Sustainability, Innovation, and Green Chemistry in the Production and Valorization of Phenolic Extracts from Olea europaea L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Plant Material, and Instruments
2.2. Extraction of Low-Molecular Weight Phenolic Compounds
2.3. Fractionating of the Phenolic Extracts
2.4. Isolation of HTyr-Enriched Fractions
2.5. Evaluation of the Antiradical Activity of Soft Extract Olea GL, Soft Extract Olea DL, and Soft Extract Olea HTyr
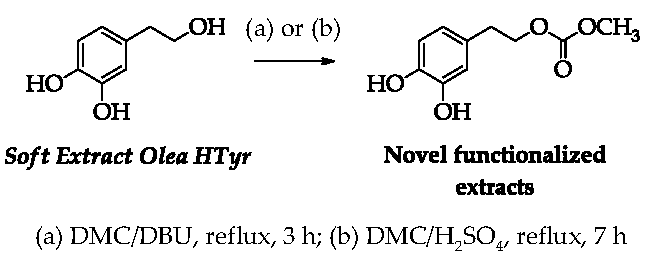
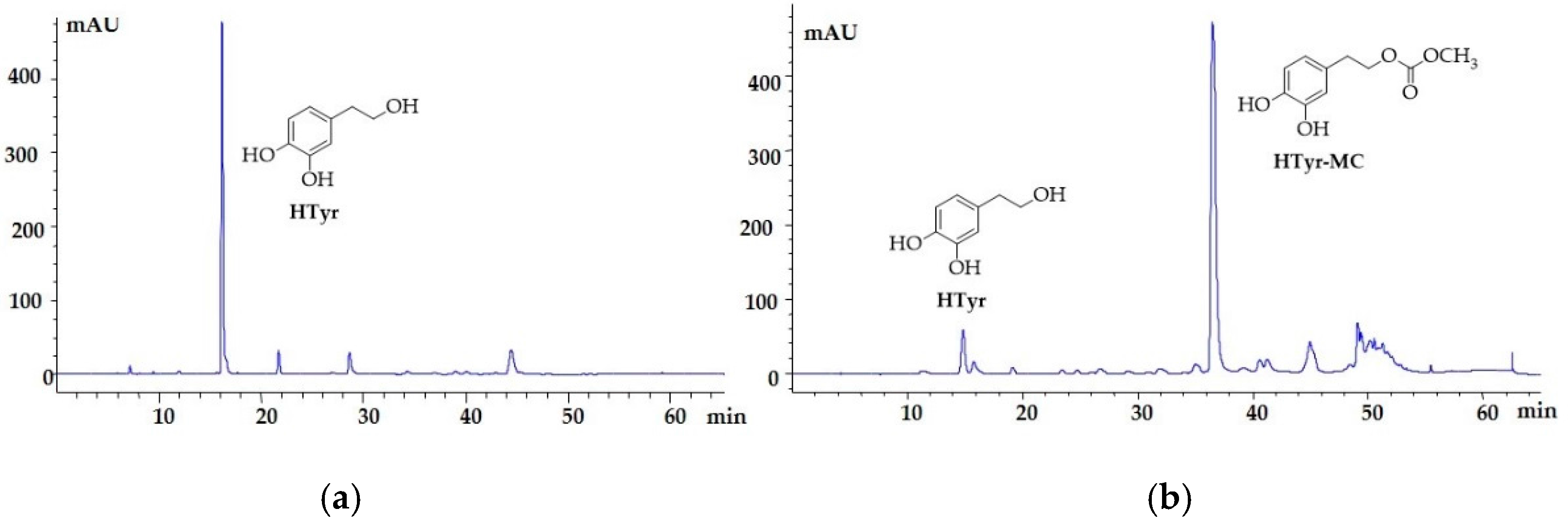
2.6. Functionalization of the HTyr-Enriched Fraction (Soft Extract Olea HTyr)
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HTyr | Hydroxytyrosol |
| HTyr-MC | Hydroxytyrosol methyl carbonate |
| GL | Green leaves |
| DL | Dried leaves |
| Soft Extract Olea GL | Olea europaea fraction deriving from green leaves |
| Soft Extract Olea DL | Olea europaea fraction deriving from dried leaves |
| Soft Extract Olea HTyr | Olea europaea fraction deriving from pitted olive pulp |
| HPLC/DAD | High Performance Liquid Chromatography/Diode Array Detector |
References
- Stahel, W.R. The circular economy. Nature 2016, 531, 435–438. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Circular Economy Strategy. Available online: http://ec.europa.eu/environment/circular-economy/index_en.htm (accessed on 26 June 2016).
- Donnelly, D.; Clancy, M.; Hart, J. Developing a sustainable business model. In Proceedings of the 9th World Congress on Polyphenols Applications, St Julian’s, Malta, 3–5 June 2015.
- Romani, A.; Scardigli, A.; Campo, M.; Romani, M.; Pinelli, P. Sustainable productive processes of polyphenol standardized fractions. In Proceedings of the 9th ISANH Congress Polyphenols Applications, St. Julian’s, Malta, 3–5 June 2015.
- Morand, C.; Sies, H. Polyphenols and health (Special Issue). Arch. Biochem. Biophys. 2016. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Martin-Cabrejas, M.A.; Gonzalez de Mejia, E. Phenolic compounds in fruits and beverages consumed as part of the Mediterranean diet: Their role in prevention of chronic diseases. Phytochem. Rev. 2016, 15, 405–423. [Google Scholar] [CrossRef]
- Franconi, F.; Coinu, R.; Carta, S.; Urgeghe, P.P.; Ieri, F.; Mulinacci, N.; Romani, A. Antioxidant effect of two virgin olive oils depends on the concentration and composition of minor polar compounds. J. Agric. Food Chem. 2006, 54, 3121–3125. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; Gentili, A.; Isidori, M.; Monaco, P.; Nardelli, A.; Parrella, A.; Temussi, F. Environmental effects caused by olive mill wastewaters: Toxicity comparison of low-molecular-weight phenol components. J. Agric. Food Chem. 2003, 51, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Della Greca, M.; Previtera, L.; Temussi, F.; Zarrelli, A. Low-molecular weight components of olive oil mill wastewaters. Phytochem. Anal. 2004, 15, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Gambacorta, A.; Tofani, D.; Bernini, R.; Migliorini, A. High yielding preparation of a stable precursor of hydroxytyrosol by total synthesis and from the natural glucoside oleuropein. J. Agric. Food Chem. 2007, 55, 3386–3391. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Galli, C. Olive oil phenols and their potential effects on human health. J. Agric. Food Chem. 1998, 46, 4292–4296. [Google Scholar] [CrossRef]
- Aunon-Calles, D.; Giordano, D.; Bohnenbergerc, S.; Visioli, F. Hydroxytyrosol is not genotoxic in vitro. Pharm. Res. 2013, 74, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Merendino, N.; Romani, A.; Velotti, F. Naturally occurring hydroxytyrosol: Synthesis and anticancer potential. Curr. Med. Chem. 2013, 20, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortunõ, A.; del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Medina, E.; de Castro, A.; Romero, C.; Ramírez, E.; Brenes, M. Effect of antimicrobial compounds from olive products on microorganisms related to health, food and agriculture. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; Volume 2, pp. 1087–1094. [Google Scholar]
- Romero, C.; Medina, E.; Vargas, J.; Brenes, M.; de Castro, A. In vitro activity of olive oil polyphenols against Helicobacter pylori. J. Agric. Food Chem. 2007, 55, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Meneguzzo, F.; Fidalgo, A.; Ilharco, L.M.; Pagliaro, M. Extraction, benefits and valorization of olive polyphenols. Eur. J. Lipid Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Takac, S.; Karakaya, A. Recovery of phenolic compounds from olive mill wastewaters. Recent Pat. Chem. Eng. 2009, 2, 230–237. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Giorno, L.; Drioli, E. Fractionation of olive mill wastewaters by membrane separation techniques. J. Hazard. Mat. 2013, 248–249, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Castello, E.; Cassano, A.; Criscuoli, A.; Conidi, C.; Drioli, E. Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system. Water Res. 2010, 44, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Ochando-Pulido, J.M.; Martinez-Ferez, A. On the recent use of membrane technology for olive mill wastewater purification. Membranes 2015, 5, 513–531. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Mincione, E.; Barontini, M.; Crisante, F. Convenient synthesis of hydroxytyrosol and its lipophilic derivatives from tyrosol or homovanillyl alcohol. J. Agric. Food Chem. 2008, 56, 8897–8904. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Mincione, E.; Barontini, M.; Crisante, F. Method for Preparing Hydroxytyrosol Derivatives and Hydroxytyrosol. WO2008110908 A1, 18 September 2008. [Google Scholar]
- Pinelli, P.; Galardi, C.; Mulinacci, N.; Vincieri, F.F.; Cimato, A.; Romani, A. Minor polar compound and fatty acid analyses in monocultivar virgin olive oils from Tuscany. Food Chem. 2003, 80, 331–336. [Google Scholar] [CrossRef]
- Pizzichini, D.; Russo, C.; Vitagliano, M.; Pizzichini, M.; Romani, A.; Ieri, F.; Pinelli, P.; Vignolini, P. Process for Producing Concentrated and Refined Actives from Tissues and Byproducts of Olea europaea with Membrane Technologies. EP 2338500 A1, 29 June 2011. [Google Scholar]
- Pizzichini, M.; Romani, A.; Pizzichini, D.; Russo, C.; Pinelli, P. Process for Producing Refined Nutraceutic Extracts from Artichoke Waste and from Other Plants of the Cynara Genus. EP 2131681 B1, 4 March 2010. [Google Scholar]
- Romani, A.; Lapucci, C.; Cantini, C.; Ieri, F.; Mulinacci, N.; Visioli, F. Evolution of minor polar compounds and antioxidant capacity during storage of bottled extra-virgin olive oil. J. Agric. Food Chem. 2007, 55, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Mincione, E.; Crisante, F.; Barontini, M.; Fabrizi, G.; Gentili, P. Chemoselective and efficient carboxymethylation of the alcoholic chain of phenols by dimethyl carbonate (DMC). Tetrahedron Lett. 2007, 48, 7000–7003. [Google Scholar] [CrossRef]
- Romani, A.; Scardigli, A.; Pinelli, P. An environmentally friendly process for the production of extracts rich in phenolic antioxidants from Olea europaea L. and Cynara scolymus L. matrices. Int. J. Food Sci. Technol. 2016. submitted. [Google Scholar]
- Forte, V.T.; Cazzato, A.R.; Borsacchi, L.; Romani, A. Listeria Nelle Carni. Nuove Prospettive per la Stabilizzazione. Le Proprietà Degli Estratti Naturali Nelle Preparazioni di Carne Fresca. Available online: http://www.pointvet.it/web/index.php?com=ermes&option=index&id=909 (accessed on 9 October 2016). (In Italian)
- Romani, A.; Pinelli, P.; Campo, M.; Corgna, M. Characterization of dietary supplements and their antioxidant activity in human serum. In Proceedings of the XXVIIth International Conference on Polyphenols & Tannin Conference, Nagoya, Japan, 2–6 September 2014.
- Bargiacchi, E.; Bellotti, P.; Costa, G.; Miele, S.; Pinelli, P.; Romani, A.; Scardigli, A.; Zambelli, P. Use of Chestnut Tannin Extract as Antioxidant, Antimicrobial Additive and for Reducing Nitrosamines and Mycotoxins. EP 2904910 A1, 12 August 2015. [Google Scholar]
- Bernini, R.; Mincione, E.; Barontini, M.; Crisante, F.; Gambacorta, A. Dimethyl carbonate: An environmentally friendly solvent for hydrogen peroxide (H2O2)/methyltrioxorhenium (CH3ReO3, MTO) catalytic oxidations. Tetrahedron 2007, 63, 6895–6900. [Google Scholar] [CrossRef]
- Bernini, R.; Crisante, F.; Ginnasi, M.C. A convenient and safe O-methylation of flavonoids with dimethyl carbonate (DMC). Molecules 2011, 16, 1418–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernini, R.; Crisante, F.; Barontini, M.; Tofani, D.; Balducci, V.; Gambacorta, A. Synthesis and structure/antioxidant activity relationship of novel catecholic antioxidants structurally analogues to hydroxytyrosol and its lipophilic esters. J. Agric. Food Chem. 2012, 60, 7408–7416. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Luzi, F.; Dugo, L.; Fanali, C.; Tripodo, G.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R. Effect of hydroxytyrosol methyl carbonate on the thermal, migration and antioxidant properties of PVA based films for active food packaging. Polym. Int. 2016. [Google Scholar] [CrossRef]
- Fuggetta, M.P.; Cottarelli, A.; Lanzilli, G.; Tricarico, M.; Bernini, R. In vitro antitumor activity of olive oil tyrosol and hydroxytyrosol and their methyl carbonate. Med. Aromat. Plant Sci. Biotechnol. 2012, 6, 25–30. [Google Scholar]
- Bernini, R.; Cacchi, S.; Fabrizi, G.; Filisti, E. 2-Arylhydroxytyrosol derivatives via Suzuki-Miyaura cross-coupling. Org. Lett. 2008, 10, 3457–3460. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Gilardini Montani, M.S.; Merendino, N.; Romani, A.; Velotti, F. Hydroxytyrosol-derived compounds: A basis for the creation of new pharmacological agents for cancer prevention and therapy. J. Med. Chem. 2015, 58, 9089–9107. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Crisante, F.; Merendino, N.; Molinari, R.; Soldatelli, M.C.; Velotti, F. Synthesis of a novel ester of hydroxytyrosol and alpha-lipoic acid exhibiting an antiproliferative effect on human colon cancer HT-29 cells. Eur. J. Med. Chem. 2011, 46, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Crisante, F.; Fabrizi, G.; Gentili, P. Convenient synthesis of 1-aryl-dihydroxyisochromans exhibiting antioxidant activity. Curr. Org. Chem. 2012, 16, 1051–1057. [Google Scholar] [CrossRef]
- Bernini, R.; Fabrizi, G.; Pouysegu, L.; Deffieux, D.; Quideau, S. Synthesis of biologically active catecholic compounds via ortho-selective oxygenation of phenolic compounds using hypervalent iodine(V) reagents. Curr. Org. Synth. 2012, 9, 650–669. [Google Scholar] [CrossRef]


| Samples | Extracted Leaves (%) | Extraction Time (min) | Total Polyphenols and Oleuropein 1,2 | |
|---|---|---|---|---|
| entry 1 | GL | 15 | 30 | 13.6 (38.5) |
| entry 2 | GL | 15 | 60 | 17.8 (43.9) |
| entry 3 | DL, rt, 15 days | 15 | 30 | 12.3 (19.5) |
| entry 4 | DL, rt, 15 days | 15 | 60 | 16.1 (22.0) |
| entry 5 | DL, ventilated stove, 40 °C, 3 days | 15 | 30 | 11.4 (26.0) |
| entry 6 | DL, ventilated stove, 40 °C, 3 days | 15 | 60 | 16.8 (41.0) |
| Compounds | Soft Extract Olea GL | Soft Extract Olea DL | Soft Extract Olea HTyr | |
|---|---|---|---|---|
| entry 1 | Hydroxytyrosol derivatives | 24.69 ± 3.47 | 25.21 ± 1.56 | 279.89 ± 18.24 |
| entry 2 | Secoiridoid derivatives | 164.19 ± 1.47 | 11.09 ± 0.45 | nd |
| entry 3 | Elenolic acid derivatives | 28.34 ± 0.43 | 7.54 ± 0.40 | 0.51 ± 0.04 |
| entry 4 | Flavonoids | 1.42 ± 0.06 | 4.30 ± 0.31 | 7.83 ± 0.25 |
| entry 5 | Verbascoside | 1.27 ± 0.01 | 1.00 ± 0.41 | 1.69 ± 0.17 |
| entry 6 | Lignans | 6.76 ± 0.10 | 5.85 ± 1.05 | nd |
| entry 7 | Total Polyphenols | 244.15 ± 5.54 | 57.63 ± 4.42 | 289.93 ± 18.70 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romani, A.; Pinelli, P.; Ieri, F.; Bernini, R. Sustainability, Innovation, and Green Chemistry in the Production and Valorization of Phenolic Extracts from Olea europaea L. Sustainability 2016, 8, 1002. https://doi.org/10.3390/su8101002
Romani A, Pinelli P, Ieri F, Bernini R. Sustainability, Innovation, and Green Chemistry in the Production and Valorization of Phenolic Extracts from Olea europaea L. Sustainability. 2016; 8(10):1002. https://doi.org/10.3390/su8101002
Chicago/Turabian StyleRomani, Annalisa, Patrizia Pinelli, Francesca Ieri, and Roberta Bernini. 2016. "Sustainability, Innovation, and Green Chemistry in the Production and Valorization of Phenolic Extracts from Olea europaea L." Sustainability 8, no. 10: 1002. https://doi.org/10.3390/su8101002
APA StyleRomani, A., Pinelli, P., Ieri, F., & Bernini, R. (2016). Sustainability, Innovation, and Green Chemistry in the Production and Valorization of Phenolic Extracts from Olea europaea L. Sustainability, 8(10), 1002. https://doi.org/10.3390/su8101002







