Effect of Topping Trees on Biomass and Nitrogen Removal in the Thinning of Norway Spruce Stands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Stand Characteristics
2.3. Estimation of Logging Residues after Conventional FT-Cable Yarding
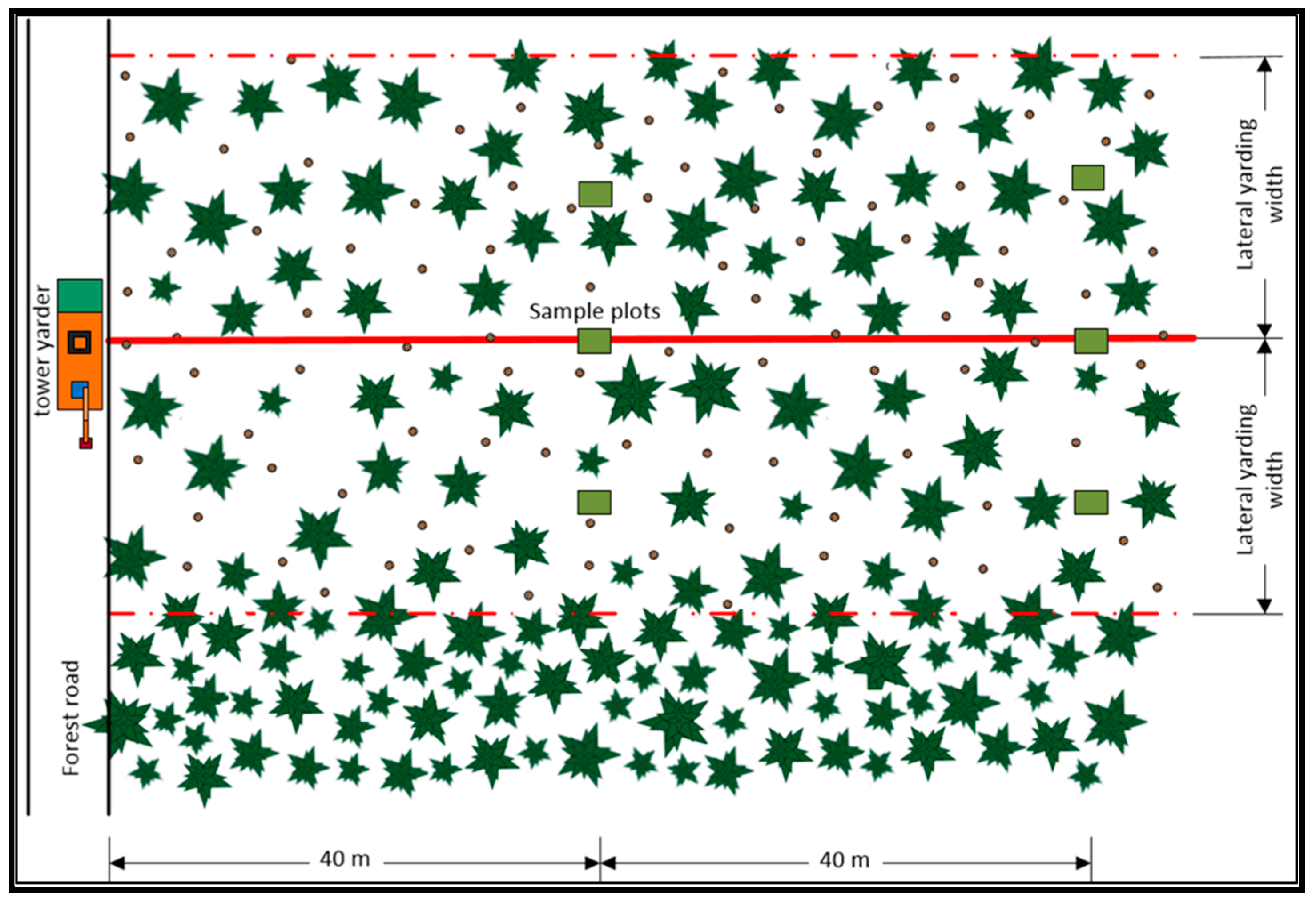
2.3.1. Harvesting Operations
2.3.2. Estimation of Logging Residues
2.4. Estimation of Total-Tree and Tree-Top Biomass
2.4.1. Stand Inventory
2.4.2. Felled-Tree Sampling
2.4.3. Tree Biomass Equations
2.4.4. Tree-Top Biomass Equations
2.5. Estimation of Biomass and Nitrogen Removal
- branches and foliage, which break off during felling and extraction (Section 2.3);
- biomass of tree-tops, which remain after topping the trees at a certain diameter (Section 2.4).
3. Results
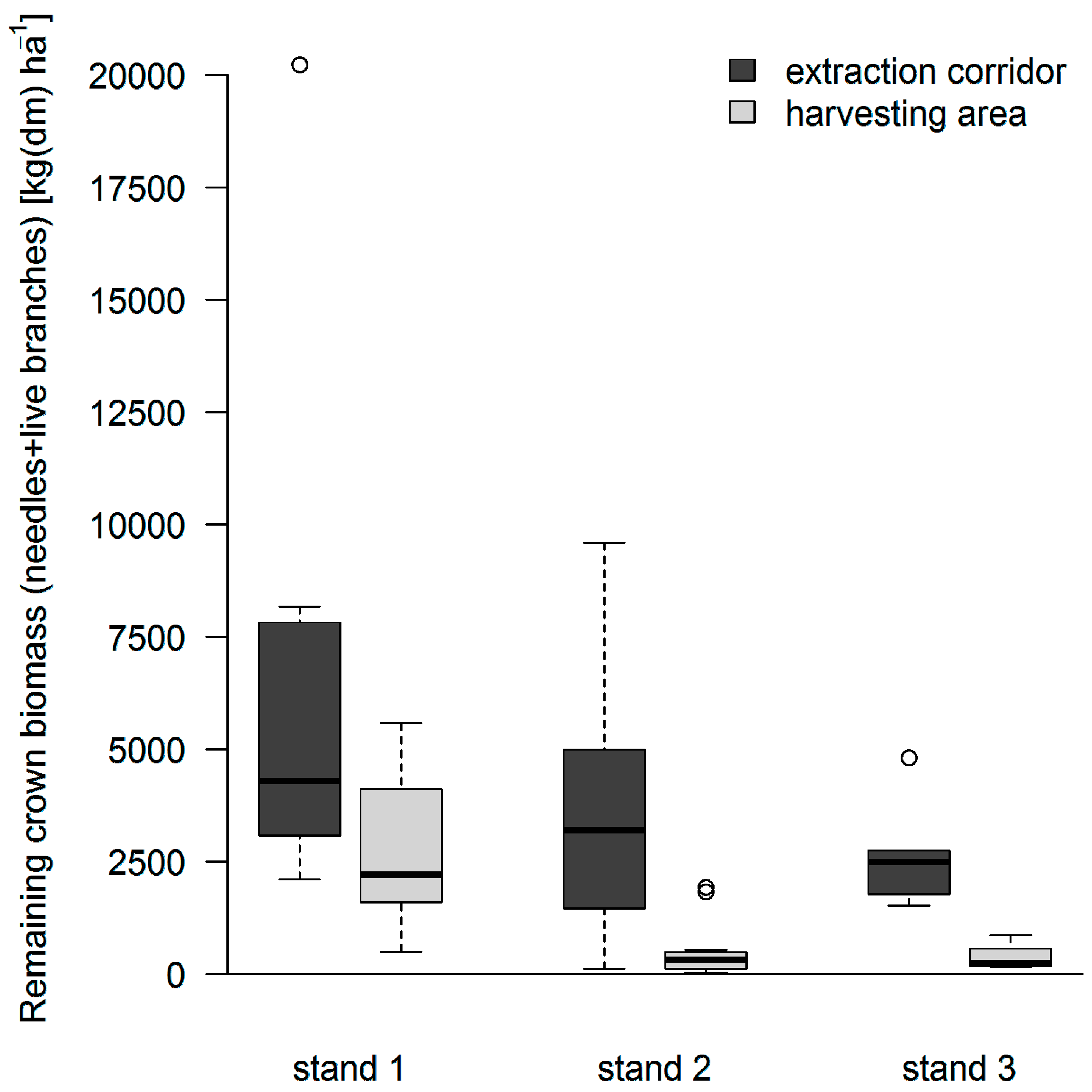
3.1. Logging Residues after Conventional FT Cable Yarding
3.2. Distribution of Logging Residues after Conventional FT Cable Yarding
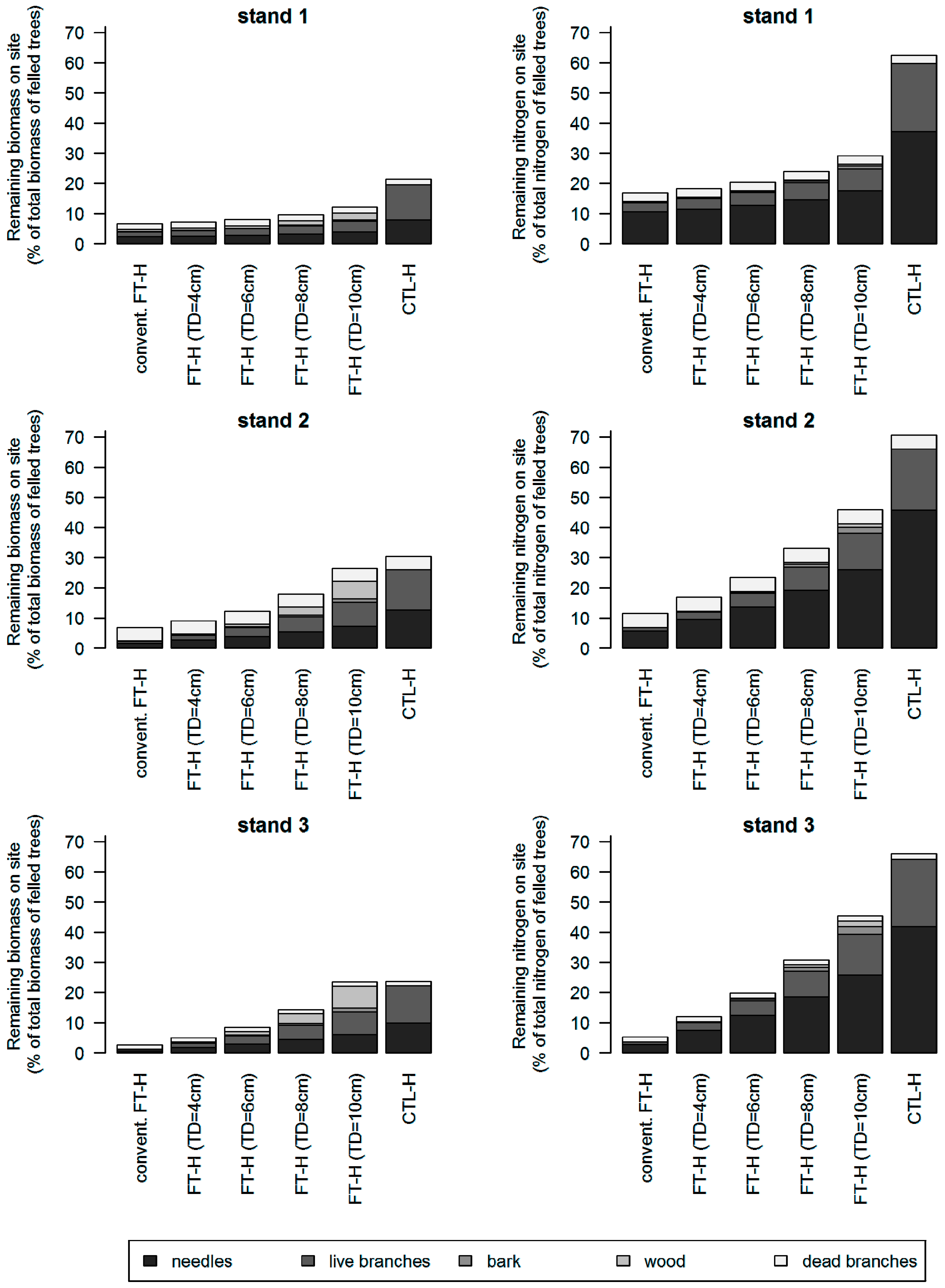
3.3. Additional Effect of Topping on the Amount of Logging Residues
3.3.1. Tree Biomass Models
3.3.2. Tree-Top Biomass Models
3.4. Scenario Analyses
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Heinimann, H.R.; Stampfer, K.; Loschek, J.; Caminada, L. Perspectives on Central European Cable Yarding Systems. Aust. J. For. Sci. 2006, 123, 121–139. [Google Scholar]
- European Parliament and Council. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the promotion of the use of energy from renewable sources and amending and subsequently repealing directives 2001/77/EC and 2003/30/EC, Public Law 140. 2009. Available online: https://www.ecolex.org/details/legislation/directive-200928ec-of-the-european-parliament-and-of-the-council-on-the-promotion-of-the-use-of-energy-from-renewable-sources-and-amending-and-subsequently-repealing-directives-200177ec-and-200330ec-lex-faoc088009/ (accessed on 16 October 2017).
- Krapfenbauer, A. Rationalisierungsbestrebungen und Standortsproduktivität. Allg. Forstzeitung. 1968, 79, 128–130. [Google Scholar]
- Kreutzer, K. Der Einfluss moderner Holzernteverfahren auf die Ökologie des Waldes. Verhandlungen der Gesellschaft für Ökologie 1980, 8, 229–233. [Google Scholar]
- Helmisaari, H.-S.; Hanssen, K.H.; Jacobson, S.; Kukkola, M.; Luiro, J.; Saarsalmi, A.; Tamminen, P.; Tveite, B. Logging residue removal after thinning in Nordic boreal forests: Long term impact on tree growth. For. Ecol. Manag. 2011, 261, 1919–1927. [Google Scholar] [CrossRef]
- Sterba, H. Increment losses by full-tree harvesting in Norway spruce (Picea abies). For. Ecol. Manag. 1988, 24, 283–292. [Google Scholar] [CrossRef]
- Proe, M.F.; Dutch, J. Impact of whole-tree harvesting on second-rotation growth of Sitka spruce: The first 10 years. For. Ecol. Manag. 1994, 66, 39–54. [Google Scholar] [CrossRef]
- Egnell, G.; Leijon, B. Effects of different levels of biomass removal in thinning on short–term production of pinus sylvestris and picea abies stands. Scand. J. For. Res. 1997, 12, 17–26. [Google Scholar] [CrossRef]
- Egnell, G. Is the productivity decline in Norway spruce following whole-tree harvesting in the final felling in boreal Sweden permanent or temporary? For. Ecol. Manag. 2011, 261, 148–153. [Google Scholar] [CrossRef]
- Tveite, B.; Hanssen, K.H. Whole-tree thinnings in stands of Scots pine (Pinus sylvestris) and Norway spruce (Picea abies): Short- and long-term growth results. For. Ecol. Manag. 2013, 298, 52–61. [Google Scholar] [CrossRef]
- Brandtberg, P.-O.; Olsson, B.A. Changes in the effects of whole-tree harvesting on soil chemistry during 10 years of stand development. For. Ecol. Manag. 2012, 277, 150–162. [Google Scholar] [CrossRef]
- Akselsson, C.; Westling, O.; Sverdrup, H.; Holmqvist, J.; Thelin, G.; Uggla, E.; Malm, G. Impact of harvest intensity on long-term base cation budgets in Swedish forest soils. Water Air Soil Poll. 2007, 7, 201–210. [Google Scholar] [CrossRef]
- Ranger, J.; Turpault, M.-P. Input-output nutrient budgets as a diagnostic tool for sustainable forest management. For. Ecol. Manag. 1999, 122, 139–154. [Google Scholar] [CrossRef]
- Palviainen, M.; Finér, L. Estimation of nutrient removals in stem-only and whole-tree harvesting of Scots pine, Norway spruce, and birch stands with generalized nutrient equations. Europ. J. For. Res. 2012, 131, 945–964. [Google Scholar] [CrossRef]
- Briedis, J.I.; Wilson, J.S.; Benjamin, J.G.; Wagner, R.G. Biomass retention following whole-tree, energy wood harvests in central Maine: Adherence to five state guidelines. Biomass Bionenerg. 2011, 35, 3552–3560. [Google Scholar] [CrossRef]
- Hytönen, J.; Moilanen, M. Effect of harvesting method on the amount of logging residues in the thinning of Scots pine stands. Biomass Bioenerg. 2014, 67, 347–353. [Google Scholar] [CrossRef]
- Ghaffariyan, M.R.; Apolit, R. Harvest residues assessment in pine plantations harvested by whole tree and cut-to-length harvesting methods (a case study in Queensland, Australia). Silva Balc. 2015, 16, 113–122. [Google Scholar]
- Kizha, A.R.; Han, H.-S. Forest residues recovered from whole-tree timber harvesting operations. Eur. J. For. Eng. 2015, 1, 46–55. [Google Scholar]
- Young, H.E.; Carpenter, P.M. Weight, Nutrient Element and Productivity Studies of Seedlings and Saplings of Eight tree Species in Natural Ecosystems. Maine Agricultural Experiment Station Technical Bulletin 28; University of Maine: Orono, ME, USA, 1967. [Google Scholar]
- Lick, E. Untersuchungen zur Problematik des Biomassen- und Nährelemententzuges bei der Erstdurchforstung eines Zentralalpinen Fichtenbestandes. Ph.D. Thesis, University of Natural Resources and Life Sciences, Vienna, Austria, 1989. [Google Scholar]
- Hendrickson, O.Q.; Chatarpaul, L.; Burgess, D. Nutrient cycling following whole-tree and conventional harvest in northern mixed forest. Can. J. For. Res. 2016, 19, 725–735. [Google Scholar] [CrossRef]
- Krapfenbauer, A.; Buchleitner, E. Holzernte, Biomassen- und Nährstoffaustrag, Nährstoffbilanz eines Fichtenbestandes. Aust. J. For. Sci. 1981, 98, 193–223. [Google Scholar]
- Huber, C.; Stampfer, K. Efficiency of topping trees in cable yarding operations. Croat. J. For. Eng. 2015, 36, 321–331. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models, R-package Version 3.1-128. 2016. Available online: https://cran.r-project.org/web/packages/nlme/nlme.pdf (accessed on 16 May 2016).
- R Core Team. A Language and Environment of Statistical Computing; R Foundation of Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Ring, E.; Högbom, L.; Jansson, G. Effects of previous nitrogen fertilization on soil-solution chemistry after final felling and soil scarification at two nitrogen-limited forest sites. Can. J. For. Res. 2013, 43, 396–404. [Google Scholar] [CrossRef]
- Page, J.M.; Gustafson, M.L. Equipment for Forest Fertilization. SAE Technical Paper 690553 1969. Available online: https://doi.org/10.4271/690553 (accessed on 16 May 2016).
- Väätäinen, K.; Sirparanta, E.; Räisänen, M.; Tahvanainen, T. The costs and profitability of using granulated wood ash as a forest fertilizer in drained peatland forests. Biomass Bioenerg. 2011, 35, 3335–3341. [Google Scholar] [CrossRef]
- Huber, C.; Stampfer, K. Evaluation of a modified centrifugal spreader to apply nutrient-rich fine 432 fractions from woodchips as a fertilizer to cutover areas in steep terrain. In Proceedings of the 39th 433 Annual Meeting of the Council of Forest Engineering (COFE), Vancouver, BC, Canada, 19–21 September 2016. [Google Scholar]
- Pasztor, F.; Matulla, C.; Rammer, W.; Lexer, M.J. Drivers of the bark beetle disturbance regime in Alpine forests in Austria. For. Ecol. Manag. 2014, 318, 349–358. [Google Scholar] [CrossRef]
- Seidl, R.; Baier, P.; Rammer, W.; Schopf, A.; Lexer, M.J. Modelling tree mortality by bark beetle infestation in Norway spruce forests. Ecol. Model. 2007, 206, 383–399. [Google Scholar] [CrossRef]
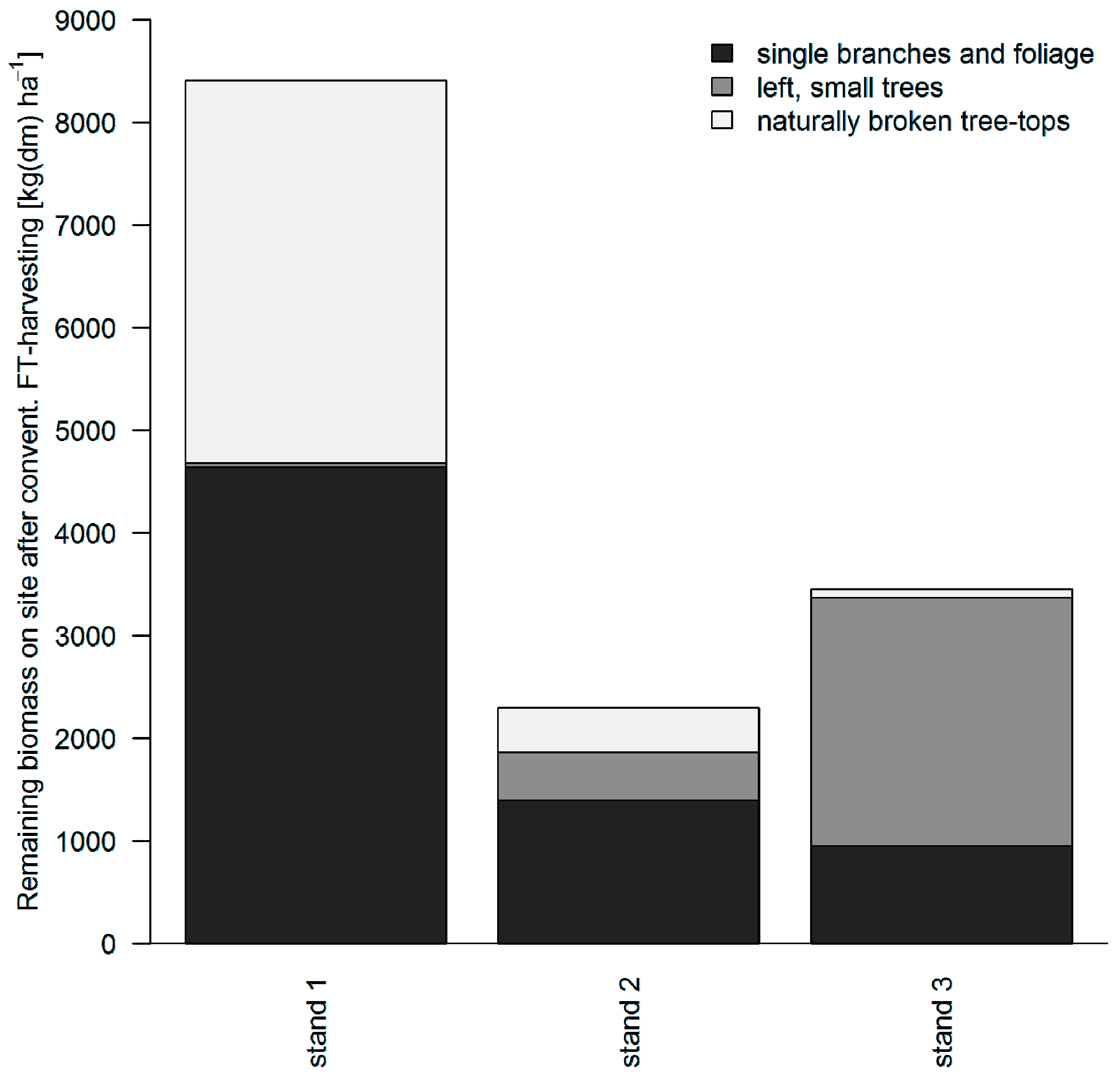
| Parameter | Study Sites (Stands) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Harvesting operation | Second thinning | First thinning | First thinning | |
| Previous operation | First thinning | Pre-commercial thinning | - | |
| Harvesting area (m2) | 10,200 | 11,700 | 6800 | |
| Number of cable lines | 2 | 2 | 2 | |
| Yarding distance (m) | 170 | 250 | 125 | |
| Extraction direction | uphill | uphill | uphill | |
| Stand age (years) | 58 | 38 | 34 | |
| Average slope (%) | 53 | 61 | 70 | |
| Species composition | 85% spruce | 83% spruce | 80% spruce | |
| 9% larch | 9% larch | 10% birch | ||
| 6% others | 8% others | 10% others | ||
| Stand density 1 (trees ha−1) | Before harvesting | 728 | 979 | 1667 |
| After harvesting | 320 | 454 | 500 | |
| Basal area 1 (m² ha−1) | Before harvesting | 52.4 | 26.4 | 34.2 |
| After harvesting | 31.9 | 15.7 | 17.1 | |
| Stand volume 1 (m³ ha−1) | Before harvesting | 727 | 271 | 204 |
| After harvesting | 451 | 163 | 109 | |
| Attribute | Mean | SD 1 | Min | Max |
|---|---|---|---|---|
| Stand 1—Second Thinning (n = 8) | ||||
| DBH (cm) | 29.9 | 8.7 | 16.0 | 41.9 |
| height (m) | 29.1 | 3.8 | 22.1 | 34.1 |
| crown length (m) | 13.8 | 1.9 | 10.3 | 16.7 |
| crown ratio (%) | 47 | 4 | 39 | 54 |
| Stand 2—First Thinning (n = 8) | ||||
| DBH (cm) | 17.4 | 4.5 | 10.4 | 23.0 |
| height (m) | 18.3 | 3.0 | 12.4 | 20.8 |
| crown length (m) | 9.2 | 2.4 | 6.3 | 12.7 |
| crown ratio (%) | 50 | 8 | 36 | 61 |
| Stand 3—First Thinning (n = 9) | ||||
| DBH (cm) | 16.5 | 4.8 | 9.1 | 22.4 |
| height (m) | 16.5 | 4.3 | 7.1 | 21.0 |
| crown length (m) | 7.8 | 2.6 | 2.5 | 10.3 |
| crown ratio (%) | 46 | 8 | 35 | 62 |
| Component | n | Parameter Estimates | RMSE | AIC | cf | ||||
|---|---|---|---|---|---|---|---|---|---|
| β0 | β1 | β2 | β3 | β4 | |||||
| ln(Needles) | 25 | −9.748 c | 2.507 c | - | 1.219 b | - | 0.244 | 22.2 | 1.007 |
| ln(Live branches) | 25 | −5.059 c | 2.613 c | - | - | - | 0.308 | 28.5 | 1.026 |
| ln(Dead branches) | 25 | 1.645 | 2.539 c | - | −1.961 b | - | 0.386 | 45.2 | 1.059 |
| ln(Bark) | 25 | −5.054 c | 1.719 c | 0.795 a | - | - | 0.160 | 1.5 | 1.015 |
| ln(Wood) | 25 | −4.131 c | 1.885 c | 1.018 c | - | - | 0.137 | −8.4 | 0.976 |
| Component | n | Parameter estimates | RMSE | AIC | cf | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β0 | β1 | β2 | β3 | β4 | β5 | |||||
| ln(Needles) | 125 | −7.324 c | - | 1.553 c | - | - | 1.906 c | 0.402 | 164.4 | 1.037 |
| ln(Live branches) | 125 | −6.031 c | 0.942 c | - | - | - | 2.223 c | 0.399 | 152.7 | 1.089 |
| ln(Bark) | 125 | −7.565 c | −0.914 c | 0.996 b | - | - | 3.247 c | 0.399 | 126.4 | 0.928 |
| ln(Wood) | 125 | −6.427 c | −1.213 c | 1.141 c | - | - | 3.669 c | 0.336 | 92.5 | 0.936 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huber, C.; Kastner, M.; Hochbichler, E.; Stampfer, K. Effect of Topping Trees on Biomass and Nitrogen Removal in the Thinning of Norway Spruce Stands. Sustainability 2017, 9, 1856. https://doi.org/10.3390/su9101856
Huber C, Kastner M, Hochbichler E, Stampfer K. Effect of Topping Trees on Biomass and Nitrogen Removal in the Thinning of Norway Spruce Stands. Sustainability. 2017; 9(10):1856. https://doi.org/10.3390/su9101856
Chicago/Turabian StyleHuber, Christoph, Maximilian Kastner, Eduard Hochbichler, and Karl Stampfer. 2017. "Effect of Topping Trees on Biomass and Nitrogen Removal in the Thinning of Norway Spruce Stands" Sustainability 9, no. 10: 1856. https://doi.org/10.3390/su9101856
APA StyleHuber, C., Kastner, M., Hochbichler, E., & Stampfer, K. (2017). Effect of Topping Trees on Biomass and Nitrogen Removal in the Thinning of Norway Spruce Stands. Sustainability, 9(10), 1856. https://doi.org/10.3390/su9101856





