Managing Cuscuta gronovii (Swamp Dodder) in Cranberry Requires an Integrated Approach
Abstract
:1. Introduction

2. Cranberry and Dodder Phenology
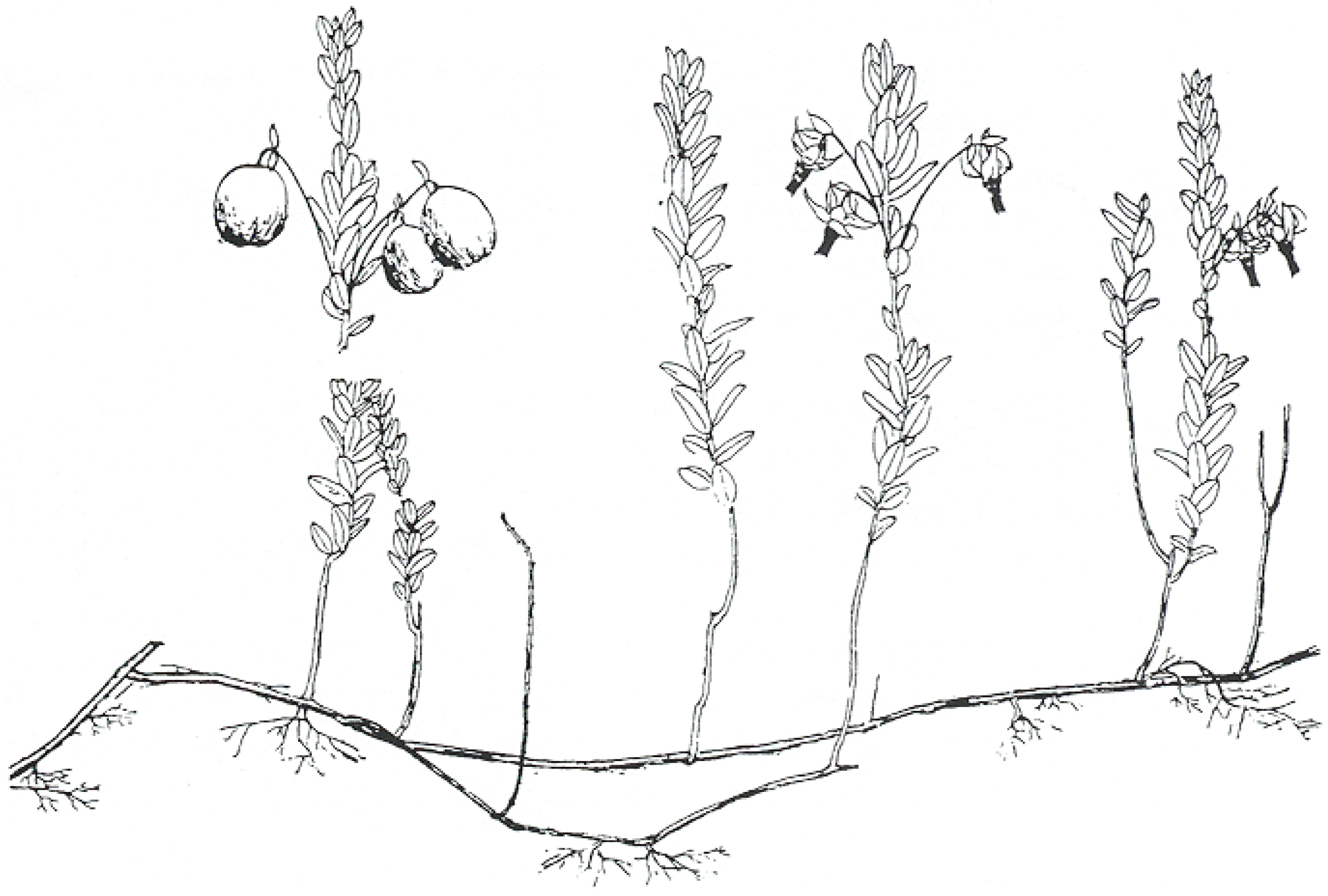
3. Host Preference, Seeds, and Taxonomy
3.1. Host Choice and Preferences for Dodder
3.2. Seed Longevity and Factors Affecting Emergence
3.3. Taxonomy of Dodder Species in Cranberry
4. Current Chemical Options
4.1. Preemergence Options
| Host | Herbicide |
|---|---|
| alfalfa | trifluralin, pronamide, pendimethalin, ethalfluralin, glyphosate, N-phuric acid |
| onion | DCPA |
| tomatoes | DCPA, rimsulfuron (suppression); glyphosate. |
| sugar beets | glyphosate, ethofumesate, metolachlor, trifluralin, pronamide |
| carrot | pendimethalin, paraquat dichloride |
| blueberry | dichlobenil |
| cranberry | dichlobenil, mesotrione |

4.2. Postemergence Herbicides
4.3. Plant Growth Regulators (PGRs) and Other Products
4.4. Phenolics and Metabolites
5. Current Nonchemical Methods
5.1. Mechanical or Hand Removal
5.2. Flooding
5.3. Sanding
5.4. Flame Cultivation and Solarization
5.5. Dodder Resistant Varieties
6. Biological Control with Fungi, Insects, and Bacteria
7. Conclusions
Prevent movement of seeds onto farm.
Scout for newly emerged seedlings.
Use preemergence herbicides.
Manage early season weed hosts.
Use short-term spring floods.
Remove seedlings and infected hosts by hand.
Apply postemergence herbicides.
Rake heavy dodder infestations.
Remove seed pods in harvest floods.
Clean equipment between infested and clean farms.
Apply uniform applications of sand.
Future additions:
Use of plant growth regulators? New herbicides? Flame cultivation?
Unconventional (household) products? Biological control products?
Late water floods have not proven effective against dodder.
The efficacy of fall floods has not been tested but the general inclination is that they would not be effective at all. The seed is already formed (by late August-September) and is quite capable of surviving winter floods.
Smolder, a mycoherbicide formulated with the fungus, Alternaria destruens, performed poorly in 2 years of field tests conducted in both Wisconsin and in Massachusetts and is not being pursued as a viable option.
Napropamide and norflurazon are not effective preemergence herbicides.
Acknowledgements
References and Notes
- Parker, C.; Riches, C.R. Parasitic Weeds of the World-Biology and Control; CAB International: Oxon, UK, 1993. [Google Scholar]
- Chiu, S.B.; Shen, H. Growth studies of Cuscuta spp. (dodder parasitic plant) on Mikania micrantha and Asystasia intrusa. Planter 2004, 80, 31–36. [Google Scholar]
- Yu, H.; Liu, J.; He, W.; Miao, S.; Dong, M. Restraints on Mikania micrantha by Cuscuta campetris facilitates restoration of the disturbed ecosystem. Biodiversity 2009, 10, 72–78. [Google Scholar]
- Grewell, B.J. Parasite facilitates plant species coexistence in a coastal wetland. Ecology 2008, 89, 1481–1488. [Google Scholar]
- Pennings, S.C.; Callaway, R.M. Impact of a parasitic plant on the structure and dynamics of salt marsh vegetation. Ecology 1996, 77, 1410–1419. [Google Scholar]
- Dean, H.L. Dodder overwintering as haustorial tissue within Cuscuta galls. Proc. Iowa Acad. Sci. 1954, 61, 99–196. [Google Scholar]
- Meulebrouck, K.; Ameloot, E.; Brys, R.; Tanghe, L.; Verheyen, K.; Hermy, M. Hidden in the host—unexpected vegetative hibernation of the holoparasite Cuscuta epithymum (L.) L. and its implications for population persistence. Flora 2009, 204, 306–315. [Google Scholar]
- Stevens, O.A. Weights of seed and numbers per plant. Weeds 1957, 5, 46–55. [Google Scholar] [CrossRef]
- Sandler, H.A.; Ghantous, K. Germination patterns of swamp dodder seeds planted near a commercial cranberry farm. Proc. Northeast. Weed Sci. Soc. 2007, 61, 65. [Google Scholar]
- Sandler, H.A.; Else, M.J.; Sutherland, M. Application of sand for inhibition of swamp dodder (Cuscuta gronovii) seedling emergence and survival on cranberry (Vaccinium macrocarpon) bogs. Weed Technol. 1997, 11, 318–323. [Google Scholar]
- Wolswinkel, P.; Ammerlaan, A.; Peters, H.F.C. Phloem unloading of amino acids at the site of attachment of Cuscuta europaea. Plant Physiol. 1984, 75, 13–20. [Google Scholar]
- Truscott, F.H. On the regeneration of new shoots from isolated dodder haustoria. Amer. J. Bot. 1958, 45, 169–177. [Google Scholar] [CrossRef]
- Pattee, H.; Allred, K.R.; Wiebe, H. Photosynthesis in dodder. Weed Sci. 1965, 13, 193–194. [Google Scholar]
- Hibberd, J.M.; Bungard, R.A.; Press, M.C.; Jeschke, W.D.; Scholes, J.D.; Quick, W.P. Localization of photosynthetic metabolism in the parasitic angiosperm Cuscuta reflexa. Planta 1998, 205, 506–513. [Google Scholar]
- Sherman, T.D.; Pettigrew, W.T.; Vaughn, K.C. Structural and immunological characterization of the Cuscuta pentagona L. chloroplast. Plant Cell Phys. 1999, 40, 592–603. [Google Scholar] [CrossRef]
- Gharib, C.; Haidar, M.A.; Sleiman, F.T.; Sidahmed, M. Germination and Viability of Cuscuta spp. (Dodder) Seeds after Digestion in Sheep Rumen. 2007. Available online: http://www.cpe.vt.edu/wcopp/Abstracts_Final.pdf (accessed on 24 June 2009).
- Bunch, H.D.; Moore, C.E.; Grisez, J.F. Magnetic seed cleaners. Crops Soils 1959, 11, 20–21. [Google Scholar]
- Devlin, R.M.; Deubert, K.H. Control of swamp dodder (Cuscuta gronovii) on cranberry bogs with butralin. Proc. Northeast. Weed Sci. Soc. 1980, 34, 399–405. [Google Scholar]
- Bewick, T.A.; Binning, L.K.; Dana, M.N. Control of swamp dodder in cranberry. HortScience 1989, 24, 850. [Google Scholar]
- Else, M.J.; Sandler, H.A.; Schluter, S. Weed mapping as a component of integrated pest management in cranberry production. HortTechnology 1995, 5, 302–305. [Google Scholar]
- Cranberry Production: A Guide for Massachusetts, CP-08; Sandler, H.A.; DeMoranville, C.J. (Eds.) University of Massachusetts Cranberry Station Extension Publication: East Wareham, MA, USA, 2008.
- Averill, A.L.; Sylvia, M.M. Cranberry Insects of the Northeast; University of Massachusetts Cranberry Station Extension Publication: East Wareham, MA, USA, 1998. [Google Scholar]
- Franklin, H.J. Cranberry Insects in Massachusetts, Parts II–VII; Massachusetts Agricultural Experiment Station: East Wareham, MA, USA, 1951. [Google Scholar]
- Beckwith, C.S. Cranberry Growing in New Jersey; Circular No. 246; New Jersey Agricultural Experiment Station: New Brunswick, NJ, USA, 1931. [Google Scholar]
- Meulebrouck, K.; Verheyen, K.; Brys, R.; Hermy, M. Limited by the host: Host age hampers establishment of holoparasite Cuscuta epithymum. Acta Oecologica 2009, 35, 533–540. [Google Scholar] [CrossRef]
- DeMoranville, C.J. Flood management. In Cranberry Production: A Guide for Massachusetts, CP-08; Sandler, H.A., DeMoranville, C.J., Eds.; University of Massachusetts Cranberry Station Extension Publication: East Wareham, MA, USA, 2008; pp. 66–71. [Google Scholar]
- DeMoranville, C.J. Cultural practices in cranberry production: Sanding and pruning. In Cranberry Production: A Guide for Massachusetts (CP-08); Sandler, H.A., DeMoranville, C.J., Eds.; University of Massachusetts Cranberry Station Extension Publication: East Wareham, MA, USA, 2008; pp. 16–21. [Google Scholar]
- Mason, J.; Sandler, H.A.; Hunsberger, L.K. Evaluation of sand stockpiles as potential sources of cranberry weeds. Weed Technol. 2006, 20, 58–66. [Google Scholar]
- Yuncker, T.G. North American flora II—Cuscuta (monograph). N. Amer. Flora, Ser. 2 1965, 4, 1–51. [Google Scholar]
- Gaertner, E.E. Studies of seed germination, seed identification, and host relationships in dodders, Cuscuta spp. Cornell Exp. Sta. Mem. 1950, 294, 1–56. [Google Scholar]
- Dawson, J.H.; Musselman, L.J.; Wolswinkel, P.; Dorr, I. Biology and control of Cuscuta. Rev. Weed Sci. 1994, 6, 265–317. [Google Scholar]
- Lanini, W.T.; Kogan, M. Biology and management of Cuscuta in crops. Cienca e Investigacion Agraria 2005, 32, 165–179. [Google Scholar]
- Farah, A.F.; Al-Abulsalam, M.A. Effect of field dodder (Cuscuta campetris Yuncker) on some legume crops. Sci. J. King Faisal Univ. 2004, 5, 103–112. [Google Scholar]
- Costea, M.; Tardif, F.J. The biology of Canadian weeds. 133. Cuscuta campetris Yuncker, C. gronovii Willd. ex. Schult., C. umbrosa Beyr. ex. Hook., C. epithymum (L.) L. and C. epilinium Weihe. Can. J. Plant Sci. 2006, 86, 293–316. [Google Scholar]
- Tsivion, Y. Possible role of cytokinins in nonspecific recognition of a host and in early growth of haustoria in the parasitic plant. Cuscuta campestris. Bot. Gaz. 1978, 139, 27–31. [Google Scholar]
- Benvenuti, S.; Dinelli, G.; Bonetti, A.; Catizone, P. Germination ecology, emergence and host detection in Cuscuta campestris. Weed Res. 2005, 45, 270–278. [Google Scholar]
- Runyon, J.B.; Mescher, M.C.; De Moraes, C.M. Volatile chemical cues guide host location and host selection by parasitic plants. Science 2006, 313, 1964–1967. [Google Scholar]
- Smith, J.L.; De Moraes, C.M.; Mescher, M.C. Jasmonate- and salicylate-mediated plant defense responses to insect herbivores, pathogens, and parasitic plants. Pest Man. Sci. 2009, 65, 497–503. [Google Scholar] [CrossRef]
- Kelly, C.K. Plant foraging: a marginal value model and coiling response in Cuscuta subinclusa. Ecology 1990, 71, 1916–1925. [Google Scholar]
- Kelly, C.K. Resource choice in Cuscuta europaea. Proc. Nat. Acad. Sci. USA 1992, 89, 12194–12197. [Google Scholar] [CrossRef]
- Alers-Garcia, J. Active Host Choice and Parasitism by Cuscuta gronovii: Its Effects on Host Individuals, Populations, and Mutualistic Interactions. Ph.D. Thesis, Department of Biology, Indiana University, Bloomington, IN, USA, 2005. [Google Scholar]
- Sandler, H.A. Weed management. In Cranberry Chart Book-Management Guide for Massachusetts; Sylvia, M.M., Guerin, N., Eds.; University of Massachusetts Cranberry Station Extension Publication: East Wareham, MA, USA, 2009; pp. 21–41. [Google Scholar]
- Bewick, T.A.; Binning, L.K.; Yandell, B. A degree day model for predicting the emergence of swamp dodder in cranberry. J. Amer. Soc. Hort. Sci. 1988, 113, 839–841. [Google Scholar]
- Sandler, H.A.; Else, M.J. Prediction of Dodder Emergence Based on a Degree Day Model; Cranberry Agricultural Research Progress Reports; Ocean Spray Cranberries: Lakeville-Middleboro, MA, USA, 1995. [Google Scholar]
- Karapetyan, N.O. The effects of the depth and duration of burial of dodder seeds in the soil on their germination. Izvestiya Sel’skokhozyaistvennykh Nauk 1972, 5, 49–54. [Google Scholar]
- Buhler, D.D.; Hoffman, M.L. Andersen’s Guide to Practical Methods of Propagating Weeds and Other Plants; Allen Press: Lawrence, KS, USA, 1999. [Google Scholar]
- Lyshede, B.O. Seed structure and germination in Cuscuta pedicellata with some notes on Cuscuta campetris. Nordic J. Bot. 1984, 4, 669–674. [Google Scholar]
- Peterson, B.S.; Cross, C.E.; Tilden, N. The Cranberry Industry in Massachusetts; Bulletin No. 201; Division of Markets, Massachusetts Department of Agriculture: Boston, MA, USA, 1968.
- O’Connell, J.M.; Sandler, H.A.; Adler, L.S.; Caruso, F.L. Evaluating flood duration and initiation under controlled conditions for dodder management in cranberries. Proc. Northeast. Weed Sci. Soc. 2009, 63, 91. [Google Scholar]
- Sandler, H.A.; Mason, J. Efficacy of flooding for the control of dodder (Cuscuta gronovii) and several broadleaf species in commercial cranberry production in Southeastern Massachusetts: a two-year study. Proc. Northeast. Weed Sci. Soc. 2004, 58, 163. [Google Scholar]
- Peachey, E. Pacific Northwest Weed Management Handbook. 2009. Available online: http://weeds.ippc.orst.edu/pnw/weeds?14W_ALFS04.dat (accessed on 16 September 2009).
- Sandler, H.A.; Ghantous, K.M. Management practices and obstacles for dodder control in cranberries. Fruit Grower News 2007, 46, 17–18. [Google Scholar]
- Dawson, J.H. Dodder control in alfalfa with dichlobenil. Weed Sci. 1970, 18, 225–230. [Google Scholar]
- Sandler, H.A.; Mason, J.; Autio, W.R.; Bewick, T.A. Effects of repeat applications of dichlobenil on weed populations and yield components of cranberry (Vaccinium macrocarpon). Weed Technol. 2004, 18, 648–657. [Google Scholar]
- Sandler, H.A.; O’Connell, J.M. Use of mesotrione for postemergence dodder control in Massachusetts cranberry production. WSSA Abstracts 2010, 50. (in press). [Google Scholar]
- Majek, B.A.; Ayeni, A.O. The phytotoxicity and utility of quinclorac, chlorimuron, and mesotrione in cranberries. Proc. Northeast. Weed Sci. Soc. 2003, 57, 94. [Google Scholar]
- Bewick, T.A.; Binning, L.K.; Balke, N.E. Absorption and translocation of glyphosate by carrot infected by swamp dodder. HortScience 1991, 116, 1035–1039. [Google Scholar]
- Baye, Y. Eastern Dodder (Cuscuta monogyna) Control by Glyphosate in Citrus and Olive Orchards. 2009. Available online: http://www.ppws.vt.edu/IPPS/IPPS%20Turkey%20Abstract%20Book.pdf (accessed on 9 October 2009).
- Hock, S.M.; Wiecko, G.; Knezevic, S.Z. Glyphosate dose affected control of field dodder (Cuscuta campestris) in the tropics. Weed Technol. 2008, 22, 151–155. [Google Scholar]
- Raven, P.H.; Evert, R.F.; Eichhorn, S.E. Biology of Plants, 6th ed.; W.H. Freeman: New York, NY, USA, 1999. [Google Scholar]
- Else, M.J.; Butkewich, S. Potential of using ethephon as a postemergence herbicide for control of dodder (Cuscuta gronovii) in cranberries. WSSA Abstracts 1995, 35, 97. [Google Scholar]
- Haidar, M.A.; Orr, G.L.; Westra, P. The response of dodder (Cuscuta spp.) seedlings to phytohormones under various light conditions. Ann. Appl. Biol. 1998, 132, 331–338. [Google Scholar] [CrossRef]
- Ramasubramanian, T.S.; Paliyath, G.; Rajagopal, I.; Maheshwari, R.; Mahadevan, S. Hormones and Cuscuta development. In vitro induction of haustoria by cytokinin and its inhibition by other hormones. J. Plant Growth Reg. 1988, 7, 133–144. [Google Scholar]
- Tronchet, J. The effect of 0.2% 2,4-D on young seedlings of Cuscuta gronovii Willd: intense elongation, inverted geotropism, and development of internodes. Compte Rendu Hebdomadaire des Seances de l’Academie des Sciences 1958, 246, 1811–1813. [Google Scholar]
- Gimesi, A. Chemical control of Cuscuta species. In Parasitic Flowering Plants, Proceedings of the 4th ISPFP, Marburg, Germany, August 1987; pp. 249–252.
- Maheshwari, R.; Shailini, C.; Veluthambi, K.; Mahadevan, S. Interaction of gibberellic acid and indole-3-acetic acid in the growth of excised Cuscuta shoot tips in vitro. Plant Physiol. 1980, 65, 186–192. [Google Scholar] [CrossRef]
- Eaton, G.W. Floral induction and biennial bearing in the cranberry. Fruit Var. J. 1978, 32, 58–60. [Google Scholar]
- Devlin, R.M.; Demoranville, I.E. Influence of gibberellic acid and Gibrel on fruit set and yield in Vaccinium macrocarpon cv. Early Black. Physiol. Planta. 1967, 20, 587–592. [Google Scholar] [CrossRef]
- Paclobutrazol Summary Document; 2007. Available online: http://www.epa.gov/oppsrrd1/registration_review/paclobutrazol/index.htm (accessed on 14 January 2010).
- Serres, R.; McCown, B. Rapid flowering of microcultured cranberry plants. HortScience 1994, 29, 159–161. [Google Scholar]
- Morrison, J.R.; Sandler, H.A.; Romaneo, L.K. Management of swamp dodder (Cuscuta gronovii Willd.) in cranberry may be enhanced by the integration of a nontoxic household cleaner. Crop Prot. 2005, 24, 1–6. [Google Scholar]
- O’Connell, J.M.; Sandler, H.A.; Adler, L.S.; Caruso, F.L. Short-term floods and chemical controls: developing an integrated program for dodder control in cranberry. Proc. Northeast. Weed Sci. Soc. 2010, 64, 14. [Google Scholar]
- Habib, S.A.; Rahman, A.A.A. Evaluation of some weed extracts against field dodder on alfalfa (Medicago sativa). J. Chem. Ecol. 1988, 14, 443–452. [Google Scholar]
- Vurro, M.; Boari, A.; Evidente, A.; Andolfi, A.; Zermane, N. Natural metabolites for parasitic weed management. Pest Man. Sci. 2008, 65, 566–571. [Google Scholar] [CrossRef]
- Vurro, M.; Boari, A.; Pilgeram, A.L.; Sands, D.C. Exogenous amino acids inhibit seed germination and tubercle formation by Orobanche ramosa (broomrape): potential application for management of parasitic weeds. Bio. Cont. 2006, 36, 258–265. [Google Scholar]
- Cudney, D.W.; Orloff, S.B.; Reints, J.S. An integrated weed management procedure for the control of dodder (Cuscuta indecora) in alfalfa (Medicago sativa). Weed Technol. 1992, 6, 603–606. [Google Scholar]
- Hunsberger, L.K.; Autio, W.R.; DeMoranville, C.J.; Sandler, H.A. Mechanical removal of summer dodder infestations and impacts on cranberry yield. HortTechnology 2006, 16, 78–82. [Google Scholar]
- Franklin, H.J. Cranberry Insects in Massachusetts; Bulletin No. 445; Massachusetts Agricultural Experiment Station: East Wareham, MA, USA, 1948. [Google Scholar]
- Averill, A.L.; Sylvia, M.M.; Kusek, C.C.; DeMoranville, C.J. Flooding in cranberry to minimize insecticide and fungicide inputs. Amer. J. Alt. Agric. 1997, 12, 50–54. [Google Scholar] [CrossRef]
- DeMoranville, C.J.; Sandler, H.A.; Shumaker, D.E.; Averill, A.L.; Caruso, F.L.; Sylvia, M.M.; Pober, D.M. Fall flooding for management of cranberry fruitworm (Acrobasis vaccinii) and dewberry (Rubus hispidus) in Massachusetts cranberry production. J. Crop Prot. 2005, 24, 999–1006. [Google Scholar] [CrossRef]
- Blake, G.; Sandler, H.A.; Coli, W.; Pober, D.M.; Coggins, C. An assessment of grower perceptions and factors influencing adoption of IPM in commercial cranberry production. Renew. Agric. Food Syst. 2007, 22, 134–144. [Google Scholar] [CrossRef]
- Botelho, M.R.; Vanden Heuvel, J.E. Harvest flooding affects seasonal pattern of carbohydrate accumulation in cranberry uprights. HortScience 2005, 40, 498. [Google Scholar]
- Vanden Heuvel, J.E.; Goffinet, M.C. The effects of flood initiation timing and water temperature during flooding on nonstructural carbohydrate concentration and anatomy of cranberry. HortScience 2008, 43, 338–345. [Google Scholar]
- Gealy, D. Differential response of palmleaf morningglory (Ipomoea wrightii) and pitted morning glory (Ipomoea lacunosa) to flooding. Weed Sci. 1998, 46, 217–224. [Google Scholar]
- Rice Production Handbook, MP 192; Helms, R.S. (Ed.) University of Arkansas Cooperative Extension Service: Little Rock, AR, USA, 1994.
- Waddington, D.V.; Beegle, D.B.; Gover, A.E. Nutrient concentrations of turfgrass and soil test levels as affected by soil media and fertilizer rate and placement. Comm. Soil Sci. Plant Anal. 1994, 25, 1957–1990. [Google Scholar] [CrossRef]
- Williamson, R.C.; Potter, D.A. Nocturnal activity and movement of black cutworms (Lepidoptera: Noctuidae) and response to cultural manipulations on golf course putting greens. J. Econ. Entomol. 1997, 90, 1283–1289. [Google Scholar]
- DeMoranville, C.J.; Sandler, H.A.; Bicki, T. Sanding. 1996. Available online: http://www.umass.edu/cranberry/services/bmp (accessed on 24 January 2006).
- Tomlinson, B. Proper sanding of great importance in good bog management. Cranberries 1937, 1, 4, 8–11. [Google Scholar]
- Hunsberger, L.K.; DeMoranville, C.J.; Autio, W.R.; Sandler, H.A. Uniformity of sand deposition on cranberry farms and implications for swamp dodder control. HortTechnology 2006, 16, 488–492. [Google Scholar]
- Lukovin, S.K.; Rudenko, A.A. Dodder destruction by flaming. Zashchita Rastenii 1975, 20, 47. [Google Scholar]
- Gristsenko, T.G. Flaming lucerne stubble. Khlopkovodstvo 1968, 18, 20. [Google Scholar]
- Goksel, N. Investigations on chemical and mechanical methods of controlling Cuscuta in lucerne. Turkiye Ziraat. Mecmuasi 1958, 42, 44–54. [Google Scholar]
- Ghantous, K.M.; Sandler, H.A.; Jeranyama, P.; Autio, W.R. Response of cranberry vines to hand-held flame cultivators-initial year evaluation. Proc. Northeast. Weed Sci. Soc. 2009, 63, 2. [Google Scholar]
- Ghantous, K.M.; Sandler, H.A.; Autio, W.R.; Jeranyama, P. Response of Smilax sp., Rubus spp. and cranberry vines to infrared and open flame weed control. Proc. Northeast. Weed Sci. Soc. 2010, 64, 19. [Google Scholar]
- Haidar, M.A.; Iskandarani, N.; Sidahmed, M.; Baalbaki, R. Response of field dodder (Cuscuta campestris) seeds to soil solarization and chicken manure. Crop Prot. 1999, 18, 253–258. [Google Scholar]
- Goldwasser, Y.; Lanini, W.T.; Wrobel, R.L. Tolerance of tomato varieties to lespedeza dodder. Weed Sci. 2001, 49, 520–523. [Google Scholar]
- Hembree, K.J.; Lanini, W.T.; Va, N. Tomato varieties show promise of dodder control. Proc. California Weed Sci. Soc. 1999, 51, 205–206. [Google Scholar]
- Rao, P.N.; Reddy, A.R.S. Effect of china dodder on two pulses: green gram and cluster bean—the latter a possible trap crop to manage china dodder. In Parasitic Flowering Plants, Proceedings of the 4th ISPFP, Marburg, Germany, August 1987; pp. 665–674.
- Konieczka, C.M.; Colquhoun, J.B.; Rittmeyer, R.A. Swamp dodder (Cuscuta gronovii) applied ecology in carrot production. Weed Technol. 2009, 23, 175–178. [Google Scholar]
- Arnaud, M.C.; Thalouarn, P.; Fer, A. Characterization of mechanisms related to the resistance of crops to two parasitic higher plants (Cuscuta reflexa and Striga hermonthica). Comptes Rendus des Seances de la Societe de Biologie et de ses Filiales 1998, 192, 101–119. [Google Scholar]
- Farah, A.F. The Response of Two Legume Crops (Hyacinth Bean and Kidney Bean) to the Parasitism of Field Dodder (Cuscuta campestris). 2009. Available online: http://www.ppws.vt.edu/IPPS/IPPS%20Turkey%20Abstract%20Book.pdf (accessed on 9 October 2009).
- Goldwasser, Y.; Sibony, M.; Rubin, B. Screening of Chickpea (Cicer arietinum) Genotypes for Field Dodder (Cuscuta campestris) Resistance. 2009. Available online: http://www.ppws.vt.edu/IPPS/IPPS%20Turkey%20Abstract%20Book.pdf (accessed on 9 October 2009).
- Reddy, A.R.S.; Rao, P.N. Cluster bean—a possible herbicidal source for managing China dodder. In Proceedings of the 11th Asian Pacific Weed Science Society, Taipei, Taiwan, 29 November–5 December 1987; pp. 265–270.
- Bleischwitz, M.; Rehker, J.; Albert, M.; Lachnit, M.; Kaldenhoff, R. Generating parasitic plant resistant crops using a Cuscuta cysteine protease and a parasite inducible promoter. In Proceedings of the 10th World Congress on Parasitic Plants, Kusadasi, Turkey, 8–12 June 2009; p. 114.
- Bewick, T.A. Biology and Control of Swamp Dodder (Cuscuta gronovii). Ph.D. Thesis, Department of Horticulture, University of Wisconsin, Madison, WI, USA, 1987. [Google Scholar]
- Hopen, H.J.; Caruso, F.L.; Bewick, T.A. Control of dodder in cranberry (Vaccinium macrocarpon) with a pathogen-based herbicide. Acta Hort. 1997, 446, 427–428. [Google Scholar]
- Bewick, T.A.; Cascino, J. Development of a Biological Herbicide for Control of Cuscuta spp. 2007. Available online: http://www.cpe.vt.edu/wcopp/ (accessed on 3 June 2009).
- Sandler, H.A.; Caruso, F.L.; Mika, J.S.; Colquhoun, J.B.; Cascino, J. Results from a pilot program using Smolder (Alternaria destruens) as a biological control agent for dodder. Proc. Northeast. Weed Sci. Soc. 2010, 64, 56–59. [Google Scholar]
- Cook, J.C.; Charudattan, R.; Zimmerman, T.W.; Rosskopf, E.; Stall, W.M.; MacDonald, G.E. Effects of Alternaria destuens, glyphosate, and ammonium sulfate individually and integrated for control of dodder (Cuscuta pentagona). Weed Technol. 2009, 23, 550–555. [Google Scholar]
- Templeton, G.E. Use of Colletotrichum strains as mycoherbicides. In Colletotrichum: Biology, Pathology and Control; Bailey, J.A., Jeger, M.J., Eds.; CAB International: Wallingford, UK, 1992; pp. 358–380. [Google Scholar]
- Boyetchko, S.M.; Peng, G. Challenges and strategies for development of mycohericides. In Fungal Biotechnology in Agricultural, Food and Environmental Applications; Arora, D.K., Ed.; Marcel Dekker: New York, NY, USA, 2004; Volume 21, pp. 111–121. [Google Scholar]
- Leach, C.M. A disease of dodder caused by the fungus Colletotrichum destructivum. Plant Dis. Rep. 1958, 42, 827–829. [Google Scholar]
- Gao, Z.Y.; Gan, J. Biological control of dodder: a review on research progress of the bioherbicide Lu Bao No. 1. Chin. J. Bio. Cont. 1992, 8, 173–175. [Google Scholar]
- Cartwright, D.K.; Templeton, G.E. Preliminary evaluation of a dodder anthracnose fungus from China as a mycoherbicide for dodder control in the U.S. Proc. Arkansas Acad. Sci. 1989, 43, 15–18. [Google Scholar]
- Mika, J.S.; Caruso, F.L. The use of Colletotrichum gloeosporioides to control swamp dodder (Cuscuta gronovii Willd.). Proc. Northeast. Weed Sci. Soc. 1999, 53, 56. [Google Scholar]
- Baloch, G.M.; Mohyuddin, A.I.; Ghani, M.A. Biological control of Cuscuta sp. II. Biology and host-plant range of Melanagromyza cuscutae Hering (Dipt. Agromyzidae). Entomophaga 1967, 12, 481–489. [Google Scholar] [CrossRef]
- Marikovskii, P.; Ivannikov, A. Natural enemies of dodder in Kazakhstan. Zashchita Rastenii 1966, 11, 27–28. [Google Scholar]
- Baloch, G.M. Possibilities for biological control of some species of Cuscuta (Convolvulaceae). Pest Articles News Sum. 1968, 14, 27–33. [Google Scholar]
- Tyurebaev, S.S. The use of the dodder gall beetle for the biological control of dodder. Vestnik Sel’skokhozyaistvennoi Nauki Kazakhstana 1977, 20, 116–117. [Google Scholar]
- Shinkarenko, V.A. Phytophagous enemies of dodder. Zashchita Rastenii 1982, 2, 29–30. [Google Scholar]
- Shimi, P.; Bayat Asadi, H.; Rezapanah, M.R.; Koliaii, R. A study of Smicronyx robustus Faust (Curculionidae) as a biological control agent of eastern dodder (Cuscuta monogyna Vahl.) in Iran. J. Agric. Sci. Islamic Azad Univ. 1995, 1, Pe43–Pe51. [Google Scholar]
- Toth, P.; Tothova, M.; Cagan, L. Potential biological control agents of field bindweed, common teasel and field dodder from Slovakia. In Proceedings of the XII International Symposium on Biological Control of Weeds, La Grande Motte, France, 22–27 April 2007; pp. 216–220.
- Craemer, C.; Neser, S.; Smith Meyer, M.K.P. Eriphyid mites (Acari: Eriophyoidea: Eriophyidae) as possible control agents of undesirable introduced plants in South Africa. Suid-Afrikaanse Tydskrif vir Natuurwetenskap en Tegnologie 1997, 1, 99–109. [Google Scholar]
- Saric, M.; Vrbnicanin, S.; Bozic, D.; Raicevic, V. Effect of Plant Growth-Promoting Rhizobacteria on the Germination of Cuscuta campestris Yunck. 2009. Available online: http://www.ppws.vt.edu/IPPS/IPPS%20Turkey%20Abstract%20Book.pdf (accessed on 9 October 2009).
- Ozdemir, S.; Erilmez, S.; Kacan, K. Detection of Tomato Spotted Wilt Virus and Cucumber Mosaic Virus on Cuscuta sp. in Denizli Province of Turkey. 2009. Available online: http://www.ppws.vt.edu/IPPS/IPPS%20Turkey%20Abstract%20Book.pdf (accessed on 9 October 2009).
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sandler, H.A. Managing Cuscuta gronovii (Swamp Dodder) in Cranberry Requires an Integrated Approach. Sustainability 2010, 2, 660-683. https://doi.org/10.3390/su2020660
Sandler HA. Managing Cuscuta gronovii (Swamp Dodder) in Cranberry Requires an Integrated Approach. Sustainability. 2010; 2(2):660-683. https://doi.org/10.3390/su2020660
Chicago/Turabian StyleSandler, Hilary A. 2010. "Managing Cuscuta gronovii (Swamp Dodder) in Cranberry Requires an Integrated Approach" Sustainability 2, no. 2: 660-683. https://doi.org/10.3390/su2020660
APA StyleSandler, H. A. (2010). Managing Cuscuta gronovii (Swamp Dodder) in Cranberry Requires an Integrated Approach. Sustainability, 2(2), 660-683. https://doi.org/10.3390/su2020660





