Sustainable PH3 Purification over MOF-Derived Ce-Doped CuO Materials: Enhanced Performance and Closed-Loop Resource Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Adsorbents
2.2. Activity Tests
2.3. Characterization Methods
3. Results and Discussion
3.1. Characterization of Adsorbents
3.2. Adsorbents Performance
3.3. Mechanism of PH3 Adsorption–Oxidation over Cu1Ce0.2O
3.4. Recycling of the Exhausted Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Widera, A.; Thöny, D.; Aebli, M.; Oppenheim, J.J.; Andrews, J.L.; Eiler, F.; Wörle, M.; Schönberg, H.; Weferling, N.; Dinca, M.; et al. Solid-State Investigation, Storage, and Separation of Pyrophoric PH3 and P2H4 with α-Mg Formate. Angew. Chem.-Int. Ed. 2023, 62, e202217534. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, S.G.; Yao, X.F. Addition of reactive oxygen scavenger to enhance PH3 biopurification: Process and mechanism. Process Saf. Environ. Prot. 2020, 142, 118–125. [Google Scholar] [CrossRef]
- Ma, J.L.; Chen, W.Y.; Niu, X.J.; Fan, Y.M. The relationship between phosphine, methane, and ozone over paddy field in Guangzhou, China. Glob. Ecol. Conserv. 2019, 17, e00581. [Google Scholar] [CrossRef]
- Huang, B.Y.; Wang, F.; Li, H.Y.; Li, Y.; Li, K. Synergistic role of Co and CoP on highly dispersed cobalt nanoparticles embeded in porous carbon for efficient PH3 decomposition. Sep. Purif. Technol. 2024, 348, 10. [Google Scholar] [CrossRef]
- Yi, H.H.; Yang, L.P.; Tang, X.L.; Yu, Q.F.; Yang, L. Adsorption of Phosphine in Yellow Phosphorus Tail Gas by Impregnated Activated Carbon. In Proceedings of the Asia-Pacific Power and Energy Engineering Conference (APPEEC), Chengdu, China, 28–31 March 2010. [Google Scholar]
- Lin, T.; Chen. Oxidative Removal of PH3 and H2S from Yellow Phosphorus Tail Gas by JC Series Catalysts. Nat. Gas Chem. Ind. 2004, 29, 19–23. [Google Scholar]
- Yi, H.H.; Yang, L.N.; Tang, X.L.; Yu, L.L.; Wang, H.Y. Purifying PH3 with Cu2+ Absorption Solution. In Proceedings of the Asia-Pacific Power and Energy Engineering Conference (APPEEC), Chengdu, China, 28–31 March 2010. [Google Scholar]
- Hsu, J.N.; Bai, H.L.; Li, S.N.; Tsai, C.J. Copper Loaded on Sol-Gel-Derived Alumina Adsorbents for Phosphine Removal. J. Air Waste Manag. Assoc. 2010, 60, 629–635. [Google Scholar] [CrossRef]
- Feng, J.Y.; Wang, F.; Wang, C.; Li, K.; Ning, P.; Sun, X.; Jia, L.J. Ce-doping CuO/HZSM-5 as a regenerable sorbent for Adsorption-Oxidation removal of PH3 at low temperature. Sep. Purif. Technol. 2021, 277, 10. [Google Scholar] [CrossRef]
- Tang, Y.; Feng, J.Y.; Ning, P.; Wang, F.; Sun, X.; Li, K. Low-temperature efficient removal of PH3 over novel Cu-based adsorbent in an anaerobic environment. Chem. Eng. J. 2023, 461, 11. [Google Scholar] [CrossRef]
- Wang, Y.W.; Ning, P.; Zhao, R.H.; Li, K.; Wang, C.; Sun, X.; Song, X.; Lin, Q. A Cu-modified active carbon fiber significantly promoted H2S and PH3 simultaneous removal at a low reaction temperature. Front. Env. Sci. Eng. 2021, 15, 10. [Google Scholar] [CrossRef]
- Yu, Q.F.; Li, M.; Ning, P.; Yi, H.H.; Tang, X.L. Characterization of Metal Oxide-modified Walnut-shell Activated Carbon and Its Application for Phosphine Adsorption: Equilibrium, Regeneration, and Mechanism Studies. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2019, 34, 487–495. [Google Scholar] [CrossRef]
- Song, X.; Li, S.; Li, K.; Ning, P.; Wang, C.; Sun, X.; Wang, Y.W. Preparation of Cu-Fe composite metal oxide loaded SBA-15 and its capacity for simultaneous catalytic oxidation of hydrogen sulfide and phosphine. Microporous Mesoporous Mat. 2018, 259, 89–98. [Google Scholar] [CrossRef]
- Li, S.; Li, K.; Hao, J.M.; Ning, P.; Tang, L.H.; Sun, X. Acid modified mesoporous Cu/SBA-15 for simultaneous adsorption/oxidation of hydrogen sulfide and phosphine. Chem. Eng. J. 2016, 302, 69–76. [Google Scholar] [CrossRef]
- Luo, Z.; Liang, Q.X.; Qi, Y.S.; Huang, G.Q. The Cu/TiO2 adsorbent modified by Ce-doping removes trace phosphorus impurities from circular hydrogen of a polysilicon chemical vapor deposition furnace. Sep. Purif. Technol. 2024, 344, 11. [Google Scholar] [CrossRef]
- Xu, B.W.; Qu, J.X.; Wang, X.Q.; Wang, L.L.; Pu, Y.; Ning, P.; Xie, Y.B.; Ma, Y.X.; Ma, Q. Unravelling the nature of the active species as well as the Mn doping effect over γ-Al203 catalyst for eliminating AsH3 and PH3. J. Environ. Sci. 2024, 136, 213–225. [Google Scholar] [CrossRef]
- Ren, Z.D.; Quan, S.S.; Zhu, Y.C.; Chen, L.; Deng, W.X.; Zhang, B.A. Purification of yellow phosphorus tail gas for the removal of PH3 on the spot with flower-shaped CuO/AC. Rsc Adv. 2015, 5, 29734–29740. [Google Scholar] [CrossRef]
- Bhoria, N.; Basina, G.; Pokhrel, J.; Reddy, K.S.K.; Anastasiou, S.; Balasubramanian, V.V.; AlWahedi, Y.F.; Karanikolos, G.N. Functionalization effects on HKUST-1 and HKUST-1/graphene oxide hybrid adsorbents for hydrogen sulfide removal. J. Hazard. Mater. 2020, 394, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Yang, C.; Geng, Q.; Fan, H.L.; Wang, B.J.; Wu, M.M.; Tian, Z. Adsorption of hydrogen sulfide by amine-functionalized metal organic framework (MOF-199): An experimental and simulation study. Appl. Surf. Sci. 2019, 497, 10. [Google Scholar] [CrossRef]
- Peng, Z.W.; Wang, S.X.; Wu, Y.H.; Liu, X.; Liu, H.L.; Zhang, D.K.; Fu, L.K. Synthesis of novel MOF for adsorption of germanium: Kinetics, isotherm and thermodynamics. Microporous Mesoporous Mat. 2024, 363, 11. [Google Scholar] [CrossRef]
- Jiang, N.; Du, B.Y.; Gao, D.; Chai, Z.B.; Liu, C.; Zhu, X. Effective As(V) removal using in situ grown Ti-based MOFs on ZnAl-LDHs. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2024, 303, 12. [Google Scholar] [CrossRef]
- Duan, R.D.; Feng, Y.J.; Gu, J.L.; Wang, M.H.; He, B.N.; Liu, L.L.; Zhu, B.J.; Wang, X.D. Ultralow loading of Cu2O/CuO nanoparticles on metal-organic framework-derived carbon octahedra and activated semi-coke for highly efficient SO2 removal. J. Clean. Prod. 2022, 341, 11. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, H.; Wang, Y.; He, C.; Wang, Y.; Zhang, X. Synthesis of octahedral like Cu-BTC derivatives derived from MOF calcined under different atmosphere for application in CO oxidation. J. Solid State Chem. 2018, 258, 582–587. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, X.; Yao, Y.; Xin, J.G.; Xie, L.L.; Han, Y.T.; Zhu, Z.G. Preparation of a high-performance H2S gas sensor based on CuO/Co3O4 composite derived from bimetallic MOF. Nanotechnology 2024, 35, 10. [Google Scholar] [CrossRef] [PubMed]
- Zamaro, J.M.; Pérez, N.C.; Miró, E.E.; Casado, C.; Seoane, B.; Téllez, C.; Coronas, J. HKUST-1 MOF: A matrix to synthesize CuO and CuO-CeO2 nanoparticle catalysts for CO oxidation. Chem. Eng. J. 2012, 195, 180–187. [Google Scholar] [CrossRef]
- Feng, C.; Xiong, G.; Wang, Y.; Pan, Y.; Liu, Y. Synthesis of CuO-Cu1.5Mn1.5O4 Composite Oxide by Using a Bimetallic Organic Framework for Catalytic Propane Total Oxidation. Environ. Eng. 2022, 40, 69–77. [Google Scholar]
- Hoot, N.; Sheikhhosseini, E.; Ahmadi, S.A.; Ghazizadeh, M.; Malekshahi, M.; Yahyazadehfar, M. Synthesis of pyridone derivatives using 2D rod like bifunctional Fe based MOF and CuO nanocomposites as a novel heterogeneous catalyst. Sci. Rep. 2023, 13, 14. [Google Scholar] [CrossRef]
- Salehi, S.; Anbia, M. High CO2 Adsorption Capacity and CO2/CH4 Selectivity by Nanocomposites of MOF-199. Energy Fuels 2017, 31, 5376–5384. [Google Scholar] [CrossRef]
- Yang, X.; Liu, W.; Tan, F.H.; Zhang, Z.X.; Chen, X.D.; Liang, T.D.; Wu, C.D. A robust strategy of homogeneously hybridizing silica and Cu3(BTC)2 to in situ synthesize highly dispersed copper catalyst for furfural hydrogenation. Appl. Catal. A-Gen. 2020, 596, 11. [Google Scholar] [CrossRef]
- Maciel, C.G.; Silva, T.D.; Hirooka, M.I.; Belgacem, M.N.; Assaf, J.M. Effect of nature of ceria support in CuO/CeO2 catalyst for PROX-CO reaction. Fuel 2012, 97, 245–252. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, C.; Xiao, W.M.; Xu, L.; Zhang, N. CuO/CeO2 catalysts with well-dispersed active sites prepared from Cu3(BTC)2 metal-organic framework precursor for preferential CO oxidation. Catal. Commun. 2012, 26, 25–29. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, K.; Xiong, J.X.; Ren, Q.M.; Zhong, J.P.; Cai, H.D.; Huang, H.M.; Chen, P.R.; Wu, J.L.; Chen, L.M.; et al. Static and dynamic quantification tracking of asymmetric oxygen vacancies in copper-ceria catalysts with superior catalytic activity. Appl. Catal. B-Environ. 2022, 316, 13. [Google Scholar] [CrossRef]
- Li, L.; Zhan, Y.Y.; Zheng, Q.; Zheng, Y.H.; Chen, C.Q.; She, Y.S.; Lin, X.Y.; Wei, K.M. Water-Gas Shift Reaction over CuO/CeO2 Catalysts: Effect of the Thermal Stability and Oxygen Vacancies of CeO2 Supports Previously Prepared by Different Methods. Catal. Lett. 2009, 130, 532–540. [Google Scholar] [CrossRef]
- Martínez-Arias, A.; Fernández-García, M.; Gálvez, O.; Coronado, J.M.; Anderson, J.A.; Conesa, J.C.; Soria, J.; Munuera, G. Comparative study on redox properties and catalytic behavior for CO oxidation of CuO/CeO2 and CuO/ZrCeO4 catalysts. J. Catal. 2000, 195, 207–216. [Google Scholar] [CrossRef]
- Wang, F.; Büchel, R.; Savitsky, A.; Zalibera, M.; Widmann, D.; Pratsinis, S.E.; Lubitz, W.; Schüth, F. In Situ EPR Study of the Redox Properties of CuO-CeO2 Catalysts for Preferential CO Oxidation (PROX). ACS Catal. 2016, 6, 3520–3530. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Zhang, C.; Duan, M.; Wang, H.; Fan, H.; Li, Y.; Shangguan, J.; Lin, J. Effect of hierarchical porous MOF-199 regulated by PVP on their ambient desulfurization performance. Fuel 2022, 319, 123845. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Zhang, Z.-R.; Yang, C.; Ling, L.-X.; Wang, B.-J.; Fan, H.-L. A Computational Study of the Adsorptive Removal of H2S by MOF-199. J. Inorg. Organomet. Polym. Mater. 2018, 28, 694–701. [Google Scholar] [CrossRef]
- Das, R.; Pachfule, P.; Banerjee, R.; Poddar, P. Metal and metal oxide nanoparticle synthesis from metal organic frameworks (MOFs): Finding the border of metal and metal oxides. Nanoscale 2012, 4, 591–599. [Google Scholar] [CrossRef]
- Banerjee, A.; Singh, U.; Aravindan, V.; Srinivasan, M.; Ogale, S. Synthesis of CuO nanostructures from Cu-based metal organic framework (MOF-199) for application as anode for Li-ion batteries. Nano Energy 2013, 2, 1158–1163. [Google Scholar] [CrossRef]
- Liu, G.; Mba Wright, M.; Zhao, Q.; Brown, R.C. Engineering, Hydrocarbon and ammonia production from catalytic pyrolysis of sewage sludge with acid pretreatment. ACS Sustain. Chem. 2016, 4, 1819–1826. [Google Scholar] [CrossRef]
- Gupta, N.K.; Bae, J.; Kim, K.S. From MOF-199 Microrods to CuO Nanoparticles for Room-Temperature Desulfurization: Regeneration and Repurposing Spent Adsorbents as Sustainable Approaches. ACS Omega 2021, 6, 25631–25641. [Google Scholar] [CrossRef]
- Li, R.; Huang, Y.; Zhu, D.D.; Ho, W.K.; Cao, J.J.; Lee, S.C. Improved Oxygen Activation over a Carbon/Co3O4 Nanocomposite for Efficient Catalytic Oxidation of Formaldehyde at Room Temperature. Environ. Sci. Technol. 2021, 55, 4054–4063. [Google Scholar] [CrossRef]
- Cheng, W.W.; Guan, W.J.; Lin, Y.J.; Lu, C. Rapid Discrimination of Adsorbed Oxygen and Lattice Oxygen in Catalysts by the Cataluminescence Method. Anal. Chem. 2022, 94, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Worayingyong, A.; Kangvansura, P.; Ausadasuk, S.; Praserthdam, P. The effect of preparation:: Pechini and Schiff base methods, on adsorbed oxygen of LaCoO3 perovskite oxidation catalysts. Colloids Surf. A-Physicochem. Eng. Asp. 2008, 315, 217–225. [Google Scholar] [CrossRef]
- Zhu, H.J.; Chen, Y.Y.; Gao, Y.B.; Liu, W.X.; Wang, Z.P.; Cui, C.C.; Liu, W.; Wang, L.G. Catalytic oxidation of CO on mesoporous codoped ceria catalysts: Insights into correlation of physicochemical property and catalytic activity. J. Rare Earths 2019, 37, 961–969. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Zhang, J.B.; Liu, Y.J. Promoted low-temperature CO oxidation activity of CeO2 by Cu doping: The important role of oxygen vacancies. Fuel 2024, 359, 10. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, J.; Tian, J.T.; Xie, Z.H.; Liu, Y.; Liu, P.; Ye, D.Q. Insight into catalytic performance and reaction mechanism for toluene total oxidation over Cu-Ce supported catalyst. J. Environ. Sci. 2025, 149, 476–487. [Google Scholar] [CrossRef]
- Yu, L.Z.; Sun, X.H.; Wang, F.; Xue, B.; Xu, J. Acid-base bifunctional ZnNbCl/eg-C3N4 materials towards catalytic synthesis of dimethyl carbonate via transesterification of ethylene carbonate. Appl. Catal. A-Gen. 2023, 666, 119432. [Google Scholar] [CrossRef]
- Zhao, L.; Kang, Q.; Guan, X.F.; Martyniuk, C.J. Hydrotalcite-based CeNiAl mixed oxides for SO2 adsorption and oxidation. Environ. Technol. 2019, 40, 3678–3688. [Google Scholar] [CrossRef]
- Feng, R.M.; Yang, X.J.; Ji, W.J.; Chen, Y.; Au, C.T. VPO catalysts supported on H3PO4-treated ZrO2 highly active for n-butane oxidation. J. Catal. 2007, 246, 166–176. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wang, J.L.; Zhang, Y.H.; Jiao, Y.; Ren, C.J.; Gong, M.C.; Chen, Y.Q. A study on H2-TPR of Pt/Ce0.27Zr0.73O2 and Pt/Ce0.27Zr0.70La0.03Ox for soot oxidation. Appl. Surf. Sci. 2016, 377, 48–55. [Google Scholar] [CrossRef]
- Shi, H.C.; Zheng, T.; Zuo, Y.H.; Wu, Q.M.; Zhang, Y.; Fan, Y.; Tontiwachwuthikul, P. Synthesis of Cu3P/SnO2 composites for degradation of tetracycline hydrochloride in wastewater. RSC Adv. 2021, 11, 33471–33480. [Google Scholar] [CrossRef]
- Zhao, R.B.; Geng, Q.; Chang, L.; Wei, P.P.; Luo, Y.L.; Shi, X.F.; Asiri, A.M.; Lu, S.Y.; Wang, Z.M.; Sun, X.P. Cu3P nanoparticle-reduced graphene oxide hybrid: An efficient electrocatalyst to realize N2-to-NH3conversion under ambient conditions. Chem. Commun. 2020, 56, 9328–9331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Zhan, Z.B.; Li, Z.; Di, L.B. Thermal Activation of CuBTC MOF for CO Oxidation: The Effect of Activation Atmosphere. Catalysts 2017, 7, 11. [Google Scholar] [CrossRef]
- Liu, H.Y.; Xie, J.W.; Liu, P.; Dai, B. Effect of Cu+/Cu2+ Ratio on the Catalytic Behavior of Anhydrous Nieuwland Catalyst during Dimerization of Acetylene. Catalysts 2016, 6, 120. [Google Scholar] [CrossRef]
- Vineesh, T.V.; Yarmiayev, V.; Zitoun, D. Tailoring the electrochemical hydrogen evolution activity of Cu3P through oxophilic surface modification. Electrochem. Commun. 2020, 113, 106691. [Google Scholar] [CrossRef]
- Wang, L.; Peng, H.; Shi, S.L.; Hu, Z.; Zhang, B.Z.; Ding, S.M.; Wang, S.H.; Chen, C. Metal-organic framework derived hollow CuO/CeO2 nano-sphere: To expose more highly dispersed Cu-O-Ce interface for enhancing preferential CO oxidation. Appl. Surf. Sci. 2022, 573, 12. [Google Scholar] [CrossRef]
- Cai, W.Q.; Zhang, F.J.; Wang, Y.R.; Li, D.C. A novel I-type 0D/0D ZnS@Cu3P heterojunction for photocatalytic hydrogen evolution. Inorg. Chem. Commun. 2021, 134, 8. [Google Scholar] [CrossRef]
- Ge, G.Y.; Yuan, S.T.; Liu, Q.Z.; Yang, D.F.; Shi, J.S.; Lan, X.F.; Xiao, K.F. Insight into the function of noble-metal free Cu3P decorated Zn0.5Cd0.5S for enhanced photocatalytic hydrogen evolution under visible light irradiation-mechanism for continuous increasing activity. Appl. Surf. Sci. 2022, 597, 9. [Google Scholar] [CrossRef]
- Slimani, Y.; Khan, A.; Sivakumar, R.; Nawaz, M.; Thakur, A. Synergistic impact of lanthanum and cerium co-doping ZnO for improved photocatalytic rhodamine B degradation efficiency under UV light. J. Mol. Struct. 2025, 1322, 17. [Google Scholar] [CrossRef]
- Thi, L.N.; Phan, T.T.T.; Tan, L.N.; Le, T.L.T.; Nguyen, L.; Hoang, N.T.; Vo, V.; Khieu, D.Q. Activated carbon/g-C3N4/H2O2 system with enhanced photocatalytic activity for Rhodamine-B degradation under the visible light. Environ. Eng. Res. 2025, 30, 11. [Google Scholar] [CrossRef]
- Bekchanov, D.; Mukhamediev, M.; Inkhonova, A.; Eshtursunov, D.; Babojonova, G.; Rajabov, O.; Khalilov, U.; Yusupov, M.; Lieberzeit, P. Magnetic and reusable Fe3O4/PPE-2 functional material for efficient photodegradation of organic dye. Environ. Res. 2025, 269, 13. [Google Scholar] [CrossRef]
- Shi, K.R.; Li, X.Y.; Tian, Z.Q.; Luo, Y.R.; Ding, R.M.; Zhu, Y.S.; Yao, H.Q. Synergistic and efficient photocatalytic degradation of rhodamine B and tetracycline in wastewater based on novel S-scheme heterojunction phosphotungstic Acid@MIL-101(Cr). J. Environ. Manag. 2025, 373, 12. [Google Scholar] [CrossRef] [PubMed]
- Raguram, T.; Rajni, K.S.; Kanchana, D.; Jose, S.-E.; Granados-Tavera, K.; Cardenas-Jiron, G.; Shobana, M.; Meher, S.R. Exploring structural and optical properties of iodine-doped TiO2 nanoparticles in Rhodamine-B dye degradation: Experimental and theoretical investigation. Chemosphere 2024, 364, 143183. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.K.; Zhang, Y.J.; Zhang, J.L.; Li, Z.Y.; Hu, F.Y.; Pan, D.; Melhi, S.; Shi, X.T.; Amin, M.A.; El-Bahy, Z.M.; et al. Electrochemically synthesized Tin micro-nanometer powders for visible light photocatalytic degradation of Rhodamine B dye from polluted water. Adv. Compos. Hybrid Mater. 2024, 7, 12. [Google Scholar] [CrossRef]


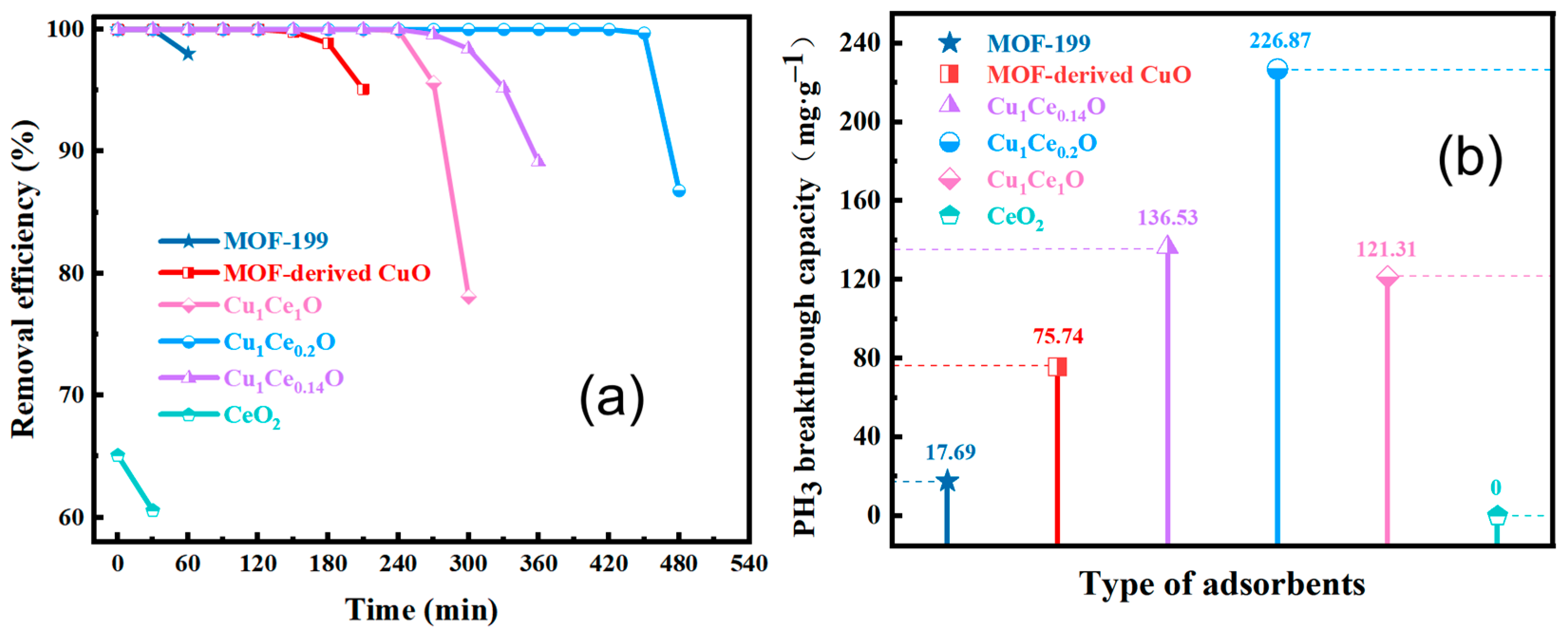
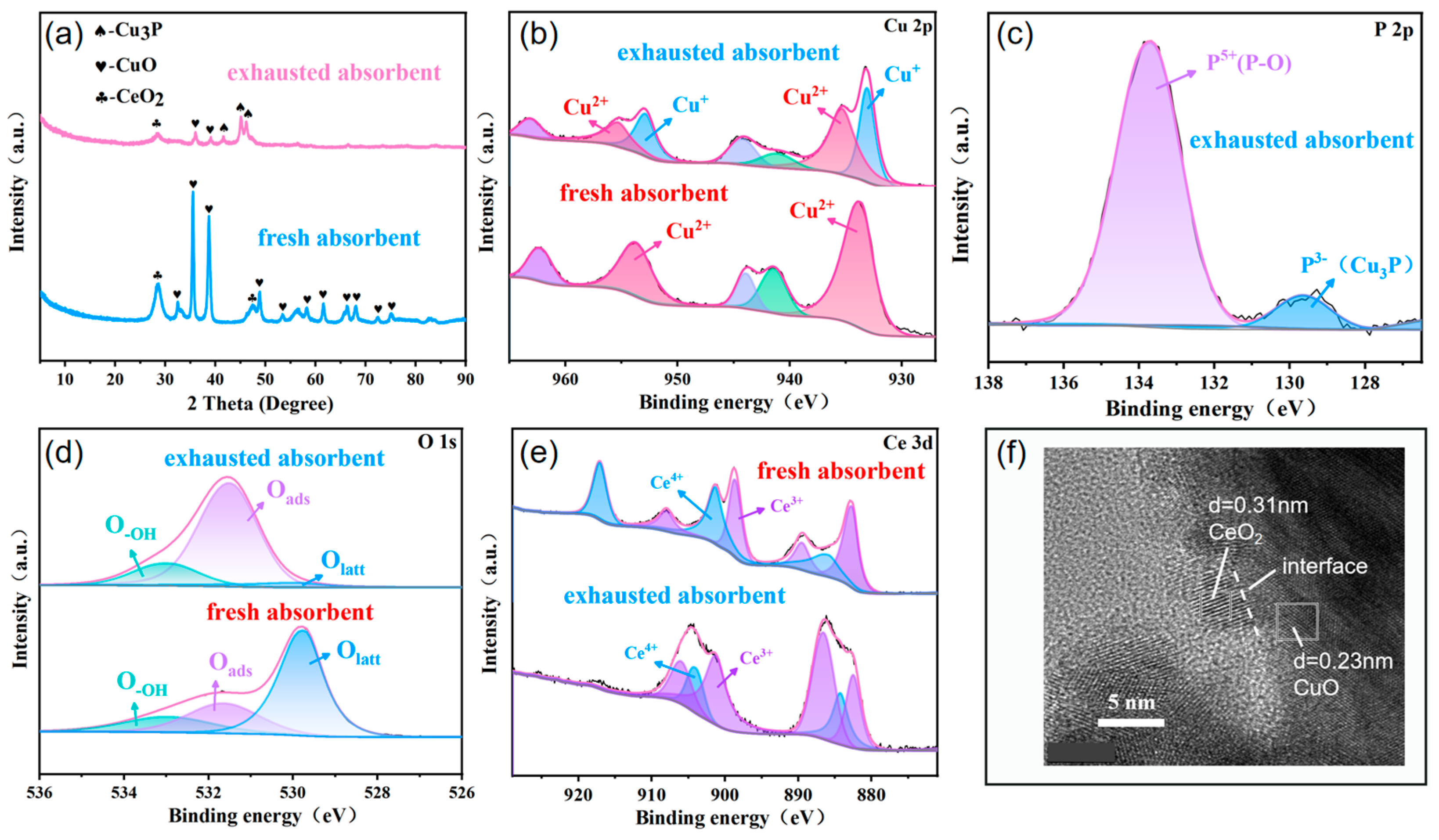

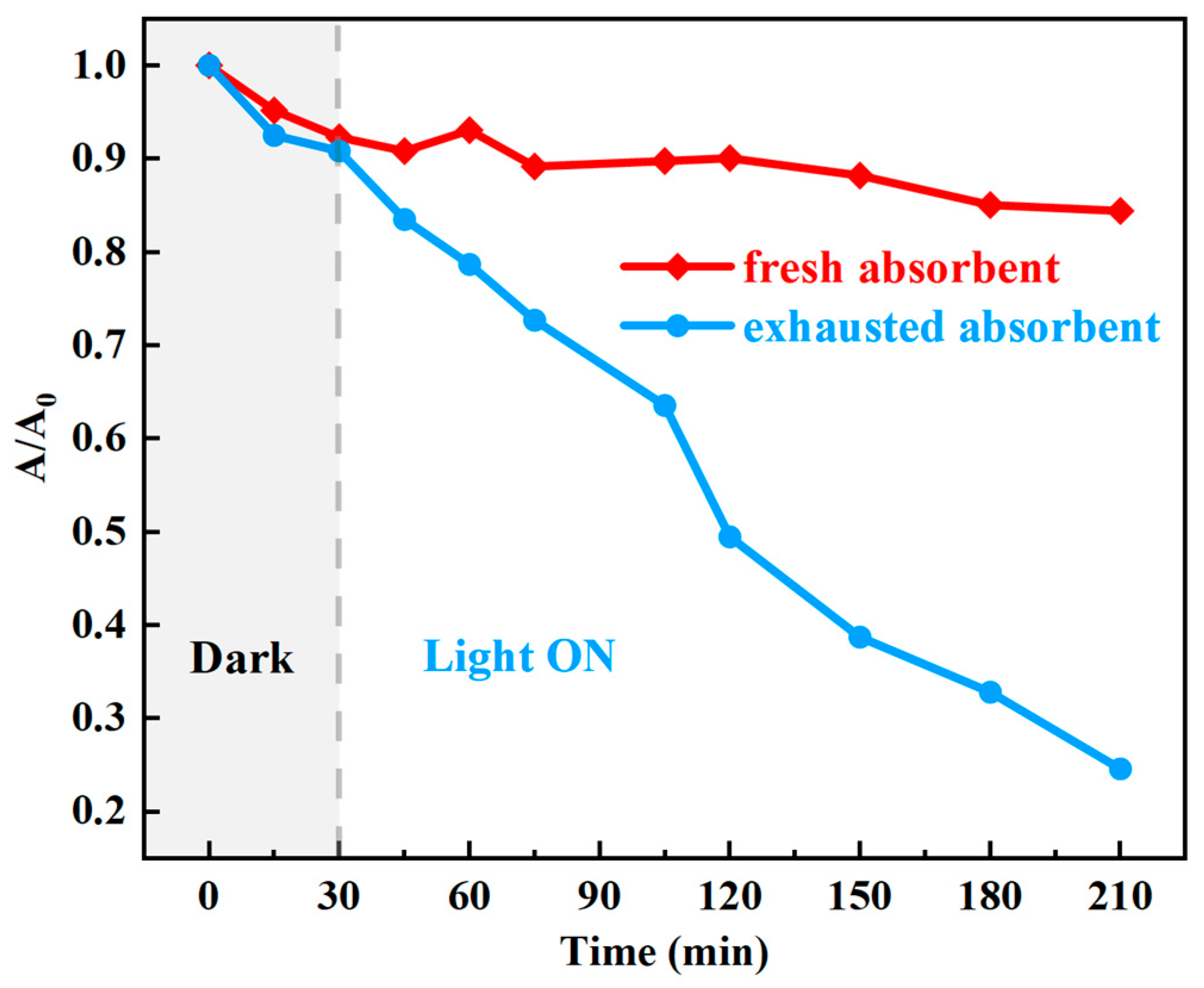
| Samples | SBET (m2·g−1) | Pore Volume (mL·g−1) | Average Pore Size (nm) |
|---|---|---|---|
| MOF-199 | 1109.41 | 0.5980 | 1.078 |
| MOF-derived CuO | 6.51 | 0.0105 | 3.234 |
| Cu1Ce0.14O | 20.37 | 0.0374 | 3.672 |
| Cu1Ce0.2O | 28.01 | 0.0579 | 4.136 |
| Cu1Ce1O | 70.20 | 0.0980 | 2.791 |
| exhausted Cu1Ce0.2O | 13.82 | 0.0232 | 3.360 |
| CeO2 | 77.48 | 0.2195 | 5.667 |
| Samples | Oads (%) | Olatt (%) | O-OH (%) |
|---|---|---|---|
| MOF-derived CuO | 18.87 | 60.34 | 20.79 |
| Cu1Ce0.14O | 21.05 | 65.45 | 13.50 |
| Cu1Ce0.2O | 25.71 | 57.99 | 16.30 |
| Cu1Ce1O | 19.83 | 64.16 | 16.01 |
| Samples | Experimental Condition | Time (min) | Removal (%) | Ref. |
|---|---|---|---|---|
| Fe3O4/PPE-2 | ultraviolet light, 50 mg catalyst, RhB 50 mg·L−1 | 160 | 98.2 | [62] |
| PTA@MIL-101 (Cr) | visible light, 30 mg catalyst, RhB 30 mg·L−1 | 180 | 97.81 | [63] |
| Iodine-doped TiO2 nanoparticles | visible light, 100 mg catalyst, RhB 50 mg·L−1 | 140 | 82.36 | [64] |
| Sn/SnO2 | visible light, 50 mg catalyst, RhB 10 mg·L−1 | 300 | 90 | [65] |
| Exhausted Cu1Ce0.2O | visible light, 30 mg catalyst, RhB 50 mg·L−1 | 180 | 80 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, H.; Li, K.; Li, B.; Wang, C.; Li, K.; Ning, P. Sustainable PH3 Purification over MOF-Derived Ce-Doped CuO Materials: Enhanced Performance and Closed-Loop Resource Recovery. Sustainability 2025, 17, 4084. https://doi.org/10.3390/su17094084
Yi H, Li K, Li B, Wang C, Li K, Ning P. Sustainable PH3 Purification over MOF-Derived Ce-Doped CuO Materials: Enhanced Performance and Closed-Loop Resource Recovery. Sustainability. 2025; 17(9):4084. https://doi.org/10.3390/su17094084
Chicago/Turabian StyleYi, Haoyang, Kai Li, Bo Li, Chi Wang, Kunlin Li, and Ping Ning. 2025. "Sustainable PH3 Purification over MOF-Derived Ce-Doped CuO Materials: Enhanced Performance and Closed-Loop Resource Recovery" Sustainability 17, no. 9: 4084. https://doi.org/10.3390/su17094084
APA StyleYi, H., Li, K., Li, B., Wang, C., Li, K., & Ning, P. (2025). Sustainable PH3 Purification over MOF-Derived Ce-Doped CuO Materials: Enhanced Performance and Closed-Loop Resource Recovery. Sustainability, 17(9), 4084. https://doi.org/10.3390/su17094084





