino-pilosa | 448–514, 581–707, 737–780 | 780–886, 1174–1400 | 1400–2500 |
| Festuca picta | 350–735 | 929–1400 | 1400–2500 |
| Juncus trifidus | 350–585, 621–702, 711–780 | 780–1370 | 1409–1529, 1829–2145, 2348–2465, 2470–2471, 2491–2500 |
| Agrostis rupestris | 351–356, 363–364, 422–524, 568–709, 722–780 | 780–1133, 1356–1400 | 1400–1835, 1845–1862, 1879–2500 |
| Oreochloa disticha | 353–365, 368–369, 371, 373–376, 598, 608–693 | 1360–1400 | 1400–2500 |
| common to all species | 621–639 | 1360–1372 | 1409–1529, 1829–1835, 1845–1862, 1879–2145, 2348–2465, 2470–2471, 2491–2500 |
| AR_R | AR_T | LAP_R | LAP_T | JT_R | JT_T | FP_R | FP_T | OD_R | OD_T | % | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mNDVI 705 | 0.52 ** | 0.47 ** | 0.58 ** | 0.53 ** | 0.61 ** | 0.50 ** | 0.52 ** | 0.47 ** | 0.50 | 0.49 | 2–18 |
| PRI | −0.03 ** | −0.04 ** | −0.02 ** | −0.03 ** | −0.03 ** | −0.08 ** | −0.03 | −0.04 | −0.03 | −0.04 | 33–67 |
| SIPI 1 | 1.08 ** | 1.14 ** | 1.01 ** | 1.04 ** | 1.04 ** | 1.11 ** | 1.07 ** | 1.10 ** | 1.08 ** | 1.11 ** | 3–7 |
| NDLI | 0.06 ** | 0.05 ** | 0.06 | 0.06 | 0.05 ** | 0.05 ** | 0.06 ** | 0.06 ** | 0.05 | 0.05 | 17 |
| PSRI 1 | 0.05 ** | 0.08 ** | 0.00 ** | 0.03 ** | 0.03 ** | 0.08 ** | 0.04 ** | 0.06 ** | 0.05 ** | 0.06 ** | 20–67 |
| CRI 1 | 5.25 ** | 3.94 ** | 8.15 ** | 5.80 ** | 7.33 ** | 8.96 ** | 6.43 ** | 4.02 ** | 5.37 | 4.76 | 11–37 |
| CRI 2 | 6.20 ** | 5.15 ** | 9.35 | 7.12 | 8.26 ** | 12.05 ** | 7.41 ** | 5.20 ** | 6.80 | 6.07 | 11–46 |
| ARI1 | 0.95 ** | 1.21 ** | 1.21 ** | 1.32 ** | 0.92 ** | 3.09 ** | 0.97 ** | 1.17 ** | 1.43 | 1.31 | 8–36 |
| ARI 2 | 0.50 ** | 0.69 ** | 0.48 ** | 0.74 ** | 0.55 ** | 1.42 ** | 0.52 ** | 0.64 ** | 0.73 | 0.68 | 7–58 |
| MSI 1 | 0.60 ** | 0.69 ** | 0.51 ** | 0.59 ** | 0.51 ** | 0.62 ** | 0.57 ** | 0.63 ** | 0.59 ** | 0.66 ** | 11–22 |
| NDII 1 | 0.21 ** | 0.15 ** | 0.28 ** | 0.22 ** | 0.28 ** | 0.20 ** | 0.23 ** | 0.18 ** | 0.21 ** | 0.16 ** | 21–29 |
| WBI 1 | 1.02 ** | 1.00 ** | 1.04 ** | 1.02 ** | 1.04 ** | 1.01 ** | 1.02 ** | 1.01 ** | 1.02 ** | 1.01 ** | 1–3 |
| NDWI 1 | 0.02 ** | −0.01 ** | 0.04 ** | 0.01 ** | 0.05 ** | 0.00 ** | 0.02 ** | 0.00 ** | 0.01 ** | 0.00 ** | 50–100 |
| SAVI 1 | 0.62 ** | 0.55 ** | 0.72 ** | 0.67 ** | 0.68 ** | 0.60 ** | 0.62 ** | 0.58 ** | 0.59 ** | 0.56 ** | 5–12 |
| WRDVI 1 | 0.69 ** | 0.62 ** | 0.79 ** | 0.74 ** | 0.77 ** | 0.71 ** | 0.70 ** | 0.64 ** | 0.68 ** | 0.65 ** | 4–10 |
| Green NDVI | 0.59 ** | 0.56 ** | 0.65 ** | 0.63 ** | 0.67 | 0.66 | 0.60 ** | 0.56 ** | 0.60 | 0.58 | 1–7 |
| TCARI | 0.21 ** | 0.17 ** | 0.28 ** | 0.25 ** | 0.18 ** | 0.15 ** | 0.21 | 0.21 | 0.19 ** | 0.17 ** | 10–19 |
| MCARI | 0.11 ** | 0.09 ** | 0.11 | 0.10 | 0.09 ** | 0.08 ** | 0.10 ** | 0.11 ** | 0.09 ** | 0.09 ** | 9–18 |
| NPCI | 0.27 ** | 0.30 ** | 0.19 ** | 0.23 ** | 0.22 ** | 0.36 ** | 0.27 | 0.27 | 0.28 | 0.29 | 3–64 |
| SRPI 1 | 0.65 ** | 0.55 ** | 0.92 ** | 0.77 ** | 0.71 ** | 0.49 ** | 0.64 ** | 0.62 ** | 0.61 ** | 0.56 ** | 3–31 |
| NDNI 1 | 0.20 ** | 0.19 ** | 0.22 ** | 0.22 ** | 0.19 ** | 0.18 ** | 0.19 ** | 0.20 ** | 0.19 ** | 0.18 ** | 5–5 |
| CAI | −0.01 ** | 0.00 ** | −0.01 | −0.01 | −0.01 ** | −0.01 ** | −0.01 | 0.00 | −0.01 ** | −0.01 ** | 0–100 |
| NPQI | −0.06 ** | −0.07 ** | −0.01 ** | −0.02 ** | −0.03 ** | −0.05 ** | −0.06 | −0.05 | −0.07 | −0.07 | 16–100 |
| XES | 0.14 | 0.14 | 0.13 | 0.13 | 0.10 ** | 0.09 ** | 0.13 ** | 0.15 ** | 0.12 | 0.12 | 10–16 |
| GI 1 | 1.61 ** | 1.28 ** | 2.40 ** | 1.88 ** | 1.77 ** | 1.36 ** | 1.67 ** | 1.40 ** | 1.49 ** | 1.35 ** | 9–23 |
| REPI | 720.71 | 720.87 | 720.48 ** | 719.94 ** | 721.64 ** | 720.85 ** | 720.71 ** | 720.10 ** | 720.04 | 720.30 | 0.02–0.11 |
| CCI | ts-ta | fAPAR | |
|---|---|---|---|
| Luzula alpino-pilosa T | SIPI [0.81] * | WBI [0.58] * | NPQI [−0.72] * |
| Luzula alpino-pilosa R | XES [0.54] | GI [0.62] * | SAVI [0.49] |
| Festuca picta T | - | WBI [0.60] | mNDVI 705 [−0.83] * |
| Festuca picta R | - | TCARI [−0.78] * | NDII [−0.81] * |
| Juncus trifidus T | - | WBI [−0.85] * | PRI [−0.39] |
| Juncus trifidus R | - | MCARI [0.42] | CRI1 [0.68] * |
| Agrostis rupestris T | GNDVI [−0.67] | NDNI [−0.58] * | NPCI [0.68] * |
| Agrostis rupestris R | GNDVI [0.71] | NDWI [−0.75] * | NDWI [−0.69] * |
| Oreochloa disticha T | - | NDWI [−0.71] | NPQI [−0.72] * |
| Oreochloa disticha R | - | NDWI [0.49] | mNDVI 705 [0.49] |
| Plant Species | Trampled Buffer | Reference Buffer | ||
|---|---|---|---|---|
| Fv/Fm | t½ ms | Fv/Fm | t½ ms | |
| Juncus trifidus | 0.496 | 145 | 0.549 | 173 |
| Luzula alpino-pilosa | 0.596 * | 116 | 0.627 * | 79 |
| Agrostis rupestris | 0.471 * | 113 | 0.597 * | 113 |
| Oreochloa disticha | 0.520 | 126 | 0.598 | 119 |
| Festuca picta | 0.572 * | 123 | 0.496 * | 121 |
| Fv/Fm | t½ | |
|---|---|---|
| Luzula alpino-pilosa T | REPI [0.36] | REPI [0.46] |
| Luzula alpino-pilosa R | WBI [−0.67] * | PRI [−0.52] |
| Festuca picta T | GNDVI [0.83] * | SIPI [0.82] * |
| Festuca picta R | PRI [0.54] | SIPI [0.49] |
| Juncus trifidus T | GNDVI [−0.66] * | ARI1 [−0.63] * |
| Juncus trifidus R | CAI [−0.62] | mNDVI705 [0.66] * |
| Agrostis rupestris T | TCARI [0.63] * | mNDVI705 [0.67] * |
| Agrostis rupestris R | mNDVI 705 [0.56] | PRI [0.74] * |
| Oreochloa disticha T | CAI [−0.77] | GNDVI [0.37] |
| Oreochloa disticha R | SIPI [0.77] | GNDVI [0.75] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kycko, M.; Zagajewski, B.; Lavender, S.; Romanowska, E.; Zwijacz-Kozica, M. The Impact of Tourist Traffic on the Condition and Cell Structures of Alpine Swards. Remote Sens. 2018, 10, 220. https://doi.org/10.3390/rs10020220
Kycko M, Zagajewski B, Lavender S, Romanowska E, Zwijacz-Kozica M. The Impact of Tourist Traffic on the Condition and Cell Structures of Alpine Swards. Remote Sensing. 2018; 10(2):220. https://doi.org/10.3390/rs10020220
Chicago/Turabian StyleKycko, Marlena, Bogdan Zagajewski, Samantha Lavender, Elżbieta Romanowska, and Magdalena Zwijacz-Kozica. 2018. "The Impact of Tourist Traffic on the Condition and Cell Structures of Alpine Swards" Remote Sensing 10, no. 2: 220. https://doi.org/10.3390/rs10020220
APA StyleKycko, M., Zagajewski, B., Lavender, S., Romanowska, E., & Zwijacz-Kozica, M. (2018). The Impact of Tourist Traffic on the Condition and Cell Structures of Alpine Swards. Remote Sensing, 10(2), 220. https://doi.org/10.3390/rs10020220