Urban, Architectural, and Socioeconomic Factors Contributing to the Concentration of Potential Arbovirus Vectors and Arbovirosis in Urban Environments from a One Health Perspective: A Systematic Review
Abstract
1. Introduction
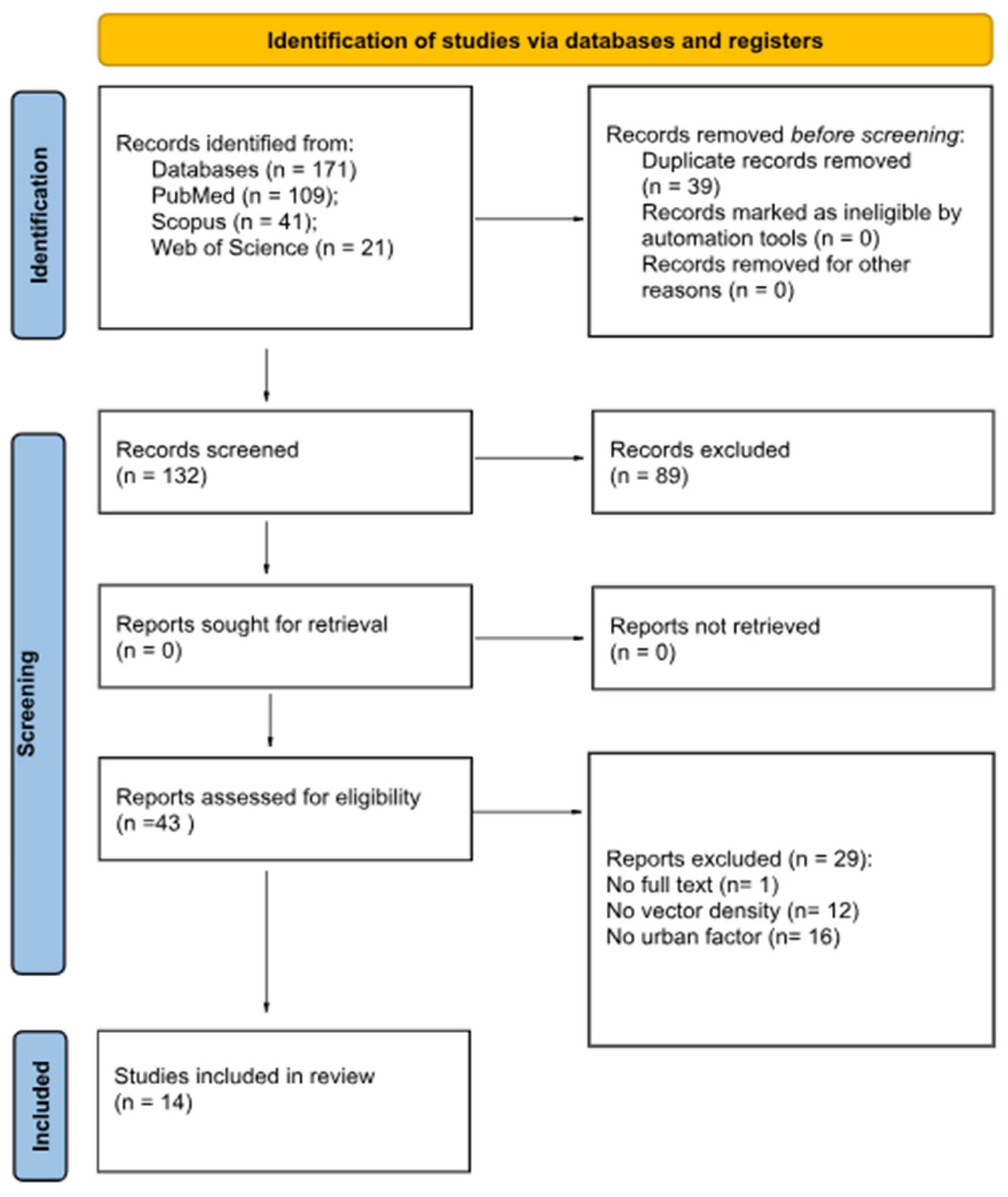
2. Materials and Methods
2.1. Selection Protocol and Search Strategy
2.2. Inclusion and Exclusion Criteria for the Study
2.3. Data Extraction and Quality Assessment
3. Results
- ○
- Socio-demographic factors: 11 studies;
- ○
- Urban–architectural factors: 13 studies;
- ○
- Natural factors: 12 studies (including temperature and humidity).
| Authors Year Country | Socioeconomic and Demographic Factors | Urban and Architectural Factors | Natural Factors | Carrier Types/Species | Type of Measurement (Direct, Estimated, Indirect) | Quality |
|---|---|---|---|---|---|---|
| Liu et al. 2013 China [28] | Low socioeconomic level and high population density associated with higher carrier density | Leakage from pipes and wetlands associated with higher presence; tree-lined courtyards, moderate presence; absence of greenery but with drainage systems very low presence; affluent residential area but with private courtyards and proximity to large park, high vector density | Vegetation and lake with fish associated with lower occurrence; trees and irrigated areas associated with higher occurrence | Culex pipiens complex | Direct measurement (bed net trap; light trap; labour hour method) | Good |
| Wu et al. 2020 China [29] | Low socioeconomic status and overcrowding, high carrier density | Heavily urbanised areas, lower density; sparsely urbanised areas with haphazard urbanisation, overcrowding, and density with low socioeconomic level, very high density | Proximity to waterways but with low socioeconomic level, very high vector density; vegetated areas, intermediate vector density; unused areas (unfinished construction sites, wasteland, or vacant lots), urban—assimilated to urban voids—and suburban lowest density of all | Aedes albopictus | Direct measurement: Breteau Index (BI); Standard Space Index (SSI); Adult Density Index (ADI) | Good |
| Romeo-Aznar et al. 2018 India [41] | Low socioeconomic level and high population density with overcrowding, high carrier density | High building density in deprived areas (and thus poor quality), sheet metal, use of plastic roofing, lack of planning and drainage systems, functional mix (although it is correlated with poor control, open water containers), heat islands, and shaded areas (narrow alleys or lots of vegetation), high vector density | Not described | Aedes aegypti | Indirect estimation (from dengue incidence) | Good |
| Santos J.P.C. et al. 2020 Brazil [30] | Net demographic density | Percentage of occupied area; percentage of residential area; percentage of area of substandard clusters; percentage of area with strategic points; mean verticalisation; number of neighbouring districts; border perimeter with neighbouring districts; percentage of households with unpaved streets; percentage of households with no tree-lined streets; percentage of households with streets without manholes; percentage of households with streets with exposed trash; percentage of households with streets with open sewage | Percentage of vegetation; mean daytime surface temperature; mean night-time surface temperature; monthly cumulative rainfall | Incidence of dengue cases and Aedes Egg Density Index (EDI) | Mean number of incident cases in the study period to the mean population over the same period multiplied by 100,000 inhabitants. The Aedes Egg Density Index (EDI) is calculated for each neighbourhood from 2013 to 2014 through entomological monitoring by ovitraps. | Good |
| French M.A. et al. 2021 Australia [31] | Head of household; number of members, number living in household; name, date of birth, sex, marital status, relationship to head of household, education level, literacy; symptoms, healthcare utilisation, mental health; height and weight for children; haemoglobin; soil-transmitted helminths; individual primary activities and time use, household assets, self-assessed socioeconomic status and life satisfaction | Floor, wall, and roof materials; number of rooms; land ownership/tenure, occupation tenure; water sources, access, treatment methods, and cost; sanitation access, type, ownership, and disposal; garbage disposal; local flooding events; child environmental exposure | E. coli, nitrogen, pH, temperature, turbidity, conductivity, dissolved oxygen in water; E. Coli in soil; local agricultural (foodstock), domestic, or feral animals in animal faeces; temperature and humidity in thermal environment; mosquito species and relative abundance; small mammalian pest species and relative abundance | Culex quinquefasciatus (94⋅7%) and Aedes aegypti (4.9%). Small numbers of other species were caught, including Anopheles species, Aedes albopictus, Culex sitiens, a Uranotaenia species, and a Mansonia species | Counting and identification of mosquitoes caught in traps | Good |
| Kigozi S.P. et al. 2015 Uganda [32] | Number of participants; mean age in years during follow-up; total number of routine blood slides; parasite prevalence; person-years of observation; total episodes of malaria; incidence of malaria per person-year | Household density, and night-time light brightness | Land cover, vegetation amount | Anopheles mosquitoes | Household density of mosquitoes (number of female Anopheles mosquitoes captured) | Good |
| Wai et al. 2012 India, Myanmar, Thailand, Indonesia, Philippines, Sri Lanka [33] | Subjects aged >25 years. Middle class neighbourhoods and good to reasonable access to public services | Rainwater reservoirs, unplanned construction, low buildings in deprived areas, accumulation of materials in streets, unmaintained public or private spaces, all associated with higher vector density | Annual rainfall data | Aedes aegypti | Direct measurement: Breteau Index (BI); House Index (HI), Container Index (CI), pupae per person index (PPI) | Good |
| Kang et al. 2018 Uganda [34] | Not described | Concrete walls, brick floors, screened gutters and vents, tiled roofs, protective factors; incomplete houses, exposed brick, architectural elements that promote stagnation and vector density | Temperature, humidity, rainfall | Anopheles spp. | Direct measurement (trap) | Good |
| Ferdousi et al. 2015 Bangladesh [40] | Not described | Brick or concrete houses, brick houses and bamboo roofs, shacks, 111 different types of indoor, outdoor, or rooftop water containers | Not described | Aedes aegypti | Estimated | Good |
| Xavier et al., 2023 Brazil [35] | Not described | Mostly single-family houses with basic sanitation and mutual geographic isolation: about 2.5 km apart and separated by stretches of unbuilt environment | Possible temporal variations due to monthly weather conditions (precipitation or temperature) or insecticide spraying are considered | Aedes aegypti e Culex quinquefasciatus | The density of adult mosquitoes measured directly by active aspiration of adult mosquitoes and indices based on the presence of eggs of Aedes females in traps and the abundance of eggs in traps | Fair |
| Micanaldo E.F. et al. 2021 Philippines [36] | Low to middle, high population density, inadequate sanitary conditions | Entomological, epidemiological, and landscape data from the rainy season; lack of drainage infrastructure, which contributes to flooding deteriorating infrastructure, agriculture, water bodies, commercial, residential | Landscape and climate variables, strong monsoon rain and tropical storms. Heavy rain | Aedes aegypti and Aedes albopictus | Direct and indirect | Good |
| Gómez-Vargas W. et al. 2024 Colombia [37] | High population density, low to middle | Unregulated urbanisation, regulated urbanisation deteriorating infrastructure, agriculture, water bodies, commercial, residential, drainage system, 3 different municipalities | Landscape, monthly records of precipitation (mm), relative humidity (%), and temperature (˚C) | Aedes aegypti | Direct and indirect. Mean density of Ae. aegypti was 1.47 females/dwelling and 0.51 females/inhabitant, Prokopack aspirators | Good |
| Telle O. et al. 2021 India [38] | Low socioeconomic level and high population density associated with higher carrier density | Taller buildings (in affluent neighbourhoods) are associated with lower larval presence, building quality is associated with lower larval presence (less accumulation on sheet metal and uncovered tanks), higher maintenance, access to potable water | Absence of manicured vegetation in deprived areas creates favourable microclimate for mosquitoes | Aedes aegypti | House Index, Container Index, Breteau Index (BI) | Good |
| Fuentes-Vallejo M et al. 2015 Colombia [39] | High housing density in deprived areas increased vector density and lower land care and poor building quality | Favourable elements are unplanned urbanisation (coincides with proximity to waterways), low and close buildings (favourable microclimate), irregularly shaped yards (water stagnation), unmaintained common areas, lack of drainage | Proximity to waterways is associated with poor urban quality and poor drainage but also with alluvial features that promote water accumulation | Aedes aegypti | Pupae per person index (PPI), Breteau Index (BI) | Good |
4. Discussion
5. Limitations and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- United Nations. World Urbanization Prospects: The 2014 Revision, Highlights (ST/ESA/SER.A/352); United Nations: New York, NY, USA, 2014. [Google Scholar]
- Lenzi, A. Why urbanisation and health? Acta Biomed. 2019, 90, 181–183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harpham, T. Urban health in developing countries:what do we know and where do we go? Health Place 2009, 15, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Kolimenakis, A.; Heinz, S.; Wilson, M.L.; Winkler, V.; Yakob, L.; Michaelakis, A.; Papachristos, D.; Richardson, C.; Horstick, O. The role of urbanisation in the spread of Aedes mosquitoes and the diseases they transmit—A systematic review. PLOS Negl. Trop. Dis. 2021, 15, e0009631. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Tu, W.; Chiu, M.; Kuo, M.; Cheng, H.; Chan, C.; Dai, S. Joint influence of architectural and spatiotemporal factors on the presence of Aedes aegypti in urban environments. Pest Manag. Sci. 2023, 79, 4367–4375. [Google Scholar] [CrossRef]
- World Health Organization (WHO); UNICEF; UNDP; World Bank. Special Programme for Research and Training in Tropical Diseases; Global Vector Control Response 2017–2030; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Bellini, R.; Michaelakis, A.; Petrić, D.; Schaffner, F.; Alten, B.; Angelini, P.; Aranda, C.; Becker, N.; Carrieri, M.; Di Luca, M.; et al. Practical management plan for invasive mosquito species in Europe: I. Asian tiger mosquito (Aedes albopictus). Travel Med. Infect. Dis. 2020, 35, 101691. [Google Scholar] [CrossRef] [PubMed]
- Soto, S.M. Human migration and infectious diseases. Clin. Microbiol. Infect. 2009, 15 (Suppl. 1), 26–28. [Google Scholar] [CrossRef] [PubMed]
- Vora, N. Impact of anthropogenic environmental alterations on vector-borne diseases. Medscape J. Med. 2008, 10, 238. [Google Scholar] [PubMed]
- Whiteman, A.; Loaiza, J.R.; Yee, D.A.; Poh, K.C.; Watkins, A.S.; Lucas, K.J.; Rapp, T.J.; Kline, L.; Ahmed, A.; Chen, S.; et al. Do socioeconomic factors drive Aedes mosquito vectors and their arboviral diseases? A systematic review of dengue, chikungunya, yellow fever, and Zika Virus. One Health 2020, 11, 100188. [Google Scholar] [CrossRef]
- Little, E.; Biehler, D.; Leisnham, P.T.; Jordan, R.; Wilson, S.; LaDeau, S.L. Socio-Ecological Mechanisms Supporting High Densities of Aedes albopictus (Diptera: Culicidae) in Baltimore, MD. J. Med. Entomol. 2017, 54, 1183–1192. [Google Scholar] [CrossRef]
- Yitbarek, S.; Chen, K.; Celestin, M.; McCary, M. Urban mosquito distributions are modulated by socioeconomic status and environmental traits in the USA. Ecol. Appl. A Publ. Ecol. Soc. America 2023, 33, e2869. [Google Scholar] [CrossRef]
- Jones, R.; Kulkarni, M.A.; Davidson, T.M.; Radam-Lac Research Team; Talbot, B. Arbovirus vectors of epidemiological concern in the Americas: A scoping review of entomological studies on Zika, dengue and chikungunya virus vectors. PLoS ONE 2020, 15, e0220753. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.V.; Hintz, C.W.; Jones, L.; Shiau, J.; Solano, N.; Drake, J.M.; Murdock, C.C. Microclimate and Larval Habitat Density Predict Adult Aedes albopictus Abundance in Urban Areas. Am. J. Trop. Med. Hyg. 2019, 101, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global change and the ecology of cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Romanello, M.; Di Napoli, C.; Drummond, P.; Green, C.; Kennard, H.; Lampard, P.; Scamman, D.; Arnell, N.; Ayeb-Karlsson, S.; Ford, L.B.; et al. The 2022 report of the Lancet Countdown on health and climate change: Health at the mercy of fossil fuels. Lancet 2022, 400, 1619–1654, Erratum in Lancet 2022, 400, 1680. Erratum in Lancet 2022, 400, 1766. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zardini, A.; Menegale, F.; Gobbi, A.; Manica, M.; Guzzetta, G.; D’Andrea, V.; Marziano, V.; Trentini, F.; Montarsi, F.; Caputo, B.; et al. Estimating the potential risk of transmission of arboviruses in the Americas and Europe: A modelling study. Lancet Planet. Health 2024, 8, e30–e40. [Google Scholar] [CrossRef]
- Caputo, A.; Garavelli, P.L. Climate, environment and transmission of malaria. Infez. Med. 2016, 24, 93–104. [Google Scholar] [PubMed]
- Leung, X.Y.; Islam, R.M.; Adhami, M.; Ilic, D.; McDonald, L.; Palawaththa, S.; Diug, B.; Munshi, S.U.; Karim, N. A systematic review of dengue outbreak prediction models: Current scenario and future directions. PLOS Negl. Trop. Dis. 2023, 17, e0010631. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Lillepold, K.; Semenza, J.C.; Tozan, Y.; Quam, M.B.; Rocklöv, J. Reviewing estimates of the basic reproduction number for dengue, Zika and chikungunya across global climate zones. Environ. Res. 2020, 182, 109114. [Google Scholar] [CrossRef]
- Marinho, R.d.S.S.; Duro, R.L.S.; Mota, M.T.d.O.; Hunter, J.; Diaz, R.S.; Kawakubo, F.S.; Komninakis, S.V. Environmental Changes and the Impact on the Human Infections by Dengue, Chikungunya and Zika Viruses in Northern Brazil, 2010–2019. Int. J. Environ. Res. Public Health 2022, 19, 12665. [Google Scholar] [CrossRef]
- Osman, S.; Preet, R. Dengue, chikungunya and Zika in GeoSentinel surveillance of international travellers: A literature review from 1995 to 2020. J. Travel Med. 2020, 27, taaa222. [Google Scholar] [CrossRef]
- Paull, S.H.; Song, S.; McClure, K.M.; Sackett, L.C.; Kilpatrick, A.M.; Johnson, P.T. From super-spreaders to disease hotspots: Linking transmission acrosshosts and space. Front. Ecol. Environ. 2012, 10, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, M.A.; Prosper, O.; Lopiano, K.; Ruktanonchai, N.; Caughlin, T.T.; Martcheva, M.; Osenberg, C.W.; Smith, D.L. Spatial heterogeneity, host movement and mosquito-borne disease transmission. PLoS ONE 2015, 10, e0127552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vazquez-Prokopec, G.M.; Morrison, A.C.; Paz-Soldan, V.; Stoddard, S.T.; Koval, W.; Waller, L.A.; Perkins, T.A.; Lloyd, A.L.; Astete, H.; Elder, J.; et al. Inapparent infections shape the transmission heterogeneity of dengue. PNAS Nexus 2023, 2, pgad024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Palmieri, V.; Colamesta, V.; La Torre, G. Evaluation of methodological quality of studies. Senses Sci. 2016, 3, 235–241. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, X.; Cirendunzhu; Woodward, A.; Pengcuociren; Bai, L.; Baimaciwang; Sang, S.; Dazhen; Wan, F.; et al. Mosquitoes established in Lhasa city, Tibet, China. Parasites Vectors 2013, 6, 224. [Google Scholar] [CrossRef]
- Wu, S.; Ren, H.; Chen, W.; Li, T. Neglected urban villages in current vector surveillance system: Evidences in Guangzhou, China. Int. J. Environ. Res. Public Health 2020, 17, 2. [Google Scholar] [CrossRef]
- Santos, J.P.C.; Honório, N.A.; Barcellos, C.; Nobre, A.A. A perspective on inhabited urban space: Land use and occupation, heat islands, and precarious urbanization as determinants of territorial receptivity to dengue in the city of Rio de Janeiro. Int. J. Environ. Res. Public Health 2020, 17, 6537. [Google Scholar] [CrossRef]
- French, M.A.; Barker, S.F.; Taruc, R.R.; Ansariadi, A.; Duffy, G.A.; Saifuddaolah, M.; Agussalim, A.Z.; Awaluddin, F.; Zainal, Z.; Wardani, J.; et al. A planetary health model for reducing exposure to faecal contamination in urban informal settlements: Baseline findings from Makassar, Indonesia. Environ. Int. 2021, 155, 106679. [Google Scholar] [CrossRef]
- Kigozi, S.P.; Pindolia, D.K.; Smith, D.L.; Arinaitwe, E.; Katureebe, A.; Kilama, M.; Nankabirwa, J.; Lindsay, S.W.; Staedke, S.G.; Dorsey, G.; et al. Associations between urbaniity and malaria at local scales in Uganda. Malar. J. 2015, 14, 374. [Google Scholar] [CrossRef]
- Wai, K.T.; Arunachalam, N.; Tana, S.; Espino, F.; Kittayapong, P.; Abeyewickreme, W.; Hapangama, D.; Tyagi, B.K.; Htun, P.T.; Koyadun, S.; et al. Estimating dengue vector abundance in the wet and dry season: Implications for targeted vector control in urban and peri-urban Asia. Ann. Trop. Med. Parasitol. 2012, 106, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Battle, K.E.; Gibson, H.S.; Cooper, L.V.; Maxwell, K.; Kamya, M.; Lindsay, S.W.; Dorsey, G.; Greenhouse, B.; Rodriguez-Barraquer, I.; et al. Heterogeneous exposure and hotspots for malaria vectors at three study sites in Uganda. Gates Open Res. 2018, 2, 32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xavier, A.; Bonfim, C.; Júnior, W.B.; Bezerra, G.; Oliveira, C.; Uchikawa, R.; da Silva, F.; Aguiar-Santos, A.; Medeiros, Z. Influence of social and environmental factors for Culex quinquefasciatus distribution in Northeastern Brazil: A risk index. Int. J. Environ. Health Res. 2022, 33, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Francisco, M.E.; Carvajal, T.M.; Ryo, M.; Nukazawa, K.; Amalin, D.M.; Watanabe, K. Dengue disease dynamics are modulated by the combined influences of precipitation and landscape: A machine learning approach. Sci. Total. Environ. 2021, 792, 148406. [Google Scholar] [CrossRef]
- Gómez-Vargas, W.; Ríos-Tapias, P.A.; Marin-Velásquez, K.; Giraldo-Gallo, E.; Segura-Cardona, A.; Arboleda, M. Density of Aedes aegypti and dengue virus transmission risk in two municipalities of Northwestern Antioquia, Colombia. PLoS ONE 2024, 19, e0295317. [Google Scholar] [CrossRef]
- Telle, O.; Nikolay, B.; Kumar, V.; Benkimoun, S.; Pal, R.; Nagpal, B.; Paul, R.E. Social and environmental risk factors for dengue in Delhi city: A retrospective study. PLOS Negl. Trop. Dis. 2021, 15, e0009024. [Google Scholar] [CrossRef]
- Fuentes-Vallejo, M.; Higuera-Mendieta, D.R.; García-Betancourt, T.; Alcalá-Espinosa, L.A.; García-Sánchez, D.; Munévar-Cagigas, D.A.; Brochero, H.L.; González-Uribe, C.; Quintero, J. Territorial analysis of Aedes aegypti distribution in two Colombian cities: A chorematic and ecosystem approach. Cad. Saúde Pública 2015, 31, 517–530. [Google Scholar] [CrossRef]
- Ferdousi, F.; Yoshimatsu, S.; Ma, E.; Sohel, N.; Wagatsuma, Y. Identification of essential containers for Aedes larval breeding to control dengue in Dhaka, Bangladesh. Trop. Med. Health 2015, 43, 253–264. [Google Scholar] [CrossRef]
- Romeo-Aznar, V.; Paul, R.; Telle, O.; Pascual, M. Mosquito-borne transmission in urban landscapes: The missing link between vector abundance and human density. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180826. [Google Scholar] [CrossRef]
- Bowman, L.R.; Runge-Ranzinger, S.; McCall, P.J. Assessing the relationship between vector indices and dengue transmission: A systematic review of the evidence. PLOS Negl. Trop. Dis. 2014, 8, e2848. [Google Scholar] [CrossRef]
- Kamalipour, H. Informal urban design: Forms of informal settlement. In Research Handbook on Urban Design; Edward Elgar Publishing: Cheltenham, UK, 2024. [Google Scholar] [CrossRef]
- Braveman, P.; Gottlieb, L. The Social Determinants of Health: It’s Time to Consider the Causes of the Causes. Public Health Rep. 2014, 129 (Suppl. 2), 19–31. [Google Scholar] [CrossRef] [PubMed]
- Sanya, T.; Lewis, C.A.; Mogola, I. Guidelines for Water-Sensitive Informal Settlement Upgrading in the Global South. In The Palgrave Encyclopedia of Urban and Regional Futures; Brears, R.C., Ed.; Palgrave Macmillan: Cham, Germany, 2022. [Google Scholar] [CrossRef]
- Wang, J.; Kuffer, M.; Sliuzas, R.; Kohli, D. The exposure of slums to high temperature: Morphology-based local scale thermal patterns. Sci. Total Environ. 2019, 650, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Abdulai, A.; Owusu-Asenso, C.M.; Haizel, C.; Mensah, S.K.E.; Sraku, I.K.; Halou, D.; Doe, R.T.; Mohammed, A.R.; Akuamoah-Boateng, Y.; Forson, A.O.; et al. The Role of Car Tyres in the Ecology of Aedes aegypti Mosquitoes in Ghana. Curr. Res. Parasitol. Vector-Borne Dis. 2024, 5, 100176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gibb, R.; Colón-González, F.J.; Lan, P.T.; Huong, P.T.; Nam, V.S.; Duoc, V.T.; Hung, D.T.; Dong, N.T.; Chien, V.C.; Trang, L.T.T.; et al. Interactions between climate change, urban infrastructure and mobility are driving dengue emergence in Vietnam. Nat. Commun. 2023, 14, 8179. [Google Scholar] [CrossRef]
- Power, G.M.; Vaughan, A.M.; Qiao, L.; Clemente, N.S.; Pescarini, J.M.; Paixão, E.S.; Lobkowicz, L.; Raja, A.I.; Souza, A.P.; Barreto, M.L.; et al. Socioeconomic risk markers of arthropod-borne virus (arbovirus) infections: A systematic literature review and meta-analysis. BMJ Glob. Health 2022, 7, e007735. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kagaba Amina, G.; Kampala International University IX. Socio-Economic Determinants and Malaria Risk: Assessing the Impact of Poverty, Housing Conditions, and Healthcare Accessibility in High-Incidence Regions. Newport Int. J. Res. Med. Sci. 2024, 5, 120–124. [Google Scholar]
- Tsiotas, D.; Dialesiotis, S.; Christopoulou, O. Examining the relationship between regional economic resilience and epidemiologic spread of COVID-19: Evidence from Greece. Environ. Dev. Sustain. 2023, 27, 8433–8469. [Google Scholar] [CrossRef]
- Herath, S.; Mansour, A.; Bentley, R. Urban density, household overcrowding and the spread of COVID-19 in Australian cities. Health Place 2024, 89, 103298. [Google Scholar] [CrossRef]
- Alrasheed, H.; Alballa, N.; Al-Turaiki, I.; Almutlaq, F.; Alabduljabbar, R. City Transmission Networks: Unraveling Disease Spread Dynamics. ISPRS Int. J. Geo-Inf. 2024, 13, 283. [Google Scholar] [CrossRef]
- Román-Pérez, S.; Aguirre-Gómez, R.; Hernández-Ávila, J.E.; Íñiguez-Rojas, L.B.; Santos-Luna, R.; Correa-Morales, F. Identification of Risk Areas of Dengue Transmission in Culiacan, Mexico. ISPRS Int. J. Geo-Inf. 2023, 12, 221. [Google Scholar] [CrossRef]
- Chaves, L.S.M.; Bergo, E.S.; Conn, J.E.; Laporta, G.Z.; Prist, P.R.; Sallum, M.A.M. Anthropogenic landscape decreases mosquito biodiversity and drives malaria vector proliferation in the Amazon rainforest. PLoS ONE 2021, 16, e0245087. [Google Scholar] [CrossRef] [PubMed]
- Perrin, A.; Glaizot, O.; Christe, P. Worldwide impacts of landscape anthropization on mosquito abundance and diversity: A meta-analysis. Glob. Change Biol. 2022, 28, 6857–6871. [Google Scholar] [CrossRef] [PubMed]
- Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sdgs.un.org/2030agenda (accessed on 21 February 2025).
- World Health Organization; UNICEF. “Global Vector Control Response 2017–2030.” Global Vector Control Response 2017–2030. 2017. Available online: https://www.who.int/publications/i/item/9789241512978 (accessed on 21 February 2025).
- Di Napoli, C.; McGushin, A.; Romanello, M.; Ayeb-Karlsson, S.; Cai, W.; Chambers, J.; Dasgupta, S.; Escobar, L.E.; Kelman, I.; Kjellstrom, T.; et al. Tracking the impacts of climate change on human health via indicators: Lessons from the Lancet Countdown. BMC Public Health 2022, 22, 663. [Google Scholar] [CrossRef]
- Programme for the Environment and Climate Action (LIFE). Available online: https://commission.europa.eu/funding-tenders/find-funding/eu-funding-programmes/programme-environment-and-climate-action-life_en (accessed on 21 February 2025).
- European Environment Agency. European Climate Risk Assessment; Publications Office of the European Union: Luxembourg, 2024. [Google Scholar] [CrossRef]
- Capolongo, S.; Rebecchi, A.; Dettori, M.; Appolloni, L.; Azara, A.; Buffoli, M.; Capasso, L.; Casuccio, A.; Conti, G.O.; D’amico, A.; et al. Healthy Design and Urban Planning Strategies, Actions, and Policy to Achieve Salutogenic Cities. Int. J. Environ. Res. Public Health 2018, 15, 2698. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cofone, L.; Sabato, M.; Di Paolo, C.; Di Giovanni, S.; Donato, M.A.; Paglione, L. Urban, Architectural, and Socioeconomic Factors Contributing to the Concentration of Potential Arbovirus Vectors and Arbovirosis in Urban Environments from a One Health Perspective: A Systematic Review. Sustainability 2025, 17, 4077. https://doi.org/10.3390/su17094077
Cofone L, Sabato M, Di Paolo C, Di Giovanni S, Donato MA, Paglione L. Urban, Architectural, and Socioeconomic Factors Contributing to the Concentration of Potential Arbovirus Vectors and Arbovirosis in Urban Environments from a One Health Perspective: A Systematic Review. Sustainability. 2025; 17(9):4077. https://doi.org/10.3390/su17094077
Chicago/Turabian StyleCofone, Luigi, Marise Sabato, Carolina Di Paolo, Stefano Di Giovanni, Maria Assunta Donato, and Lorenzo Paglione. 2025. "Urban, Architectural, and Socioeconomic Factors Contributing to the Concentration of Potential Arbovirus Vectors and Arbovirosis in Urban Environments from a One Health Perspective: A Systematic Review" Sustainability 17, no. 9: 4077. https://doi.org/10.3390/su17094077
APA StyleCofone, L., Sabato, M., Di Paolo, C., Di Giovanni, S., Donato, M. A., & Paglione, L. (2025). Urban, Architectural, and Socioeconomic Factors Contributing to the Concentration of Potential Arbovirus Vectors and Arbovirosis in Urban Environments from a One Health Perspective: A Systematic Review. Sustainability, 17(9), 4077. https://doi.org/10.3390/su17094077






