Development of a Sustainable Process for the Solid-Liquid Extraction of Antioxidants from Oat
Abstract
:1. Introduction
2. Experimental Section
2.1. Laboratory Experimentation
2.1.1. Materials and Methods
2.1.2. Milling and Sieving

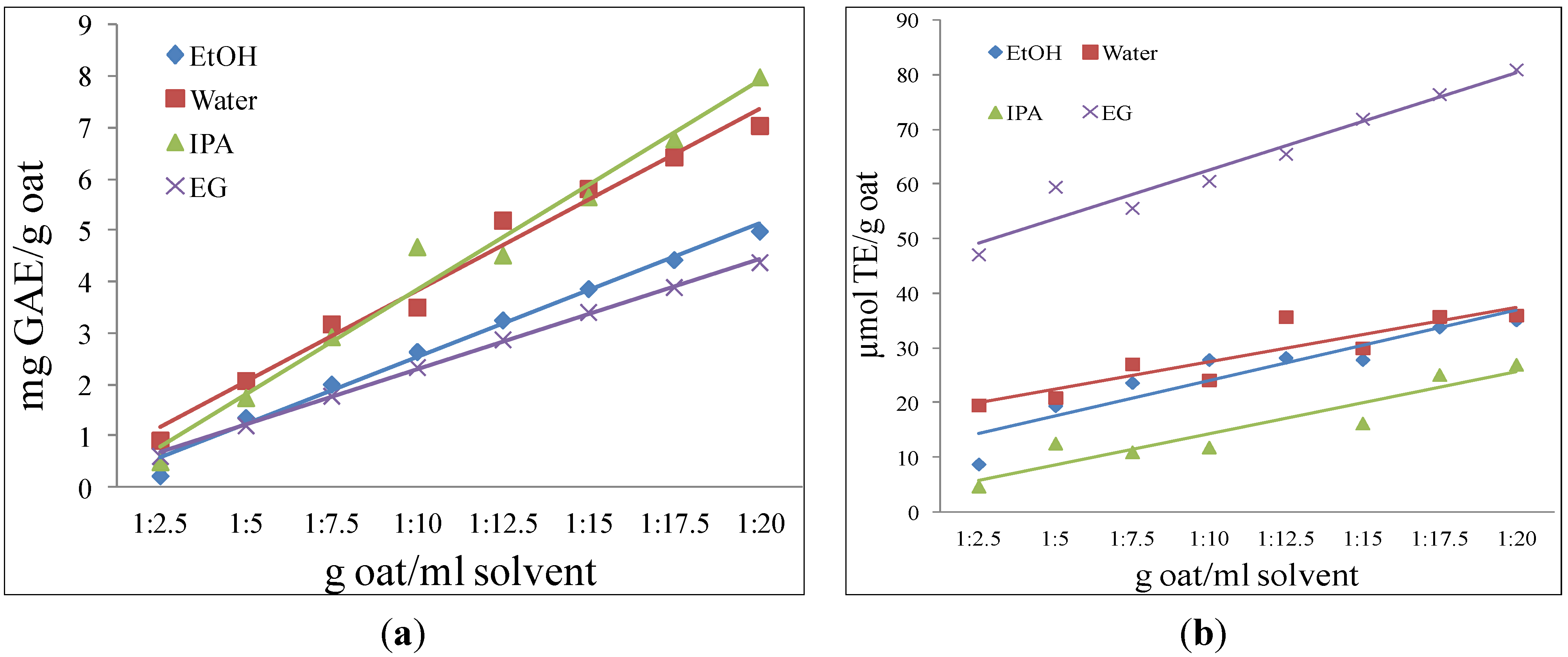
2.1.3. Solvent Selection
2.1.4. Solvent Extraction
2.1.5. Total Polyphenol Content (TPC)
| Factorial 1 | Factorial 2 | ||||
|---|---|---|---|---|---|
| Slope | Intercept | R2 | Slope | Intercept | R2 |
| 0.0008 | −0.0505 | 0.9943 | 0.0009 | −0.0362 | 0.9909 |
2.1.6. Oxygen Radical Absorption Capacity (ORAC)
| Factorial 1 | Factorial 2 | ||||||
|---|---|---|---|---|---|---|---|
| Solvent | Slope | Intercept | R2 | Solvent | Slope | Intercept | R2 |
| IPA | 0.7789 | 14.1939 | 0.9796 | EtOH | 0.6526 | 14.0051 | 0.9627 |
| Water | 0.9996 | −0.7754 | 0.9836 | EG | 0.6779 | 13.3367 | 0.9432 |
2.1.7. Design of Experiment
| Variable | Factorial 1 | Factorial 2 | ||
|---|---|---|---|---|
| Low | High | Low | High | |
| Particle size (mm) | 0.5 | 2 | 0.075 | 0.5 |
| Temperature (°C) | 30 | 50 | 30 | 50 |
| Time (min) | 20 | 60 | 20 | 60 |
| Solvent | IPA | Water | EtOH | EG |
2.1.8. Solubility Behavior
2.2. Process Development and Sustainability Analysis
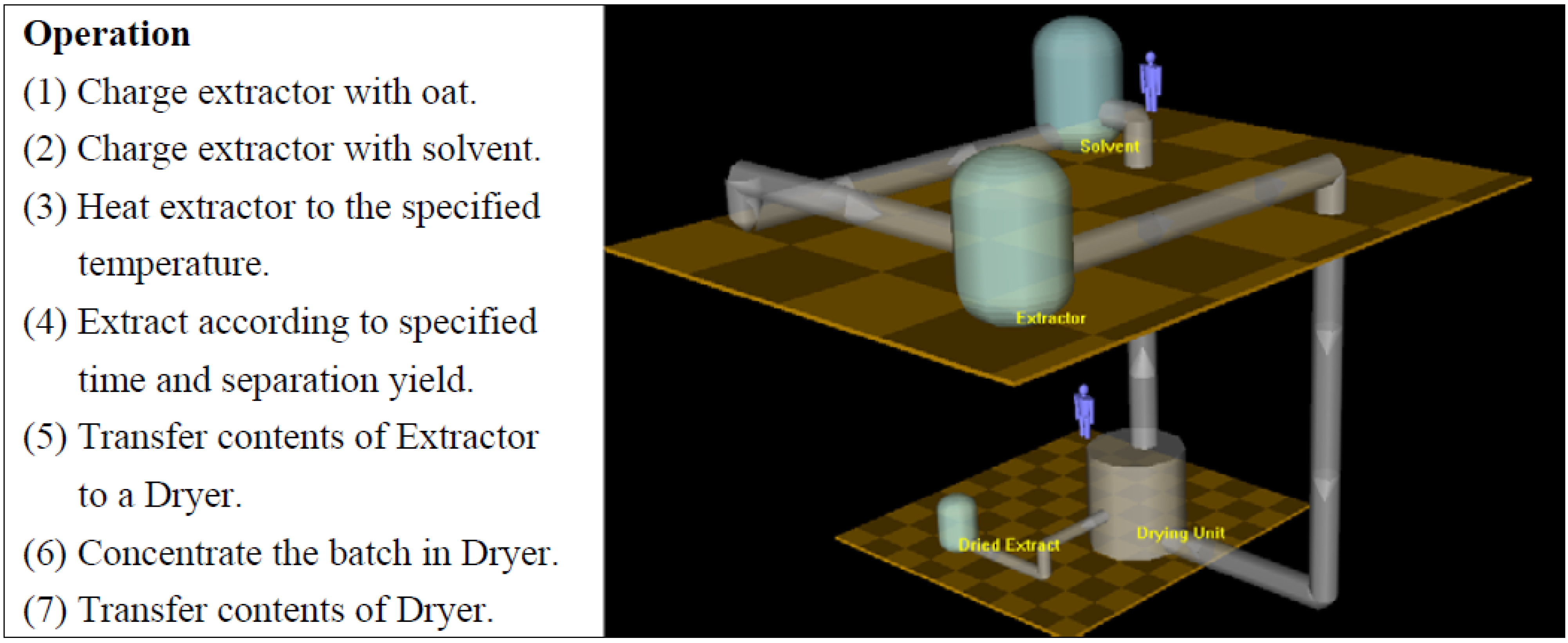
3. Results and Discussion
| TPC | ORAC | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | DF | SS | F | p | Source | DF | SS | F | p |
| Main Effects | 4 | 5.338 | 120.100 | <0.001 | Main Effects | 4 | 1963.170 | 53.540 | <0.001 |
| A | 1 | 0.016 | 1.420 | 0.251 | A | 1 | 16.120 | 1.760 | 0.203 |
| B | 1 | 2.025 | 182.250 | <0.001 | B | 1 | 855.530 | 93.330 | <0.001 |
| C | 1 | 0.046 | 4.140 | 0.059 | C | 1 | 37.240 | 4.060 | 0.061 |
| D | 1 | 3.251 | 292.600 | <0.001 | D | 1 | 1054.280 | 115.020 | <0.001 |
| 2- Interactions | 6 | 1.388 | 20.820 | <0.001 | 2- Interactions | 6 | 312.520 | 5.680 | 0.003 |
| AB | 1 | 0.045 | 4.000 | 0.063 | AB | 1 | 0.130 | 0.010 | 0.905 |
| AC | 1 | 0.034 | 3.100 | 0.097 | AC | 1 | 56.640 | 6.180 | 0.024 |
| AD | 1 | 0.007 | 0.640 | 0.436 | AD | 1 | 2.090 | 0.230 | 0.640 |
| BC | 1 | 0.002 | 0.220 | 0.649 | BC | 1 | 55.420 | 6.050 | 0.026 |
| BD | 1 | 1.297 | 116.760 | <0.001 | BD | 1 | 188.510 | 20.570 | <0.001 |
| CD | 1 | 0.002 | 0.190 | 0.669 | CD | 1 | 9.730 | 1.060 | 0.318 |
| 3-Interactions | 4 | 0.102 | 2.300 | 0.103 | 3-Interactions | 4 | 45.82 | 1.25 | 0.33 |
| ABC | 1 | 0.006 | 0.530 | 0.478 | ABC | 1 | 10.950 | 1.190 | 0.291 |
| ABD | 1 | 0.058 | 5.200 | 0.037 | ABD | 1 | 24.460 | 2.670 | 0.122 |
| ACD | 1 | 0.008 | 0.690 | 0.417 | ACD | 1 | 10.140 | 1.110 | 0.308 |
| BCD | 1 | 0.031 | 2.790 | 0.114 | BCD | 1 | 0.260 | 0.030 | 0.868 |
| 4-Interactions | 1 | 0.000 | 0.000 | 0.974 | 4-Interactions | 1 | 1.780 | 0.190 | 0.665 |
| ABCD | 1 | 0.000 | 0.000 | 0.974 | ABCD | 1 | 1.780 | 0.190 | 0.665 |
| Residual Error | 16 | 0.178 | Residual Error | 16 | 146.660 | ||||
| Total | 31 | 7.006 | Total | 31 | 2469.960 | ||||
| R2 = 0.98 | R2 = 0.94 | ||||||||
| TPC | ORAC | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | DF | SS | F | p | Source | DF | SS | F | p |
| Main Effects | 4 | 5.343 | 30.760 | <0.001 | Main Effects | 4 | 3551.070 | 21.130 | <0.001 |
| A | 1 | 0.032 | 0.750 | 0.400 | A | 1 | 10.660 | 0.250 | 0.621 |
| B | 1 | 0.049 | 1.140 | 0.302 | B | 1 | 1.720 | 0.040 | 0.842 |
| C | 1 | 0.078 | 1.810 | 0.198 | C | 1 | 2.170 | 0.050 | 0.823 |
| D | 1 | 5.183 | 119.360 | <0.001 | D | 1 | 3536.530 | 84.170 | <0.001 |
| 2- Interactions | 6 | 1.188 | 4.560 | 0.007 | 2-Interactions | 6 | 562.360 | 2.230 | 0.094 |
| AB | 1 | 0.057 | 1.310 | 0.270 | AB | 1 | 20.170 | 0.480 | 0.498 |
| AC | 1 | 0.051 | 1.170 | 0.296 | AC | 1 | 0.380 | 0.010 | 0.925 |
| AD | 1 | 0.262 | 6.030 | 0.026 | AD | 1 | 105.850 | 2.520 | 0.132 |
| BC | 1 | 0.654 | 15.060 | <0.001 | BC | 1 | 44.650 | 1.060 | 0.318 |
| BD | 1 | 0.081 | 1.860 | 0.192 | BD | 1 | 189.450 | 4.510 | 0.050 |
| CD | 1 | 0.084 | 1.940 | 0.182 | CD | 1 | 201.860 | 4.800 | 0.044 |
| 3-Interactions | 4 | 1.869 | 10.760 | <0.001 | 3-Interactions | 4 | 75.580 | 0.450 | 0.771 |
| ABC | 1 | 0.588 | 13.530 | 0.002 | ABC | 1 | 5.550 | 0.130 | 0.721 |
| ABD | 1 | 0.061 | 1.400 | 0.255 | ABD | 1 | 2.180 | 0.050 | 0.823 |
| ACD | 1 | 0.055 | 1.260 | 0.278 | ACD | 1 | 0.010 | 0.000 | 0.988 |
| BCD | 1 | 1.166 | 26.860 | <0.001 | BCD | 1 | 67.850 | 1.610 | 0.222 |
| 4-Interactions | 1 | 0.533 | 12.270 | 0.003 | 4-Interactions | 1 | 28.370 | 0.680 | 0.423 |
| ABCD | 1 | 0.533 | 12.270 | 0.003 | ABCD | 1 | 28.370 | 0.680 | 0.423 |
| Residual Error | 16 | 0.695 | Residual Error | 16 | 672.280 | ||||
| Total | 31 | 9.6288 | Total | 31 | 4889.670 | ||||
| R = 0.93 | R = 0.86 | ||||||||
| Definition | Coefficients | |
|---|---|---|
| Factorial 1 | Factorial 2 | |
| Constant | 27.3536 | 14.7806 |
| Temperature | –0.3793 | 0.2560 |
| Particle Size | –9.2789 | 20.5945 |
| Time | –0.2975 | –0.0020 |
| Solvent | 8.1849 | 17.1912 |
| Temperature*Particle Size | 0.1473 | –0.7656 |
| Temperature*Time | 0.0115 | –0.0023 |
| Temperature*Solvent | 0.0200 | –0.1117 |
| Particle Size*Time | 0.0683 | –0.1140 |
| Particle Size*Solvent | –5.6240 | –65.5139 |
| Time*Solvent | 0.2113 | –0.2312 |
| Temperature*Particle Size*Time | –0.0039 | 0.0098 |
| Temperature*Particle Size*Solvent | 0.0537 | 1.0090 |
| Temperature*Time*Solvent | –0.0048 | 0.0065 |
| Particle Size*Time*Solvent | –0.0569 | 1.2288 |
| Temperature*Particle Size*Time*Solvent | 0.0016 | –0.0222 |
3.1. First Factorial
3.2. Second Factorial
3.3. Extraction Optimization
| Solvent | Temperature | Particle Size | Time | ORAC |
|---|---|---|---|---|
| (°C) | (mm) | (min) | (µmol TE/g oat) | |
| Water | 50 | 0.5 | 60 | 27.0621 |
| IPA | 50 | 0.5 | 60 | 13.6600 |
| EG | 50 | 0.075 | 60 | 37.5267 |
| EtOH | 30 | 0.5 | 20 | 20.5623 |
3.4. Solubility Behavior

3.5 Process Simulation
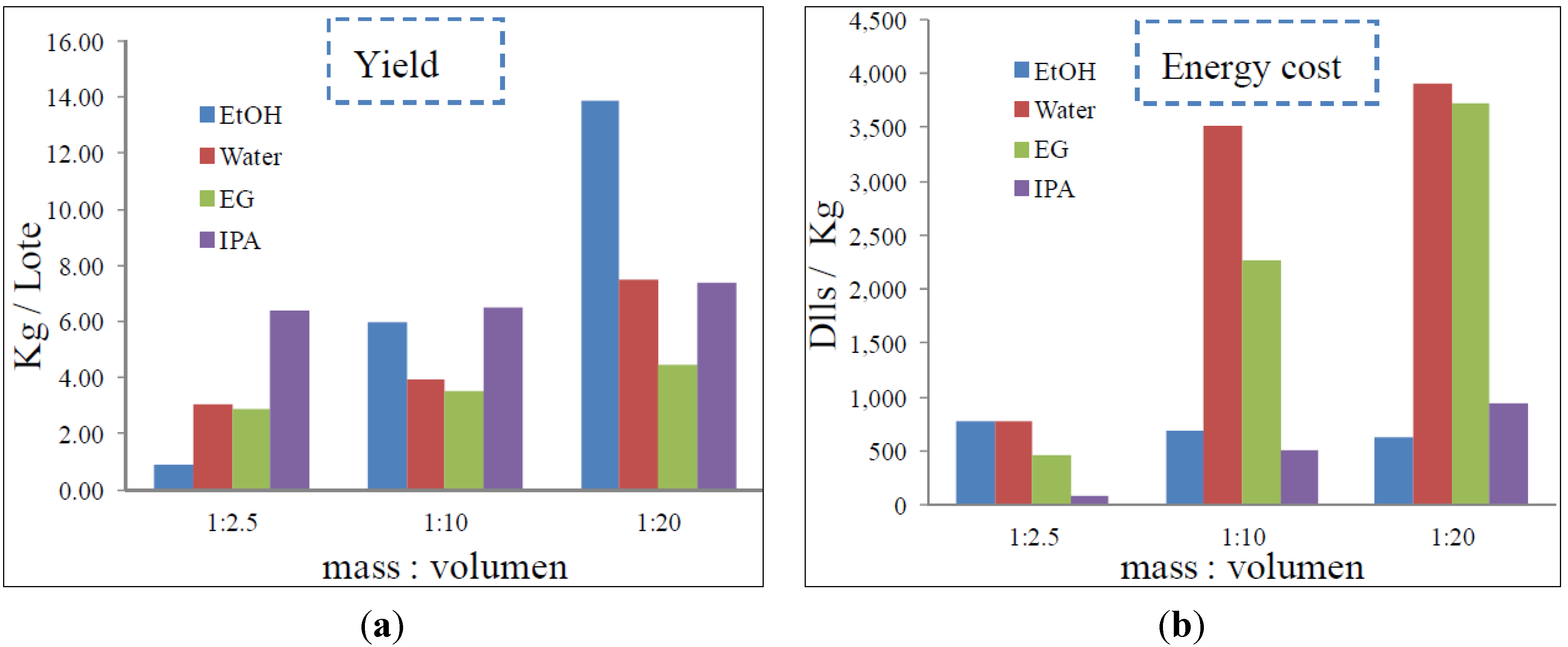
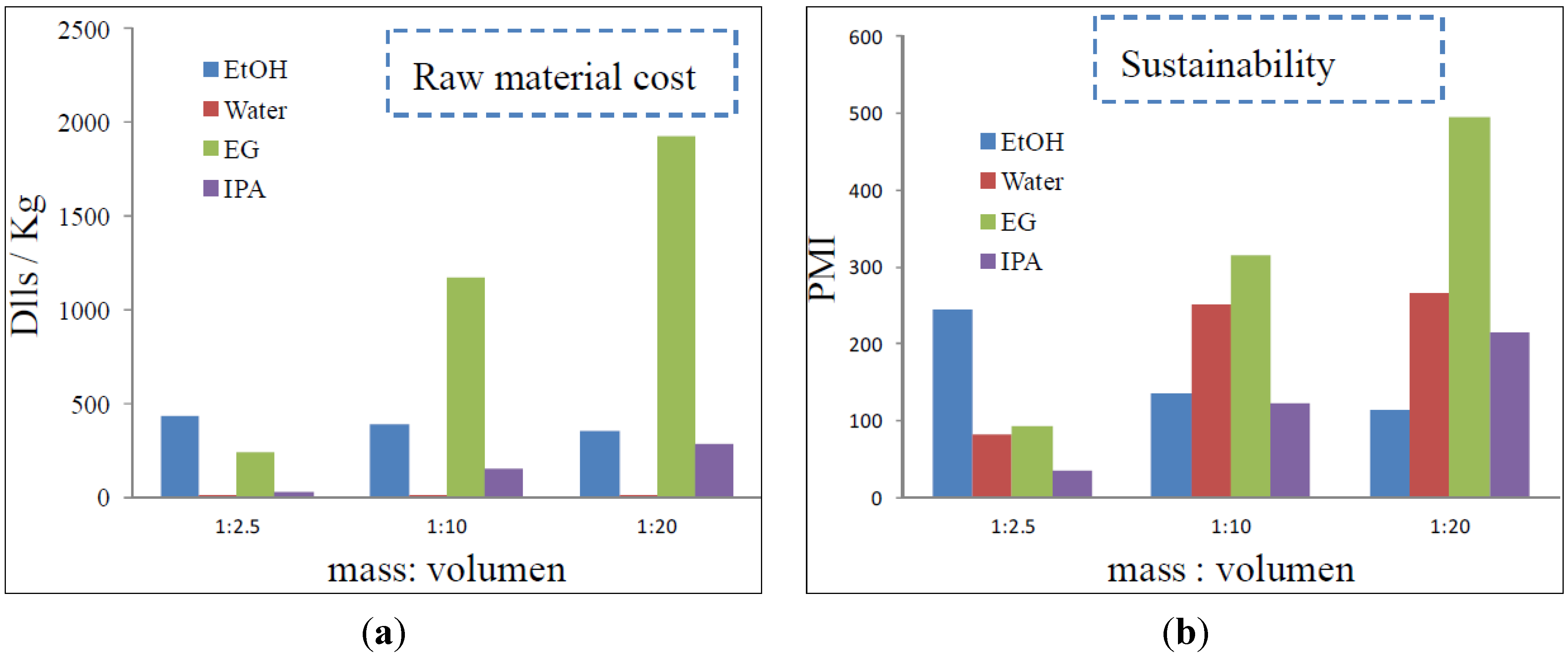
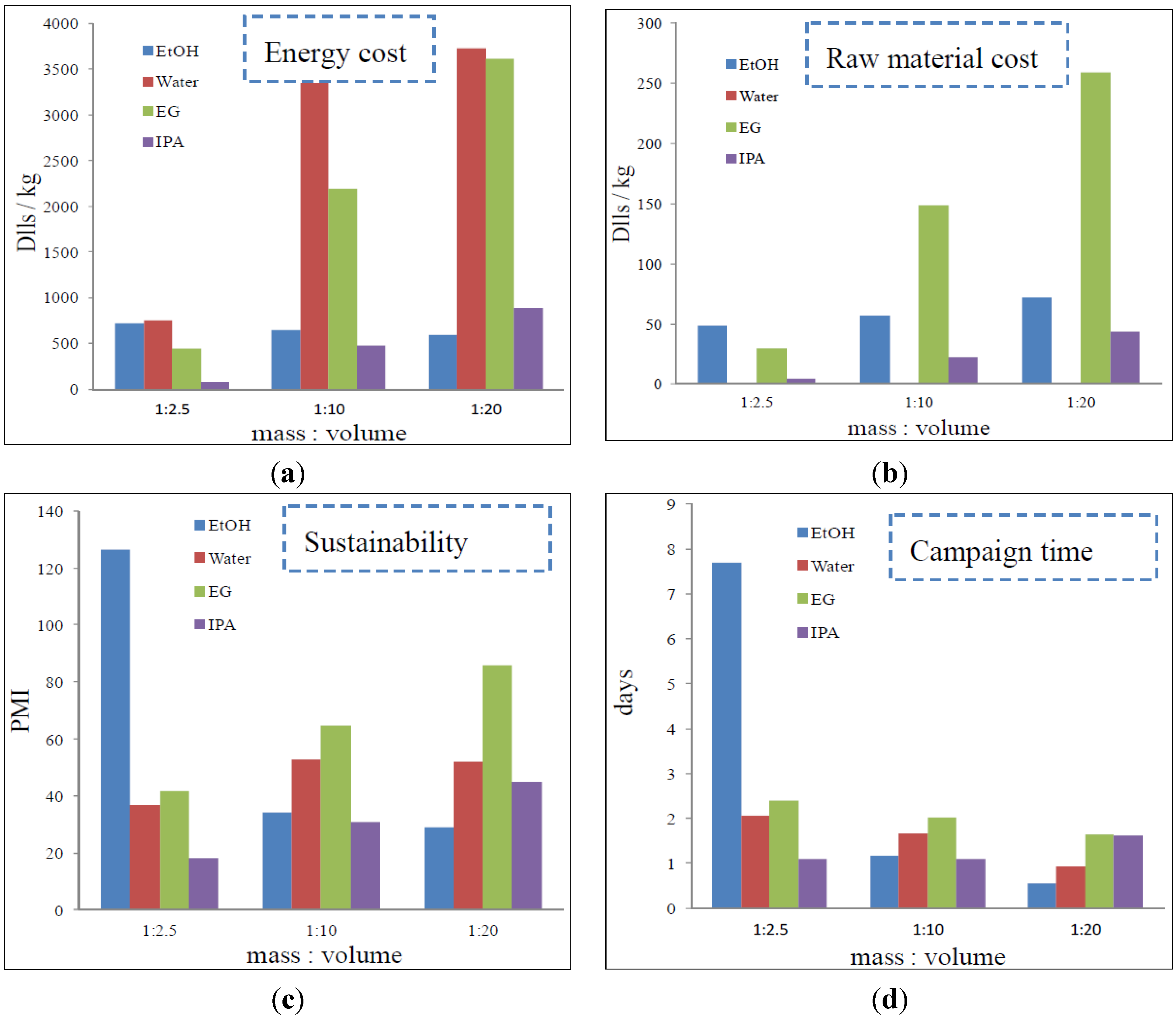
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nie, L.; Wise, M.L.; Peterson, D.M.; Meydani, M. Avenanthramide, a polyphenol from oats, inhibits vascular smooth muscle cell proliferation and enhances nitric oxide production. Atherosclerosis 2006, 186, 260–266. [Google Scholar] [CrossRef]
- Peterson, D.M.; Dimberg, L.H. Avenanthramide concentrations and hydroxycinnamoyl-CoA: hydroxyanthranilate N-hydroxycinnamoyltransferase activities in developing oats. J. Cereal Sci. 2008, 47, 101–108. [Google Scholar] [CrossRef]
- Emmons, L.; Peterson, M. Antioxidant Activity and Phenolic Contents of Oat Groats and Hulls. Ceral Chem. 1999, 76, 902–906. [Google Scholar] [CrossRef]
- Peterson, M. Oat Antioxidants. J. Cereal Sci. 2001, 33, 115–129. [Google Scholar] [CrossRef]
- Sen, L.; Nan, Y.; Zhao-hua, H.; Yang, Y.; Li, L.; Xian-rong, Z.; Gui-xing, R. Antioxidant Effects of Oats Avenanthramides on Human Serum. Agr. Sci. China 2011, 10, 1301–1305. [Google Scholar] [CrossRef]
- Chu, Y.-F.; Wise, M.L.; Gulvady, A.A.; Chang, T.; Kendra, D.F.; van Klinken, B.J.-W.; Shi, Y.; O’Shea, M. In vitro antioxidant capacity and anti-inflammatory activity of seven common oats. Food Chem. 2013, 139, 426–431. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar]
- Verardo, V.; Serea, C.; Segal, R.; Fiorenza, C. Free and bound minor polar compounds in oats: Different extraction methods and analytical determinations. J. Cereal Sci. 2011, 54, 211–217. [Google Scholar] [CrossRef]
- Karen, D.; Pamela, W. Extraction and identification of antioxidants in oats. J. Am. Oil Chem. Soc. 1991, 68, 365–370. [Google Scholar] [CrossRef]
- Curzons, A.D.; Constable, D.; Mortimer, D.; Cunningham, V. So you think your process is green, how do you know? Using principles of sustainability to determine what is green—A corporate perspective. Green Chem. 2001, 3, 1–6. [Google Scholar]
- Smallwood, I.M. Solvent Recovery Handbook, 2nd ed.; Blackwell Science: Oxford, UK, 2002. [Google Scholar]
- Jimenez-Gonzalez, C.; Poechlauer, P.; Broxterman, Q.B.; Yang, B.-S.; am Ende, D.; Baird, J.; Bertsch, C.; Hannah, R.E.; Dell’Orco, P.; Noorman, H.; et al. Key Green Engineering Research Areas for Sustainable Manufacturing: A Perspective from Pharmaceutical and Fine Chemicals Manufacturers. Org. Process Res. Dev. 2011, 15, 900–911. [Google Scholar] [CrossRef]
- Zilic, S.; Sukalovicb, V.H.-T.; Dodiga, D.; Maksimovi, V.; Maksimovi, M.; Basi, Z. Antioxidant activity of small grain cereals caused by phenolics and lipid soluble antioxidants. J. Cereal Sci. 2011, 54, 417–424. [Google Scholar] [CrossRef]
- Handelman, G.; Cao, G.; Walter, M.; Nightingale, Z.; Paul, G.; Prior, R.; Blumberg, J. Antioxidant Capacity of Oat (Avena sativa L.) Extracts. 1. Inhibition of Low-Density Lipoprotein Oxidation and Oxygen Radical Absorbance Capacity. J. Agric. Food Chem. 1999, 48, 4888–4893. [Google Scholar]
- Lehtinen, P.; Laakso, S. Effect of Extraction Conditions on the Recovery and Potency of Antioxidants in Oat Fiber. J. Agric. Food. Chem. 1998, 46, 4842–4845. [Google Scholar] [CrossRef]
- Bryngelsson, S.; Dimberg, L.H.; Eldin, A.K. Effects of Commercial Processing on Levels of Antioxidants in Oats (Avena sativa L.). J. Agric. Food Chem. 2002, 50, 1890–1896. [Google Scholar] [CrossRef]
- Anderson, N.G. Practical Process Research & Development, 2nd ed.; Academic Press: Oxford, UK, 2012. [Google Scholar]
- Perez-Vega, S.; Peter, S.; Salmeron-Ochoa, I.; Nieva-de la Hidalga, A.; Sharratt, P.N. Analytical hierarchy processes (AHP) for the selection of solvents in early stages of pharmaceutical process development. Process Saf. Environ. Prot. 2011, 89, 261–267. [Google Scholar]
- Wypych, G. (Ed.) Handbook of Solvents; ChemTec Publishing: Toronto, Canada, 2001.
- Curzons, A.D.; Constable, D.C.; Cunningham, V.L. Solvent selection guide: A guide to the integration of environmental, health and safety criteria into the selection of solvents. Clean Technol. Environ. Pol. 1999, 1, 82–90. [Google Scholar] [CrossRef]
- Constable, D.J.C.; Jimenez-Gonzalez, C.; Henderson, R.K. Perspective on Solvent Use in the Pharmaceutical Industry. Org. Process Res. Dev. 2007, 11, 133–137. [Google Scholar] [CrossRef]
- Singleton, V.; Orthofer, R.; Lamuelas-Raventos, R. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 229, 152–178. [Google Scholar]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food. Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 5th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Aspen Batch Plus. Available online: http://www.aspentech.com/products/aspen-batch-plus.aspx (accessed on 1 June 2013).
- Peterson, D.; Wesenbergb, D.; Burrupb, D.; Charles, A. Relationships among Agronomic Traits and Grain Composition in Oat Genotypes Grown in Different Environments. Crop Sci. 2005, 45, 1249–1255. [Google Scholar] [CrossRef]
- Sultana, B.; Anwa, F.; Ashraf, M. Effect of Extraction Solvent/Technique on the Antioxidant Activity of Selected Medicinal Plant Extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Speisky, H.; López-Alarcó, C.; Gómez, M.; Fuentes, J.; Sandoval-Acuña, C. First Web-Based Database on Total Phenolics and Oxygen Radical Absorbance Capacity (ORAC) of Fruits Produced and Consumed within the South Andes Region of South America. J. Agric. Food. Chem. 2012, 60, 8851–8859. [Google Scholar] [CrossRef]
- Bratt, K.; Sunnerheim, K.; Brynglesson, S.; Fagerlund, A.; Engman, L.; Andersson, R.E.; Dimberg, L.H. Avenanthramides in Oats (Avena sativa L.) and Structure-Antioxidant Activity Relationships. J. Agric. Food. Chem. 2003, 51, 594–600. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R. Lipophilic and Hydrophilic Antioxidant Capacities of Common Foods in the United States. J. Agric. Food. Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef]
- Cuppett, S.; Schnepf, M.; Hall, C. Natural antioxidants—Are they a reality. In Natural Antioxidants, Chemistry, Health Effects and Applications; Shahidi, F., Ed.; AOCS Press: Champaign, IL, USA, 1997; pp. 12–24. [Google Scholar]
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food. Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food. Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Orozco-Mena, R.; Salmerón-Ochoa, I.; Ortega-Rivas, E.; Perez-Vega, S. Development of a Sustainable Process for the Solid-Liquid Extraction of Antioxidants from Oat. Sustainability 2014, 6, 1504-1520. https://doi.org/10.3390/su6031504
Orozco-Mena R, Salmerón-Ochoa I, Ortega-Rivas E, Perez-Vega S. Development of a Sustainable Process for the Solid-Liquid Extraction of Antioxidants from Oat. Sustainability. 2014; 6(3):1504-1520. https://doi.org/10.3390/su6031504
Chicago/Turabian StyleOrozco-Mena, Raul, Iván Salmerón-Ochoa, Enrique Ortega-Rivas, and Samuel Perez-Vega. 2014. "Development of a Sustainable Process for the Solid-Liquid Extraction of Antioxidants from Oat" Sustainability 6, no. 3: 1504-1520. https://doi.org/10.3390/su6031504
APA StyleOrozco-Mena, R., Salmerón-Ochoa, I., Ortega-Rivas, E., & Perez-Vega, S. (2014). Development of a Sustainable Process for the Solid-Liquid Extraction of Antioxidants from Oat. Sustainability, 6(3), 1504-1520. https://doi.org/10.3390/su6031504





