Can UAV-Based Infrared Thermography Be Used to Study Plant-Parasite Interactions between Mistletoe and Eucalypt Trees?
Abstract
1. Introduction
- What is the extent of the mistletoe infection of the studied CPW remnant woodland?
- Can UAV thermal imagery be applied to study differences in surface temperature between mistletoes, infected and uninfected trees?
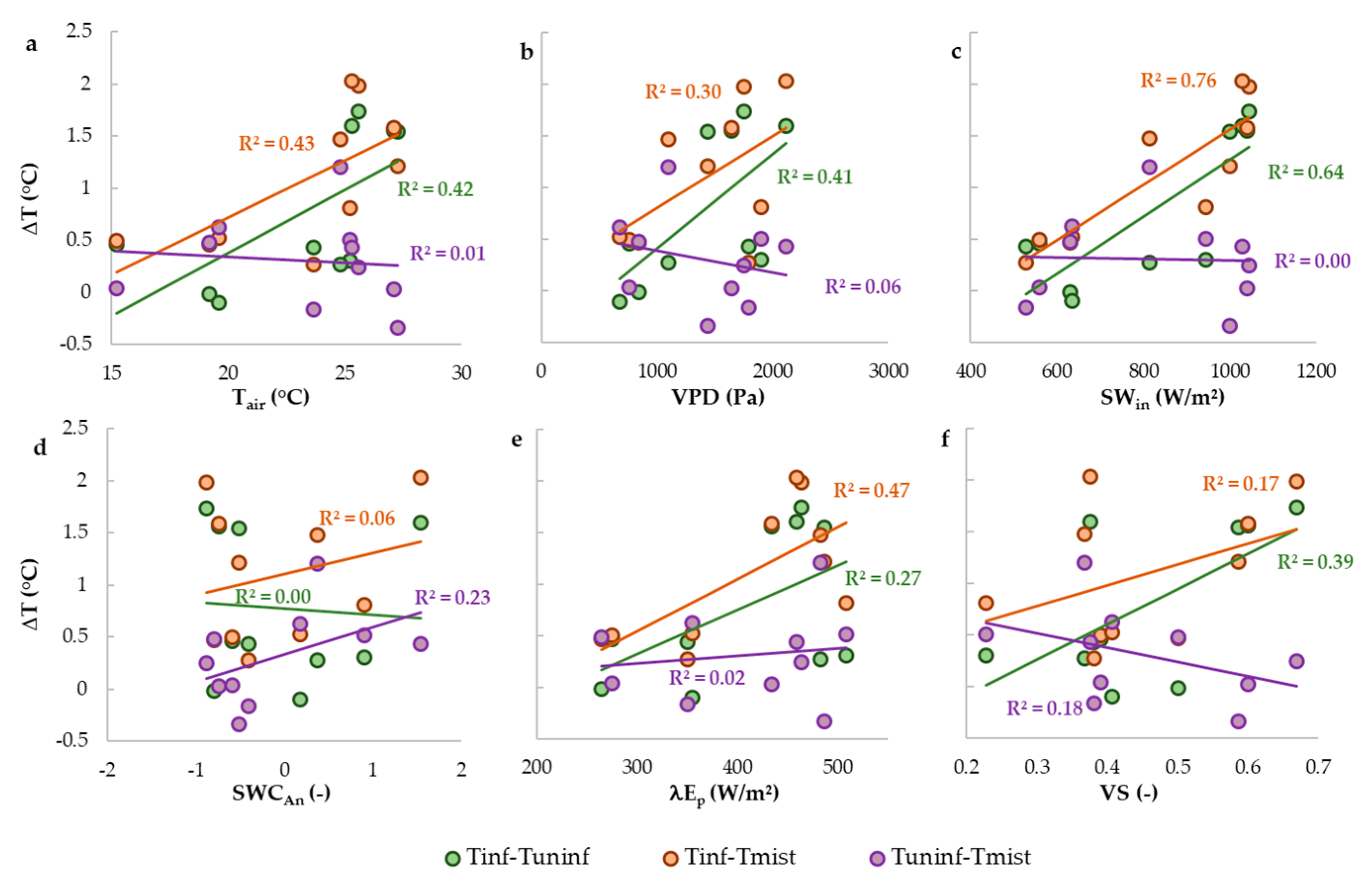
- What are the drivers of temperature differences between mistletoe and foliage of infected and uninfected trees?
2. Materials and Methods
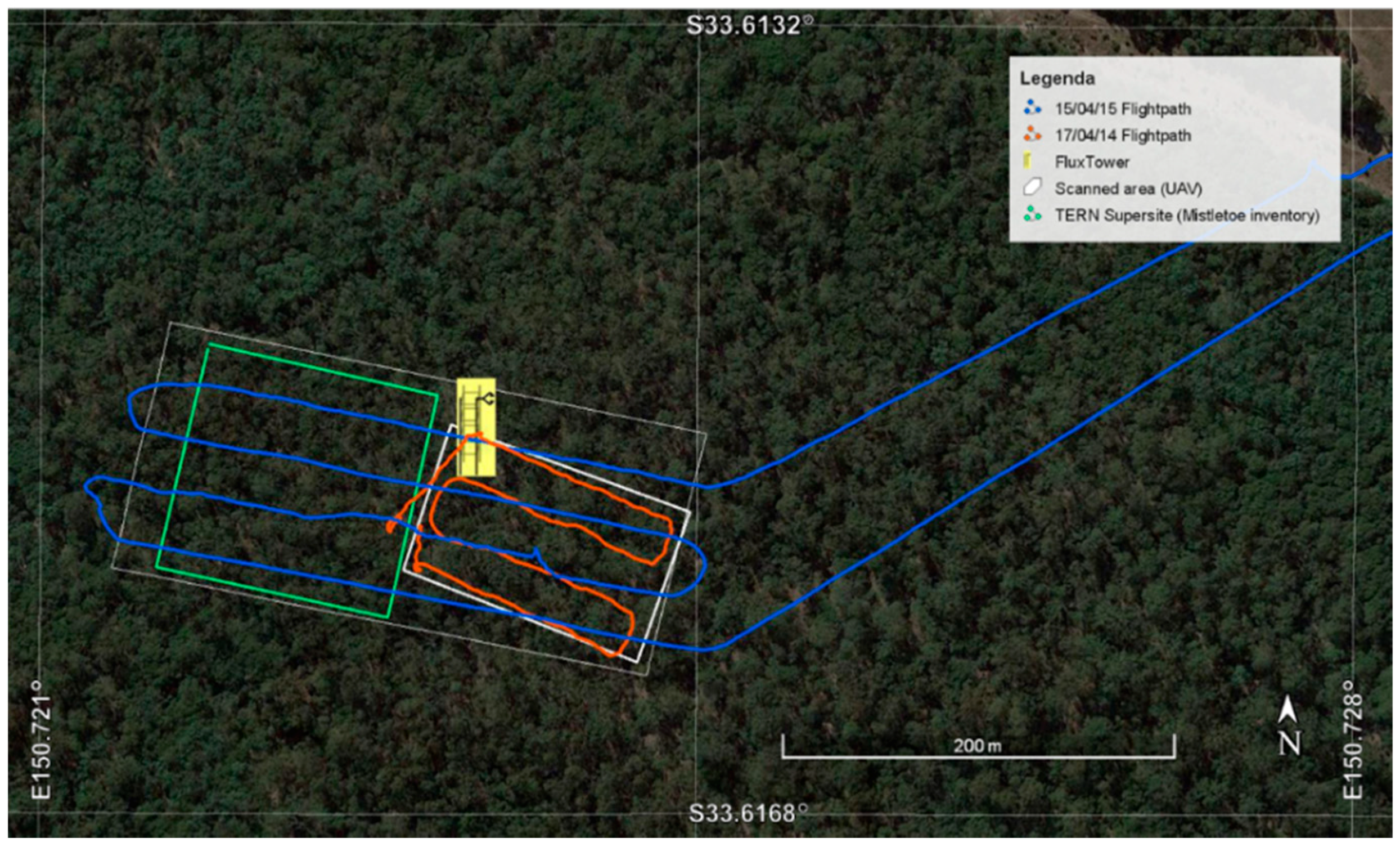
2.1. Study Site
2.2. UAV Flight Campaign
2.3. Data Processing
2.4. Calculations of Anomaly in Soil Water Content, Potential Evaporation and Vegetation Stress
2.5. Statistical Analysis
3. Results
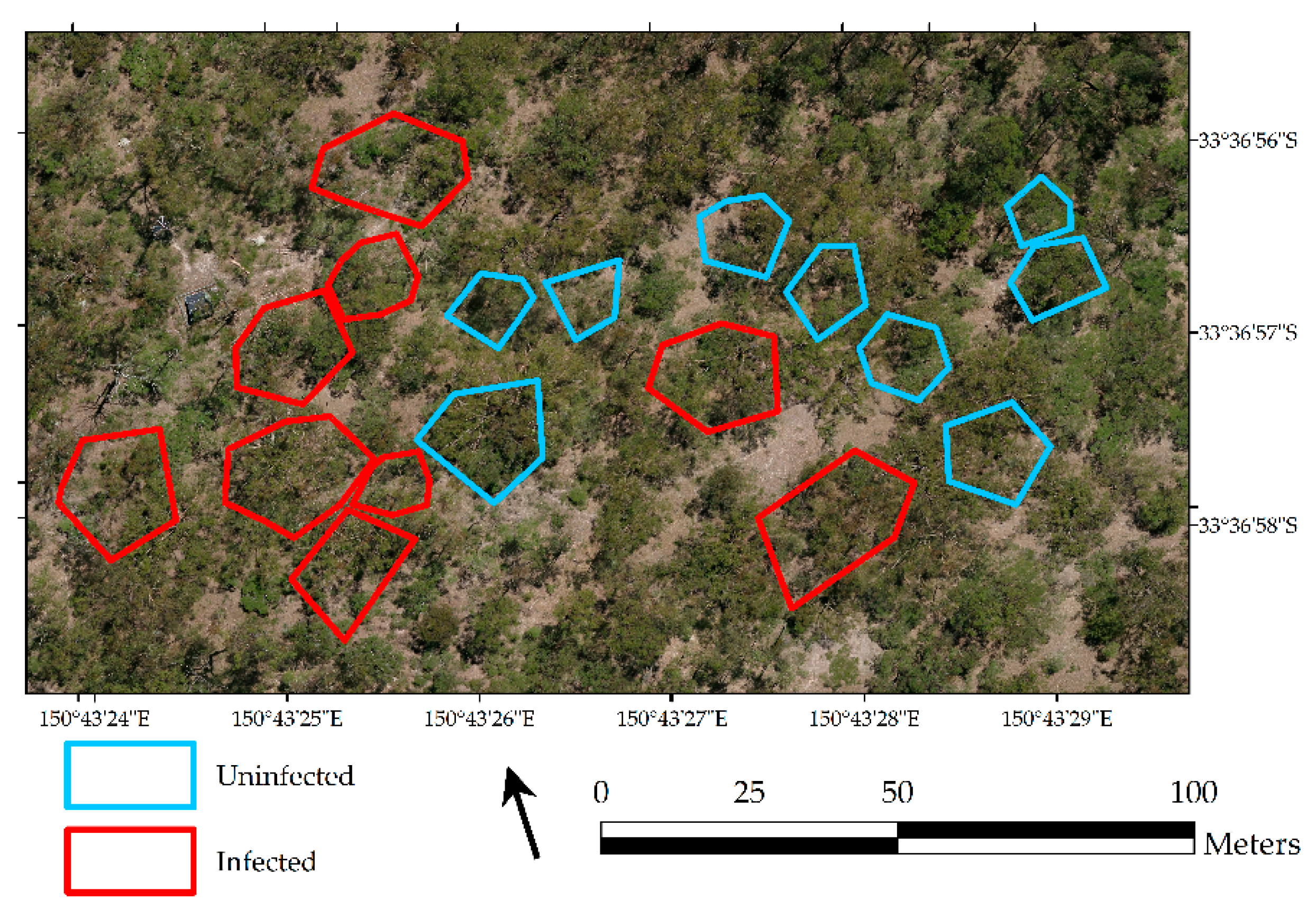
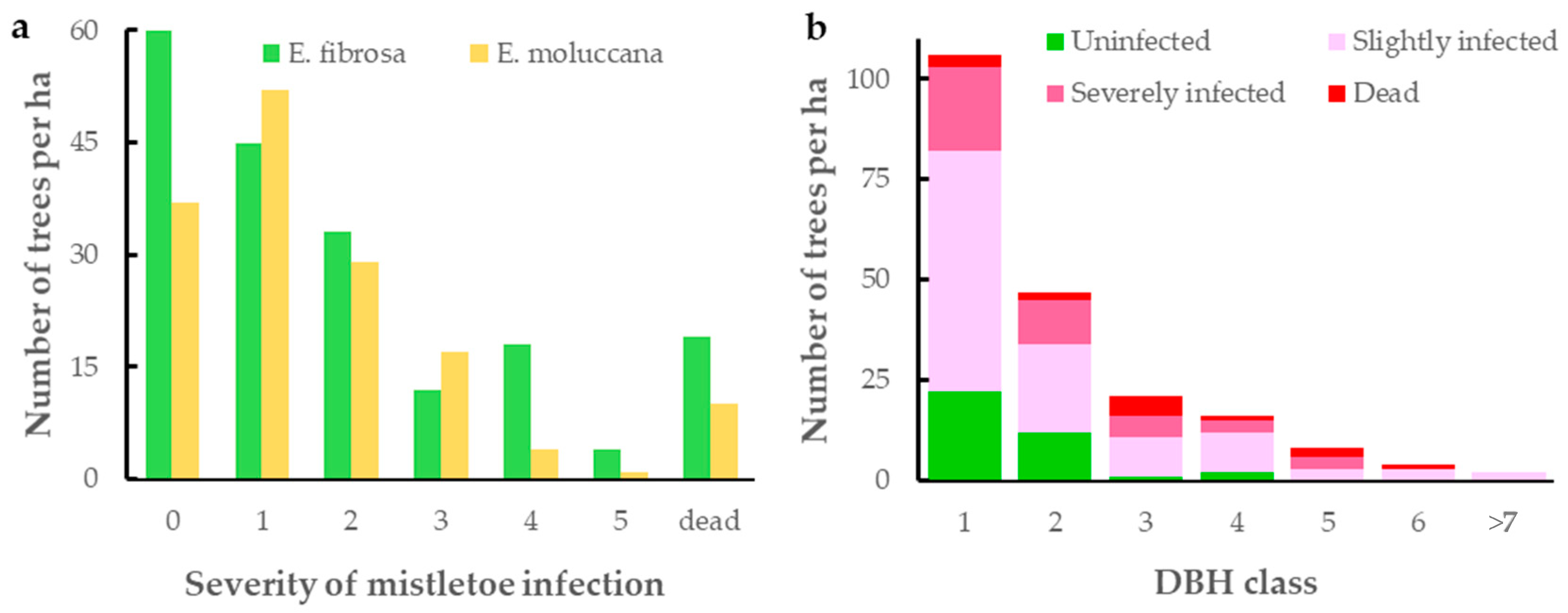
3.1. Extent of Mistletoe Infection
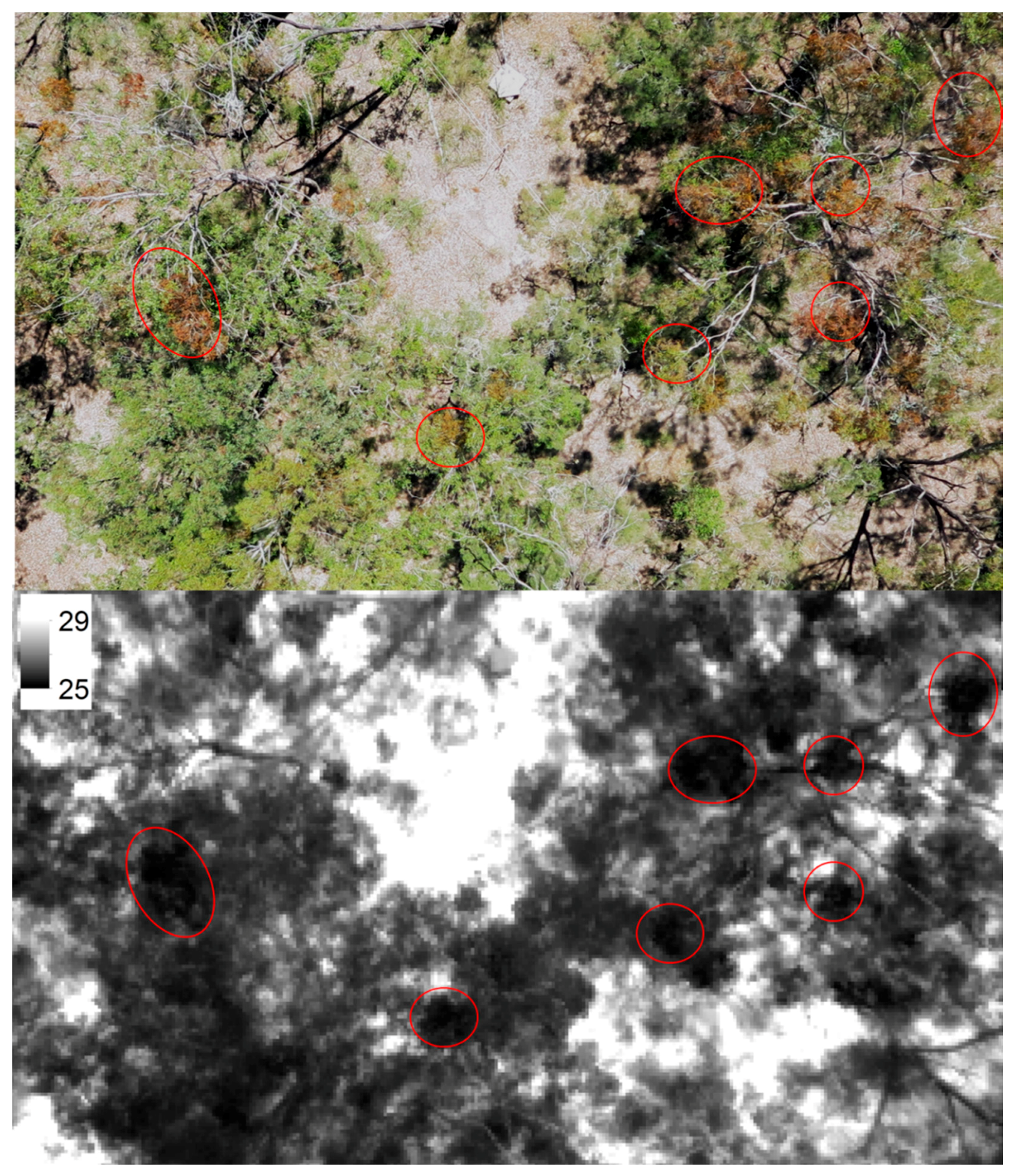
3.2. Comparison of Canopy Temperature of Infected and Uninfected Eucalypt Foliage and of Mistletoe
4. Discussion
4.1. Extent of Mistletoe Infection
4.2. Evaluation of Thermal Remote Sensing for Studying Mistletoe-Host Interactions
4.3. Consequences of Differences in Foliage Temperature between Infected and Uninfected Foliage and Mistletoe
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maes, W.H.; Steppe, K. Estimating evapotranspiration and drought stress with ground-based thermal remote sensing in agriculture: A review. J. Exp. Bot. 2012, 63, 4671–4712. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.; Tanner, C.B. Infrared thermometry of vegetation. Agron. J. 1966, 58, 597–601. [Google Scholar] [CrossRef]
- Maes, W.H.; Steppe, K. Perspectives for remote sensing with unmanned aerial vehicles in precision agriculture. Trends Plant Sci. 2018, 24, 45. [Google Scholar] [CrossRef]
- Khanal, S.; Fulton, J.; Shearer, S. An overview of current and potential applications of thermal remote sensing in precision agriculture. Comput. Electron. Agric. 2017, 139, 22–32. [Google Scholar] [CrossRef]
- Maes, W.H.; Minchin, P.E.H.; Snelgar, W.P.; Steppe, K. Early detection of psa infection in Kiwifruit by means of infrared thermography at leaf and orchard scale. Funct. Plant Biol. 2014, 41, 1207–1220. [Google Scholar] [CrossRef]
- López-López, M.; Calderón, R.; González-Dugo, V.; Zarco-Tejada, P.; Fereres, E. Early detection and quantification of Almond red leaf blotch using high-resolution hyperspectral and thermal imagery. Remote Sens. 2016, 8, 276. [Google Scholar] [CrossRef]
- Elarab, M.; Ticlavilca, A.M.; Torres-Rua, A.F.; Maslova, I.; McKee, M. Estimating chlorophyll with thermal and broadband multispectral high resolution imagery from an unmanned aerial system using relevance vector machines for precision agriculture. Int. J. Appl. Earth Obs. Geoinf. 2015, 43, 32–42. [Google Scholar] [CrossRef]
- Maimaitijiang, M.; Ghulam, A.; Sidike, P.; Hartling, S.; Maimaitiyiming, M.; Peterson, K.; Shavers, E.; Fishman, J.; Peterson, J.; Kadam, S.; et al. Unmanned aerial system (UAS)-based phenotyping of soybean using multi-sensor data fusion and extreme learning machine. ISPRS J. Photogramm. Remote Sens. 2017, 134, 43–58. [Google Scholar] [CrossRef]
- Kefauver, S.C.; Vicente, R.; Vergara-Diaz, O.; Fernandez-Gallego, J.A.; Kerfal, S.; Lopez, A.; Melichar, J.P.E.; Molins, M.D.S.; Araus, J.L. Comparative UAV and field phenotyping to assess yield and nitrogen use efficiency in hybrid and conventional barley. Front. Plant Sci. 2017, 8, 1733. [Google Scholar] [CrossRef]
- Tattaris, M.; Reynolds, M.P.; Chapman, S.C. A direct comparison of remote sensing approaches for high-throughput phenotyping in plant breeding. Front. Plant Sci. 2016, 7, 1131. [Google Scholar] [CrossRef]
- Cleverly, J.; Eamus, D.; Restrepo Coupe, N.; Chen, C.; Maes, W.; Li, L.; Faux, R.; Santini, N.S.; Rumman, R.; Yu, Q.; et al. Soil moisture controls on phenology and productivity in a semi-arid critical zone. Sci. Total Environ. 2016, 568, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Smigaj, M.; Gaulton, R.; Barr, S.L.; Suarez, J.C. Uav-borne thermal imaging for forest health monitoring: Detection of disease-induced canopy temperature increase. In Proceedings of the ISPRS Geospatial Week 2015, La Grande Motte, France, 28 September–3 October 2015; Mallet, C., Paparoditis, N., Dowman, I., Elberink, S.O., Raimond, A.M., Rotensteiner, F., Yang, M., Christophe, S., Coltekin, A., Bredif, M., Eds.; Copernicus Gesellschaft Mbh: Gottingen, Germany, 2015; Volume 40, pp. 349–354. [Google Scholar]
- Yu, M.; Ding, G.; Gao, G.; Zhao, Y.; Sai, K.J.F. Leaf temperature fluctuations of typical psammophytic plants and their application to stomatal conductance estimation. Forests 2018, 9, 313. [Google Scholar] [CrossRef]
- Scherrer, D.; Bader, M.K.F.; Korner, C. Drought-sensitivity ranking of deciduous tree species based on thermal imaging of forest canopies. Agric. For. Meteorol. 2011, 151, 1632–1640. [Google Scholar] [CrossRef]
- Junttila, S.; Vastaranta, M.; Hamalainen, J.; Latva-kayra, P.; Holopainen, M.; Clemente, R.H.; Hyyppa, H.; Navarro-Cerrillo, R.M. Effect of forest structure and health on the relative surface temperature captured by airborne thermal imagery—Case study in Norway spruce-dominated stands in southern finland. Scand. J. For. Res. 2017, 32, 154–165. [Google Scholar] [CrossRef]
- Idso, S.B.; Jackson, R.D.; Pinter, P.J.; Reginato, R.J.; Hatfield, J.L. Normalizing the stress-degree-day parameter for environmental variability. Agric. Meteorol. 1981, 24, 45–55. [Google Scholar] [CrossRef]
- Meron, M.; Tsipris, J.; Charitt, D. Remote mapping of crop water status to assess spatial variability of crop stress. In Precision Agriculture, Proceedings of the 4th European Conference on Precision Agriculture, Berlin, Germany, 15–19 June 2003; Stafford, J., Werner, A., Eds.; Academic Publishers: Wageningen, The Netherlands, 2003; pp. 405–410. [Google Scholar]
- Maes, W.H.; Baert, A.; Huete, A.R.; Minchin, P.E.H.; Snelgar, W.P.; Steppe, K. A new wet reference target method for continuous infrared thermography of vegetations. Agric. For. Meteorol. 2016, 226–227, 119–131. [Google Scholar] [CrossRef]
- Grant, O.M.; Ochagavia, H.; Baluja, J.; Diago, M.P.; Tardaguila, J. Thermal imaging to detect spatial and temporal variation in the water status of grapevine (Vitis vinifera L.). J. Horticult. Sci. Biotechnol. 2016, 91, 43–54. [Google Scholar] [CrossRef]
- Gonzalez-Dugo, V.; Goldhamer, D.; Zarco-Tejada, P.; Fereres, E. Improving the precision of irrigation in a pistachio farm using an unmanned airborne thermal system. Irrig. Sci. 2015, 33, 43–52. [Google Scholar] [CrossRef]
- Egea, G.; Padilla-Díaz, C.M.; Martinez-Guanter, J.; Fernández, J.E.; Pérez-Ruiz, M. Assessing a crop water stress index derived from aerial thermal imaging and infrared thermometry in super-high density olive orchards. Agric. Water Manag. 2017, 187, 210–221. [Google Scholar] [CrossRef]
- Leinonen, I.; Grant, O.M.; Tagliavia, C.P.P.; Chaves, M.M.; Jones, H.G. Estimating stomatal conductance with thermal imagery. Plant Cell Environ. 2006, 29, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.G.; Archer, N.; Rotenberg, E. Thermal radiation, canopy temperature and evaporation from forest canopies. In Forests at the Land-Atmosphere Interface; Mencuccini, M., Grace, J., Moncrieff, J., McNaughton, K.G., Eds.; CABI Publishing: Wallingford, UK, 2003; pp. 123–144. [Google Scholar]
- Maes, W.H.; Pashuysen, T.; Trabucco, A.; Veroustraete, F.; Muys, B. Does energy dissipation increase with ecosystem succession? Testing the ecosystem exergy theory combining theoretical simulations and thermal remote sensing observations. Ecol. Model. 2011, 23–24, 3917–3941. [Google Scholar] [CrossRef]
- Leuzinger, S.; Körner, C. Tree species diversity affects canopy leaf temperatures in a mature temperate forest. Agric. For. Meteorol. 2007, 146, 29–37. [Google Scholar] [CrossRef]
- Kim, Y.; Still, C.J.; Hanson, C.V.; Kwon, H.; Greer, B.T.; Law, B.E.J.A. Canopy skin temperature variations in relation to climate, soil temperature, and carbon flux at a ponderosa pine forest in central Oregon. Agric. For. Meteorol. 2016, 226, 161–173. [Google Scholar] [CrossRef]
- Maes, W.; Huete, A.; Steppe, K. Optimizing the processing of UAV-based thermal imagery. Remote Sens. 2017, 9, 476. [Google Scholar] [CrossRef]
- Ribeiro-Gomes, K.; Hernandez-Lopez, D.; Ortega, J.F.; Ballesteros, R.; Poblete, T.; Moreno, M.A. Uncooled thermal camera calibration and optimization of the photogrammetry process for uav applications in agriculture. Sensors 2017, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, P.H.; Burns, K.C. Mistletoe macroecology: Spatial patterns in species diversity and host use across Australia. Biol. J. Linn. Soc. 2012, 106, 459–468. [Google Scholar] [CrossRef]
- Griebel, A.; Watson, D.; Pendall, E. Mistletoe, friend and foe: Synthesizing ecosystem implications of mistletoe infection. Environ. Res. Lett. 2017, 12, 115012. [Google Scholar] [CrossRef]
- Ziegler, H.; Weber, J.; Lüttge, U.E. Thermal dissipation probe measurements of sap flow in the xylem of trees documenting dynamic relations to variable transpiration given by instantaneous weather changes and the activities of a mistletoe xylem parasite. Trees 2009, 23, 441–450. [Google Scholar] [CrossRef]
- Urban, J.; Gebauer, R.; Nadezhdina, N.; Čermák, J. Transpiration and stomatal conductance of mistletoe (Loranthus europaeus) and its host plant, Downy oak (Quercus pubescens). Biologia 2012, 67, 917–926. [Google Scholar] [CrossRef]
- Yang, D.; Goldstein, G.; Wang, M.; Zhang, W.-W.; Wang, A.-Y.; Liu, Y.-Y.; Hao, G.-Y. Microenvironment in the canopy rivals the host tree water status in controlling sap flow of a mistletoe species. Tree Physiol. 2017, 37, 501–510. [Google Scholar] [CrossRef]
- Zweifel, R.; Bangerter, S.; Rigling, A.; Sterck, F.J. Pine and mistletoes: How to live with a leak in the water flow and storage system? J. Exp. Bot. 2012, 63, 2565–2578. [Google Scholar] [CrossRef] [PubMed]
- Küppers, M.; Küppers, B.I.; Neales, T.F.; Swan, A.G. Leaf gas exchange characteristics, daily carbon and water balances of the host/mistletoe pair Eucalyptus behriana f. Muell. and Amyema miquelii (lehm. Ex miq.) tiegh. at permanently low plant water status in the field. Trees 1992, 7, 1–7. [Google Scholar]
- Strong, G.L.; Bannister, P. Water relations of temperate mistletoes on various hosts. Funct. Plant Biol. 2002, 29, 89–96. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Schulze, E.D.; Ziegler, H.; Lange, O.L.; Farquhar, G.D.; Cowar, I.R. Xylem-tapping mistletoes—Water or nutrient parasites. Science 1985, 227, 1479–1481. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Cook, C.S.; Tieszen, L.L. Comparative water-use and nitrogen relationships in a mistletoe and its host. Oecologia 1986, 68, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Garkoti, S.; Akoijam, S.; Singh, S. Ecology of water relations between mistletoe (Taxillus vestitus) and its host oak (Quercus floribunda). Trop. Ecol. 2002, 43, 243–249. [Google Scholar]
- Cernusak, L.A.; Pate, J.S.; Farquhar, G.D. Oxygen and carbon isotope composition of parasitic plants and their hosts in southwestern Australia. Oecologia 2004, 139, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Raftoyannis, Y.; Radoglou, K.; Bredemeier, M. Effects of mistletoe infestation on the decline and mortality of Abies cephalonica in Greece. Ann. For. Res. 2015, 58, 55. [Google Scholar] [CrossRef]
- Scalon, M.C.; Rossatto, D.R.; Domingos, F.M.C.B.; Franco, A.C. Leaf morphophysiology of a neotropical mistletoe is shaped by seasonal patterns of host leaf phenology. Oecologia 2016, 180, 1103–1112. [Google Scholar] [CrossRef]
- Schulze, E.D.; Turner, N.; Glatzel, G. Carbon, water and nutrient relations of two mistletoes and their hosts: A hypothesis. Plant Cell Environ. 1984, 7, 293–299. [Google Scholar]
- Marshall, J.D.; Ehleringer, J.R. Are xylem-tapping mistletoes partially heterotrophic? Oecologia 1990, 84, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Scalon, M.C.; Wright, I.J. Leaf trait adaptations of xylem-tapping mistletoes and their hosts in sites of contrasting aridity. Plant Soil 2017, 415, 117–130. [Google Scholar] [CrossRef]
- Galiano, L.; Martínez-Vilalta, J.; Lloret, F. Carbon reserves and canopy defoliation determine the recovery of Scots pine 4 year after a drought episode. New Phytol. 2011, 190, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Meinzer, F.; Woodruff, D.; Shaw, D. Integrated responses of hydraulic architecture, water and carbon relations of Western hemlock to Dwarf mistletoe infection. Plant Cell Environ. 2004, 27, 937–946. [Google Scholar] [CrossRef]
- Rigling, A.; Eilmann, B.; Koechli, R.; Dobbertin, M. Mistletoe-induced crown degradation in Scots pine in a xeric environment. Tree Physiol. 2010, 30, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, S.; Osma, E.; Ilhan, V.; Turkoglu, H.I.; Atici, O. Mistletoe (Viscum album) reduces the growth of the Scots pine by accumulating essential nutrient elements in its structure as a trap. Trees 2016, 30, 815–824. [Google Scholar] [CrossRef]
- Dobbertin, M.; Rigling, A. Pine mistletoe (Viscum album ssp. Austriacum) contributes to Scots pine (Pinus sylvestris) mortality in the Rhone valley of Switzerland. For. Pathol. 2006, 36, 309–322. [Google Scholar] [CrossRef]
- Scott, J.M.; Mathiasen, R.L. Assessing growth and mortality of Bristlecone pine infected by Dwarf mistletoe using dendrochronology. For. Sci. 2012, 58, 366–376. [Google Scholar] [CrossRef]
- Tozer, M. The native vegetation of the Cumberland plain, western Sydney: Systematic classification and field identification of communities. Cunninghamia 2003, 8, 1–75. [Google Scholar]
- Hill, S.J.; Tung, P.J.; Leishman, M.R. Relationships between anthropogenic disturbance, soil properties and plant invasion in endangered Cumberland plain woodland, Australia. Aust. Ecol. 2005, 30, 775–788. [Google Scholar] [CrossRef]
- Renchon, A.A.; Griebel, A.; Metzen, D.; Williams, C.A.; Medlyn, B.; Duursma, R.A.; Barton, C.V.; Maier, C.; Boer, M.M.; Isaac, P. Upside-down fluxes down under: CO2 net sink in winter and net source in summer in a temperate evergreen broadleaf forest. Biogeosciences 2018, 15, 3703–3716. [Google Scholar] [CrossRef]
- Karan, M.; Liddell, M.; Prober, S.M.; Arndt, S.; Beringer, J.; Boer, M.; Cleverly, J.; Eamus, D.; Grace, P.; Van Gorsel, E. The Australian supersite network: A continental, long-term terrestrial ecosystem observatory. Sci. Total Environ. 2016, 568, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Boer, M.D. Vascular Plant Data, Direct Measure of Stems, above Ground Biomass, Cumberland Plain Supersite, Core 1 ha, 4th ed.; TERN Australian SuperSite Network: Richmond NSW, Australia, 2016. [Google Scholar]
- Maes, W.H.; Gentine, P.; Verhoest, N.E.C.; Miralles, D.G. Potential evaporation at eddy-covariance sites across the globe. Hydrol. Earth Syst. Sci. Discuss. 2018. [Google Scholar] [CrossRef]
- Jurskis, V.; Turner, R.; Jurskis, D. Mistletoes increasing in ‘undisturbed’ forest: A symptom of forest decline caused by unnatural exclusion of fire? Aust. For. 2005, 68, 221–226. [Google Scholar] [CrossRef]
- Blick, R.A.J.; Burns, K.C.; Moles, A.T. Dominant network interactions are not correlated with resource availability: A case study using mistletoe host interactions. Oikos 2013, 122, 889–895. [Google Scholar] [CrossRef]
- Turner, R.J.; Smith, P. Mistletoes increasing in eucalypt forest near Eden, New South Wales. Aust. J. Bot. 2016, 64, 171–179. [Google Scholar] [CrossRef]
- Ward, M.J. Patterns of Box mistletoe Amyema miquelii infection and Pink gum Eucalyptus fasciculosa condition in the mount lofty ranges, south Australia. For. Ecol. Manag. 2005, 213, 1–14. [Google Scholar] [CrossRef]
- Bowen, M.E.; McAlpine, C.A.; House, A.P.N.; Smith, G.C. Agricultural landscape modification increases the abundance of an important food resource: Mistletoes, birds and brigalow. Biol. Conserv. 2009, 142, 122–133. [Google Scholar] [CrossRef]
- Baker, F.A.; Knowles, K.R. Case study: 36 years of Dwarf mistletoe in a regenerating Black spruce stand in Northern Minnesota. North. J. Appl. For. 2004, 21, 150–153. [Google Scholar]
- Varga, I.; Poczai, P.; Tiborcz, V.; Aranyi, N.R.; Baltazar, T.; Bartha, D.; Pejchal, M.; Hyvonen, J. Changes in the distribution of European mistletoe (Viscum album) in Hungary during the last hundred years. Folia Geobot. 2014, 49, 559–577. [Google Scholar] [CrossRef]
- Diaz-Limon, M.P.; Cano-Santana, Z.; Queijeiro-Bolanos, M.E. Mistletoe infection in an urban forest in Mexico City. Urban For. Urban Green. 2016, 17, 126–134. [Google Scholar] [CrossRef]
- Rist, L.; Shaanker, R.U.; Ghazoul, J. The spatial distribution of mistletoe in a Southern Indian tropical forest at multiple scales. Biotropica 2011, 43, 50–57. [Google Scholar] [CrossRef]
- MacRaild, L.M.; Radford, J.Q.; Bennett, A.F. Non-linear effects of landscape properties on mistletoe parasitism in fragmented agricultural landscapes. Landsc. Ecol. 2010, 25, 395–406. [Google Scholar] [CrossRef]
- Ritter, S.M.; Hoffman, C.M.; Stewart, J.E.; Zimmerman, T. The influence of prescribed crown fire on Lodgepole pine dwarf mistletoe (Arceuthobium americanum) populations 33 years post-fire. For. Pathol. 2018, 48, e12419. [Google Scholar] [CrossRef]
- Aukema, J.E.; Del Rio, C.M. Variation in mistletoe seed deposition: Effects of intra-and interspecific host characteristics. Ecography 2002, 25, 139–144. [Google Scholar] [CrossRef]
- Roxburgh, L.; Nicolson, S.W. Differential dispersal and survival of an African mistletoe: Does host size matter? Plant Ecol. 2008, 195, 21–31. [Google Scholar] [CrossRef]
- Maes, W.H.; Achten, W.M.J.; Reubens, B.; Muys, B. Monitoring stomatal conductance of Jatropha curcas seedlings under different levels of water shortage with infrared thermography. Agric. For. Meteorol. 2011, 151, 554–564. [Google Scholar] [CrossRef]
- Galiano, L.; Martinez-Vilalta, J.; Lloret, F. Drought-induced multifactor decline of Scots pine in the Pyrenees and potential vegetation change by the expansion of co-occurring oak species. Ecosystems 2010, 13, 978–991. [Google Scholar] [CrossRef]
- Bowie, M.; Ward, D. Water and nutrient status of the mistletoe Plicosepalus acaciae parasitic on isolated Negev desert populations of Acacia raddiana differing in level of mortality. J. Arid Environ. 2004, 56, 487–508. [Google Scholar] [CrossRef]

| Date | Hour | Tair (°C) | VPD (kPa) | SWin (W/m²) | SWC (-) | λE (W/m²) | λEp (W/m²) | VS (-) |
|---|---|---|---|---|---|---|---|---|
| 07/02/2014 | 12:25 | 25.6 | 1.75 | 1045 | 0.08 | 183 | 464 | 0.33 |
| 20/02/2014 | 11:52 | 25.2 | 1.90 | 944 | 0.22 | 491 | 509 | 0.77 |
| 05/03/2014 | 11:59 | 24.8 | 1.10 | 814 | 0.18 | 299 | 483 | 0.63 |
| 17/04/2014 | 16:14 | 23.7 | 1.79 | 529 | 0.12 | 223 | 350 | 0.62 |
| 15/05/2014 | 13:34 | 19.2 | 0.84 | 629 | 0.09 | 131 | 265 | 0.50 |
| 16/06/2014 | 13:09 | 15.2 | 0.76 | 559 | 0.10 | 189 | 275 | 0.61 |
| 22/12/2014 | 11:58 | 27.3 | 1.44 | 1001 | 0.11 | 244 | 487 | 0.41 |
| 08/01/2015 | 12:13 | 27.1 | 1.65 | 1039 | 0.09 | 319 | 435 | 0.40 |
| 30/01/2015 | 14:45 | 25.3 | 2.12 | 1028 | 0.27 | 395 | 459 | 0.62 |
| 15/04/2015 | 11:30 | 19.6 | 0.68 | 634 | 0.16 | 208 | 355 | 0.59 |
| Date | Tmist | Tinf | Tuninf | Tinf-Tmist | Tinf-Tuninf | Tuninf-Tmist |
|---|---|---|---|---|---|---|
| 07/02/14 | 29.1 ± 0.9 | 31.1 ± 1.5 | 29.3 ± 0.7 | 2.0 | 1.8 | 0.2 |
| 20/02/14 | 26.3 ± 0.6 | 27.1 ± 0.9 | 26.8 ± 0.7 | 0.8 | 0.3 | 0.5 |
| 05/03/14 | 25.6 ± 0.4 | 27.1 ± 0.6 | 26.8 ± 0.4 | 1.5 | 0.3 | 1.2 |
| 17/04/14 | 24.5 ± 0.7 | 24.8 ± 1.0 | 24.4 ± 0.4 | 0.3 | 0.4 | −0.1 |
| 15/05/14 | 20.2 ± 0.7 | 20.7 ± 0.7 | 20.7 ± 0.9 | 0.5 | 0.0 | 0.5 |
| 16/06/14 | 16.1 ± 0.5 | 16.6 ± 0.6 | 16.1 ± 0.7 | 0.5 | 0.5 | 0.0 |
| 22/12/14 | 31.3 ± 0.9 | 32.5 ± 1.1 | 31.0 ± 1.7 | 1.2 | 1.5 | −0.3 |
| 08/01/15 | 31.1 ± 0.7 | 32.7 ± 1.3 | 31.1 ± 0.7 | 1.6 | 1.6 | 0.0 |
| 30/01/15 | 29.4 ± 0.8 | 31.4 ± 0.8 | 29.8 ± 0.7 | 2.0 | 1.6 | 0.4 |
| 15/04/15 | 21.2 ± 0.5 | 21.7 ± 0.5 | 21.8 ± 0.6 | 0.5 | -0.1 | 0.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maes, W.H.; Huete, A.R.; Avino, M.; Boer, M.M.; Dehaan, R.; Pendall, E.; Griebel, A.; Steppe, K. Can UAV-Based Infrared Thermography Be Used to Study Plant-Parasite Interactions between Mistletoe and Eucalypt Trees? Remote Sens. 2018, 10, 2062. https://doi.org/10.3390/rs10122062
Maes WH, Huete AR, Avino M, Boer MM, Dehaan R, Pendall E, Griebel A, Steppe K. Can UAV-Based Infrared Thermography Be Used to Study Plant-Parasite Interactions between Mistletoe and Eucalypt Trees? Remote Sensing. 2018; 10(12):2062. https://doi.org/10.3390/rs10122062
Chicago/Turabian StyleMaes, Wouter H., Alfredo R. Huete, Michele Avino, Matthias M. Boer, Remy Dehaan, Elise Pendall, Anne Griebel, and Kathy Steppe. 2018. "Can UAV-Based Infrared Thermography Be Used to Study Plant-Parasite Interactions between Mistletoe and Eucalypt Trees?" Remote Sensing 10, no. 12: 2062. https://doi.org/10.3390/rs10122062
APA StyleMaes, W. H., Huete, A. R., Avino, M., Boer, M. M., Dehaan, R., Pendall, E., Griebel, A., & Steppe, K. (2018). Can UAV-Based Infrared Thermography Be Used to Study Plant-Parasite Interactions between Mistletoe and Eucalypt Trees? Remote Sensing, 10(12), 2062. https://doi.org/10.3390/rs10122062







