Abstract
Following the critical analysis of the concept of “sustainability”, developed on the basis of exergy considerations in previous works, an analysis of possible species “behavior” is presented and discussed in this paper. Once more, we make use of one single axiom: that resource consumption (material and immaterial) can be quantified solely in terms of exergy flows. This assumption leads to a model of population dynamics that is applied here to describe the general behavior of interacting populations. The resulting equations are similar to the Lotka-Volterra ones, but more strongly coupled and intrinsically non-linear: as such, their solution space is topologically richer than those of classical prey-predator models. In this paper, we address an interesting specific problem in population dynamics: if a species assumes a commensalistic behavior, does it gain an evolutionary advantage? And, what is the difference, in terms of the access to the available exergy resources, between mutualism and commensalism? The model equations can be easily rearranged to accommodate both types of behavior, and thus only a brief discussion is devoted to this facet of the problem. The solution space is explored in the simplest case of two interacting populations: the model results in population curves in phase space that can satisfactorily explain the evolutionistic advantages and drawbacks of either behavior and, more importantly, identify the presence or absence of a “sustainable” solution in which both species survive.
List of Symbols
| aji | Discharge from “j” used by “i” |
| ci | Minimum consumption for survival of “i”, W |
| Ė | Exergy flow rate, W |
| Ėwi | Exergy flow rate discharged by “i”, W |
| Ėδi | Exergy flow rate destroyed by “i”, W |
| N | Population numerosity |
| pi | Global discharge coefficient of “i” |
| ri | Limit growth rate (births-deaths) of “i”, 1/y |
| wi | Exergy discharge coefficient of “i” |
Greek symbols
| βi | Dimensionless exergy capture coefficient of “i” |
| γi | Inflow capture coefficient, W/person of “i” |
| δi | Exergy destruction coefficient of “i” |
 i i | Coupling terms in Equation (7), W/person |
| θ | Parameter in Equation (15) |
| λi | Lyapunov exponents, 1/y |
| μi | Intrinsic mortality rate of “i”, 1/y |
1. Introduction
The concept of sustainability is based on the qualitative or quantitative assessment of environmental interactions between a system and its environment at large, and implies the identification of a certain number of natural—and possibly finite—resources so that the dynamic interplay among species in a given ecological niche can be modeled to study the time evolution of the system. A first problem is thus that of “measuring” the resources, i.e., of finding a quantifier that is at the same time useful, convenient and general enough to represent material and immaterial fluxes. A second problem is posed by the empirical observation that most existing species display a multitude of “modes” of natural interrelation, like indifference, competition, cooperation, antagonism, adaptation, etc. A third problem is the “flexible attitude” exhibited by most species towards the substitutability of certain resources [1,2,3].
Since the very first attempts to construct behavioral and evolutionary models of living organisms, two substantially different approaches have been taken:
- a) An ad hoc approach that focuses on the particular dynamics of a single species or of a particular, generally small, ecological niche: models of such kind strongly depend on a set of very stringent initial assumptions, but thanks to their “specificity” have enjoyed a remarkable degree of success in reproducing experimental field data and—to a lesser extent—to predict future trends;
- b) The opposite approach is also possible: one tries to understand the population dynamics in a global sense, emphasizing and exploiting similarities in the behavior of different species in different environmental conditions and within different trophic chains. An original model of this second type has been proposed in previous papers by the present authors [4,5], and is based on the generally accepted principle that population dynamics depend substantially on the availability of primary resource and on the axiom that the consumption of such resources can be measured by an environmental indicator called extended exergy, EE in the following [6]. Extended Exergy is a physical quantity (measured in J, its fluxes in W) that explicitly measures the different forms in which natural resources are “available”, has the logical attributes of a “cost”, and thus is amenable to a resource accounting procedure -the method in fact goes under the name of Extended Exergy Accounting, EEA. Extended exergy is additive, explicitly includes irreversible losses, and its formulation covers the so-called “externalities”: using a somewhat anthropological expression, it can be rightly said that the EE of a commodity is a measure of the biosphere “effort” to convert low-entropy into high-entropy resources while generating that commodity. (Note: The conceptual novelty of Extended Exergy is its inclusion of the equivalent Capital, Labor and Environmental Remediation costs into the exergy “embodied” in a product of whatever material- or energy conversion chain. In the context of this paper, Capital and Labor are of course absent, and the difference between the use of exergy and extended exergy consists in the inclusion in the latter of the amount of natural exergy resources required of the environment to “biodegrade” the environmental damage produced by a species. To provide a simple example, the restoration of the trees destroyed by the Southern Pine Beetle [3] requires a certain amount of cumulative exergy, in the form of solar irradiation and nutrients, that represents the “cost” for the biosphere to recover its previous state. Similarly, the oxidation of pollutants in a water basin requires solar irradiation, microbic action, etc., all of which can be expressed in terms of “exergy inflow”).
Nature’s intertwined trophic chains are far too complex a system to be amenable to a tractable comprehensive mathematical approach: for example, it is impossible to classify within general schemes voluntary or instinctive behaviors that, especially in superior species, can be treated only by recurring to species-specific, and therefore non-general, assumptions. Reasoning in an ethologic sense, though, it is legitimate to posit [7] the dependence of the dynamics of a certain species on the available resources (measured by extended exergy), formulate a proper mathematical model on its basis, and apply the model to predict the dynamics of a single species (local level) or of an entire ecological niche (global level): since a large amount of experimental data are available on the dynamics of certain populations, the results can subsequently be validated and verified. It was shown in two previous papers [4,5] that such an analysis of the sustainability concept leads to credible results. In the model adopted in those papers, “competition” was among the species and for the available resources. The underlying assumptions are quite simple:
- 1) The number N of individuals in a species at a given time depends on the global exergy resources a population can avail itself of.
- 2) These resources may be quantified in terms of Extended Exergy (i.e., by their primary resource equivalent), and expressed as a flux of exergy that each species may tap.
The resulting equation for a single population (for the complete derivation, see [4,5]) couples the population size (its numerosity N) with the exergy rate Ė(t) of the global exergy resources exploited by the population in its ecological niche:
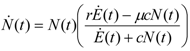
where the dot denotes the time derivative, the constant r is the limit growth rate of the population (in the case of infinitely abundant flow of resources) and μ is the intrinsic mortality rate of the species (calculated in the case in which the availability of resources drops to zero). It is useful to briefly discuss the behavior of the solutions of this equation in a very simple but realistic situation. Suppose that the incoming exergy rate is constant in time: this means the population N(t) can avail itself of the same exergy inflow at every instant of time. What one expects is then a “logistic” behavior, in the sense that, after an initial transient, the solution N(t) approaches a limit depending on the parameters of the model and on the available exergy flow. Indeed in this simple case the equation possesses an implicit solution given by:

where N0 = N(t = t0) and  . It is clear that as t → ∞, N(t) → N*. The behavior is indeed similar to that of a logistic-type equation under a carrying capacity constraint: here however the specific exergy consumption rate and the parameters of the model exactly define the carrying capacity. Note that in this case, under a constant influx condition (and therefore an infinite amount of cumulative exergy) at its disposal, the numerosity can sustain itself to the value N* for an infinite time. Obviously this is no longer true when
. It is clear that as t → ∞, N(t) → N*. The behavior is indeed similar to that of a logistic-type equation under a carrying capacity constraint: here however the specific exergy consumption rate and the parameters of the model exactly define the carrying capacity. Note that in this case, under a constant influx condition (and therefore an infinite amount of cumulative exergy) at its disposal, the numerosity can sustain itself to the value N* for an infinite time. Obviously this is no longer true when  describes a finite amount of cumulative exergy (see [4,5]). Notice also that the carrying capacity N* is proportional to Ė but also inversely proportional to c, a model constant representing the specific exergy consumption rate corresponding to“minimum survival” of the population; indeed if in a given interval of time the total energy consumption rate Ė(t) of the population is much higher than cN(t) then the population increases in that interval, while in the opposite situation the population would decrease: this means that the curve N(t) “follows” in some sense the curve Ė(t). A last remark: in the previous simple situation when Ė(t) is constant we can have two qualitative different behavior of the solution N(t) (see e.g. [4]): if at t = t0 the initial population size is larger than N*, then the population will monotonically (and exponentially) decrease to the value N*, while if the population size at t = t0 is smaller than N*, then the population will increase to the value N*. Some plots of the transient N(t) are provided in Figure 1: the gray curves represent the first case, the black ones the latter.
describes a finite amount of cumulative exergy (see [4,5]). Notice also that the carrying capacity N* is proportional to Ė but also inversely proportional to c, a model constant representing the specific exergy consumption rate corresponding to“minimum survival” of the population; indeed if in a given interval of time the total energy consumption rate Ė(t) of the population is much higher than cN(t) then the population increases in that interval, while in the opposite situation the population would decrease: this means that the curve N(t) “follows” in some sense the curve Ė(t). A last remark: in the previous simple situation when Ė(t) is constant we can have two qualitative different behavior of the solution N(t) (see e.g. [4]): if at t = t0 the initial population size is larger than N*, then the population will monotonically (and exponentially) decrease to the value N*, while if the population size at t = t0 is smaller than N*, then the population will increase to the value N*. Some plots of the transient N(t) are provided in Figure 1: the gray curves represent the first case, the black ones the latter.
 . It is clear that as t → ∞, N(t) → N*. The behavior is indeed similar to that of a logistic-type equation under a carrying capacity constraint: here however the specific exergy consumption rate and the parameters of the model exactly define the carrying capacity. Note that in this case, under a constant influx condition (and therefore an infinite amount of cumulative exergy) at its disposal, the numerosity can sustain itself to the value N* for an infinite time. Obviously this is no longer true when
. It is clear that as t → ∞, N(t) → N*. The behavior is indeed similar to that of a logistic-type equation under a carrying capacity constraint: here however the specific exergy consumption rate and the parameters of the model exactly define the carrying capacity. Note that in this case, under a constant influx condition (and therefore an infinite amount of cumulative exergy) at its disposal, the numerosity can sustain itself to the value N* for an infinite time. Obviously this is no longer true when  describes a finite amount of cumulative exergy (see [4,5]). Notice also that the carrying capacity N* is proportional to Ė but also inversely proportional to c, a model constant representing the specific exergy consumption rate corresponding to“minimum survival” of the population; indeed if in a given interval of time the total energy consumption rate Ė(t) of the population is much higher than cN(t) then the population increases in that interval, while in the opposite situation the population would decrease: this means that the curve N(t) “follows” in some sense the curve Ė(t). A last remark: in the previous simple situation when Ė(t) is constant we can have two qualitative different behavior of the solution N(t) (see e.g. [4]): if at t = t0 the initial population size is larger than N*, then the population will monotonically (and exponentially) decrease to the value N*, while if the population size at t = t0 is smaller than N*, then the population will increase to the value N*. Some plots of the transient N(t) are provided in Figure 1: the gray curves represent the first case, the black ones the latter.
describes a finite amount of cumulative exergy (see [4,5]). Notice also that the carrying capacity N* is proportional to Ė but also inversely proportional to c, a model constant representing the specific exergy consumption rate corresponding to“minimum survival” of the population; indeed if in a given interval of time the total energy consumption rate Ė(t) of the population is much higher than cN(t) then the population increases in that interval, while in the opposite situation the population would decrease: this means that the curve N(t) “follows” in some sense the curve Ė(t). A last remark: in the previous simple situation when Ė(t) is constant we can have two qualitative different behavior of the solution N(t) (see e.g. [4]): if at t = t0 the initial population size is larger than N*, then the population will monotonically (and exponentially) decrease to the value N*, while if the population size at t = t0 is smaller than N*, then the population will increase to the value N*. Some plots of the transient N(t) are provided in Figure 1: the gray curves represent the first case, the black ones the latter.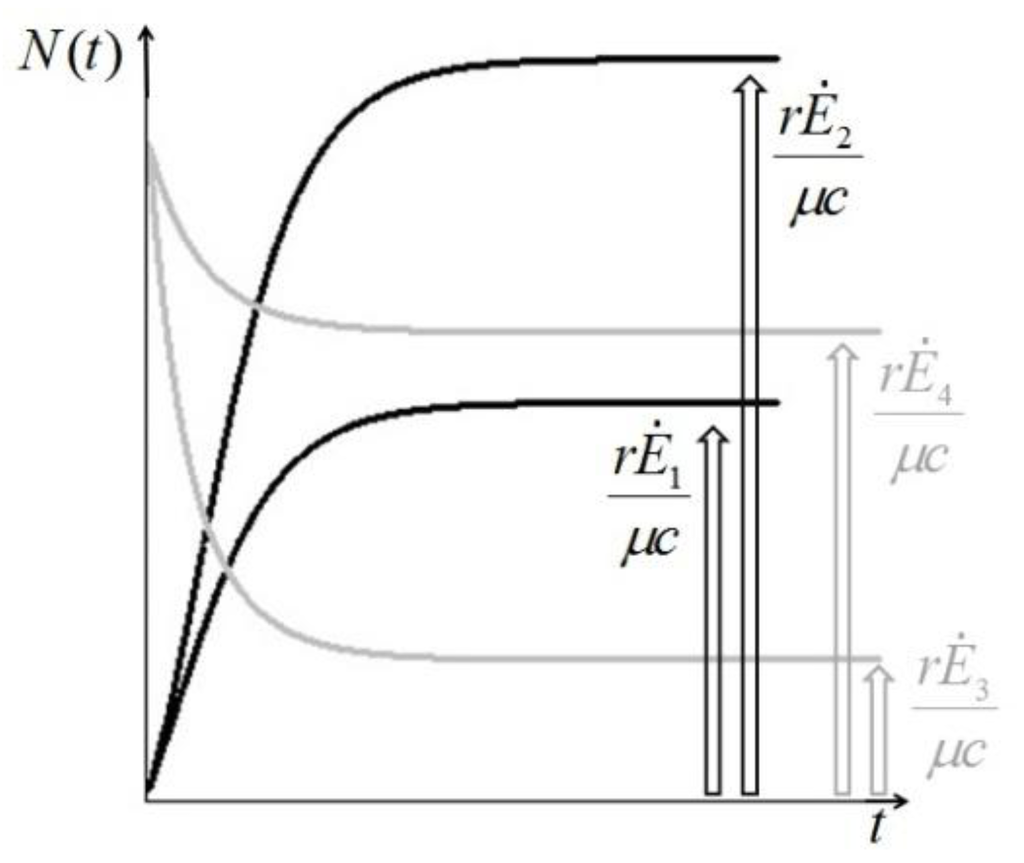
Figure 1.
Qualitative transient behavior or the solutions of Equation (3).
In the case of Z populations the previous equation generalizes to:
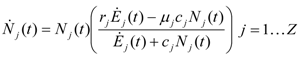
where again the rj, j=1…Z, are the limit growth rates of each population and the μj are the intrinsic mortality rate of each species. Now the “source term” Ėj(t) represents the exergy rate allocated to population j and, as such, it will depend on the strength of the mutualism and competition between populations i and j. Once the characteristics of each species have been assigned (rj, μj and Ėj(t) above), a “scenario” is completely identified by specifying the evolution of the incoming total exergy rate: for each scenario, equations (3) describe the evolution of the Z populations. For a detailed analysis on the dynamics of competing populations governed by Equation (3), we refer readers to [4]. The relative strength of the interactions between populations i and j, that we describe by means of a set of parameters characteristic of the species and of their ecological niche, determines the relative amount of the global incoming exergy allocated to each population.
In this paper we show that the system (3) is comprehensive and general enough to encompass the three following experimentally observed modes of species interaction [8,9]:
- a) Indifference: two species in the same environmental niche share the available incoming exergy flux but do not feed on each other’s discharges;
- b) Commensalism: one of the species survives solely on the resources released by its “host”;
- c) Mutualism; the two species compete for the externally available resources, but feed on each other’s discharges.
2. A Model of Two Interacting Populations Sharing a Common Renewable Resource
Consider two species that share a single renewable resource and who “feed” on their respective discharges (Figure 2). Notice that the relative strength of the interactions between populations i and j, that we describe by means of a set of parameters characteristic of the species and of their ecological niche, determines the relative amount of the global incoming exergy allocated to each population. Our model assumes that, at each instant of time, the rate of exergy accumulation or depletion (Ė1 and Ė2 here) is the force that drives the increase (or decrease) in the respective numerosities: it will be therefore regarded as “useful input”.
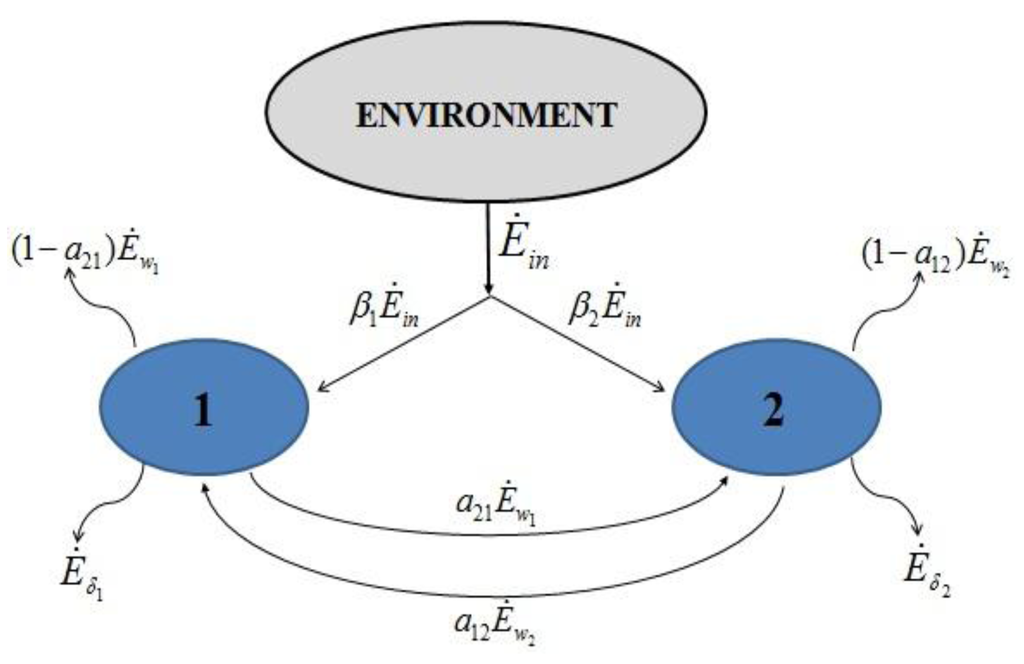
Figure 2.
Exergy “balance” for species 1 and 2.
The exergy “balances” read:
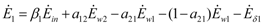
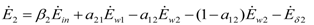
Notice that, since exergy is not a conserved quantity, in this paper the expression “exergy balance” is adopted to denote the closure of the exergy equation obtained by introducing the exergy destruction Eδ.
We assume that the allocation of the exergy flows Ė1 and Ė2 increases with the numerosity of the respective population. So we set: 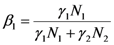 and
and  , γ1 and γ2 (both in J/individual/day) being the specific exergy consumptions of the two species (notice that β1+β2=1 and that
, γ1 and γ2 (both in J/individual/day) being the specific exergy consumptions of the two species (notice that β1+β2=1 and that  as requested by the imposed boundary conditions). In most real situations, the parameters γi could depend also on time: in this paper, for simplicity, we posit that they are constant. Note that the parameter ank is the amount of the discharge of species k “recycled” by species n: if ank=akn=0, the two populations are indifferent to each other, i.e., neither one exploits the discharges of the other as its own exergy input). Since the instantaneous rate of discharge and destruction of the exergy flow can be only a fraction of the incoming one, we set
as requested by the imposed boundary conditions). In most real situations, the parameters γi could depend also on time: in this paper, for simplicity, we posit that they are constant. Note that the parameter ank is the amount of the discharge of species k “recycled” by species n: if ank=akn=0, the two populations are indifferent to each other, i.e., neither one exploits the discharges of the other as its own exergy input). Since the instantaneous rate of discharge and destruction of the exergy flow can be only a fraction of the incoming one, we set 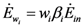 and
and 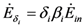 where wi and δi are two dimensionless species-specific constants measuring the portion of incoming flow being discharged and destroyed by population Ni: In the model presented here, these “discharges” are NOT equivalent to the detritus (which is a pure material flow rate): they include also any form of exergy released by species j (thermal, chemical, etc.)
where wi and δi are two dimensionless species-specific constants measuring the portion of incoming flow being discharged and destroyed by population Ni: In the model presented here, these “discharges” are NOT equivalent to the detritus (which is a pure material flow rate): they include also any form of exergy released by species j (thermal, chemical, etc.)
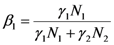 and
and  , γ1 and γ2 (both in J/individual/day) being the specific exergy consumptions of the two species (notice that β1+β2=1 and that
, γ1 and γ2 (both in J/individual/day) being the specific exergy consumptions of the two species (notice that β1+β2=1 and that  as requested by the imposed boundary conditions). In most real situations, the parameters γi could depend also on time: in this paper, for simplicity, we posit that they are constant. Note that the parameter ank is the amount of the discharge of species k “recycled” by species n: if ank=akn=0, the two populations are indifferent to each other, i.e., neither one exploits the discharges of the other as its own exergy input). Since the instantaneous rate of discharge and destruction of the exergy flow can be only a fraction of the incoming one, we set
as requested by the imposed boundary conditions). In most real situations, the parameters γi could depend also on time: in this paper, for simplicity, we posit that they are constant. Note that the parameter ank is the amount of the discharge of species k “recycled” by species n: if ank=akn=0, the two populations are indifferent to each other, i.e., neither one exploits the discharges of the other as its own exergy input). Since the instantaneous rate of discharge and destruction of the exergy flow can be only a fraction of the incoming one, we set 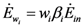 and
and 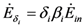 where wi and δi are two dimensionless species-specific constants measuring the portion of incoming flow being discharged and destroyed by population Ni: In the model presented here, these “discharges” are NOT equivalent to the detritus (which is a pure material flow rate): they include also any form of exergy released by species j (thermal, chemical, etc.)
where wi and δi are two dimensionless species-specific constants measuring the portion of incoming flow being discharged and destroyed by population Ni: In the model presented here, these “discharges” are NOT equivalent to the detritus (which is a pure material flow rate): they include also any form of exergy released by species j (thermal, chemical, etc.)It is clear that this interpretation for the constants wi and δi implies the constraints wi + δi ≤ 1. Equations (4) and (5) then result in:
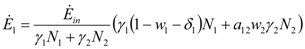

Due to the constraints wi + δi ≤ 1 we see that the above expressions are indeed, at every instant, non-negative, as requested by their physical interpretation. It is convenient to set pi = 1 − wi − δi: by substituting (6) and (7) into (3), we obtain:


Where for simplicity we set 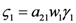 and
and 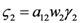 . The strong coupling between the numerosity of each species and the time evolution of ALL the participating species is apparent. Notice that in this case, Z = 2, but the formulae can be easily extended to account for whatever number Z of interacting populations, see [1]. We shall assume in the following that the exergy influx Ėin is constant and that the model coefficients γ, w, δ, r, μ and c are known for all species considered. The type and degree of interaction between the two species is represented by the terms
. The strong coupling between the numerosity of each species and the time evolution of ALL the participating species is apparent. Notice that in this case, Z = 2, but the formulae can be easily extended to account for whatever number Z of interacting populations, see [1]. We shall assume in the following that the exergy influx Ėin is constant and that the model coefficients γ, w, δ, r, μ and c are known for all species considered. The type and degree of interaction between the two species is represented by the terms  k and γk that respectively contain the “βj” (that express the exergy voracity of species j) and the “αij,” that quantify the degree of mutualism. Let us examine, on the same line as proposed in [8], the three possible general behaviors. Notice that Jørgensen [8] uses “cooperation” and “parasitism” to indicate “mutualism” and “commensalism” respectively.
k and γk that respectively contain the “βj” (that express the exergy voracity of species j) and the “αij,” that quantify the degree of mutualism. Let us examine, on the same line as proposed in [8], the three possible general behaviors. Notice that Jørgensen [8] uses “cooperation” and “parasitism” to indicate “mutualism” and “commensalism” respectively.
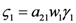 and
and 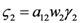 . The strong coupling between the numerosity of each species and the time evolution of ALL the participating species is apparent. Notice that in this case, Z = 2, but the formulae can be easily extended to account for whatever number Z of interacting populations, see [1]. We shall assume in the following that the exergy influx Ėin is constant and that the model coefficients γ, w, δ, r, μ and c are known for all species considered. The type and degree of interaction between the two species is represented by the terms
. The strong coupling between the numerosity of each species and the time evolution of ALL the participating species is apparent. Notice that in this case, Z = 2, but the formulae can be easily extended to account for whatever number Z of interacting populations, see [1]. We shall assume in the following that the exergy influx Ėin is constant and that the model coefficients γ, w, δ, r, μ and c are known for all species considered. The type and degree of interaction between the two species is represented by the terms  k and γk that respectively contain the “βj” (that express the exergy voracity of species j) and the “αij,” that quantify the degree of mutualism. Let us examine, on the same line as proposed in [8], the three possible general behaviors. Notice that Jørgensen [8] uses “cooperation” and “parasitism” to indicate “mutualism” and “commensalism” respectively.
k and γk that respectively contain the “βj” (that express the exergy voracity of species j) and the “αij,” that quantify the degree of mutualism. Let us examine, on the same line as proposed in [8], the three possible general behaviors. Notice that Jørgensen [8] uses “cooperation” and “parasitism” to indicate “mutualism” and “commensalism” respectively.2.1. Indifference
Species 1 and 2 display a disjointed behavior: in spite of sharing the same ecological niche, they do not interact, in the sense that neither species feeds, directly or indirectly, on the waste of the other one, which means that a21 = a12 = 0. Equations (8) and (9) simplify to:

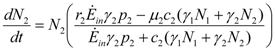
Even in this rather simple case, the strong non-linearity of the equations make them not amenable to an explicit solution. However the global behavior of the solutions, as we will see, strongly depends on the two quantities  and
and  . These two parameters contain the efficiency of each species to gain “net” exergy flows (γkpk), their “minimal survival” requests (ck) and the ratio of the growth rate to the mortality rate
. These two parameters contain the efficiency of each species to gain “net” exergy flows (γkpk), their “minimal survival” requests (ck) and the ratio of the growth rate to the mortality rate 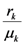 in the respective limit cases of infinite and zero resources available. For simplicity let us consider that
in the respective limit cases of infinite and zero resources available. For simplicity let us consider that  >
>  ; the symmetric results for
; the symmetric results for  >
>  will simply follow by permutation of the indices 1 ↔ 2 , and the special case
will simply follow by permutation of the indices 1 ↔ 2 , and the special case  =
=  will be discussed at the end of the section. In the state plane (N1, N2), the system of Equations 8 and 9 possesses three fixed points, listed in Table 1:
will be discussed at the end of the section. In the state plane (N1, N2), the system of Equations 8 and 9 possesses three fixed points, listed in Table 1:
 and
and  . These two parameters contain the efficiency of each species to gain “net” exergy flows (γkpk), their “minimal survival” requests (ck) and the ratio of the growth rate to the mortality rate
. These two parameters contain the efficiency of each species to gain “net” exergy flows (γkpk), their “minimal survival” requests (ck) and the ratio of the growth rate to the mortality rate 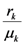 in the respective limit cases of infinite and zero resources available. For simplicity let us consider that
in the respective limit cases of infinite and zero resources available. For simplicity let us consider that  >
>  ; the symmetric results for
; the symmetric results for  >
>  will simply follow by permutation of the indices 1 ↔ 2 , and the special case
will simply follow by permutation of the indices 1 ↔ 2 , and the special case  =
=  will be discussed at the end of the section. In the state plane (N1, N2), the system of Equations 8 and 9 possesses three fixed points, listed in Table 1:
will be discussed at the end of the section. In the state plane (N1, N2), the system of Equations 8 and 9 possesses three fixed points, listed in Table 1:
Table 1.
Fixed points of the system of Equations (10–11).
| N1 | N2 | |
| a) | 0 | 0 |
| b) | 0 | 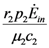 |
| c) |  | 0 |
The corresponding Lyapunov exponents are given by the following expressions:

Table 2.
Lyapunov exponents of the system of Equations (10–11) at points a, b and c.
| λ1 | λ2 | |
| a) | r1 | r2 |
| b) | 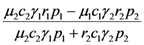 |  |
| c) |  | 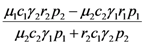 |
Point a is always unstable. The exponents λ1 and λ2 for points b and c are such that one point will be unstable and the other stable: the assumption  >
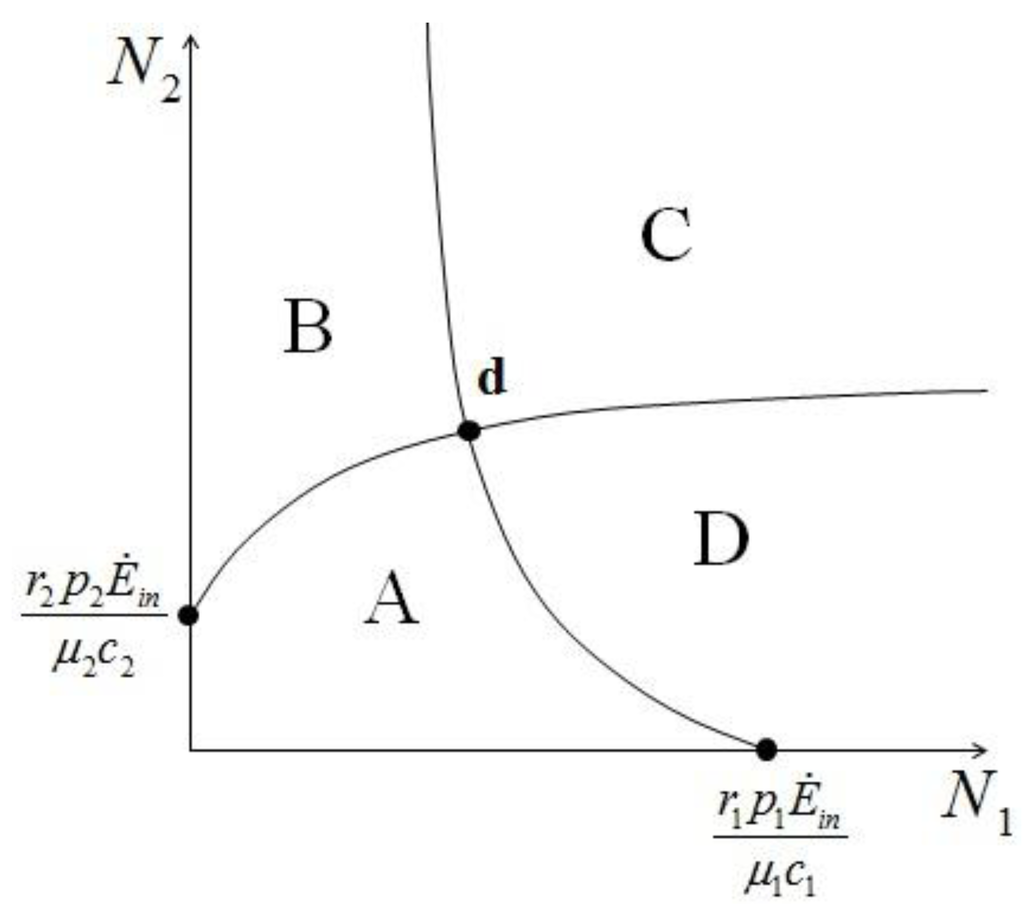
>  results in point c being asymptotically stable and point b unstable. We can divide the plane (N1, N2) into three different regions (only the semi-plane defined by N1 ≥ 0 and N2 ≥ 0 are considered here because this semiplane is invariant under the flow). The three regions are divided by the so called isoclines s1 and s2, defined as (see Figure 3):
results in point c being asymptotically stable and point b unstable. We can divide the plane (N1, N2) into three different regions (only the semi-plane defined by N1 ≥ 0 and N2 ≥ 0 are considered here because this semiplane is invariant under the flow). The three regions are divided by the so called isoclines s1 and s2, defined as (see Figure 3):
 >
>  results in point c being asymptotically stable and point b unstable. We can divide the plane (N1, N2) into three different regions (only the semi-plane defined by N1 ≥ 0 and N2 ≥ 0 are considered here because this semiplane is invariant under the flow). The three regions are divided by the so called isoclines s1 and s2, defined as (see Figure 3):
results in point c being asymptotically stable and point b unstable. We can divide the plane (N1, N2) into three different regions (only the semi-plane defined by N1 ≥ 0 and N2 ≥ 0 are considered here because this semiplane is invariant under the flow). The three regions are divided by the so called isoclines s1 and s2, defined as (see Figure 3):
On s1 we have  1 = 0 and on s2,
1 = 0 and on s2,  2 = 0. Notice that the two lines have the same angular coefficient and, since here
2 = 0. Notice that the two lines have the same angular coefficient and, since here  >
>  , line s1 is above s2.
, line s1 is above s2.
 1 = 0 and on s2,
1 = 0 and on s2,  2 = 0. Notice that the two lines have the same angular coefficient and, since here
2 = 0. Notice that the two lines have the same angular coefficient and, since here  >
>  , line s1 is above s2.
, line s1 is above s2.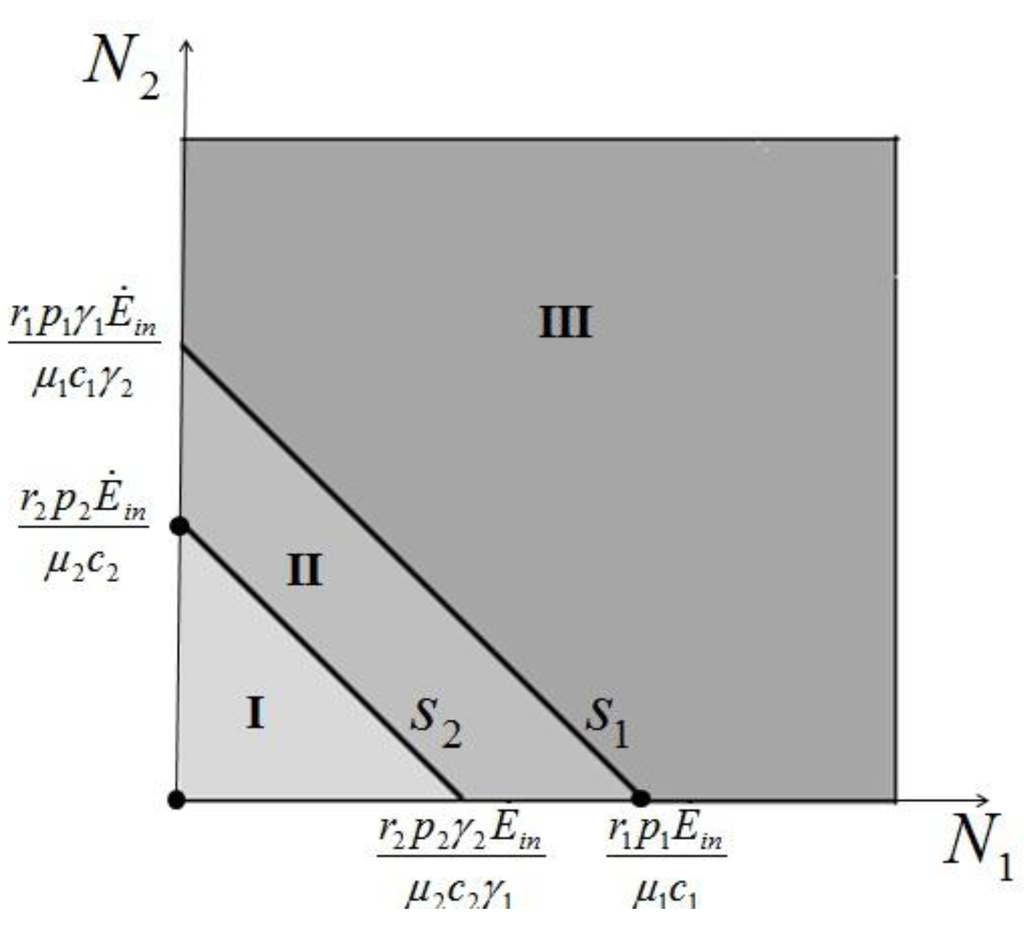
Figure 3.
The three regions defined by the two isoclines. The fixed points are marked by a dot.
In region I N1 and N2 are both increasing functions of time, in region III they are both decreasing functions of time, while in region II N1 is increasing and N2 decreasing. The behavior of the solution is characterized as follows (see Appendix 1 for the proof): if at some time t0 the solution is in region I then there exists a finite time t > t0 for which the solution will leave that region. If at some t1 the solution is in region II then it will remain in this region and asymptotically approach point c for t→∞. If at some time t3 the solution is in region III then it will either remain there for all subsequent times, asymptotically approaching c, or it will enter at some time t' > t3 into region II to approach again point c. Thus in all cases for which  >
>  is satisfied, population N2 will become extinct and population N1 will tend to the stable (sustainable) value
is satisfied, population N2 will become extinct and population N1 will tend to the stable (sustainable) value  . By the same arguments it can be shown that conversely, if
. By the same arguments it can be shown that conversely, if  <
<  , population N1 will die out and population N2 reaches a stable limit
, population N1 will die out and population N2 reaches a stable limit 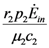 . An ensemble of plots for the case
. An ensemble of plots for the case  >
>  , corresponding to different initial conditions, is shown in Figure 4.
, corresponding to different initial conditions, is shown in Figure 4.
 >
>  is satisfied, population N2 will become extinct and population N1 will tend to the stable (sustainable) value
is satisfied, population N2 will become extinct and population N1 will tend to the stable (sustainable) value  . By the same arguments it can be shown that conversely, if
. By the same arguments it can be shown that conversely, if  <
<  , population N1 will die out and population N2 reaches a stable limit
, population N1 will die out and population N2 reaches a stable limit 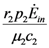 . An ensemble of plots for the case
. An ensemble of plots for the case  >
>  , corresponding to different initial conditions, is shown in Figure 4.
, corresponding to different initial conditions, is shown in Figure 4. 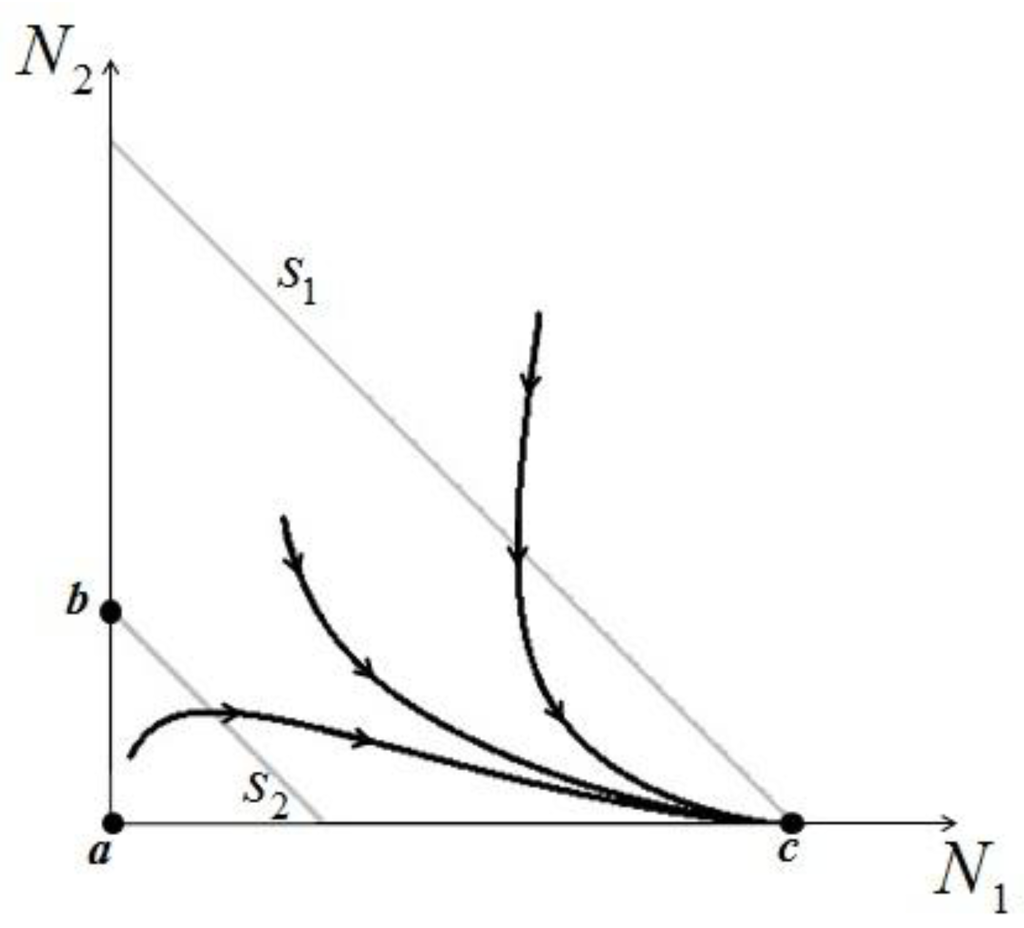
Figure 4.
Three different orbits for the system (10–11) in the case  >
>  .
.
 >
>  .
.
The special case  =
=  is very interesting. Point a is again unstable, but now the two isoclines s1 and s2 converge into a line that is itself an attractor. Both species survive and the weighted sum γ1N1 + γ2N2 reaches the carrying capacity limit for which
is very interesting. Point a is again unstable, but now the two isoclines s1 and s2 converge into a line that is itself an attractor. Both species survive and the weighted sum γ1N1 + γ2N2 reaches the carrying capacity limit for which 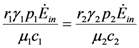 . Figure 5 shows examples of possible behaviors in phase space.
. Figure 5 shows examples of possible behaviors in phase space.
 =
=  is very interesting. Point a is again unstable, but now the two isoclines s1 and s2 converge into a line that is itself an attractor. Both species survive and the weighted sum γ1N1 + γ2N2 reaches the carrying capacity limit for which
is very interesting. Point a is again unstable, but now the two isoclines s1 and s2 converge into a line that is itself an attractor. Both species survive and the weighted sum γ1N1 + γ2N2 reaches the carrying capacity limit for which 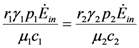 . Figure 5 shows examples of possible behaviors in phase space.
. Figure 5 shows examples of possible behaviors in phase space. 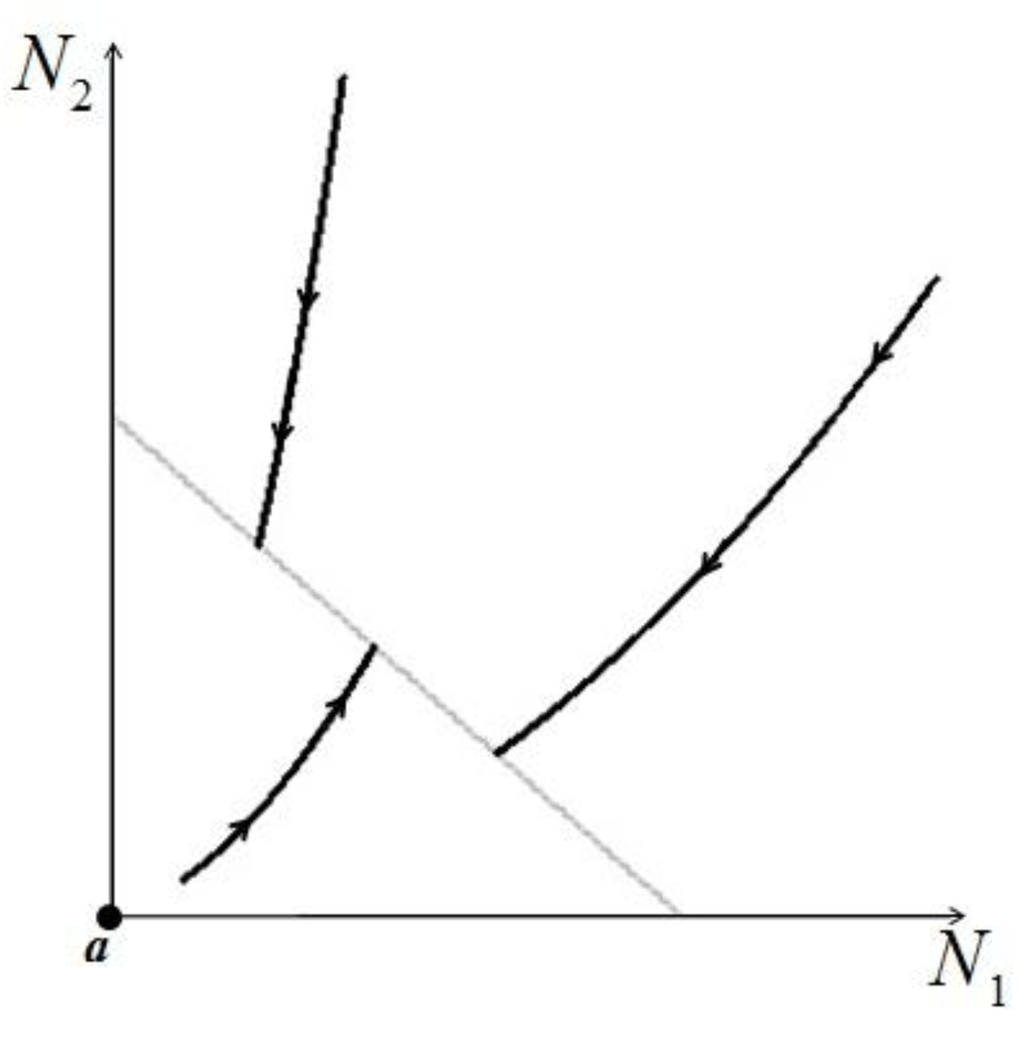
Figure 5.
The case 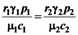 : The arrows indicate the time direction of the evolution.
: The arrows indicate the time direction of the evolution.
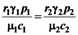 : The arrows indicate the time direction of the evolution.
: The arrows indicate the time direction of the evolution.
2.2. Commensalism
One of the two species displays a commensalistic behavior with respect to the other one, in the sense that rather than extracting its exergy feed rate from Ėin, it thrives solely on the “discharge flows” of the other species. Asymmetrically, the species “pestered” by the parasite does not feed on it—(in other words, any degree of mutualistic behavior is disregarded). Assuming species 2 to be the parasite, this implies:

Under these circumstances, (10-11) become:

Note that now the coupling between the species (or better of species 2 with species 1) is described only in terms of the two constants  1 and γ1: thus, the system (14) can be formally integrated, even if the solutions can be only written out implicitly:
1 and γ1: thus, the system (14) can be formally integrated, even if the solutions can be only written out implicitly:
 1 and γ1: thus, the system (14) can be formally integrated, even if the solutions can be only written out implicitly:
1 and γ1: thus, the system (14) can be formally integrated, even if the solutions can be only written out implicitly:
where the two values  and
and  are given by:
are given by:
 and
and  are given by:
are given by: 
These are also the asymptotic values of N1(t) and N2(t) since clearly from (15) we have that 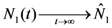 and
and 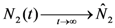 . Thus, both species reach their respective carrying capacity limit. Note that
. Thus, both species reach their respective carrying capacity limit. Note that 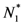 is proportional to p1, i.e. to 1 − w1 − δ1: increasing the discharged and destroyed portion of the incoming exergy flows proportionally reduces the corresponding carrying capacity value for population 1. But population 2 feeds on this discharged flow: Equation 16 shows that indeed the carrying capacity limit for N2 is proportional to w1. Note that, in contrast to the case of competing populations, and assuming w1 ≠ 0, the two populations will always both survive. In fact, species 1 meets no competition in tapping the exergy flow it needs and species 2 feeds on the “crumbs” of species 1. This result is quite obvious, but we stress that:
is proportional to p1, i.e. to 1 − w1 − δ1: increasing the discharged and destroyed portion of the incoming exergy flows proportionally reduces the corresponding carrying capacity value for population 1. But population 2 feeds on this discharged flow: Equation 16 shows that indeed the carrying capacity limit for N2 is proportional to w1. Note that, in contrast to the case of competing populations, and assuming w1 ≠ 0, the two populations will always both survive. In fact, species 1 meets no competition in tapping the exergy flow it needs and species 2 feeds on the “crumbs” of species 1. This result is quite obvious, but we stress that:
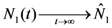 and
and 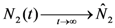 . Thus, both species reach their respective carrying capacity limit. Note that
. Thus, both species reach their respective carrying capacity limit. Note that 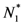 is proportional to p1, i.e. to 1 − w1 − δ1: increasing the discharged and destroyed portion of the incoming exergy flows proportionally reduces the corresponding carrying capacity value for population 1. But population 2 feeds on this discharged flow: Equation 16 shows that indeed the carrying capacity limit for N2 is proportional to w1. Note that, in contrast to the case of competing populations, and assuming w1 ≠ 0, the two populations will always both survive. In fact, species 1 meets no competition in tapping the exergy flow it needs and species 2 feeds on the “crumbs” of species 1. This result is quite obvious, but we stress that:
is proportional to p1, i.e. to 1 − w1 − δ1: increasing the discharged and destroyed portion of the incoming exergy flows proportionally reduces the corresponding carrying capacity value for population 1. But population 2 feeds on this discharged flow: Equation 16 shows that indeed the carrying capacity limit for N2 is proportional to w1. Note that, in contrast to the case of competing populations, and assuming w1 ≠ 0, the two populations will always both survive. In fact, species 1 meets no competition in tapping the exergy flow it needs and species 2 feeds on the “crumbs” of species 1. This result is quite obvious, but we stress that: - a) it is derived on exact mathematical basis.
- b) the carrying capacity limits are directly linked to the parameters of the model.
- c) Equations 14 describe also how fast these limits are reached (Figure 6).
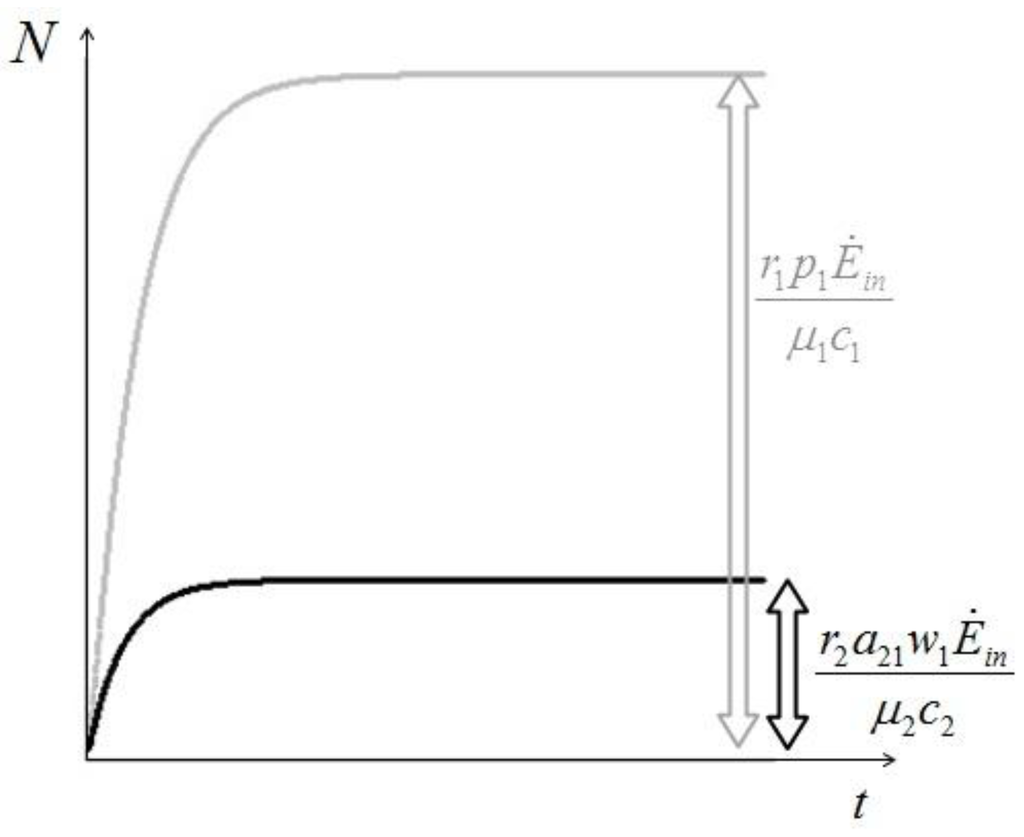
Figure 6.
An example of the evolution of the two populations in the commensalistic case.
2.3. Mutualism
This is the most general case, in which both a12 and a21 are strictly positive (>0). The model equations are 6 and 7. Again, it is convenient to examine the phase space (N1, N2), or better the invariant subspace given by {(N1, N2): N1 ≥ 0, N2 ≥ 0}. In this case, the Lyapunov coefficients assume the following values (the fixed points a, b and c are the same as in Table 1):

Table 3.
Lyapunov exponents of the system of Equations (10-11) at points a, b and c.
| λ1 | λ2 | |
| a) | r1 | r2 |
| b) | r1 |  |
| c) |  | r2 |
All three points are unstable. This means that the system tends to avoid situations in which one or both of the populations become extinct. This is an indication that in the present model, cooperation (mutualism) may lead to sustainable scenarios. Let us show the existence of another fixed point and look more closely at the dynamics.
If we consider the numerators of (8-9), repeated here for clarity in Equations 16, it is possible to verify that, for whichever value of the model parameters, the two curves described by (16) always intersect in a point (the fixed point) on the semi-plane {(N1, N2): N1 ≥ 0, N2 ≥ 0}, which we shall denote as d.
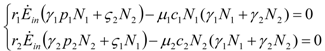
It is useful to write down the solution of this system in terms of the parameter q given by the ratio of N2 /N1:

where the parameter q solves the following quadratic equation ( 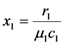 and
and  ):
):
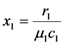 and
and  ):
):
the two solutions θ± being:
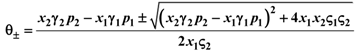
From (20) we note that θ+ is always positive and θ− always negative. The negative solution must be discarded because N1 and N2 are both positive definite. So there exists another fixed point given by:

Furthermore this point is attractive. Indeed we can label different domains in the semi-plane {(N1, N2): N1 ≥ 0, N2 ≥ 0} according to the signs of  1 and
1 and  2. Consider species 1 first. In order to have
2. Consider species 1 first. In order to have  1 ≥ 0 we need:
1 ≥ 0 we need:
 1 and
1 and  2. Consider species 1 first. In order to have
2. Consider species 1 first. In order to have  1 ≥ 0 we need:
1 ≥ 0 we need: 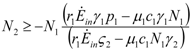
Equation 22 describes a curve dividing the semi-plane in two regions; region I, where  1 > 0, and region II, where
1 > 0, and region II, where  1 < 0. The two lines depicted in Figure 7 correspond to the two cases
1 < 0. The two lines depicted in Figure 7 correspond to the two cases  and
and 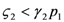 : the special case
: the special case 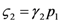 (and the equivalent case for species 2
(and the equivalent case for species 2 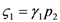 ) will be discussed at the end of this section.
) will be discussed at the end of this section.
 1 > 0, and region II, where
1 > 0, and region II, where  1 < 0. The two lines depicted in Figure 7 correspond to the two cases
1 < 0. The two lines depicted in Figure 7 correspond to the two cases  and
and 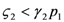 : the special case
: the special case 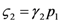 (and the equivalent case for species 2
(and the equivalent case for species 2 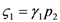 ) will be discussed at the end of this section.
) will be discussed at the end of this section. 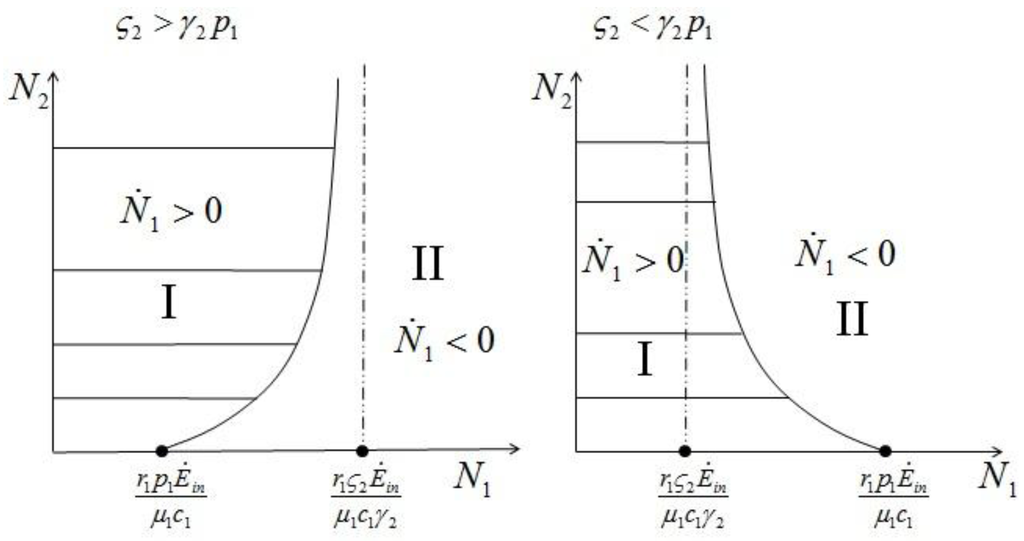
Figure 7.
The two regions identified by the curve (22) for  (left) and
(left) and 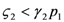 (right).
(right).
 (left) and
(left) and 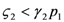 (right).
(right).
Repeating the same argument for species 2, we find two more curves dividing the semi-plane as in Figure 8, again corresponding to the two cases 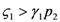 and
and  :
:
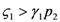 and
and  :
: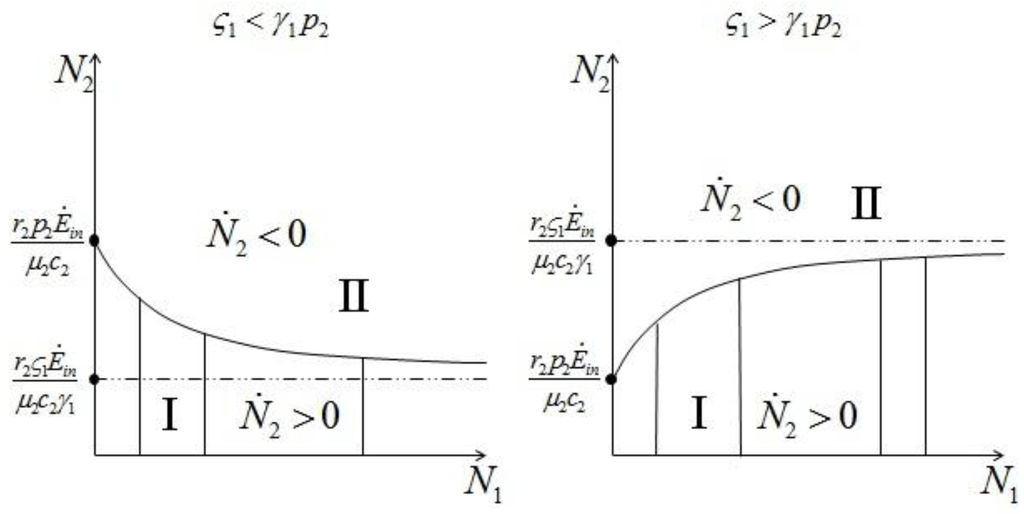
Figure 8.
The two regions symmetrically defined for  2 for
2 for  (left) and
(left) and  .
.
 2 for
2 for  (left) and
(left) and  .
.
Note that the fixed point d is given by the intersection of a curve in Figure 7 with a curve in Figure 8. For example if we have  and
and  , to find the fixed point d we can superimpose the two curves on the right in Figure 7 and Figure 8 to obtain the picture shown in Figure 9.
, to find the fixed point d we can superimpose the two curves on the right in Figure 7 and Figure 8 to obtain the picture shown in Figure 9.
 and
and 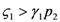 , to find the fixed point d we can superimpose the two curves on the right in Figure 7 and Figure 8 to obtain the picture shown in Figure 9.
, to find the fixed point d we can superimpose the two curves on the right in Figure 7 and Figure 8 to obtain the picture shown in Figure 9.It is also clear by geometrical considerations that the fixed point d will always lie on the positive quadrant. Note that the two curves divide this quadrant into four regions, marked with the letters A, B, C, and D in Figure 9: each region is characterized by different signs of  1 and
1 and  2. In region A both
2. In region A both  1 and
1 and  2 are positive, so N1 and N2 are both increasing functions of time. In region B N1 is increasing and N2 is decreasing. In region C N1 is decreasing and N2 increasing and finally in region D both N1 and N2 are decreasing functions of time. Thus, point d is attractive. The same argument applies for the other three possible cases (
2 are positive, so N1 and N2 are both increasing functions of time. In region B N1 is increasing and N2 is decreasing. In region C N1 is decreasing and N2 increasing and finally in region D both N1 and N2 are decreasing functions of time. Thus, point d is attractive. The same argument applies for the other three possible cases ( 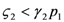 ,
, 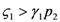 ), (
), (  ,
,  ) and (
) and ( 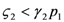 ,
,  ).
).
 1 and
1 and  2. In region A both
2. In region A both  1 and
1 and  2 are positive, so N1 and N2 are both increasing functions of time. In region B N1 is increasing and N2 is decreasing. In region C N1 is decreasing and N2 increasing and finally in region D both N1 and N2 are decreasing functions of time. Thus, point d is attractive. The same argument applies for the other three possible cases (
2 are positive, so N1 and N2 are both increasing functions of time. In region B N1 is increasing and N2 is decreasing. In region C N1 is decreasing and N2 increasing and finally in region D both N1 and N2 are decreasing functions of time. Thus, point d is attractive. The same argument applies for the other three possible cases ( 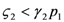 ,
, 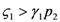 ), (
), (  ,
,  ) and (
) and ( 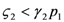 ,
,  ).
). A plot of trajectories corresponding to initial values in the four different regions is given in Figure 10.
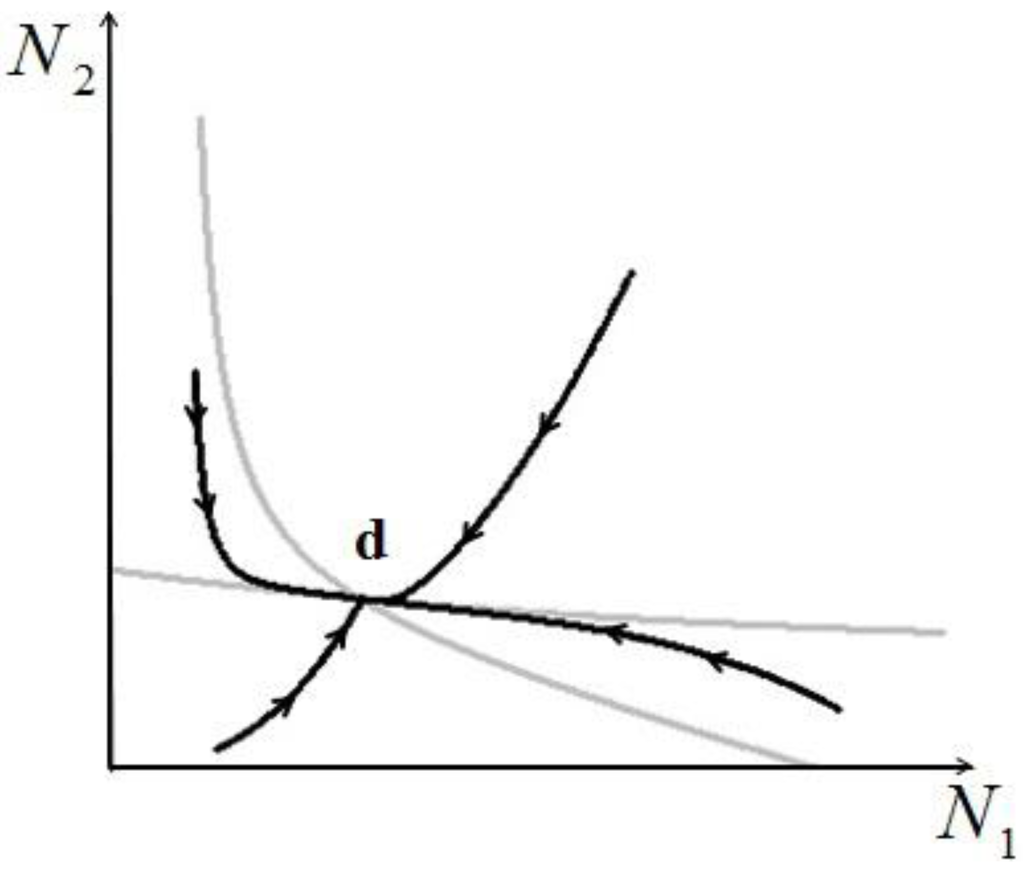
Figure 10.
Four different orbits for the general case.
It is apparent from Equation 20 that the value of θ+ is independent of Ėin: this is a strong constraint on the system because it means that the asymptotic value of the ratio 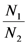 is also independent of Ėin and depends only on the parameters characterizing the species and their interaction (competition-cooperation). From a mathematically point of view this property of the solution is tautologic, but the question of whether a real system actually possesses this attribute is indeed far from obvious: since the ratio
is also independent of Ėin and depends only on the parameters characterizing the species and their interaction (competition-cooperation). From a mathematically point of view this property of the solution is tautologic, but the question of whether a real system actually possesses this attribute is indeed far from obvious: since the ratio 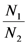 and Ėin are easily measurable in field experiments, the above constraint is suggesting a possible experimental “check” on the model. In a future paper we plan to examine some of the available experimental result in order to verify whether this statement is falsifiable.
and Ėin are easily measurable in field experiments, the above constraint is suggesting a possible experimental “check” on the model. In a future paper we plan to examine some of the available experimental result in order to verify whether this statement is falsifiable.
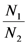 is also independent of Ėin and depends only on the parameters characterizing the species and their interaction (competition-cooperation). From a mathematically point of view this property of the solution is tautologic, but the question of whether a real system actually possesses this attribute is indeed far from obvious: since the ratio
is also independent of Ėin and depends only on the parameters characterizing the species and their interaction (competition-cooperation). From a mathematically point of view this property of the solution is tautologic, but the question of whether a real system actually possesses this attribute is indeed far from obvious: since the ratio 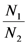 and Ėin are easily measurable in field experiments, the above constraint is suggesting a possible experimental “check” on the model. In a future paper we plan to examine some of the available experimental result in order to verify whether this statement is falsifiable.
and Ėin are easily measurable in field experiments, the above constraint is suggesting a possible experimental “check” on the model. In a future paper we plan to examine some of the available experimental result in order to verify whether this statement is falsifiable.Let us now briefly discuss the case 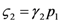 . Looking at Figure 7 now the two points marked with black dots on the N1 axis coincide, so that the curve dividing the semi-plane into two regions becomes a straight line, and the intersection point is now given by:
. Looking at Figure 7 now the two points marked with black dots on the N1 axis coincide, so that the curve dividing the semi-plane into two regions becomes a straight line, and the intersection point is now given by:
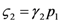 . Looking at Figure 7 now the two points marked with black dots on the N1 axis coincide, so that the curve dividing the semi-plane into two regions becomes a straight line, and the intersection point is now given by:
. Looking at Figure 7 now the two points marked with black dots on the N1 axis coincide, so that the curve dividing the semi-plane into two regions becomes a straight line, and the intersection point is now given by: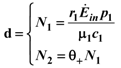
where θ+ is given by Equation 20. In this case the equation for N1 decouples, since we have:
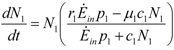
This is the same equation as the first of (14). In that case species 1 (the host), having no competitors, fed on all of the available exergy flow, so that after discharges and destructions are taken into account, its net exergy income was Ė1 = Ėinp1 (see Equation 4). On the contrary, in this case, N1 captures only a fraction of the environmental exergy at its disposal, the complementary fraction being taken by species 2; but there is another “resource” flow coming from the fraction of the exergy of species 2 discharge “recycled” from species 1 and these two flows, because of 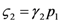 , sum up just to Ėinp1. This is a rather special situation but it is interesting to note how the equation for N1 decouples. In contrast to the second of (15), however, the equation for
, sum up just to Ėinp1. This is a rather special situation but it is interesting to note how the equation for N1 decouples. In contrast to the second of (15), however, the equation for  2 depends on both populations, so we cannot give analytical results as in that case. It is clear however that the qualitative dynamics cannot differ much from the general case.
2 depends on both populations, so we cannot give analytical results as in that case. It is clear however that the qualitative dynamics cannot differ much from the general case.
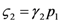 , sum up just to Ėinp1. This is a rather special situation but it is interesting to note how the equation for N1 decouples. In contrast to the second of (15), however, the equation for
, sum up just to Ėinp1. This is a rather special situation but it is interesting to note how the equation for N1 decouples. In contrast to the second of (15), however, the equation for  2 depends on both populations, so we cannot give analytical results as in that case. It is clear however that the qualitative dynamics cannot differ much from the general case.
2 depends on both populations, so we cannot give analytical results as in that case. It is clear however that the qualitative dynamics cannot differ much from the general case. 3. Conclusions
The first conclusion that can be derived from the arguments developed in this paper is that the axiom that is at the foundation of our reasoning, namely that resource consumption (material and immaterial) can be completely and unambiguously quantified in terms of exergy flows, is a convenient and fruitful working hypothesis. The model of population dynamics derived from such axiom results in strongly coupled and intrinsically non-linear evolution equations. In this paper, the time evolution patterns of two species sharing an ecological niche have been studied under three different scenarios: indifference, commensalism and mutualism. Our results indicate that:
- − if the two species just coexist in the same niche without cooperating or preying on each other, their competition invariably leads (Section 2a) to the extinction of one of them, unless their genetic (r, μ), consumption (c, γ) and efficiency (p) parameters are in a very precise and delicately tuned balance, in which case the two species may coexist sustainably;
- − if one of the two species assumes a commensalistic type of behaviour (it feeds on the other’s discharges, Section 2b), then both species reach their respective carrying capacity limits;
- − if the two species display a mutualistic type of behaviour (each one feeds partially on the other’s discharges, Section 2c), then there is always a stable sustainable point and both populations survive.
In all cases, the sustainability of the point in state-space of the surviving population(s) prescribes a certain well-identified numerosity.
In spite of the apparent success of our model, caution ought to be exercised in generalizing its results: nature is far more complex than any complicated and non-linear model makes it appear, and the results presented here are but a basis—and an incentive!—for further study. In particular, the following problems need to be carefully addressed if this type of approach is brought down from a descriptive to an applicative or predictive level:
- 1) Substitutability of exergy resources: our model explicitly lumps all of the “available” material and energy fluxes into the extended exergy inflow Ėin. This poses no problem for the energy flows, but contains the implicit assumption that ALL material flows are perfectly substitutable. In the simplest case, consider an animal species that has access to two or three different sources of vegetable food: obviously, each vegetable has a different nutritive value for the species, but also a different extended exergy content. Thus, our model would predict that the “optimal choice” for the animal would be to feed on the lower-embodied exergy food with the highest nutritive value. But this prediction disregards preferences, spatial distribution, physical accessibility, and similar factors. For more complex behaviours (for instance, related to the human species), the choice between, say, bronze and iron to build spears is even more multi-faceted than implicitly posited by the model.
- 2) Actual possibility of species reaching a stable stationary state: this is of course an extremely important point, because if two species in a single ecological niche reach a stable state (point d in Figure 10), this is by definition a sustainable situation. For larger number of species and realistic conditions, the problem of the stability of such stationary states is of paramount importance, and has not been addressed here.
- 3) Metadynamics: the model presented in this paper is spatially lumped and the niche in which the species thrive has “impermeable boundaries”. Immigration and emigration are completely neglected, as well as spatially varying distributions of Ėin. While the former could be modeled by inserting an “immigration term” in Equations (6) and (7), the spatially lumped nature of the model is difficult to modify. One solution might be that of “discretizing” the domain, and apply the equations separately to different areas, accounting for the variation of Ėin in each subdomain. This would make our model somewhat comparable to the so-called “patch” [10] or “incidence function” models [11], but would also demand for very taxing numerical simulations, not considered here.
- 4) Limiting factors (“exogenous factors” in [3]): while we maintain that (extended) exergy is a satisfactory indicator of species numerosity (in the sense that high exergy niches must logically and thermodynamically correspond to globally higher numerosities), it must be remarked that in real ecological niches there are phenomena occurring at some scales that affect the numerosity of species at a different scale [12]. Viral and fungal infections to which species N is insensitive may affect one or more of its food sources; growth of high-foliage trees with which a vegetable species does not compete for nutrients may reduce its share of solar irradiation, etc.: at the present state of development it is not clear whether and how our model may accommodate such effects (like in “tritrophic” or “multi-trophic” models [3]).
- 5) Though not addressed in this paper, the application of our model raises a question that could be of paramount importance for practical applications: is the efficiency of one single species in the niche compatible with the overall energy-conversion efficiency of the same niche, considered as a lumped system? In other words, if two or more species reach a sustainable point (“d” in Figure 10), do their respective numerosities satisfy both a local (for each species) and a global (for the entire niche) “optimal exergy use” criterion? This is a topic we have left for a future study.
Acknowledgments
One of the author (F.Z.) wishes to acknowledge the financial support received by the “Istituto Nazionale di Alta Matematica” (Italian National Institute for High Mathematics) under an INdAM-COFUND Marie Curie scholarship.
Conflict of Interest
The authors declare no conflict of interest.
Appendix 1
Suppose indeed that the solution remains always in region I. Then both N1 and N2 are bounded functions, 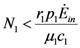 and
and 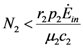 . But in this region these functions are also increasing; they must approach asymptotically a limit, say the point (x =
. But in this region these functions are also increasing; they must approach asymptotically a limit, say the point (x =  ,
,  ). This means that x is an equilibrium point. But the only equilibrium points are given in Table 1, and x cannot be any of these points since N1 and N2 are increasing functions. So the solution will leave the region I at some time t.
). This means that x is an equilibrium point. But the only equilibrium points are given in Table 1, and x cannot be any of these points since N1 and N2 are increasing functions. So the solution will leave the region I at some time t.
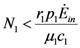 and
and 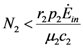 . But in this region these functions are also increasing; they must approach asymptotically a limit, say the point (x =
. But in this region these functions are also increasing; they must approach asymptotically a limit, say the point (x =  ,
,  ). This means that x is an equilibrium point. But the only equilibrium points are given in Table 1, and x cannot be any of these points since N1 and N2 are increasing functions. So the solution will leave the region I at some time t.
). This means that x is an equilibrium point. But the only equilibrium points are given in Table 1, and x cannot be any of these points since N1 and N2 are increasing functions. So the solution will leave the region I at some time t. Now suppose that for some t1 the solution is in region II and that at some subsequent time it will leave this region. To do this, it has to cross either line s1 or line s2. Suppose that it crosses s1 at t = t*. Then  1(t*) = 0. For the second derivative of
1(t*) = 0. For the second derivative of  1 at t = t* we have (since
1 at t = t* we have (since  2(t*) < 0):
2(t*) < 0):
 1(t*) = 0. For the second derivative of
1(t*) = 0. For the second derivative of  1 at t = t* we have (since
1 at t = t* we have (since  2(t*) < 0):
2(t*) < 0):
so that N1 has a minimum for t = t*. However this cannot be true because N1 is an increasing function in region II. So the hypothesis that the solution crosses the line s1 must be false. Suppose instead that it crosses the line s2 at t = t*. Then  2(t*) = 0. The second derivative of N2 at t = t* is given by:
2(t*) = 0. The second derivative of N2 at t = t* is given by:
 2(t*) = 0. The second derivative of N2 at t = t* is given by:
2(t*) = 0. The second derivative of N2 at t = t* is given by:
and is negative because of  1(t*) > 0. So N2 has a maximum at t = t*, but again this cannot be the case since N2 is a decreasing function in region II.
1(t*) > 0. So N2 has a maximum at t = t*, but again this cannot be the case since N2 is a decreasing function in region II.
 1(t*) > 0. So N2 has a maximum at t = t*, but again this cannot be the case since N2 is a decreasing function in region II.
1(t*) > 0. So N2 has a maximum at t = t*, but again this cannot be the case since N2 is a decreasing function in region II.References
- Benton, T.G.; Plaistow, S.J.; Coulson, T.N. Complex population dynamics and complex causation: Devils, details and demography. Proc. R. Soc. B 2006, 273, 1173–1181. [Google Scholar] [CrossRef]
- Hanski, I. Metapopulation Dynamics; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Turchin, P. Complex Population Dynamics: A Theoretical/Empirical Synthesis; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Sciubba, E.; Zullo, F. Exergy-based population dynamics: A thermodynamic view of the “sustainability” concept. J. Ind. Ecol. 2011, 15, 172–184. [Google Scholar] [CrossRef]
- Sciubba, E.; Zullo, F. Is sustainability a thermodynamic concept? IJEX 2011, 8, 68–85. [Google Scholar]
- Sciubba, E. Beyond thermoeconomics? The concept of extended exergy accounting and its application to the analysis and design of thermal systems. IJEX 2001, 1, 68–84. [Google Scholar]
- Sciubba, E. What did Lotka really say? Ecol. Modelling 2009, 222, 1347–1353. [Google Scholar] [CrossRef]
- Jørgensen, S.E. Fundamentals of Ecological Modeling; Fath, B., Jørgensen, S.E., Eds.; Elsevier: Oxford, UK, 2011. [Google Scholar]
- Kareiva, P.; Mullen, A.; Southwood, R. Population dynamics in spatially complex environments: Theory and data and discussion. Phil. Trans. R. Soc. Lond. 1990, 330, 175–190. [Google Scholar]
- Diamond, J.M.; May, R.M. Island Biogeography and the Design of Natural Reserves. In Theoretical Ecology; May, R.M, Ed.; Blackwell Pub.: Oxford, UK, 1981; pp. 228–252. [Google Scholar]
- Durrett, R.; Levin, S. The importance of being discrete and spatial. Theoretical Popul. Bio. 1994, 46, 363–394. [Google Scholar] [CrossRef]
- Williamson, M. The Analysis of Biological Populations; Edward Arnold Pub.: London, UK, 1971. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).