Efficiency of Iron-Based Oxy-Hydroxides in Removing Antimony from Groundwater to Levels below the Drinking Water Regulation Limits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbents
2.2. Batch Experiments
2.3. Kinetics
2.4. Column Tests
2.5. Leaching Behavior
3. Results
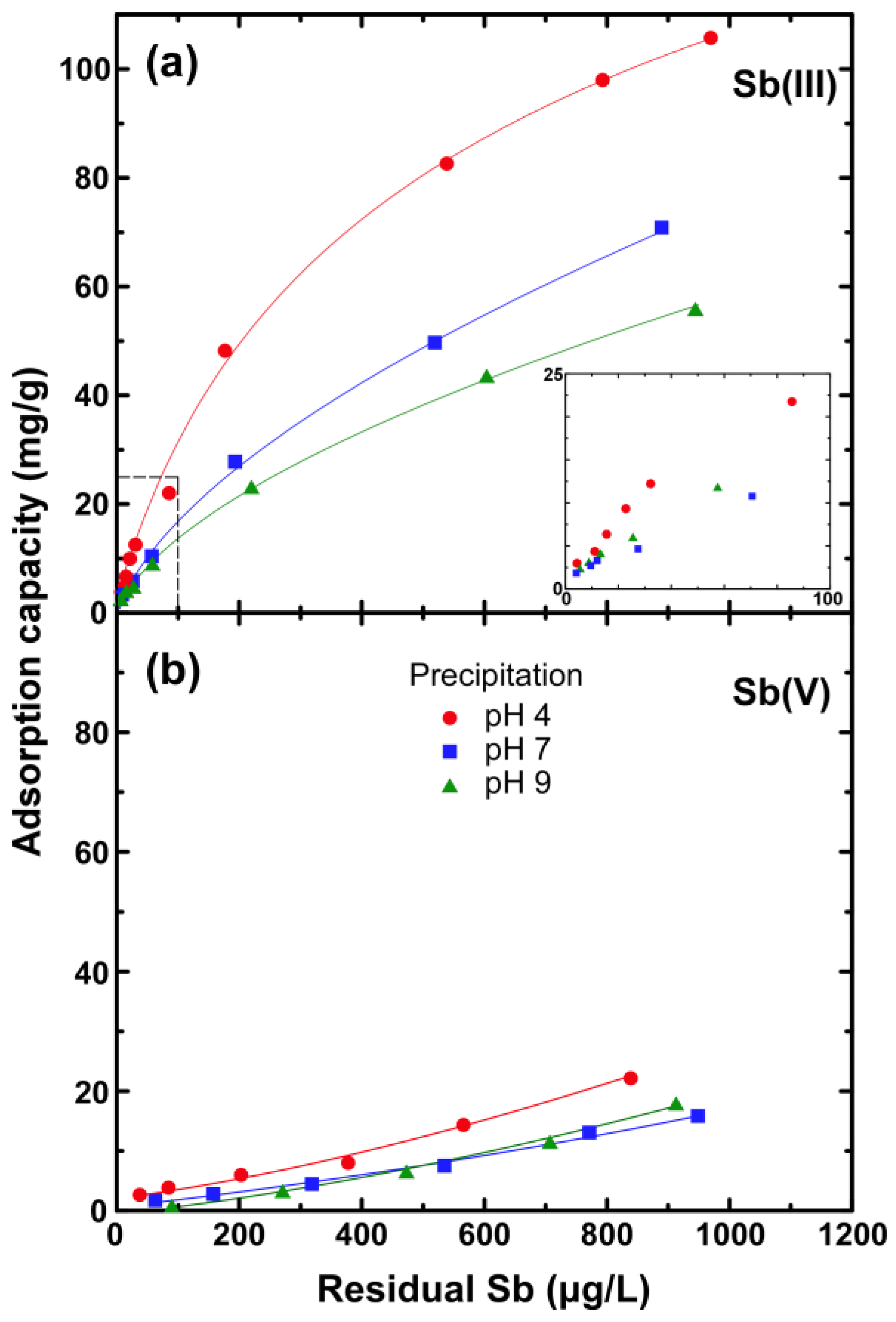
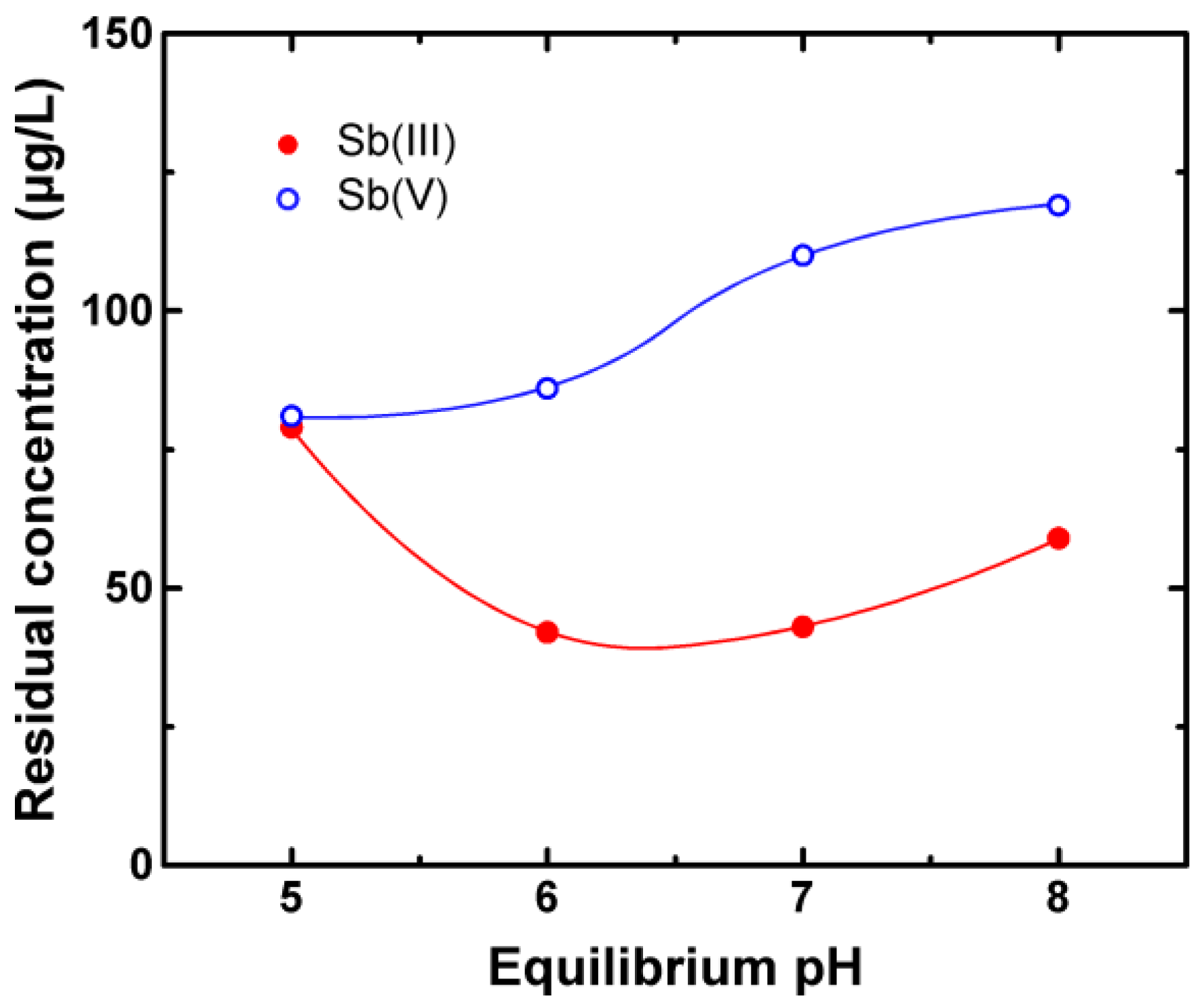
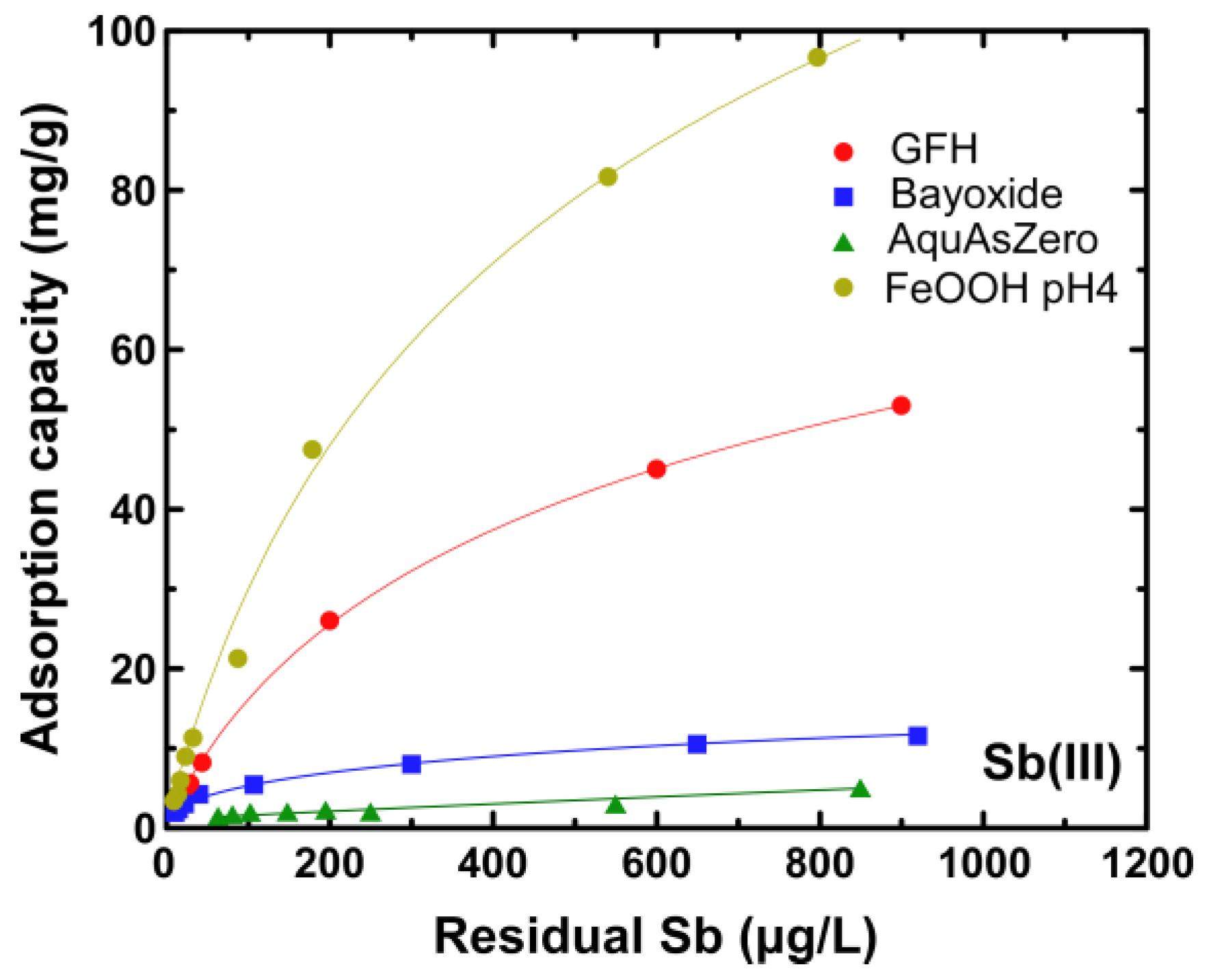
3.1. Adsorption Evaluation
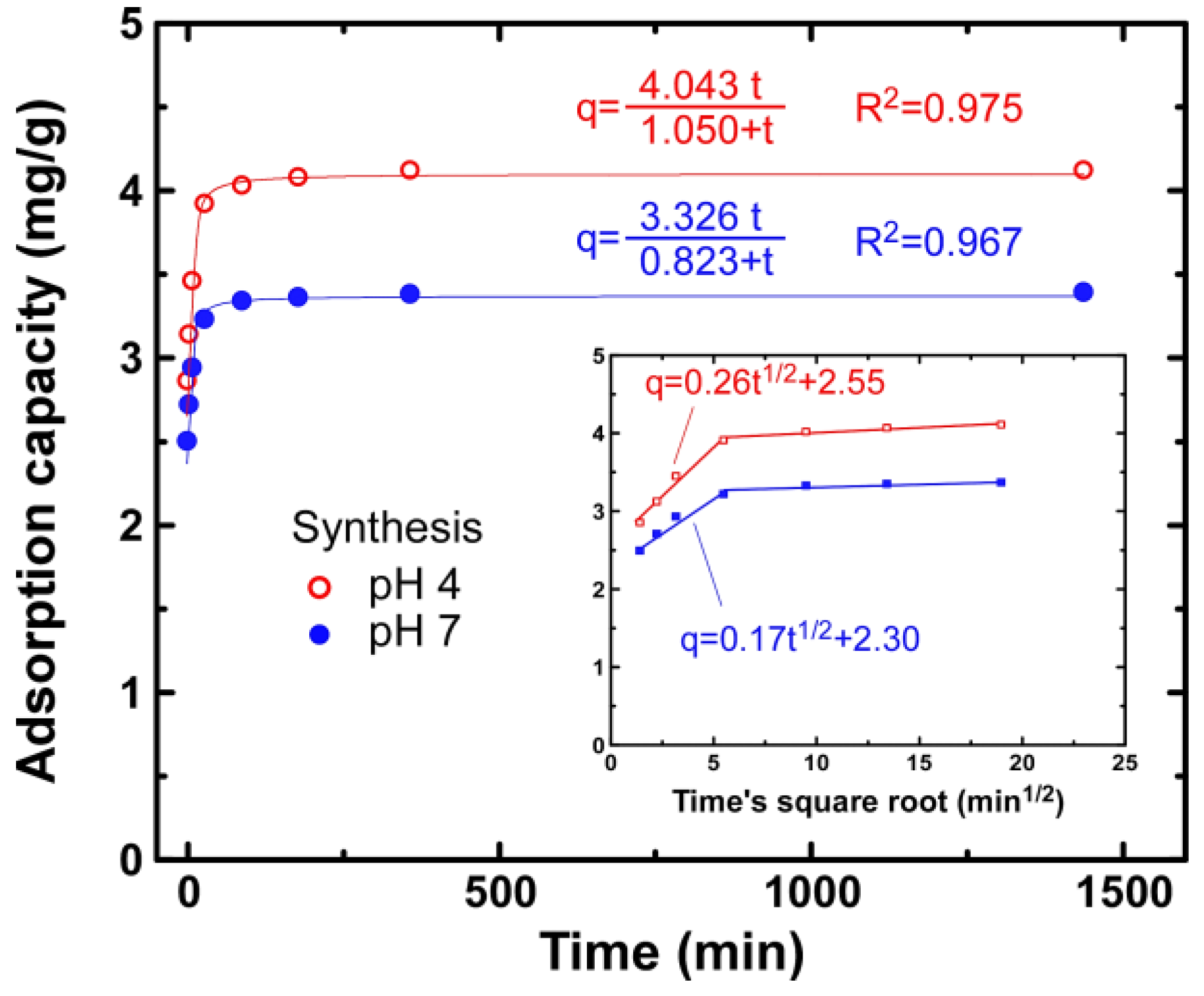
3.2. Kinetics
3.3. Column Experiments
3.4. Leaching Tests
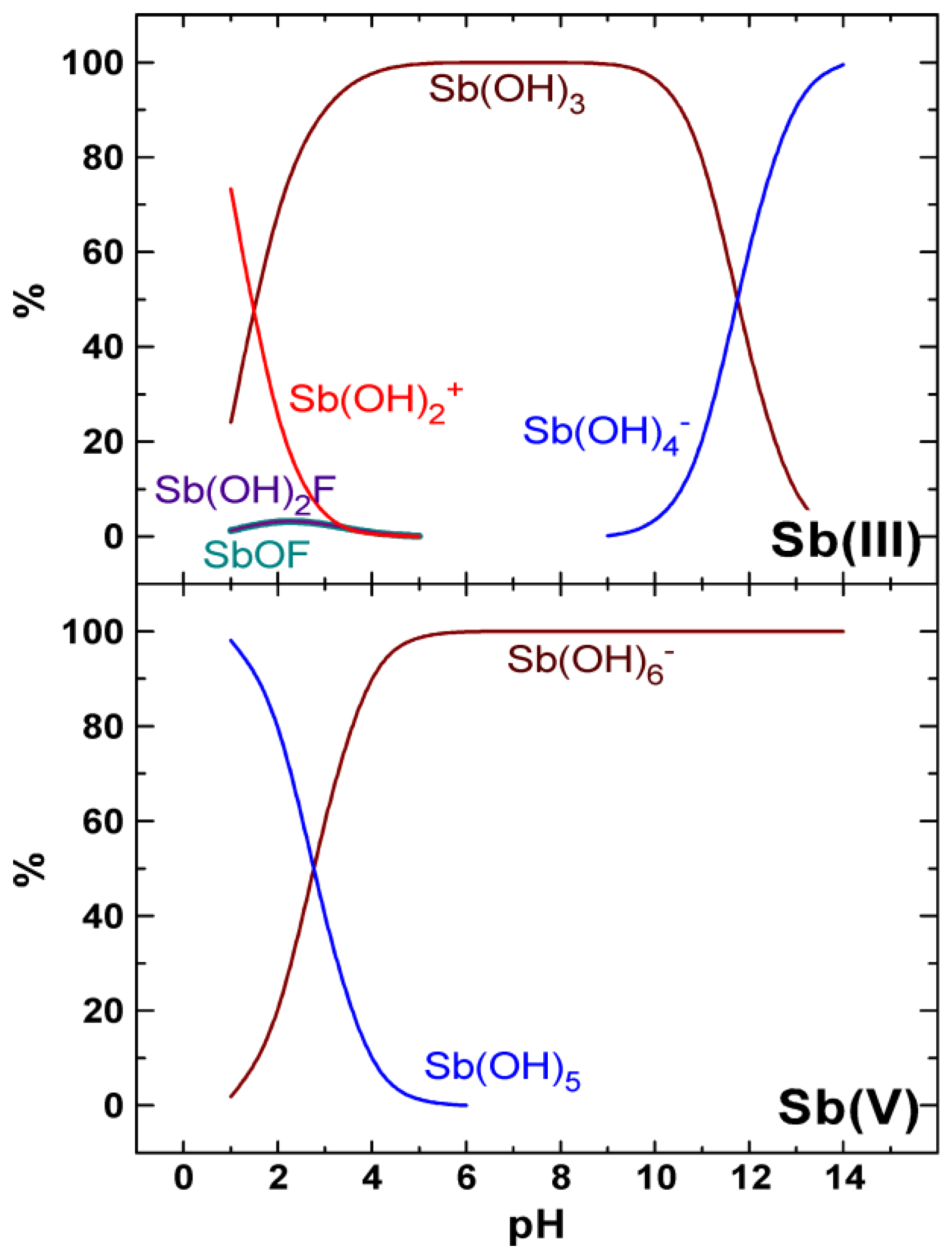
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Antimony in Drinking-Water; Background Document for Preparation of WHO Guidelines for Drinking-Water Quality; WHO/SDE/WSH/03.04/74; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- US Environmental Protection Agency. Edition of the Drinking Water Standards and Health Advisories; Office of Water, US Environmental Protection Agency: Washington, DC, USA, 2012.
- European Union. Council Directive 98/83/EC on the Quality of Water Intended for Human Consumption; L330/32; Official Journal of the European Union: Brussels, Belgium, 1998. [Google Scholar]
- Leyva, A.G.; Marrero, J.; Smichowski, P.; Cicerone, D. Sorption of antimony onto hydroxyapatite. Environ. Sci. Technol. 2001, 35, 3669–3675. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.J.; Craw, D.; Hunter, K. Antimony distribution and environmental mobility at an historic antimony smelter site, New Zealand. Environ. Pollut. 2004, 129, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption. J. Environ. Manag. 2015, 151, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, H.; Chai, L.-Y.; Mirza, N.; Yang, Z.-H.; Pervez, A.; Tariq, M.; Shaheen, S.; Mahmood, Q. Antimony (Sb)—Pollution and removal techniques—Critical assessment of technologies. Toxicol. Environ. Chem. 2015, 97, 1296–1318. [Google Scholar] [CrossRef]
- Gannon, K.; Wilson, D.J. Removal of Antimony from Aqueous Systems. Sep. Sci. Technol. 1986, 21, 475–493. [Google Scholar] [CrossRef]
- Wu, Z.; He, M.; Guo, X.; Zhou, R. Removal of antimony(III) and antimony(V) from drinking water by ferric chloride coagulation: Competing ion effect and the mechanism analysis. Sep. Purif. Technol. 2010, 76, 184–190. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Z.; He, M. Removal of antimony(V) and antimony(III) from drinking water by coagulation-flocculation-sedimentation (CFS). Water Res. 2009, 43, 4327–4335. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kamei, T.; Magara, Y. Comparing polyaluminum chloride and ferric chloride for antimony removal. Water Res. 2003, 37, 4171–4179. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, B.; Zhu, L.; Lin, S.; Sun, X.; Jiang, Z.; Tratnyek, P.G. Sequestration of Antimonite by Zerovalent Iron: Using Weak Magnetic Field Effects to Enhance Performance and Characterize Reaction Mechanisms. Environ. Sci. Technol. 2016, 50, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Guo, W.; Su, S.; Yi, C.; Xing, L. Removal of antimony(III) from aqueous solution by graphene as an adsorbent. Chem. Eng. J. 2012, 211–212, 406–411. [Google Scholar] [CrossRef]
- Dong, S.; Dou, X.; Mohan, D.; Pittman, C.U.; Luo, J. Synthesis of graphene oxide/schwertmannite nanocomposites and their application in Sb(V) adsorption from water. Chem. Eng. J. 2015, 270, 205–214. [Google Scholar] [CrossRef]
- Xu, Y.; Ohki, A.; Maeda, S. Adsorption and removal of antimony from aqueous solution by an activated Alumina. Toxicol. Environ. Chem. 2001, 80, 133–144. [Google Scholar] [CrossRef]
- Wang, L.; Wan, C.; Zhang, Y.; Lee, D.-J.; Liu, X.; Chen, X.; Tay, J.-H. Mechanism of enhanced Sb(V) removal from aqueous solution using chemically modified aerobic granules. J. Hazard. Mater. 2015, 284, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Wingenfelder, U.; Furrer, G.; Schulin, R. Sorption of antimonate by HDTMA-modified zeolite. Microporous Mesoporous Mater. 2006, 95, 265–271. [Google Scholar] [CrossRef]
- He, Z.; Liu, R.; Liu, H.; Qu, J. Adsorption of Sb(III) and Sb(V) on Freshly Prepared Ferric Hydroxide (FeOxHy). Environ. Eng. Sci. 2015, 32, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wu, Z.; He, M.; Meng, X.; Jin, X.; Qiu, N.; Zhang, J. Adsorption of antimony onto iron oxy-hydroxides: Adsorption behavior and surface structure. J. Hazard. Mater. 2014, 276, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Vithanage, M.; Rajapaksha, A.U.; Dou, X.; Bolan, N.S.; Yang, J.E.; Ok, Y.S. Surface complexation modelling and spectroscopic evidence of antimony adsorption on iron-oxide-rich red earth soils. J. Colloid Interface Sci. 2013, 406, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhu, Z.; Zhang, H.; Zhu, J.; Qiu, Y. Simultaneous removal of arsenate and antimonate in simulated and practical water samples by adsorption onto Zn/Fe layered double hydroxide. Chem. Eng. J. 2015, 276, 365–375. [Google Scholar] [CrossRef]
- Qi, P.; Pichler, T. Sequential and simultaneous adsorption of Sb(III) and Sb(V) on ferrihydrite: Implications for oxidation and competition. Chemosphere 2016, 145, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Leuz, A.K.; Mönch, H.; Johnson, C.A. Sorption of Sb(III) and Sb(V) to goethite: Influence on Sb(III) oxidation and mobilization. Environ. Sci. Technol. 2006, 40, 7277–7282. [Google Scholar] [CrossRef] [PubMed]
- Belzile, N.; Chen, Y.W.; Wang, Z. Oxidation of antimony(III) by amorphous iron and Manganese oxyhydroxides. Chem. Geol. 2001, 174, 379–387. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Liu, R.; Zhao, X.; Qu, J. The mechanism of antimony(III) removal and its reactions on the surfaces of Fe-Mn Binary Oxide. J. Colloid Interface Sci. 2011, 363, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, F.; Hu, C.; He, Z.; Liu, H.; Qu, J. Simultaneous removal of Cd(II) and Sb(V) by Fe-Mn binary oxide: Positive effects of Cd(II) on Sb(V) adsorption. J. Hazard. Mater. 2015, 300, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Sazakli, E.; Zouvelou, S.V.; Kalavrouziotis, I.; Leotsinidis, M. Arsenic and antimony removal from drinking water by adsorption on granular ferric oxide. Water Sci. Technol. 2015, 71, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Ilavský, J.; Barloková, D.; Munka, K. Antimony Removal from Water by Adsorption to Iron-Based Sorption Materials. Water Air Soil Pollut. 2015, 226, 2238. [Google Scholar] [CrossRef]
- Kolbe, F.; Weiss, H.; Morgenstern, P.; Wennrich, R.; Lorenz, W.; Schurk, K.; Stanjek, H.; Daus, B. Sorption of aqueous antimony and arsenic species onto akaganeite. J. Colloid Interface Sci. 2011, 357, 460–465. [Google Scholar] [CrossRef]
- Tresintsi, S.; Simeonidis, K.; Zouboulis, A.; Mitrakas, M. Comparative study of As(V) removal by ferric coagulation and oxy-hydroxides adsorption: Laboratory and full-scale case studies. Desalin. Water Treat. 2013, 51, 2872–2880. [Google Scholar] [CrossRef]
- Tresintsi, S.; Simeonidis, K.; Vourlias, G.; Stavropoulos, G.; Mitrakas, M. Kilogram-scale synthesis of iron oxy-hydroxides with improved arsenic removal capacity: Study of Fe(II) oxidation–precipitation parameters. Water Res. 2012, 46, 5255–5267. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Standardization. 2002. ΕΝ 12457-2: Characterization of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 2: One Stage Batch Test at Liquid to Solid Ratio of 10 L/kg for Materials with Particle Size below 4 mm (without or with Size Reduction). European Standard; European Committee for Standardization: Brussels, Belgium, 2002. [Google Scholar]
- Tripathy, S.S.; Raichur, A.M. Enhanced adsorption of activated alumina by impregnation with alum for removal of As(V) from water. Chem. Eng. J. 2008, 138, 179–186. [Google Scholar] [CrossRef]
- Cumming, L.J.; Chen, A.S.C.; Wang, L. Arsenic and Antimony Removal from Drinking Water by Adsorption Media, US EPA Demonstration Project at South Truckee Meadows General Improvement District (STMGID); NV, Final Performance Evaluation Report; EPA/600/R-09/016; US Environmental Protection Agency: Washington, DC, USA, February 2009.
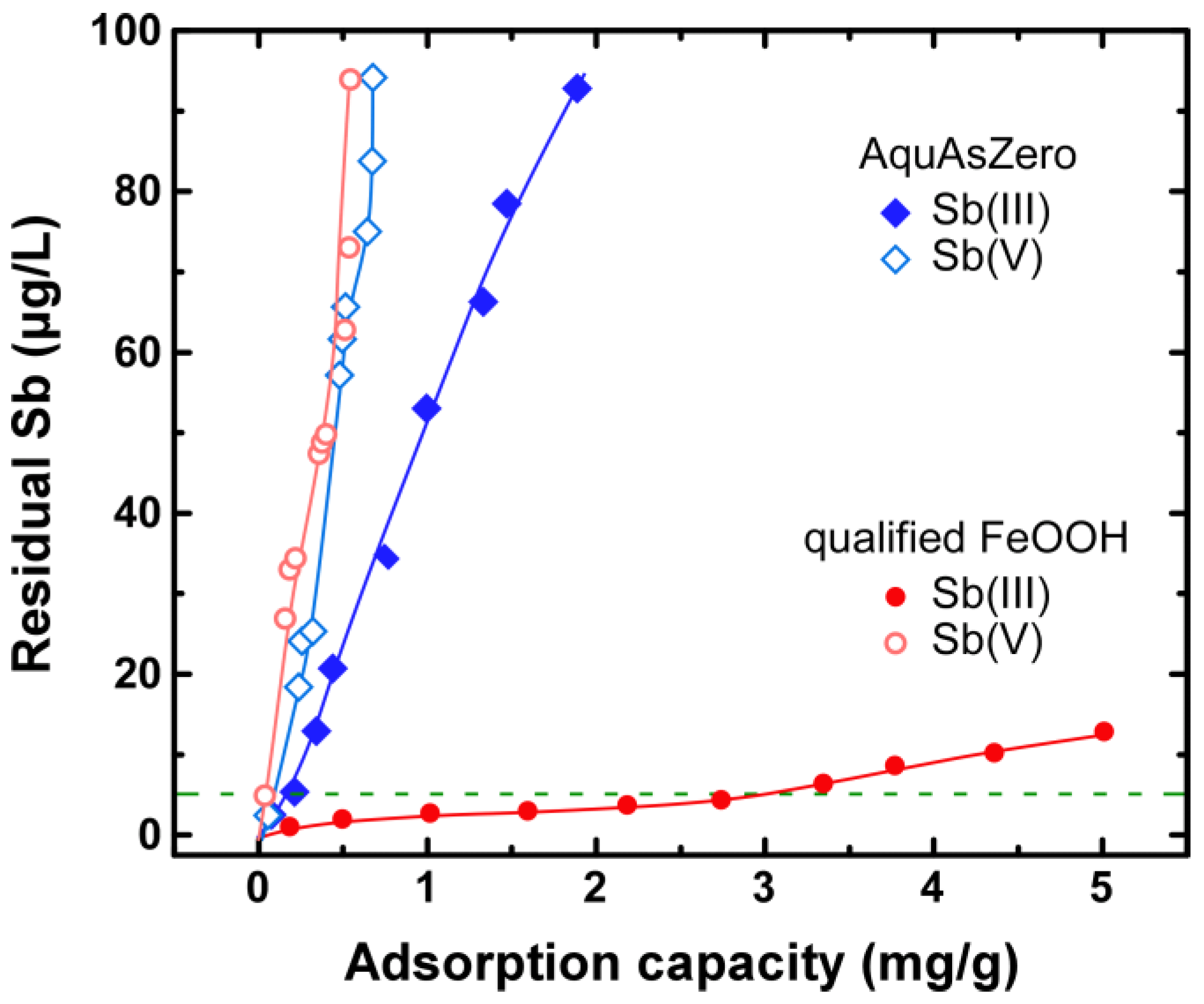

| Spent Adsorbent | Adsorption Load (mg·Sb/g) | EN12457-01 (mg/kg) 1 |
|---|---|---|
| Sb(III) | ||
| Qualified FeOOH | 5.1 | 0.05 |
| AquAsZero | 3.4 | 0.22 |
| Sb(V) | ||
| Qualified FeOOH | 0.30 | 0.55 |
| AquAsZero | 0.35 | 0.68 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simeonidis, K.; Papadopoulou, V.; Tresintsi, S.; Kokkinos, E.; Katsoyiannis, I.A.; Zouboulis, A.I.; Mitrakas, M. Efficiency of Iron-Based Oxy-Hydroxides in Removing Antimony from Groundwater to Levels below the Drinking Water Regulation Limits. Sustainability 2017, 9, 238. https://doi.org/10.3390/su9020238
Simeonidis K, Papadopoulou V, Tresintsi S, Kokkinos E, Katsoyiannis IA, Zouboulis AI, Mitrakas M. Efficiency of Iron-Based Oxy-Hydroxides in Removing Antimony from Groundwater to Levels below the Drinking Water Regulation Limits. Sustainability. 2017; 9(2):238. https://doi.org/10.3390/su9020238
Chicago/Turabian StyleSimeonidis, Konstantinos, Vasiliki Papadopoulou, Sofia Tresintsi, Evgenios Kokkinos, Ioannis A. Katsoyiannis, Anastasios I. Zouboulis, and Manassis Mitrakas. 2017. "Efficiency of Iron-Based Oxy-Hydroxides in Removing Antimony from Groundwater to Levels below the Drinking Water Regulation Limits" Sustainability 9, no. 2: 238. https://doi.org/10.3390/su9020238
APA StyleSimeonidis, K., Papadopoulou, V., Tresintsi, S., Kokkinos, E., Katsoyiannis, I. A., Zouboulis, A. I., & Mitrakas, M. (2017). Efficiency of Iron-Based Oxy-Hydroxides in Removing Antimony from Groundwater to Levels below the Drinking Water Regulation Limits. Sustainability, 9(2), 238. https://doi.org/10.3390/su9020238










