Exploring Alternative Use of Medicinal Plants for Sustainable Weed Management
Abstract
1. Introduction
2. Materials and Methods
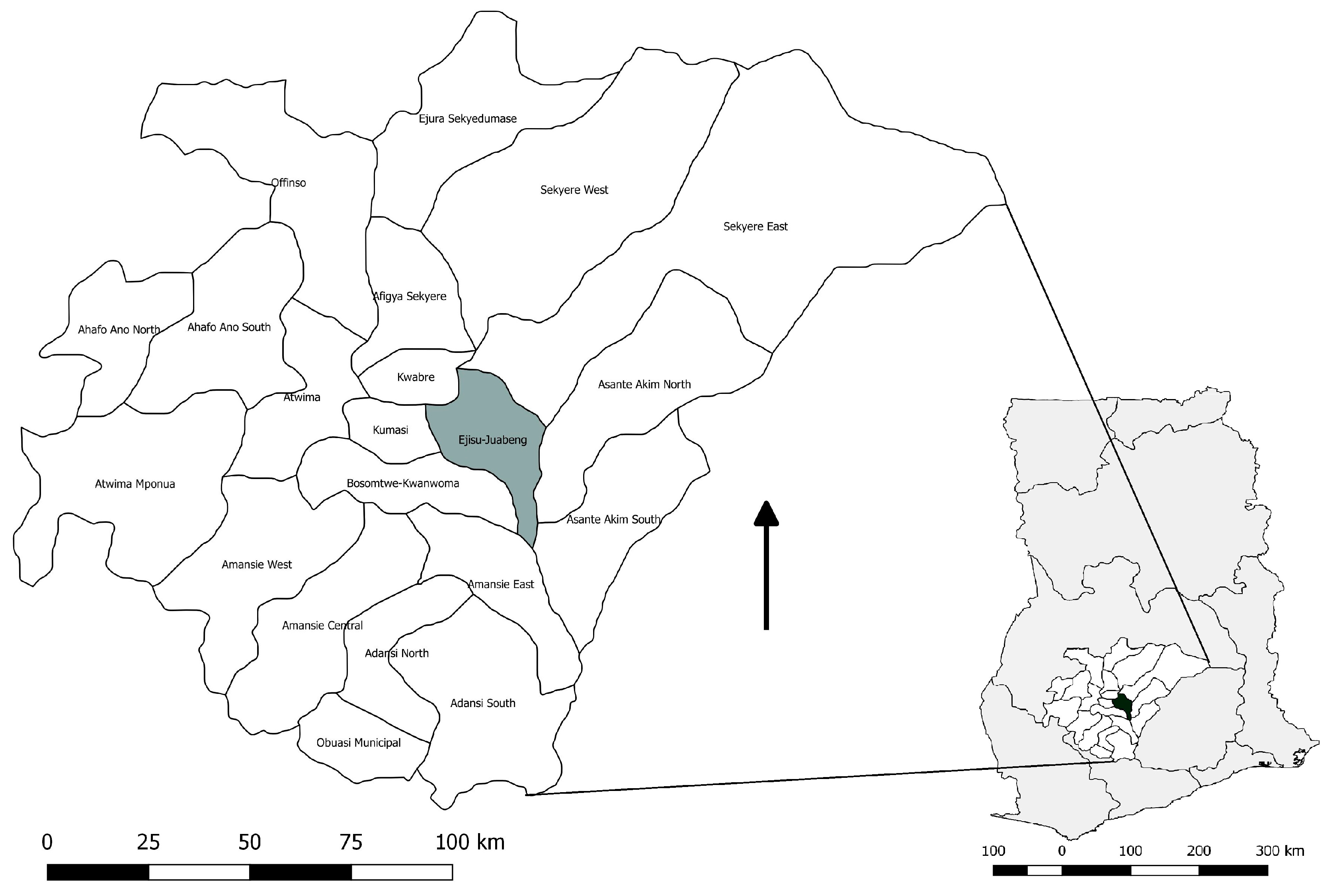
2.1. Study Area
2.2. Ethnobotanical Survey and Plants Collection
2.3. Botanical Identification
2.4. Data Analysis Using Ethnobotanical Indices
2.4.1. Use Value (UV)
2.4.2. Fidelity Level (FL)
2.4.3. Relative Frequency of Citation (RFC)
2.5. Bioassay for Potential Allelopathic Medicinal Species
2.5.1. Preparation of Plant Material
2.5.2. Sandwich Bioassay
2.5.3. Dish Pack Bioassay
2.6. Statistical Analysis
3. Results and Discussion
3.1. Profile of Informants, Medicinal Plant Diversity, and Distribution
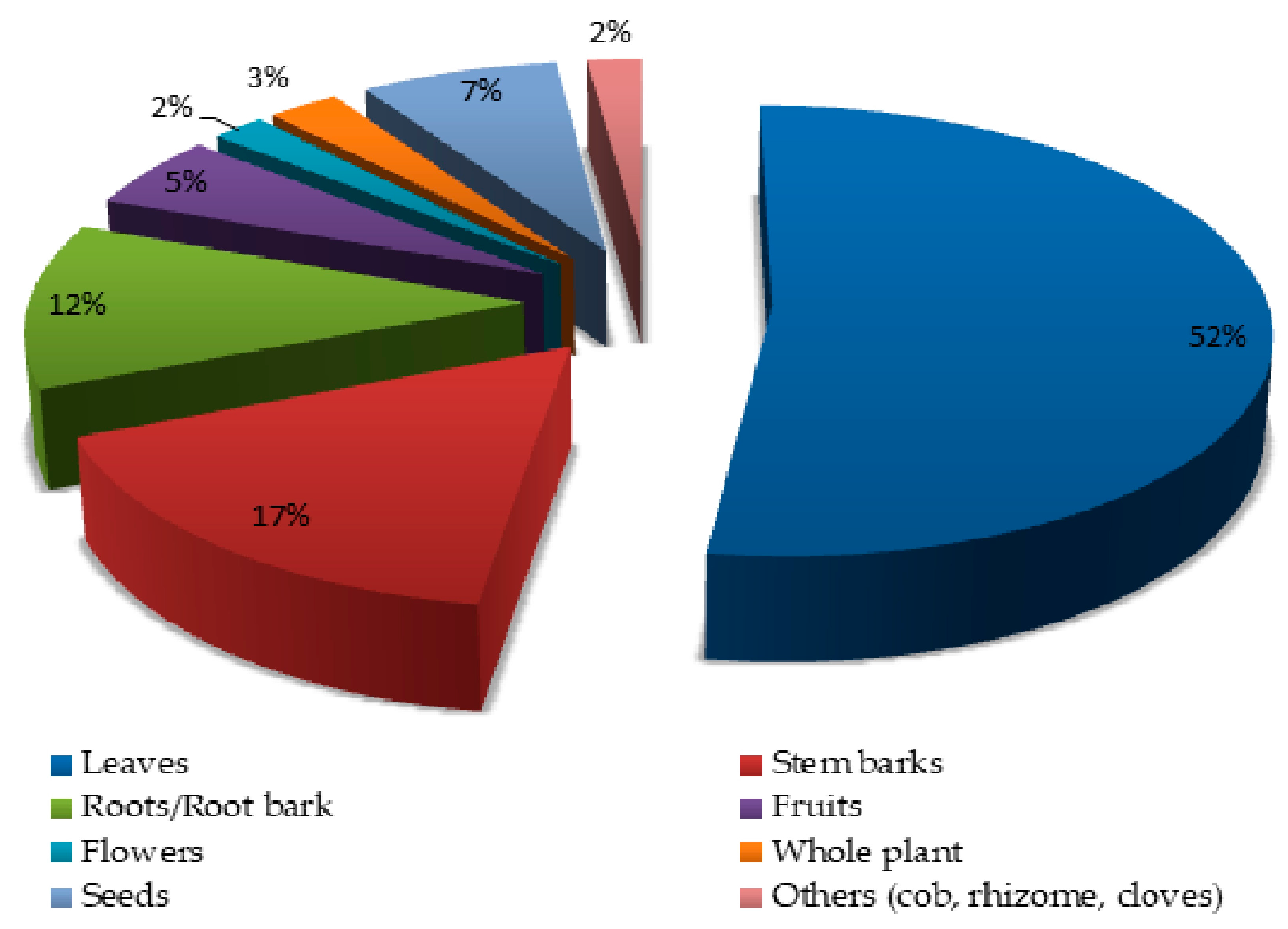
3.2. Plant Parts Used in Medicinal Remedies Preparation in the Study Area
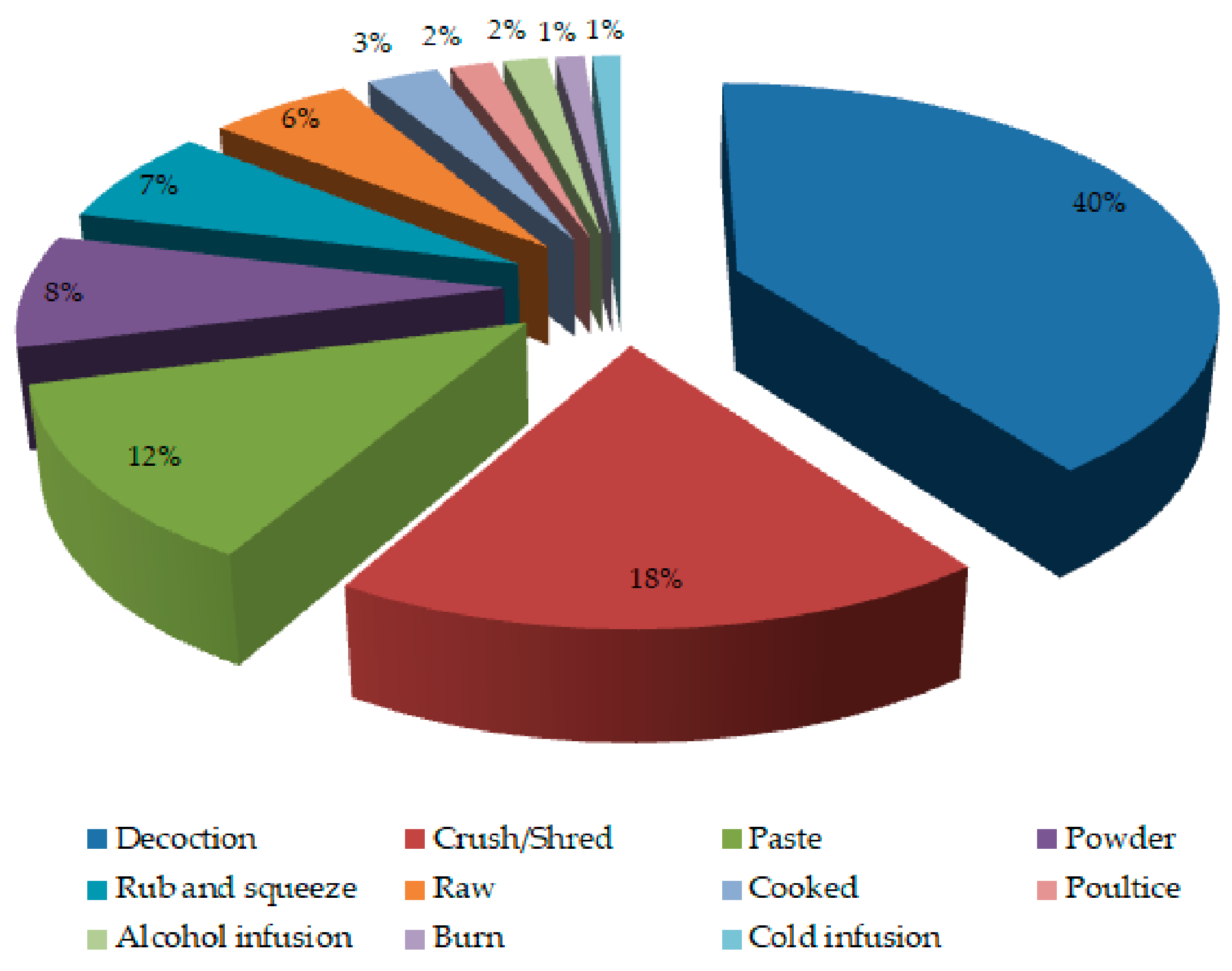
3.3. Mode of Medicinal Recipe Preparation and Administration
3.4. Ailments Treated by Medicinal Species
3.5. Comparison of Documented Data on Medicinal Plants with Previous Literature
3.6. Quantitative Ethnobotanical Indices
3.6.1. Use Value (UV)
3.6.2. Relative Frequency of Citation (RFC)
3.6.3. Fidelity Level (FL)
3.7. Allelopathy of the Medicinal Plants Collected
3.7.1. Potential Allelopathic Activity by Leachates of Medicinal Plant
3.7.2. Potential Allelopathic Effects of Volatile Constituents from Medicinal Plant
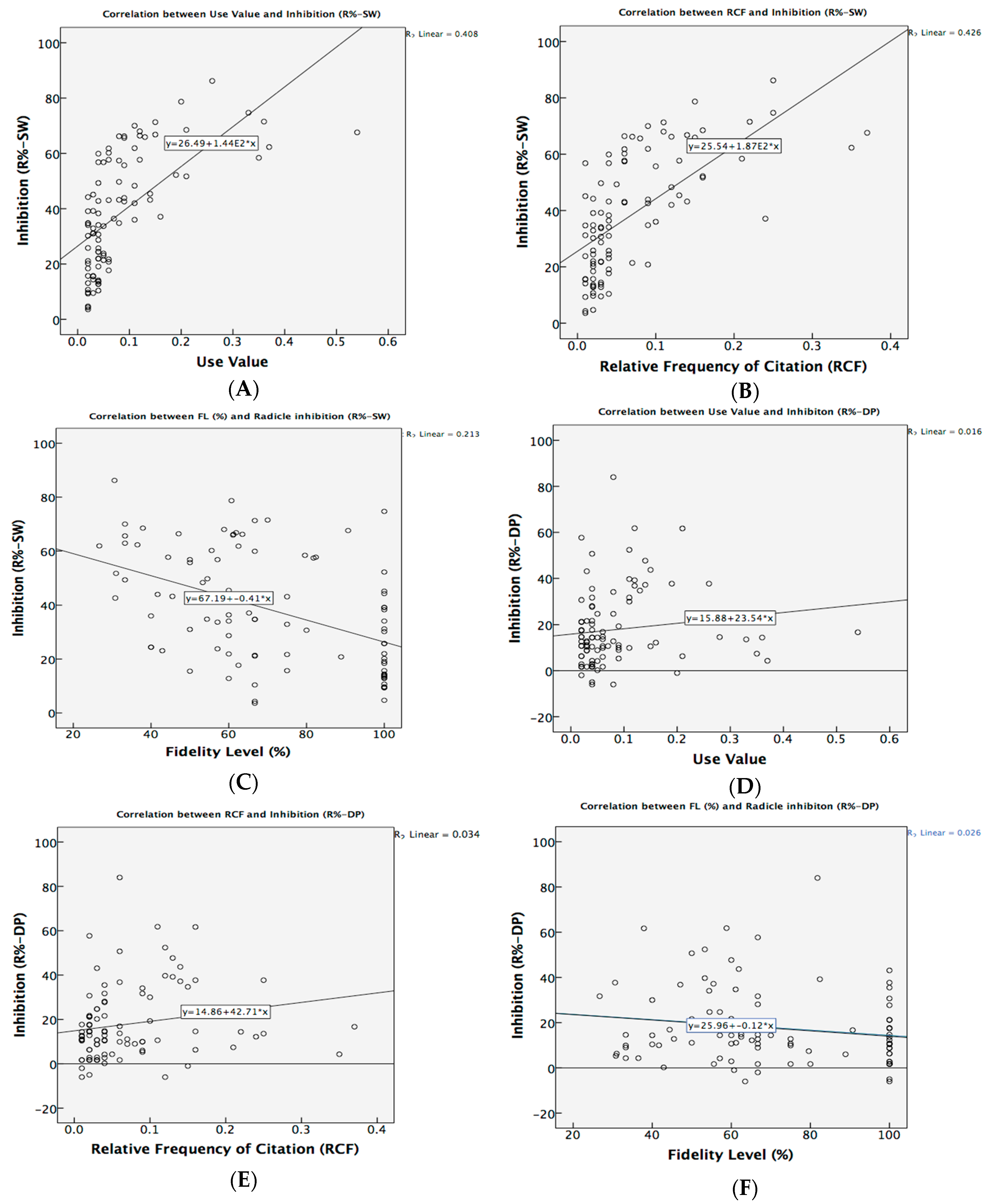
3.7.3. Correlation between Ethnobotanical Indices and Allelopathic Activity
3.7.4. Future Influence of the Present Study
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Traditional Medicine. Fact Sheet No. 134. Available online: http://www.who.int/mediacentre/factsheets/2003/fs134/en/ (accessed on 11 October 2016).
- Falodun, A. Herbal Medicine in Africa-Distribution, Standardization and Prospects. Res. J. Phytochem. 2010, 4, 154–161. [Google Scholar] [CrossRef]
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Calixto, J.B. Twenty-Five Years of Research on Medicinal Plants in Latin America: A Personal View. J. Ethnopharmacol. 2005, 100, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Hostettmann, K.; Terreaux, C. Search for New Lead Compounds from Higher Plants. Chimia 2000, 54, 652–657. [Google Scholar]
- Ghorbani, A. Studies on Pharmaceutical Ethnobotany in the Region of Turkmen Sahra, North of Iran (Part 1): General Results. J. Ethnopharmacol. 2005, 102, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Schippmann, U.; Leaman, D.J.; Cunningham, A.B. Impact of Culitvation and Gathering of Medicinal Plants on Biodiversity: Global Trends and Issues. In Proceedings of the Satellite Event on the Occasion of the Ninth Regular Session of the Commission on Genetic Resources for Food and Agriculture, Rome, Italy, 12–13 October 2002; pp. 12–13. [Google Scholar]
- Verpoorte, R. Pharmacognosy in the New Millennium: Leadfinding and Biotechnology. J. Pharm. Pharmacol. 2000, 52, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Fabricant, D.S.; Farnsworth, N.R. The Value of Plants Used in Traditional Medicine for Drug Discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A. Strategies for Discovering Drugs from Previously Unexplored Natural Products. Drug Discov. Today 2000, 5, 294–300. [Google Scholar] [CrossRef]
- Cox, P.A.; Balick, M.J. The Ethnobotanical Appproach to Drug Discovery. Sci. Am. 1994, 270, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Last 25 Years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Henkel, T.; Brunne, R.M.; Muller, H.; Reichel, F. Statistical Investigation into the Structural Complementarity of Natural Products and Synthetic Compounds. Angew. Chem. Int. Ed. 1999, 38, 675–678. [Google Scholar] [CrossRef]
- Sahoo, N.; Manchikanti, P.; Dey, S. Herbal Drugs: Standards and Regulation. Fitoterapia 2010, 81, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A. The Impact of Natural Products upon Modern Drug Discovery. Curr. Opin. Chem. Biol. 2008, 12, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Natural Products in Drug Discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.H.; Su, X. Artemisinin: Discovery from the Chinese Herbal Garden. Cell 2011, 146, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Talebzadeh, F. Anticonvulsant Evaluation of Safranal and Crocin from Crocus Sativus in Mice. Fitoterapia 2005, 76, 722–724. [Google Scholar] [CrossRef] [PubMed]
- Nöthlings, U.; Murphy, S.P.; Wilkens, L.R.; Henderson, B.E.; Kolonel, L.N. Flavonols and Pancreatic Cancer Risk: The Multiethnic Cohort Study. Am. J. Epidemiol. 2007, 166, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. Why Have No New Herbicide Modes of Action Appeared in Recent Years? Pest Manag. Sci. 2012, 68, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Bonny, S. Herbicide-Tolerant Transgenic Soybean over 15 Years of Cultivation: Pesticide Use, Weed Resistance, and Some Economic Issues. The Case of the USA. Sustainability 2011, 3, 1302–1322. [Google Scholar] [CrossRef]
- Vencill, W.K.; Nichols, R.L.; Webster, T.M.; Soteres, J.K.; Mallory-Smith, C.; Burgos, N.R.; Johnson, W.G.; McClelland, M.R. Herbicide Resistance: Toward an Understanding of Resistance Development and the Impact of Herbicide-Resistant Crops. Weed Sci. 2012, 60, 2–30. [Google Scholar] [CrossRef]
- Heap, I. Global Perspective of Herbicide-Resistant Weeds. Pest Manag. Sci. 2014, 70, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, B.C.; Sparks, T.C. Natural Products for Pest Control: An Analysis of Their Role, Value and Future. Pest Manag. Sci. 2014, 70, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Owens, D.K.; Duke, S.O. Rationale for a Natural Products Approach to Herbicide Discovery. Pest Manag. Sci. 2012, 68, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.A.; Duke, S.O. Weed and Crop Allelopathy. Crit. Rev. Plant Sci. 2003, 22, 367–389. [Google Scholar] [CrossRef]
- Zeng, R.S.; Mallik, A.U.; Luo, S.M. Allelopathy in Sustainable Agriculture and Forestry; Springer: New York, NY, USA, 2008. [Google Scholar]
- Fujii, Y. Allelopathy in the Natural and Agricultural Ecosystems and Isolation of Potent Allelochemicals from Velvet Bean (Mucuna Pruriens) and Hairy Vetch (Vicia Villosa). Biol. Sci. Space 2003, 17, 6–13. [Google Scholar] [CrossRef] [PubMed]
- International Allelopathy Society. International Allelopathy Society; What Is Allelopathy? Available online: http://allelopathy-society.osupytheas.fr/about/ (accessed on 3 July 2016).
- Weir, T.L.; Park, S.W.; Vivanco, J.M. Biochemical and Physiological Mechanisms Mediated by Allelochemicals. Curr. Opin. Plant Biol. 2004, 7, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Wang, Q.; Ruan, X.; De Pan, C.; Jiang, D.A. Phenolics and Plant Allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Fry, S.C. Potent Endogenous Allelopathic Compounds in Lepidium Sativum Seed Exudate: Effects on Epidermal Cell Growth in Amaranthus Caudatus Seedlings. J. Exp. Bot. 2012, 63, 2595–2604. [Google Scholar] [CrossRef] [PubMed]
- Mishyna, M.; Laman, N.; Prokhorov, V.; Fujii, Y. Angelicin as the Principal Allelochemical in Heracleum Sosnowskyi Fruit. Nat. Prod. Commun. 2015, 10, 1–5. [Google Scholar]
- Mishyna, M.; Laman, N.; Prokhorov, V.; Maninang, J.S.; Fujii, Y. Identification of Octanal as Plant Growth Inhibitory Volatile Compound Released from Heracleum Sosnowskyi Fruit. Nat. Prod. Commun. 2015, 10, 771–774. [Google Scholar] [PubMed]
- Mardani, H.; Azizi, M.; Sekine, T.; Mishyna, M.; Fujii, Y. Identification of Safranal as the Main Allelochemical from Saffron (Crocus Sativus). Nat. Prod. Commun. 2015, 10, 23–26. [Google Scholar]
- Rawat, L.S.; Maikhuri, R.K.; Negi, V.S.; Bahuguna, Y.M.; Pharswan, D.S.; Maletha, A. Allelopathic Performance of Medicinal Plants on Traditional Oilseed and Pulse Crop of Central Himalaya, India. Natl. Acad. Sci. Lett. 2016, 39, 141–144. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E.; Rimando, A.M.; Schrader, K.K.; Aliotta, G.; Oliva, A.; Romagni, J.G. Chemicals from Nature for Weed Management. Weed Sci. 2002, 50, 138–151. [Google Scholar] [CrossRef]
- Mitchell, G.; Bartlett, D.W.; Fraser, T.E.M.; Hawkes, T.R.; Holt, D.C.; Townson, J.K.; Wichert, R.A. Mesotrione: A New Selective Herbicide for Use in Maize. Pest Manag. Sci. 2001, 57, 120–128. [Google Scholar] [CrossRef]
- Grossmann, K.; Hutzler, J.; Tresch, S.; Christiansen, N.; Looser, R.; Ehrhardt, T. On the Mode of Action of the Herbicides Cinmethylin and 5-Benzyloxymethyl-1, 2-Isoxazolines: Putative Inhibitors of Plant Tyrosine Aminotransferase. Pest Manag. Sci. 2012, 68, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Romagni, J.G.; Dayan, F.E. Natural Products as Sources for New Mechanisms of Herbicidal Action. Crop Prot. 2000, 19, 583–589. [Google Scholar] [CrossRef]
- Hellyer, R. The Occurrence of β-Triketones in the Steam-Volatile Oils of Some Myrtaceous Australian Plants. Aust. J. Chem. 1968, 21, 2825–2828. [Google Scholar] [CrossRef]
- Cruz-Vega, D.; Verde-Star, M.J.; Salinas-Gonzalez, N.R.; Rosales-Hernandez, B.; Estrada-Garcia, I.; Mendez-Aragon, P.; Carranza-Rosales, P.; Gonzalez-Garza, M.; Castro-Garza, J. Review of Pharmacological Effects of Glycyrrhiza Radix and Its Bioactive Compounds. Zhongguo Zhong Yao Za Zhi 2009, 22, 557–559. [Google Scholar] [CrossRef]
- Amini, S.; Azizi, M.; Joharchi, M.R.; Shafei, M.N.; Moradinezhad, F.; Fujii, Y. Determination of Allelopathic Potential in Some Medicinal and Wild Plant Species of Iran by Dish Pack Method. Theor. Exp. Plant Physiol. 2014, 26, 189–199. [Google Scholar] [CrossRef]
- Appiah, K.; Li, Z.; Zeng, R.; Luo, S.; Oikawa, Y.; Fujii, Y. Determination of Allelopathic Potentials in Plant Species in Sino-Japanese Floristic Region by Sandwich Method and Dish Pack Method. Int. J. Basic Appl. Sci. 2015, 4, 381. [Google Scholar] [CrossRef]
- Bich, T.T.N.; Kato-Noguchi, H. Isolation and Identification of a Phytotoxic Substance from the Emergent Macrophyte Centrostachys Aquatica. Bot. Stud. 2014, 55, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Hamada, N.; Morita, M.; Suenaga, K. A Novel Allelopathic Substance, 13-Epi-Orthosiphol N, in Orthosiphon Stamineus. J. Plant Physiol. 2013, 170, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Pukclai, P.; Ohno, O.; Suenaga, K. Isolation and Identification of a Plant Growth Inhibitor from Tinospora Tuberculata Beumee. Acta Physiol. Plant. 2014, 36, 1621–1626. [Google Scholar] [CrossRef]
- General Social Survey. Ejisu-Juaben Municipal; District Analytical Report; General Social Survey: Chicago, IL, USA, 2014. [Google Scholar]
- Ghana Districts. Ghana Districts: A Repository of All Local Assemblies in Ghana. Available online: http://ghanadistricts.com/DistrictSublinks.aspx?s=2300&distID=18 (accessed on 4 October 2016).
- Ejisu Juaben Municipal Assembly (EJMA). Annual Progress Report for 2013; EJMA: Ejisu, Ghana, 2014. [Google Scholar]
- Martin, G.J. Ethnobotany: A Methods Manual; Chapman and Hall: London, UK, 1995. [Google Scholar]
- Alexaides, M.N. Collecting Ethnobotanical Data: An Introduction to Basic Concepts and Techniques. In Selected Guidelines for Ethnobotanical Research: A Field Manual; Alexiades, M.N., Ed.; The New York Botanical Garden: New York, NY, USA, 1996. [Google Scholar]
- Cunningham, A. Applied Ethnobotany: People, Wild Plant Use and Conservation. In People and Plants Conservation Manuals; Earthscan LLC: San Jose, CA, USA, 2001. [Google Scholar]
- Miller, A.G.; Nyberg, J.A. Collecting Herbarium Vouchers. Collect. Plant Genet. Divers. 1995, 561–573. [Google Scholar]
- Phillips, O.; Gentry, A.H.; Reynel, C.; Wilkin, P.; Galvez-Durand, B.C. Quantitative Ethnobotany and Amazonian Conservation. Conserv. Biol. 1994, 8, 225–248. [Google Scholar] [CrossRef]
- Ouelbani, R.; Bensari, S.; Mouas, T.N.; Khelifi, D. Ethnobotanical Investigations on Plants Used in Folk Medicine in the Regions of Constantine and Mila (North-East of Algeria). J. Ethnopharmacol. 2016, 194, 196–218. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Yaniv, Z.; Dafni, A.; Palewitch, D. A Preliminary Classification of the Healing Potential of Medicinal Plants, Based on a Rational Analysis of an Ethnopharmacological Field Survey among Bedouins in the Negev Desert, Israel. J. Ethnopharmacol. 1986, 16, 275–287. [Google Scholar] [CrossRef]
- Vitalini, S.; Iriti, M.; Puricelli, C.; Ciuchi, D.; Segale, A.; Fico, G. Traditional Knowledge on Medicinal and Food Plants Used in Val San Giacomo (Sondrio, Italy)—An Alpine Ethnobotanical Study. J. Ethnopharmacol. 2013, 145, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Tomè, F.; Fico, G. Traditional Uses of Medicinal Plants in Valvestino (Italy). J. Ethnopharmacol. 2009, 121, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.U.; Ijaz, F.; Afzal, A.; Iqbal, Z.; Ali, N.; Khan, S.M. Contributions to the Phytotherapies of Digestive Disorders: Traditional Knowledge and Cultural Drivers of Manoor Valley, Northern Pakistan. J. Ethnopharmacol. 2016, 192, 30–52. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Parvez, S.S.; Parvez, M.M.; Ohmae, Y.; Uda, O. Screening of 239 Medicinal Plant Species for Allelopathic Activity Using the Sandwich Method. Weed Biol. Manag. 2003, 3, 233–241. [Google Scholar] [CrossRef]
- Morikawa, C.I.O.; Miyaura, R.; de Lourdes Tapia y Figueroa, M.; Rengifo Salgado, E.L.; Fujii, Y. Screening of 170 Peruvian Plant Species for Allelopathic Activity by Using the Sandwich Method. Weed Biol. Manag. 2012, 12, 1–11. [Google Scholar] [CrossRef]
- Itani, T.; Nakahata, Y.; Kato-Noguchi, H. Allelopathic Activity of Some Herb Plant Species. Int. J. Agric. Biol. 2013, 15, 1359–1362. [Google Scholar]
- Fujii, Y.; Matsuyama, M.; Hiradate, S.; Shimozawa, H. Dish Pack Method: A New Bioassay for Volatile Allelopathy. In Establishing the Scientific Base; Wagga Wagga: New South Wales, Australia, 2005; pp. 493–497. [Google Scholar]
- Umer, A.; Yousaf, Z.; Khan, F.; Hussain, U.; Anjum, A.; Nayyab, Q.; Younas, A. Evaluation of Allelopathic Potential of Some Selected Medicinal Species. Afr. J. Biotechnol. 2010, 9, 6194–6206. [Google Scholar] [CrossRef]
- Fujii, Y.; Shibuya, T.; Nakatani, K.; Itani, T.; Hiradate, S.; Parvez, M.M. Assessment Method for Allelopathic Effect from Leaf Litter Leachates. Weed Biol. Manag. 2004, 4, 19–23. [Google Scholar] [CrossRef]
- Nadembega, P.; Boussim, J.I.; Nikiema, J.B.; Poli, F.; Antognoni, F. Medicinal Plants in Baskoure, Kourittenga Province, Burkina Faso: An Ethnobotanical Study. J. Ethnopharmacol. 2011, 133, 378–395. [Google Scholar] [CrossRef] [PubMed]
- Wodah, D.; Asase, A. Ethnopharmacological Use of Plants by Sisala Traditional Healers in Northwest Ghana. Pharm. Biol. 2012, 50, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Samoisy, A.K.; Mahomoodally, M.F. Ethnopharmacological Analysis of Medicinal Plants Used against Non-Communicable Diseases in Rodrigues Island, Indian Ocean. J. Ethnopharmacol. 2015, 173, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Asase, A.; Oteng-Yeboah, A.A.; Odamtten, G.T.; Simmonds, M.S.J. Ethnobotanical Study of Some Ghanaian Anti-Malarial Plants. J. Ethnopharmacol. 2005, 99, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.G.; Francis, A.; Kofi, A. Ethnobotanical Survey of Medicinal Plants Used for the Treatment of Diarrhoea and Skin Ulcer in the Brong Ahafo Region of Ghana. J. Med. Plants Res. 2013, 7, 3280–3285. [Google Scholar] [CrossRef]
- Ayyanar, M.; Ignacimuthu, S. Ethnobotanical Survey of Medicinal Plants Commonly Used by Kani Tribals in Tirunelveli Hills of Western Ghats, India. J. Ethnopharmacol. 2011, 134, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Hassan-Abdallah, A.; Merito, A.; Hassan, S.; Aboubaker, D.; Djama, M.; Asfaw, Z.; Kelbessa, E. Medicinal Plants and Their Uses by the People in the Region of Randa, Djibouti. J. Ethnopharmacol. 2013, 148, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Zizka, A.; Adjima, T.; Dressler, S.; Nacoulma, B.; Ouédraogo, A.; Ouédraogo, I.; Ouédraogo, O.; Zizka, G.; Hahn, K.; Schmidt, M. Traditional Plant Use in Burkina Faso (West Africa): A National-Scale Analysis with Focus on Traditional Medicine. J. Ethnobiol. Ethnomed. 2015, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Tugume, P.; Kakudidi, E.K.; Buyinza, M.; Namaalwa, J.; Kamatenesi, M.; Mucunguzi, P.; Kalema, J. Ethnobotanical Survey of Medicinal Plant Species Used by Communities around Mabira Central Forest Reserve, Uganda. J. Ethnobiol. Ethnomed. 2016, 12, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Asase, A.; Akwetey, G.A.; Achel, D.G. Ethnopharmacological Use of Herbal Remedies for the Treatment of Malaria in the Dangme West District of Ghana. J. Ethnopharmacol. 2010, 129, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Asase, A.; Asafo-Agyei, T. Plants Used for Treatment of Malaria in Communities around the Bobiri Forest Reserve in Ghana. J. Herbs Spices Med. Plants 2011, 17, 85–106. [Google Scholar] [CrossRef]
- Barku, V.Y.A.; Opoku-Boahen, Y.; Dali, G. Ethnobotanical Study of Wound Healing Plants in Kpando Traditional, Area, Ghana. Int. J. Phytomed. 2014, 6, 564–572. [Google Scholar]
- Malan, D.F.; Neuba, D.F.R. Traditional Practices and Medicinal Plants Use during Pregnancy by Anyi-Ndenye Women (Eastern Côte d’Ivoire). Afr. J. Reprod. Health 2011, 15, 85–93. [Google Scholar] [PubMed]
- Batool, A.; Shah, A.; Bahadur, A. Ethnopharmacological Relevance of Traditional Medicinal Flora from Semi-Tribal Areas in Khyber Pakhtunkhwa, Punjab, Pakistan. Pak. J. Bot. 2017, 49, 691–705. [Google Scholar]
- Giday, M.; Asfaw, Z.; Woldu, Z.; Teklehaymanot, T. Medicinal Plant Knowledge of the Bench Ethnic Group of Ethiopia: An Ethnobotanical Investigation. J. Ethnobiol. Ethnomed. 2009, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, T.; Licata, M.; Leto, C.; Savo, V.; Bonsangue, G.; Letizia Gargano, M.; Venturella, G.; La Bella, S. Ethnobotanical Investigation on Wild Medicinal Plants in the Monti Sicani Regional Park (Sicily, Italy). J. Ethnopharmacol. 2014, 153, 568–586. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.; Shrestha, S.; Koju, L.; Shrestha, K.K.; Wang, Z. Medicinal Plant Diversity and Traditional Healing Practices in Eastern Nepal. J. Ethnopharmacol. 2016, 192, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Cheikhyoussef, A.; Shapi, M.; Matengu, K.; Ashekele, H.M. Ethnobotanical Study of Indigenous Knowledge on Medicinal Plant Use by Traditional Healers in Oshikoto Region, Namibia. J. Ethnobiol. Ethnomed. 2011, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A. People, Park and Plant Use. Recommendations for Multiple-Use Zones and Development Alternatives around Bwindi Impenetrable National Park, Uganda; People and Plants Working Paper 4; UNESCO: Paris, France, 1996. [Google Scholar]
- Agyare, C.; Spiegler, V.; Sarkodie, H.; Asase, A.; Liebau, E.; Hensel, A. An Ethnopharmacological Survey and in Vitro Confirmation of the Ethnopharmacological Use of Medicinal Plants as Anthelmintic Remedies in the Ashanti Region, in the Central Part of Ghana. J. Ethnopharmacol. 2014, 158, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Chekole, G.; Asfaw, Z.; Kelbessa, E. Ethnobotanical Study of Medicinal Plants in the Environs of Tara-Gedam and Amba Remnant Forests of Libo Kemkem District, Northwest Ethiopia. J. Ethnobiol. Ethnomed. 2015, 11, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Malla, B.; Gauchan, D.P.; Chhetri, R.B. An Ethnobotanical Study of Medicinal Plants Used by Ethnic People in Parbat District of Western Nepal. J. Ethnopharmacol. 2015, 165, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M. Ethnopharmacobotanical Study on the Medicinal Plants Used by Herbalists in Sulaymaniyah Province, Kurdistan, Iraq. J. Ethnobiol. Ethnomed. 2016, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Van Andel, T.; Van Onselen, S.; Myren, B.; Towns, A.; Quiroz, D. The Medicine from behind: The Frequent Use of Enemas in Western African Traditional Medicine. J. Ethnopharmacol. 2015, 174, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Abel, C.; Busia, K. An Exploratory Ethnobotanical Study of the Practice of Herbal Medicine by the Akan Peoples of Ghana. Altern. Med. Rev. 2005, 10, 112–122. [Google Scholar] [PubMed]
- Darko, I.N. Ghanaian Indigenous Health Practices: The Use of Herbs; University of Toronto: Toronto, ON, Canada, 2009. [Google Scholar]
- Foote, K.N. Traditional Medicine in Contemporary Ghanaian Society: Practices, Problems, and Future Outlook. Available online: https://www.abibitumikasa.com/forums/showthread.php/43721-Traditional-Medicine-in-Contemporary-Ghanaian-Society-Practices-Problems-Future (accessed on 26 October 2016).
- Abo, K.A.; Fred-Jaiyesimi, A.A.; Jaiyesimi, A.E.A. Ethnobotanical Studies of Medicinal Plants Used in the Management of Diabetes Mellitus in South Western Nigeria. J. Ethnopharmacol. 2008, 115, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Asase, A.; Oteng-Yeboah, A.A. Plants Used in Wechiau Community Hippopotamus Sanctuary in Northwest Ghana. Ethnobot. Res. Appl. 2012, 10, 605–618. [Google Scholar]
- Tabuti, J.R.S. Herbal Medicines Used in the Treatment of Malaria in Budiope County, Uganda. J. Ethnopharmacol. 2008, 116, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Odugbemi, T.O.; Ibukun, O.R.A.; Aibinu, E.F.P. Medicinal Plants Useful for Malaria Therapy in Okeigbo. Ondo State, Southwest Nigeria. Afr. J. Tradit. CAM 2007, 4, 191–198. [Google Scholar] [CrossRef]
- Kirira, P.G.; Rukunga, G.M.; Wanyonyi, A.W.; Muregi, F.M.; Gathirwa, J.W.; Muthaura, C.N.; Omar, S.A.; Tolo, F.; Mungai, G.M.; Ndiege, I.O. Anti-Plasmodial Activity and Toxicity of Extracts of Plants Used in Traditional Malaria Therapy in Meru and Kilifi Districts of Kenya. J. Ethnopharmacol. 2006, 106, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Komlaga, G. Search for Antiplasmodial Compounds from Ghanaian Medicinal Plants. Ph.D. Thesis, Université Paris Saclay, Paris, France, 2015. [Google Scholar]
- Oyelakin, A.O.; Tairu, H.M.; Olawale, B.R. Ethno Botanical Survey of Plants Used For Malaria Treatment in Igboora, Ibarapa Central Local Government of Oyo State, Nigeria. Int. J. Environ. Agric. Biotechnol. 2016, 1, 411–417. [Google Scholar]
- Koné, W.M.; Koffi, A.G.; Bomisso, E.L.; Bi, T.F.H. Ethnomedical Study and Iron Content Of some Medicinal Herbs Used in Traditional Medicine in Cote d’ Ivoire For tteatment of Anemia. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Komlaga, G.; Agyare, C.; Dickson, R.A.; Mensah, M.L.K.; Annan, K.; Loiseau, P.M.; Champy, P. Medicinal Plants and Finished Marketed Herbal Products Used in the Treatment of Malaria in the Ashanti Region, Ghana. J. Ethnopharmacol. 2015, 172, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Tsabang, N.; Fokou, P.V.T.; Tchokouaha, L.R.Y.; Noguem, B.; Bakarnga-Via, I.; Nguepi, M.S.D.; Nkongmeneck, B.A.; Boyom, F.F. Ethnopharmacological Survey of Annonaceae Medicinal Plants Used to Treat Malaria in Four Areas of Cameroon. J. Ethnopharmacol. 2012, 139, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Soladoye, M.O.; Adetayo, M.O.; Chukwuma, E.C.; Adetunji, A.N. Ethnobotanical Survey of Plants Used in the Treatment of Haemorrhoids in South-Western Nigeria. Ann. Biol. Res. 2010, 1, 1–15. [Google Scholar]
- Idowu, O.A.; Soniran, O.T.; Ajana, O.; Aworinde, D.O. Ethnobotanical Survey of Antimalarial Plants Used in Ogun State, Southwest Nigeria. Afr. J. Pharm. Pharmacol. 2010, 4, 55–60. [Google Scholar]
- Mowobi, G.G.; Abubakar, S.; Osuji, C.; Etim, V.N.; Ogechi, N.; Egya, J.J. Ethnobotanical Survey of Medicinal Plants Used for the Treatment of Skin Disease in Keffi, Nigeria. Am. J. Phytomed. Clin. Ther. 2013, 4, 73–90. [Google Scholar] [CrossRef]
- Khan, M.A.; Islam, M.K.; Siraj, M.A.; Saha, S.; Barman, A.K.; Awang, K.; Rahman, M.M.; Shilpi, J.A.; Jahan, R.; Islam, E.; et al. Ethnomedicinal Survey of Various Communities Residing in Garo Hills of Durgapur, Bangladesh. J. Ethnobiol. Ethnomed. 2015, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Lans, C. Comparison of Plants Used for Skin and Stomach Problems in Trinidad and Tobago with Asian Ethnomedicine. J. Ethnobiol. Ethnomed. 2007, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Khuankaew, S.; Srithi, K.; Tiansawat, P.; Jampeetong, A.; Inta, A.; Wangpakapattanawong, P. Ethnobotanical Study of Medicinal Plants Used by Tai Yai in Northern Thailand. J. Ethnopharmacol. 2014, 151, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.L.; Wei, J.H.; Sun, W.; Li, R.T.; Liu, S.B.; Dai, H.F. Ethnobotanical Study on Medicinal Plants around Limu Mountains of Hainan Island, China. J. Ethnopharmacol. 2013, 148, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Jiofack, T.; Fokunang, C.; Guedje, N.; Kemeuze, V.; Fongnzossie, E.; Nkongmeneck, B.A.; Mapongmetsem, P.M.; Tsabang, N. Ethnobotanical Uses of Medicinal Plants of Two Ethnoecological Regions of Cameroon. Int. J. Med. Med. Sci. 2010, 2, 60–79. [Google Scholar]
- Nwauzoma, A.B.; Dappa, M.S. Ethnobotanical Studies of Port Harcourt Metropolis, Nigeria. ISRN Bot. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Ross, I.A. Chemical Constituents, Traditional and Modern Medicinal Uses; Humana Press: New York, NY, USA, 2005; Volume 3. [Google Scholar]
- Ahmad, M.; Khan, M.P.Z.; Mukhtar, A.; Zafar, M.; Sultana, S.; Jahan, S. Ethnopharmacological Survey on Medicinal Plants Used in Herbal Drinks among the Traditional Communities of Pakistan. J. Ethnopharmacol. 2016, 184, 154–186. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.S.; Abdelrasool, F.E.; Elsheikh, E.A.; Ahmed, L.A.M.N.; Mahmoud, A.L.E.; Yagi, S.M. Ethnobotanical Study of Medicinal Plants in the Blue Nile State, South-Eastern Sudan. J. Med. Plants Res. 2011, 5, 4287–4297. [Google Scholar]
- Albuquerque, U.P.; Lucena, R.F.P.; Monteiro, J.M.; Florentino, A.T.N.; Almeida, C.D.F.C.B.R. Evaluating Two Quantitative Ethnobotanical Techniques. Ethnobot. Res. Appl. 2006, 4, 51–60. [Google Scholar] [CrossRef]
- Rahman, I.U.; Ijaz, F.; Iqbal, Z.; Afzal, A.; Ali, N.; Afzal, M.; Khan, M.A.; Muhammad, S.; Qadir, G.; Asif, M. A Novel Survey of the Ethno Medicinal Knowledge of Dental Problems in Manoor Valley (Northern Himalaya), Pakistan. J. Ethnopharmacol. 2016, 194, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Correia, N.M.; Centurion, M.A.P.D.C.; Alves, P.L.D.C.A. Influência de Extratos Aquosos de Sorgo Sobre a Germinação E O Desenvolvimento de Plântulas de Soja. Ciênc. Rural 2005, 35, 498–503. [Google Scholar] [CrossRef]
- Wabo, P.J.; Ngankam, N.J.D.; Bilong, B.C.F.; Mpoame, M. A Comparative Study of the Ovicidal and Larvicidal Activities of Aqueous and Ethanolic Extracts of Pawpaw Seeds Carica Papaya (Caricaceae) on Heligmosomoides Bakeri. Asian Pac. J. Trop. Med. 2011, 4, 447–450. [Google Scholar] [CrossRef]
- Canini, A.; Alesiani, D.; D’Arcangelo, G.; Tagliatesta, P. Gas Chromatography-Mass Spectrometry Analysis of Phenolic Compounds from Carica papaya L. Leaf. J. Food Compos. Anal. 2007, 20, 584–590. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavanoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) Content of Edible Tropical Plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, B.; Barros, L.; Calhelha, R.C.; Heleno, S.; Alves, M.J.; Walcott, S.; Bittova, M.; Kuban, V.; Ferreira, I.C.F.R. Bioactive Properties and Phenolic Profile of Momordica charantia L. Medicinal Plant Growing Wild in Trinidad and Tobago. Ind. Crops Prod. 2017, 95, 365–373. [Google Scholar] [CrossRef]
- Mordue, A.J.; Simmonds, M.S.J.; Ley, S.V.; Blaney, W.M.; Mordue, W.; Nasiruddin, M.; Nisbet, A.J. Actions of Azadirachtin, a Plant Allelochemical, against Insects. Pestic. Sci. 1998, 54, 277–284. [Google Scholar] [CrossRef]
- Okoye, N.N.; Ajaghaku, D.L.; Okeke, H.N.; Ilodigwe, E.E.; Nworu, C.S.; Okoye, F.B.C. Beta-Amyrin and Alpha-Amyrin Acetate Isolated from the Stem Bark of Alstonia Boonei Display Profound Anti-Inflammatory Activity. Pharm. Biol. 2014, 52, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Adotey, J.P.K.; Adukpo, G.E.; Opoku Boahen, Y.; Armah, F.A. A Review of the Ethnobotany and Pharmacological Importance of Alstonia Boonei De Wild (Apocynaceae). ISRN Pharmacol. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Amusan, O.O.G.; Adesogan, E.K.; Makinde, J.M. Antimalarial Active Principles of Spathodea Campanulata Stem Bark. Phyther. Res. 1996, 10, 692–693. [Google Scholar] [CrossRef]
- Pianaro, A.; Pinto, J.P.; Ferreira, D.T.; Ishikawa, N.K.; Braz-Filho, R. Iridóide Glicosilado E Derivados Fenólicos Antifúngicos Isolados Das Raízes de Spathodea Campanulata. Semin. Ciênc. Agrár. 2007, 28, 251. [Google Scholar] [CrossRef]
- Dongfack, M.D.J.; Lallemand, M.-C.; Kuete, V.; Mbazoa, C.D.; Wansi, J.-D.; Trinh-van-Dufat, H.; Michel, S.; Wandji, J. A New Sphingolipid and Furanocoumarins with Antimicrobial Activity from Ficus Exasperata. Chem. Pharm. Bull. 2012, 60, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Arshad, M.; Saboor, A.; Qureshi, R.; Mustafa, G.; Sadiq, S.; Chaudhari, S.K. Ethnobotanical Appraisal and Medicinal Use of Plants in Patriata, New Murree, Evidence from Pakistan. J. Ethnobiol. Ethnomed. 2013, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Olugbemiga, O.S.; Grace, O.D.; Adeola, T.A.; Ibibia, E.-I.T.; Akhere, O.J.; Adeiza, O.D.; Oluchi, A.Y.; Obiora, N.C.; Stephen, A.O. Antidiabetic and Antidyslipidemic Effect of Ethanolic Extract of Alternanathera Pungens on Alloxan-Induced Diabetic Rats. Asian J. Biochem. 2016, 11, 82–89. [Google Scholar] [CrossRef]
- Petrus, A.; Seetharaman, T. Antioxidant Flavone C-Biosides from the Aerial Parts of Alternanthera Pungens. Indian J. Pharm. Sci. 2005, 67, 187–193. [Google Scholar]
- Bakht, J.; Islam, A.; Shafi, M. Antimicrobial Potentials of Eclipta Alba By Well Diffusion Method. Pak. J. Bot. 2011, 43, 169–174. [Google Scholar] [CrossRef]
- Mithun, N.; Shashidhara, S.; Vivek Kumar, R. Eclipta Alba (L.) A Review on Its Phytochemical and Pharmacological Profile. Pharmacologyonline 2011, 357, 345–357. [Google Scholar]
- Tomer, K.; Singh, V.; Sethiya, N.; Kumar, M.; Jaiswal, D.; Yadav, I.; Singh, H.; Chandra, D.; Jain, D. Isolation and Characterization of New Lanosteriod from Ethanolic Extract of Eclipta Linn. J. Pharm. Res. 2009, 2, 1635–1637. [Google Scholar]
- Jadhav, V.M.; Thorat, R.M.; Kadam, V.J.; Salaskar, K.P. Chemical Composition, Pharmacological Activities of Eclipta Alba. J. Pharm. Res. 2009, 2, 18–20. [Google Scholar]
- Bagavan, A.; Rahuman, A.A.; Kamaraj, C.; Kaushik, N.K.; Mohanakrishnan, D.; Sahal, D. Antiplasmodial Activity of Botanical Extracts against Plasmodium Falciparum. Parasitol. Res. 2011, 108, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Hout, S.; Chea, A.; Bun, S.S.; Elias, R.; Gasquet, M.; Timon-David, P.; Balansard, G.; Azas, N. Screening of Selected Indigenous Plants of Cambodia for Antiplasmodial Activity. J. Ethnopharmacol. 2006, 107, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Cheng, H.Y.; Lin, T.C.; Chiang, L.C.; Lin, C.C. Hippomanin A from Acetone Extract of Phyllanthus Urinaria Inhibited HSV-2 but Not HSV-1 Infection in Vitro. Phyther. Res. 2007. [Google Scholar] [CrossRef] [PubMed]
- Tieman, D.M.; Zeigler, M.; Schmelz, E.A.; Taylor, M.G.; Bliss, P.; Kirst, M.; Klee, H.J. Identification of Loci Affecting Flavour Volatile Emissions in Tomato Fruits. J. Exp. Bot. 2006, 57, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, P.M.; Diergaarde, P.J.; Ament, K.; Guerra, J.; Weidner, M.; Schutz, S.; de Both, M.T.J.; Haring, M.A.; Schuurink, R.C. The Role of Specific Tomato Volatiles in Tomato-Whitefly Interaction. Plant Physiol. 2009, 151, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Naimon, N.; Pongchairerk, U.; Suebkhampet, A. Phytochemical Analysis and Antibacterial Activity of Ethanolic Leaf Extract of Solanum Torvum Sw. Against Pathog. Bact. 2015, 523, 516–523. [Google Scholar]
- Ojewole, J.A.O.; Adewunmi, C.O. Anti-Inflammatory and Hypoglycaemic Effects of Tetrapleura Tetraptera (Taub) [Fabaceae] Fruit Aqueous Extract in Rats. J. Ethnopharmacol. 2004, 95, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Udourioh, G.; Etokudoh, M. Essential Oils and Fatty Acids Composition of Dry Fruits of Tetrapleura Tetraptera. J. Appl. Sci. Environ. Manag. 2014, 18, 419–424. [Google Scholar]
- Amjad, M.S.; Qaeem, M.; Ahmad, I.; Khan, S.U.; Chaudhari, S.K.; Malik, N.Z.; Shaheen, H.; Khan, M. Descriptive Study of Plant Resources in the Context of the Ethnomedicinal Relevance of Indigenous Flora: A Case Study from Toli Peer National Park, Azad Jammu and Kashmir, Pakistan. PLoS ONE 2017, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V.; Bezard, E.; Brotchie, J.; Calon, F.; Collingridge, G.L.; Ferger, B.; Hengerer, B.; Hirsch, E.; Jenner, P.; Novère, N.L.; et al. Novel Pharmacological Targets for the Treatment of Parkinson’s Disease. Nat. Rev. Drug Discov. 2006, 5, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Romagni, J.G.; Allen, S.N.; Dayan, F.E. Allelopathic Effects of Volatile Cineoles on Two Weedy Plant Species. J. Chem. Ecol. 2000, 26, 303–313. [Google Scholar] [CrossRef]
- Duke, S.; Vaughn, K.C.; Croom, E.M.; Elsohly, H.N. Artemisinin, A Constituent of Annual Wormwood (Artemisia Annua), Is a Selective Phytotoxin. Weed Sci. 1987, 35, 499–505. [Google Scholar]

| Category | Description | Frequency (%) |
|---|---|---|
| Age (years) | 20–30 | 14 (10) |
| 31–40 | 31 (22.1) | |
| 41–50 | 23 (16.4) | |
| 51–60 | 32 (22.9) | |
| 61+ | 40 (28.6) | |
| Gender | Male | 50 (35.7) |
| Female | 90 (64.3) | |
| Education | None | 54 (38.6) |
| Primary | 69 (49.3) | |
| Secondary | 16 (11.4) | |
| Tertiary | 1 (0.7) | |
| Religion | Christian | 125 (89.2) |
| Muslim | 9 (6.4) | |
| No religious affiliation | 6 (4.3) | |
| Ethnicity | Akan | 132 (94.3) |
| Mamprusi | 4 (2.9) | |
| Krobo | 1 (0.7) | |
| Wangala | 2 (1.4) | |
| Grusi | 1 (0.7) | |
| Occupation | Farming | 78 (54.5) |
| Trading | 35 (24.5) | |
| Teaching | 4 (2.8) | |
| Kente weaving | 3 (2.1) | |
| Traditional healer | 15 (10.5) | |
| Hairdressing | 4 (2.8) | |
| Student | 1 (0.7) |
| Scientific Name, Voucher No. (Family) | Local Names | Part(s) Used | Key Ailment(s) Treated | Preparation and Administration | FL | UV | RFC |
|---|---|---|---|---|---|---|---|
| Cleistopholis patens (Benth.) Engl. & Diels CPMR 4070 (Annonaceae) | Ngone nkyene | L/SB, F R/SB, Se | Malaria, Hernia | Decoction (oral) Paste (enema) | 90.7 | 0.54 | 0.37 |
| Ocimum gratissimum L. CPMR 4131 (Lamiaceae) | Nunum | L, Se | Diarrhoea, malaria Convulsion | Decoction (oral) Rub (topical) | 38.5 | 0.37 | 0.35 |
| Carica papaya L. CPMR 4112 (Caricaceae) | Brɔferɛ | L R, Se | Malaria Anthelmintic | Cook (oral) Raw (oral) | 30.6 | 0.26 | 0.25 |
| Azadirachta indica A. Juss. CPMR 4067 (Meliaceae) | Neem tree | L, SB | Malaria | Decoction (oral) | 100 | 0.33 | 0.25 |
| Vernonia amygdalina Delile CPMR 4088 (Asteraceae) | Awɔnwono | L | Diarrhoea, malaria | Decoction (oral) | 65.2 | 0.16 | 0.24 |
| Alstonia boonei De Wild. CPMR 4096 (Apocynaceae) | Nyamedua | SB | Measles, shingles Malaria | Crush (Topical) Decoction (oral) | 70 | 0.36 | 0.22 |
| Senna occidentalis (L.) Link CPMR 4082 (Fabaceae) | Nkwadaa brodeɛ | L R | Diarrhoea, malaria cough | Decoction (oral) Raw (oral) | 79.6 | 0.35 | 0.21 |
| Senna alata (L.) Roxb. CPMR 4083 (Fabaceae) | Sempe | L | Eczema, rashes Stomach-aches | Poultice (topical) Decoction (oral) | 33.3 | 0.28 | 0.16 |
| Alchornea cordifolia (Schumach. & Thonn.) Müll.Arg. CPMR 4065 (Euphorbiaceae) | Ogyama | L, SB SB | Constipation Fever, malaria Wounds, piles | Paste (enema) Decoction (oral) Rub (topical) | 31.0 | 0.21 | 0.16 |
| Phyllanthus muellerianus (Kuntze.) Exell. CPMR 4121 (Euphorbiaceae) | Awobɛ | L | Wounds | Squeeze (topical) | 100 | 0.03 | 0.01 |
| Eclipta alba Hassk. CPMR 4106 (Asteraceae) | Ntum | L | Eye diseases Constipation | Juice (topical) Paste (enema) | 50.0 | 0.04 | 0.11 |
| Spathodea campanulata P. Beauv. CPMR 4084 (Bignoniaceae) | Kuakuaninsuo | L, SB | Typhoid, malaria Wounds | Decoction (oral) Paste (topical) | 66.7 | 0.15 | 0.11 |
| Mangifera indica L. CPMR 4095 (Anacardiaceae) | Amango | SB L | Diarrhoea Cough, fever | Paste (Enema) Decoction (oral) | 50.0 | 0.09 | 0.1 |
| Musa paradisiaca L. CPMR 4146 (Musaceae) | Brɔdeε | L, R, Fl L, R | Wounds Fever, headache | Paste (topical) Decoction (oral) | 40.0 | 0.11 | 0.10 |
| Newbouldia laevis (P.Beauv.) Seem. CPMR 4109 (Bignoniaceae) | Sɛsɛmasa | L SB | Stomach upset Cough | Paste (enema) Raw (oral) | 26.7 | 0.11 | 0.09 |
| Family | Scientific Names | Part Used | Allelopathy (% Growth Inhibition) | |||||
|---|---|---|---|---|---|---|---|---|
| SW (10 mg/10 mL Agar) | Dish Pack (200 mg/DP) | |||||||
| R% | H% | Criteria * | R% | H% | Criteria * | |||
| Caricaceae | Carica papaya | L | 86.2 | 62.4 | **** | 37.7 | 24.8 | ** |
| Cucurbitaceae | Momordica charantia | L | 78.7 | 30.1 | *** | −1.0 | −4.0 | |
| Meliaceae | Azadirachta indica | L | 74.7 | 25.9 | *** | 13.6 | −1.0 | |
| Apocynaceae | Alstonia boonei | SB | 71.5 | 35.8 | *** | 14.4 | 8.2 | |
| Bignoniaceae | Spathodea campanulata | L | 71.3 | 16.0 | *** | 10.6 | 21.5 | |
| Menispermaceae | Ficus exasperata | L, | 70.0 | 19.5 | *** | 9.9 | 2.9 | |
| Euphorbiaceae | Phyllanthus urinaria | L | 68.5 | 47.7 | ** | 61.7 | 43.1 | **** |
| Asteraceae | Eclipta alba | L | 68.0 | 57.7 | ** | 61.8 | 47.7 | **** |
| Annonaceae | Cleistopholis patens | L | 67.6 | 12.7 | ** | 16.7 | 1.6 | |
| Crassulaceae | Kalanchoe integra | L | 66.8 | 59.8 | ** | 43.7 | 14.7 | *** |
| Amaranthaceae | Amaranthus spinosus | Wp | 66.4 | 57.3 | ** | 36.8 | 1.70 | ** |
| Asteraceae | Chromolaena odorata | L | 66.2 | 14.2 | ** | 11.1 | 1.60 | |
| Sapindaceae | Paullinia pinnata | R | 66.2 | 29.6 | ** | −6.0 | −8.0 | |
| Poaceae | Cymbopogon citratus | L | 65.9 | 47.7 | ** | 34.7 | 1.30 | ** |
| Bignoniaceae | Heliotropium indicum | L | 65.6 | −5.0 | ** | 9.0 | 4.70 | |
| Fabaceae | Senna alata | L | 62.9 | 22.6 | ** | 14.6 | 13.8 | |
| Lamiaceae | Ocimum gratissimum | L | 62.3 | −24.0 | ** | 4.3 | 18.2 | |
| Bignoniaceae | Newbouldia laevis | L | 61.9 | 49.0 | ** | 31.7 | 21.1 | * |
| Rutaceae | Zanthoxylum leprieurii | SB | 61.8 | 53.8 | ** | 13.7 | 1.70 | |
| Rutaceae | Citrus aurantiifolia | F | 60.2 | 46.7 | ** | 1.70 | −30.0 | |
| Combretaceae | Terminalia catappa | L | 59.9 | 37.8 | ** | 28.1 | 2.20 | * |
| Fabaceae | Senna occidentalis | L | 58.4 | −10.0 | * | 7.40 | 10.3 | |
| Loranthaceae | Tapinanthus bangwenis | Wp | 57.7 | 34.8 | * | 39.2 | 21.1 | ** |
| Euphorbiaceae | Manihot esculenta | L | 57.7 | 24.7 | * | 16.9 | 9.20 | |
| Amaranthaceae | Alternanthera pungens | L | 57.4 | 19.1 | *: | 84.0 | 63.0 | **** |
| Malvaceae | Sida acuta | L | 56.8 | 4.40 | * | 4.20 | 9.0 | |
| Asteraceae | Ageratum conyzoides | L | 56.8 | −2.0 | * | 17.7 | 1.80 | |
| Anacardiaceae | Mangifera indica L. | L | 55.7 | 21.1 | * | 19.3 | 17.5 | |
| Euphorbiaceae | Alchornea cordifolia | L | 51.7 | 21.8 | * | 6.30 | 7.50 | |
| Verbanaceae | Tectona grandis | L | 52.2 | 11.1 | * | 37.7 | 15.7 | ** |
| Correlations | Inhibition-SW | Inhibition-DP | UV | FL | RFC | |
|---|---|---|---|---|---|---|
| Inhibition-SW | Pearson Correlation | 1.0 | 0.186 | 0.639 ** | −0.462 ** | 0.653 ** |
| Sig. (2-tailed) | 0.071 | 0.000 | 0.000 | 0.000 | ||
| N | 95.0 | 95.0 | 95.0 | 95.0 | 95.0 | |
| Inhibition-DP | Pearson Correlation | 0.186 | 1.0 | 0.128 | −0.161 | 0.183 |
| Sig. (2-tailed) | 0.071 | 0.215 | 0.119 | 0.075 | ||
| N | 95.0 | 95.0 | 95.0 | 95.0 | 95.0 | |
| UV | Pearson Correlation | 0.639 ** | 0.128 | 1.0 | −0.242 * | 0.941 ** |
| Sig. (2-tailed) | 0.000 | 0.215 | 0.018 | 0.000 | ||
| N | 95.0 | 95.0 | 95.0 | 95.0 | 95.0 | |
| FL | Pearson Correlation | −0.462 ** | −0.161 | −0.242 * | 1 | −0.284 ** |
| Sig. (2-tailed) | 0.000 | 0.119 | 0.018 | 0.005 | ||
| N | 95.0 | 95.0 | 95.0 | 95.0 | 95.0 | |
| RFC | Pearson Correlation | 0.653 ** | 0.183 | 0.941 ** | −0.284 ** | 1.0 |
| Sig. (2-tailed) | 0.000 | 0.075 | 0.000 | 0.005 | ||
| N | 95.0 | 95.0 | 95.0 | 95.0 | 95.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appiah, K.S.; Mardani, H.K.; Osivand, A.; Kpabitey, S.; Amoatey, C.A.; Oikawa, Y.; Fujii, Y. Exploring Alternative Use of Medicinal Plants for Sustainable Weed Management. Sustainability 2017, 9, 1468. https://doi.org/10.3390/su9081468
Appiah KS, Mardani HK, Osivand A, Kpabitey S, Amoatey CA, Oikawa Y, Fujii Y. Exploring Alternative Use of Medicinal Plants for Sustainable Weed Management. Sustainability. 2017; 9(8):1468. https://doi.org/10.3390/su9081468
Chicago/Turabian StyleAppiah, Kwame Sarpong, Hossein Korrani Mardani, Asma Osivand, Sylvia Kpabitey, Christiana Adukwei Amoatey, Yosei Oikawa, and Yoshiharu Fujii. 2017. "Exploring Alternative Use of Medicinal Plants for Sustainable Weed Management" Sustainability 9, no. 8: 1468. https://doi.org/10.3390/su9081468
APA StyleAppiah, K. S., Mardani, H. K., Osivand, A., Kpabitey, S., Amoatey, C. A., Oikawa, Y., & Fujii, Y. (2017). Exploring Alternative Use of Medicinal Plants for Sustainable Weed Management. Sustainability, 9(8), 1468. https://doi.org/10.3390/su9081468






