Aloe Vera in Water Treatment: Toward a Greener Future for Environmental Engineering
Abstract
1. Introduction
2. Methodology
2.1. Preparation of the Aloe Vera-Based Natural Coagulant
2.2. Preparation of Synthetic Water
2.3. Jar Test Experiments
- Coagulation/flocculation—Stirring at 150 rpm for 1 min and 11 s to promote the formation and growth of flocs. The flocculation time selected was based on [26]. It is important to highlight that the reduced value is related to the alternative flocculation technology employed in this work.
- Sedimentation—A 60 min settling period, with sample collection at 10 min intervals for turbidity analysis.
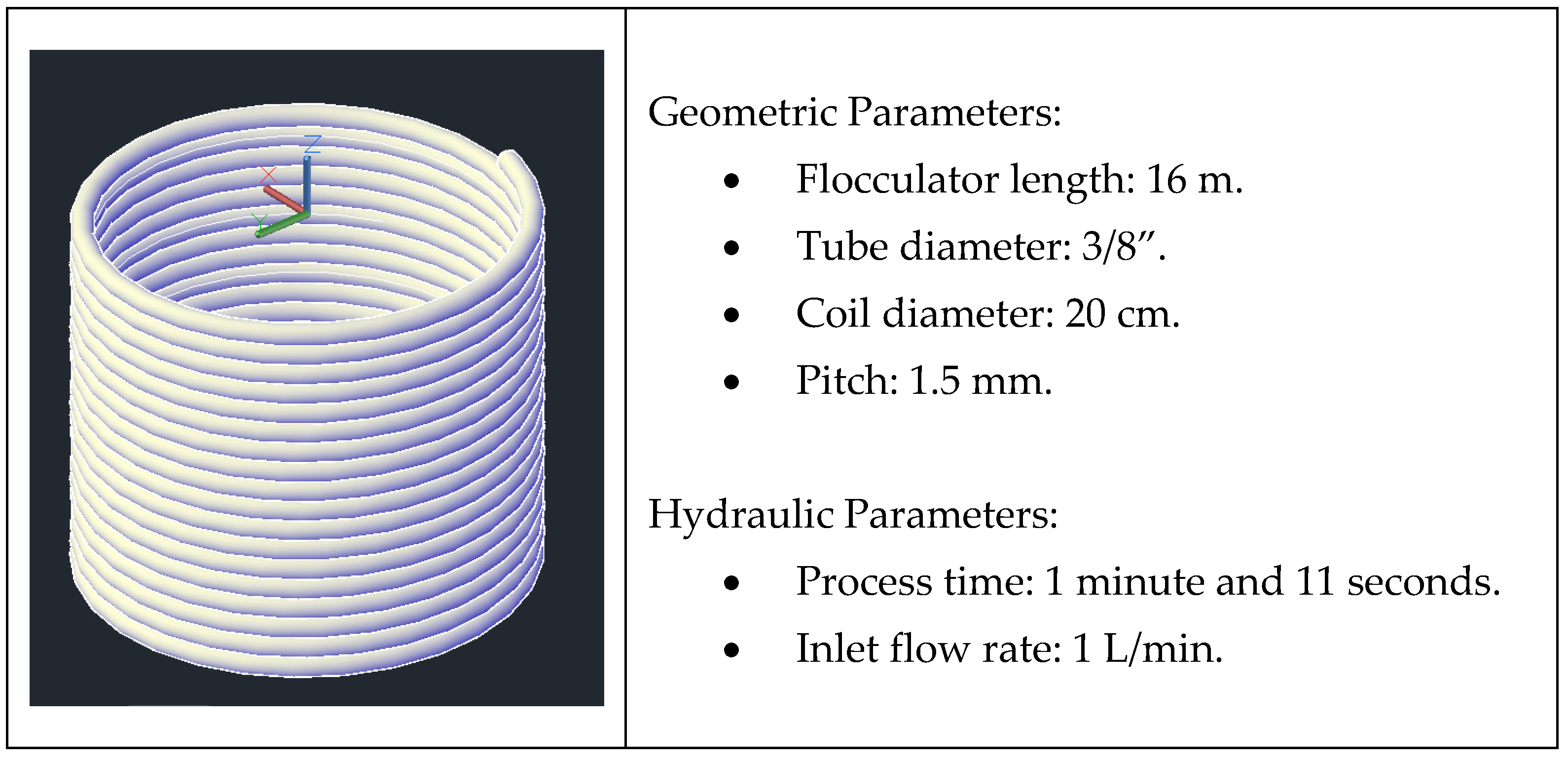
2.4. Laboratory-Scale Hydraulic System Experiments
2.5. Analysis of the Turbidity Removal Efficiency
3. Results and Discussion
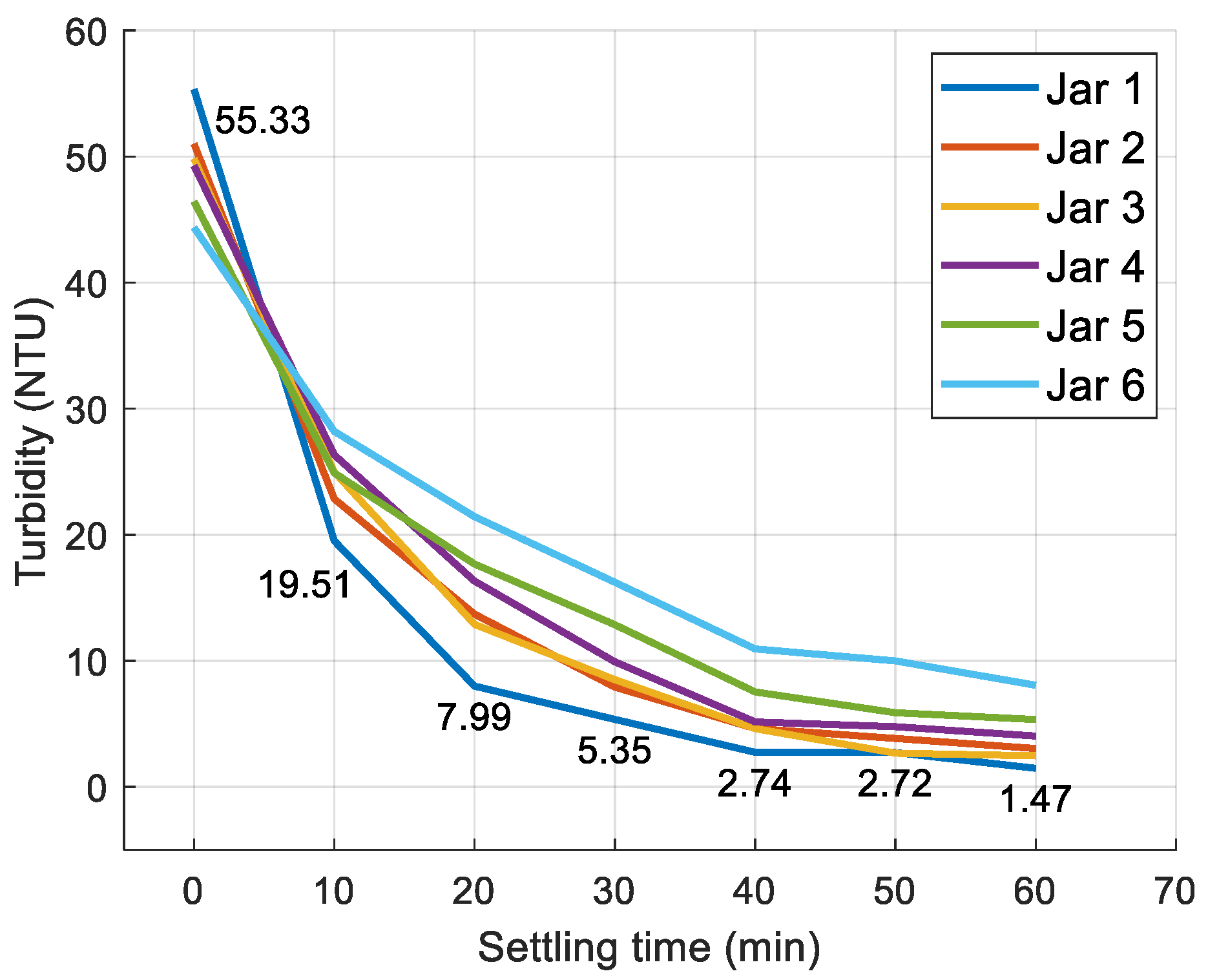
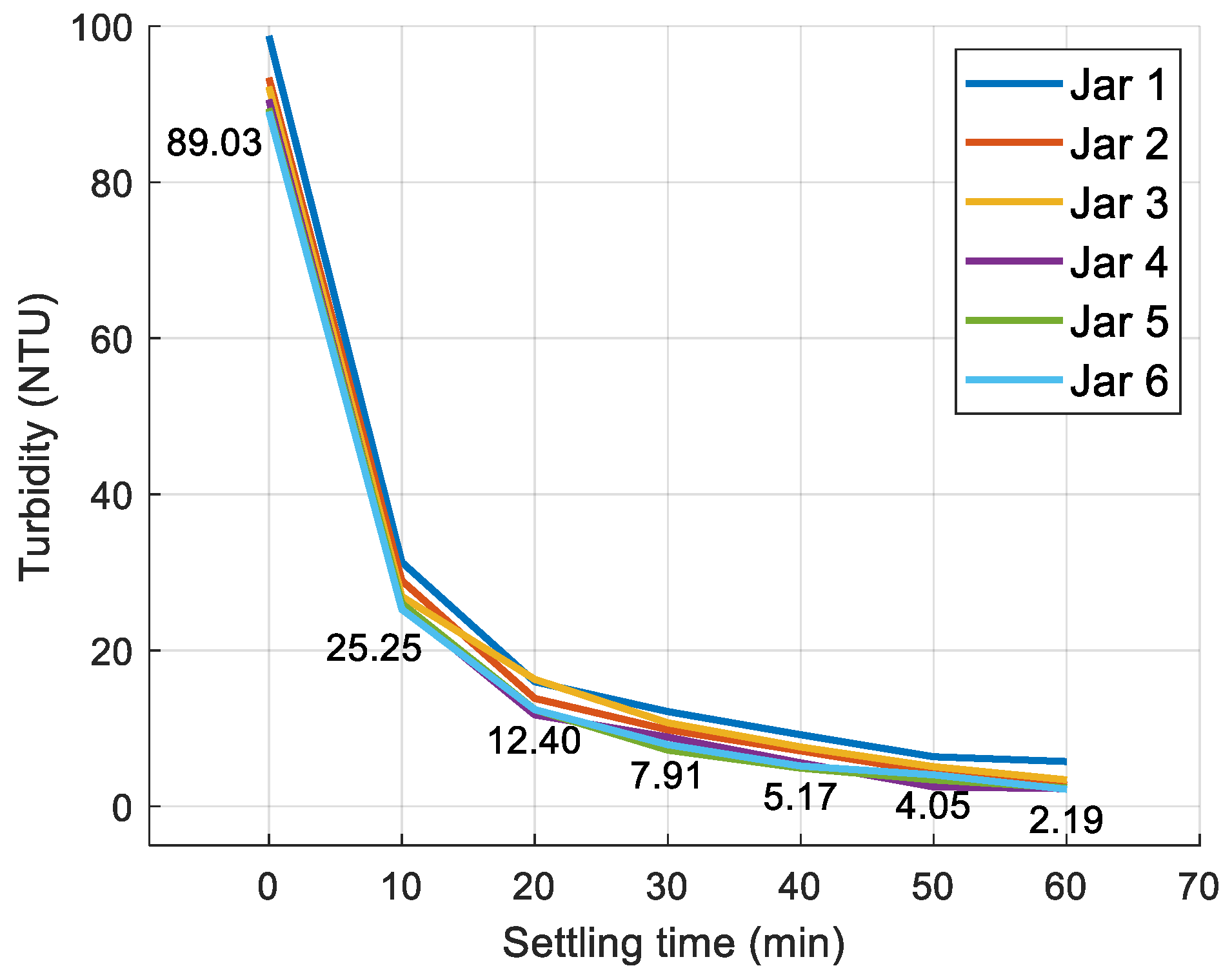
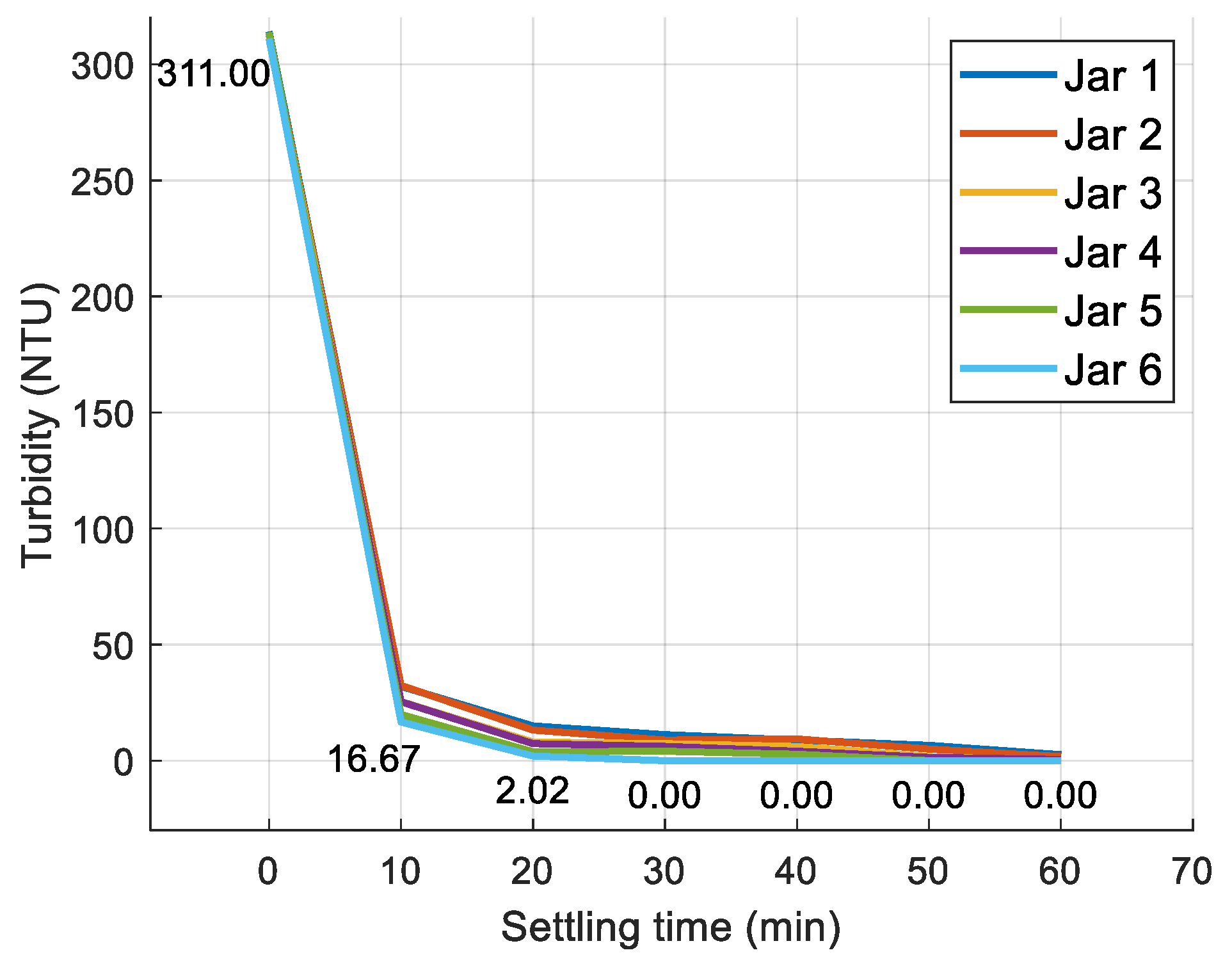
3.1. Jar Test Results
3.2. Hydraulic System Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garrick, D.; Hanemann, M.; Hepburn, C. Rethinking the economics of water: An assessment. Oxf. Rev. Econ. Policy 2020, 36, 1–23. [Google Scholar] [CrossRef]
- Mishra, B.; Kumar, P.; Saraswat, C.; Chakraborty, S.; Gautam, A. Water Security in a Changing Environment: Concept, Challenges and Solutions. Water 2021, 13, 490. [Google Scholar] [CrossRef]
- Pichel, N.; Vivar, M.; Fuentes, M. The problem of drinking water access: A review of disinfection technologies with an emphasis on solar treatment methods. Chemosphere 2019, 218, 1014–1030. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.; Graziele, I.; Marques, R.; Gonçalves, J. Investment in drinking water and sanitation infrastructure and its impact on waterborne diseases dissemination: The Brazilian case. Sci. Total Environ. 2021, 779, 146279. [Google Scholar] [CrossRef]
- Mutono, N.; Wright, J.; Mutembei, H.; Muema, J.; Thomas, M.; Mutunga, M.; Thumbi, S. The nexus between improved water supply and water-borne diseases in urban areas in Africa: A scoping review. AAS Open Res. 2021, 4, 27. [Google Scholar] [CrossRef]
- Prüss-Ustün, A.; Wolf, J.; Bartram, J.; Clasen, T.; Cumming, O.; Freeman, M.C.; Gordon, B.; Hunter, P.R.; Medlicott, K.; Johnston, R. Burden of disease from inadequate water, sanitation and hygiene for selected adverse health outcomes: An updated analysis with a focus on low- and middle-income countries. Int. J. Hyg. Environ. Health 2019, 222, 765–777. [Google Scholar] [CrossRef]
- Chu, C.; Ryberg, E.C.; Loeb, S.K.; Suh, M.-J.; Kim, J.-H. Water Disinfection in Rural Areas Demands Unconventional Solar Technologies. Acc. Chem. Res. 2019, 52, 1187–1195. [Google Scholar] [CrossRef]
- Filho, L.; Tripathi, S.; Guerra, J.A.; Giné-Garriga, R.; Lovren, O.; Willats, J. Using the sustainable development goals towards a better understanding of sustainability challenges. Int. J. Sustain. Dev. World Ecol. 2018, 26, 179–190. [Google Scholar] [CrossRef]
- Weiland, S.; Hickmann, T.; Lederer, M.; Marquardt, J.; Schwindenhammer, S. The 2030 Agenda for Sustainable Development: Transformative Change through the Sustainable Development Goals? Politics Gov. 2021, 9, 90–95. [Google Scholar] [CrossRef]
- Herrera, V. Reconciling global aspirations and local realities: Challenges facing the Sustainable Development Goals for water and sanitation. World Dev. 2019, 118, 106–117. [Google Scholar] [CrossRef]
- Rajapakse, J.; Otoo, M.; Danso, G. Progress in delivering SDG6: Safe water and sanitation. Camb. Prism. Water 2023, 1, e6. [Google Scholar] [CrossRef]
- Weststrate, J.; Dijkstra, G.; Eshuis, J.; Gianoli, A.; Rusca, M. The Sustainable Development Goal on Water and Sanitation: Learning from the Millennium Development Goals. Soc. Indic. Res. 2018, 143, 795–810. [Google Scholar] [CrossRef]
- SNIS. Thematic Diagnosis Water and Sewage Services: Technical Water Management; SNS/MDR: Brasília, Brazil, 2022; p. 56. (In Portuguese) [Google Scholar]
- Greenwood, E.E.; Lauber, T.; van den Hoogen, J.; Donmez, A.; Bain, R.E.S.; Johnston, R.; Crowther, T.W.; Julian, T.R. Mapping safe drinking water use in low- and middle-income countries. Science 2024, 385, 784–790. [Google Scholar] [CrossRef] [PubMed]
- BRAZIL. Ministry of Health. Ordinance GM/MS No. 888, of May 4, 2021. Establishes Procedures for Monitoring and Controlling the Quality of Water for Human Consumption and Its Potability Standards. In Official Gazette of the Union: Section 1, Brasília, DF, 7 May 2021. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/gm/2021/prt0888_07_05_2021.html (accessed on 12 February 2025).
- Cococeanu, A.-L.; Man, T. Methods and Characteristics of Conventional Water Treatment Technologies. In Advanced Sciences and Technologies for Security Applications; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Tian, N.; Nie, Y.; Tian, X.; Wang, Y. Current Water Treatment Technologies: An Introduction. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O.V., Torres-Martínez, L.M., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 2033–2066. [Google Scholar] [CrossRef]
- Krupińska, I. Aluminium Drinking Water Treatment Residuals and Their Toxic Impact on Human Health. Molecules 2020, 25, 641. [Google Scholar] [CrossRef]
- Koul, B.; Bhat, N.; Abubakar, M.; Mishra, M.; Arukha, A.; Yadav, D. Application of Natural Coagulants in Water Treatment: A Sustainable Alternative to Chemicals. Water 2022, 14, 3751. [Google Scholar] [CrossRef]
- Benalia, A.; Derbal, K.; Khalfaoui, A.; Bouchareb, R.; Panico, A.; Gisonni, C.; Crispino, G.; Pirozzi, F.; Pizzi, A. Use of Aloe vera as an Organic Coagulant for Improving Drinking Water Quality. Water 2021, 13, 2024. [Google Scholar] [CrossRef]
- Febrianti, N.; Permana, R.N.W.; Ariani, I.K. Utilization of aloe vera as a biocoagulant for turbidity, total dissolved solid (TDS), and iron (Fe) removal in well water. In Proceedings of the 13th International Conference of Green Technology, Malang, Indonesia, 17–18 October 2023. [Google Scholar]
- Benalia, A.; Derbal, K.; Khalfaoui, A.; Pizzi, A.; Medjahdi, G. The Use of Aloe vera as Natural Coagulant in Algerian Drinking Water Treatment Plant. J. Renew. Mater. 2021, 10, 625–637. [Google Scholar] [CrossRef]
- Katubi, K.M.; Amari, A.; Harharah, H.N.; Eldirderi, M.M.; Tahoon, M.A.; Ben Rebah, F. Aloe vera as Promising Material for Water Treatment: A Review. Processes 2021, 9, 782. [Google Scholar] [CrossRef]
- Ang, W.; Mohammad, A. State of the art and sustainability of natural coagulants in water and wastewater treatment. J. Clean. Prod. 2020, 262, 121267. [Google Scholar] [CrossRef]
- Oliveira, D.S.; Donadel, C.B. Helically Coiled Tube Flocculators in Water Clarification Systems: Optimal Length Evaluation and Process Efficiency Probabilistic Analysis. Sustainability 2024, 16, 2172. [Google Scholar] [CrossRef]
- Silva, J.R.; Oliveira, D.S. Water Treatment with Clean Technologies Using Moringa oleifera Seeds in Alternative Low-Cost Clarification Units. Clean Technol. 2024, 6, 625–645. [Google Scholar] [CrossRef]
- Oliveira, D.S.; Donadel, C.B. Global velocity gradient evaluation: An innovative approach using CFD modeling applied to water and wastewater treatment plants. J. Water Process Eng. 2019, 28, 21–27. [Google Scholar] [CrossRef]
- Oliveira, D.S.; Nascimento, R.S.; Donadel, C.B. Enhancing accessibility to water treatment: An economical and sustainable method for producing a moringa oleifera-based natural coagulant through paper filtration. MethodsX 2024, 13, 103060. [Google Scholar] [CrossRef] [PubMed]
- Sawant, M.; Deore, V.; Chaure, A.; Momin, A. Sea Water Converted into Usable and Emergency Drinkable Water. Ijraset J. Res. Appl. Sci. Eng. Technol. 2022, 10, 2997–3001. [Google Scholar] [CrossRef]
- Davies-Colley, R.; Hughes, A.; Vincent, A.; Heubeck, S. Weak numerical comparability of ISO-7027-compliant nephelometers. Ramifications for turbidity measurement applications. Hydrol. Process. 2021, 35, e14399. [Google Scholar] [CrossRef]
- Montgomery, D.C.; Runger, G.C. Applied Statistics and Probability for Engineers, 7th ed.; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]








| Container | Jar 1 | Jar 2 | Jar 3 | Jar 4 | Jar 5 | Jar 6 |
|---|---|---|---|---|---|---|
| Coagulant volume (mL) | 60 | 65 | 70 | 75 | 80 | 85 |
| Coagulant mass (g) | 2.4 | 2.6 | 2.8 | 3.0 | 3.2 | 3.4 |
| Container | Jar 1 | Jar 2 | Jar 3 | Jar 4 | Jar 5 | Jar 6 |
|---|---|---|---|---|---|---|
| Coagulant volume (mL) | 50 | 55 | 60 | 65 | 70 | 75 |
| Coagulant mass (g) | 2.0 | 2.2 | 2.4 | 2.6 | 2.8 | 3.0 |
| Container | Jar 1 | Jar 2 | Jar 3 | Jar 4 | Jar 5 | Jar 6 |
|---|---|---|---|---|---|---|
| Coagulant volume (mL) | 80 | 85 | 90 | 95 | 100 | 105 |
| Coagulant mass (g) | 3.2 | 3.4 | 3.6 | 3.8 | 4.0 | 4.2 |
| Container | Jar 1 | Jar 2 | Jar 3 | Jar 4 | Jar 5 | Jar 6 |
|---|---|---|---|---|---|---|
| Coagulant volume (mL) | 95 | 100 | 105 | 110 | 115 | 120 |
| Coagulant mass (g) | 3.8 | 4.0 | 4.2 | 4.4 | 4.6 | 4.8 |
| Test | Initial Water Turbidity (NTU) | Final Turbidity After Sedimentation (NTU) | Final Turbidity After Filtration (NTU) | Turbidity Removal Efficiency After Sedimentation (%) | Turbidity Removal Efficiency After Filtration (%) |
|---|---|---|---|---|---|
| Test 1 (100 NTU) | 99.27 | 25.88 | 0.62 | 74.0% | 99.4% |
| Test 2 (200 NTU) | 199.83 | 15.33 | 0.96 | 92.3% | 99.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, D.S.; Nascimento, R.S.; Donadel, C.B. Aloe Vera in Water Treatment: Toward a Greener Future for Environmental Engineering. Sustainability 2025, 17, 4163. https://doi.org/10.3390/su17094163
Oliveira DS, Nascimento RS, Donadel CB. Aloe Vera in Water Treatment: Toward a Greener Future for Environmental Engineering. Sustainability. 2025; 17(9):4163. https://doi.org/10.3390/su17094163
Chicago/Turabian StyleOliveira, Danieli Soares, Raynara Souza Nascimento, and Clainer Bravin Donadel. 2025. "Aloe Vera in Water Treatment: Toward a Greener Future for Environmental Engineering" Sustainability 17, no. 9: 4163. https://doi.org/10.3390/su17094163
APA StyleOliveira, D. S., Nascimento, R. S., & Donadel, C. B. (2025). Aloe Vera in Water Treatment: Toward a Greener Future for Environmental Engineering. Sustainability, 17(9), 4163. https://doi.org/10.3390/su17094163






